Abstract
Objective
We have identified a novel protein in bone marrow (BM)-derived insulin-producing cells (IPCs). Here we characterize this protein, hereby named Islet Homeostasis Protein (IHoP), in the pancreatic islet.
Methods
Detection of IHoP mRNA and protein were performed using RT-PCR, immunocytochemistry and in-situ hybridization. IHoP functions were utilizing proliferation, insulin secretion by in vitro assays, and as well as following siRNA protocols for suppression of IHoP.
Results
We found that IHoP did not homologue with known pancreatic hormones. IHoP expression was seen in both BM-derived IPCs and isolated pancreatic islets. Immunohistochemistry on pancreatic islet revealed that IHoP localized to the glucagon synthesizing α (alpha)-cells. Inhibition of IHoP by siRNA resulted in the loss of glucagon expression, which induced low blood glucose levels (63–85 mg/dL). Subsequently, cellular apoptosis was observed throughout the islet, including the insulin-producing β (beta)-cells. Islets of pre-onset diabetic patients showed normal expression of IHoP and glucagon; however IHoP was lost upon onset of the disease.
Conclusion
These data suggest that IHoP could be a new functional protein in the islet, and may play a role in islet homeostasis.
Keywords: IHoP (islet homeostasis protein), insulin, glucagon, apoptosis, Type-1 diabetes
Introduction
The pancreatic islets of Langerhans are composed of clusters of four cell types that synthesize various peptide hormones, including glucagon (α-cells), insulin (β-cells), somatostatin (δ-cells) and pancreatic polypeptide (PP-cells).1 These different cell types are in close proximity to one another and primarily produce hormones to be circulated in blood (effects of endocrine) and secretion hormones of each cell type exert actions on adjacent cells within the islet (effects of paracrine).2,3 These hormones release regulated nutrient control for management of tissue metabolism and the blood levels of glucose, fatty acids, triglycerides and amino acids. The maintenance of blood glucose levels requires production and secretion of both insulin and glucagon, which are closely regulated during glucose tolerance; these two hormones work in concert and stimulate glycogenolysis and gluconeogenesis in the presence or absence of nutrient intake.4 The function of glucagon has been opposed by the action of insulin in peripheral tissues, predominantly the liver. It also regulates both islet α-cell proliferation and survival.5 Glucagon release is normally stimulated as blood glucose concentrations fall, a response that is progressively diminished in type-1 diabetes.6,7 Pathologically, insulin deficient islets still contain a normal complement of glucagon-secreting α-cells.8 The glucagon may be the key counter regulatory hormone responsible for opposing the glucose-lowering effect of insulin, and may represent a therapeutic-target for the treatment of type-1 diabetes.9
The increasing incidence of type-1 diabetes throughout the world has generated considerable interest in developing both better diagnostic techniques and treatments that would restore glucose responsiveness and insulin secretion, as well as methods for prevention of development of diabetic mellitus by immune suppression. Several researchers have found approaches to the prevention and treatment of diabetes by using immunosuppressive and immunomodulatory agents such as insulin,10 GAD65,11 DiaPeP227,12 anti-CD3,13 mycophenolate mofetil,14 daclizumab15 and anti-CD20.16 Cell therapy using stem cells and their progeny is a promising new approach that may be capable of addressing many unmet medical needs.17 The transplantation of donor islet has been a key of treatment of type-1 diabetes mellitus, however after transplanted some patients still need to insulin injection.18 Various endeavors, including transplantation of in vitro-differentiated islet-like cells,19 transplantation of stem cells-derived insulin producing cells20,21 and combination of stem cells therapy with a pharmacological approach22 have been tested. These studies suggest that with the progression of stem cell research, new methods for the treatment of diseases such as diabetes mellitus may be possible. However the pathogenesis of type-1 diabetes as well as the mechanisms by which the above agents act is still unclear. Further study will be required to develop new approaches for the diagnosis and prevention of type-1 diabetes.
In this study, we recently identified an unknown functional protein in BM-derived IPCs and demonstrated its function. This protein was previously termed, unnamed protein product,23 but we now refer to it as Islet Homeostasis Protein, or IHoP. BM-derived IPCs and isolated pancreatic islets expressed the IHoP gene, and it co-localized within the glucagon synthesizing α-cells of the islets. We provide knockdown expression of IHoP by siRNA data indicating that IHoP’s role of glucagon synthesis in the α-cells, leads to control of insulin synthesis by β-cell. Finally, we show that IHoP positive cells were increased in the pancreatic islet of NOD mice, and similar data was obtained in pre-onset diabetic patients. However, the post-onset-type-1 diabetic islets were negative for IHoP expression. These data suggest that IHoP may work to regulate islet homeostasis by (directly or indirectly) regulating expression of other pancreatic molecules such as glucagon.
Materials and Methods
Detection of IHoP Protein and mRNA
All procedures involving animals were conducted according to institutionally approved protocols and guidelines. The detection of IHoP protein in BM-derived IPCs was accomplished as previously reported.22 Total RNA was isolated from the un-differentiated BM cells, BM-derived IPCs and isolated rat normal pancreatic islets24 using RNA-Bee (Tel-Test, Inc. Friendswood, TX). 2 μg RNA was used for cDNA synthesis via reverse transcription. Also, confirm to DNA contamination in RNA, PCR samples were run without reverse transcribed-RNA using IHoP primers (RNA-PCR). The IHoP primers used were 5′-aag ttg aac ctg gcc tcc att-3′ (sense strand) and 5′-ctt caa ggt cgt att cac cca-3′ (anti-sense strand), which delineated a 510-bp product. PCR products (30 cycles) were directly sequenced using an AmpliTaq cycle sequencing kit (Perkin-Elmer Setus, Branchburg, NJ) for genetic confirmation.
IHoP-siRNA Transduction into in vivo
The RNA interference (RNAi) has emerged as a powerful technology for studying gene functions in eukaryotes. RNAi is a post-transcriptional process triggered by the introduction of small interfering RNA (siRNA) which leads to gene silencing in a sequence-specific manner. 25–27 The design of IHoP-siRNA primers and scrambled-siRNA control were performed using a siRNA targeting program (Genescript, Piscataway, NJ). IHoP-siRNA was amplified using psiRNA-hH1GFPzeo G2 kit (Invivogen, San Diego, CA, USA). In vivo transduction of IHoP-siRNA or scrambled-siRNA (50 μg per animal) was performed per manufacturer’s instructions using the in vivo jetPEi protocol (QBiogene, Irvine, CA). Fisher F344 female rats (age 8–10 weeks, 150–200 g) were purchased from Charles River Laboratories (Wilmington, MA) and maintained on standard laboratory chow and daily cycles of alternating 12 h light and dark. The rats were divided into 3 groups (n=3) one group was a non-treated control, the second group was injected with scramble-siRNA, and the final group was injected with IHoP-siRNA, all by tail vein injection.
Western Blot Analysis and Enzyme-Linked Immunosorbent Assay (ELISA)
For testing possible cross-reactions of the IHoP antibody we prepared 1 μg each of glucagon, insulin and IHoP peptides, which were loaded and transferred onto a nylon membrane. The detection of IHoP on the membrane was followed by Western blotting, as detailed by Oh et al.21 To determine insulin secretion, the cultured conditioned media were saved from INS-1 cells following high glucose challenge with glucagon or IHoP. Secretion of insulin into cultured media was detected by ELISA. ELISA was performed on the conditioned media to determine insulin secretion using the Rat insulin ELISA kit, and following the manufacturer’s instructions (Crystal Chem Inc., Chicago, IL).
Cell Proliferation Assay
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma, St. Louis, MO] assay was performed as described previously by Mosmann.28 Briefly, INS-1 cells were inoculated in a 96-well plate (1 × 104 cells/well) and grown in INS-1 cell medium (Rosewell Park Memorial Institute-1640; Sigma) with 10% fetal bovine serum (FBS).22 After 24 hours, the medium was replaced with 10% FBS supplemented INS-1 culture medium (positive control), serum-free INS-1 culture medium containing 0.5% bovine serum albumin (BSA; negative control), or 0.5% BSA medium with glucagon (1 μM) or IHoP (1 μM). The cells were cultured with glucagon and IHoP for 24, 48 and 72 hours, and then analyzed by spectrophotometry.
In situ Hybridization with Digoxigenin Labeled DNA Probes
Isolated rat pancreatic islets were attached to slides glass and fixed for 15 min in 4% paraformaldehyde. The IHoP digoxigenin-labeled DNA probe (Roche, Indianapolis, IN) was then denatured at 80°C for 5 min and applied to sections at 52°C. The hybridization procedure was continued as previously described.21 Color development was performed at room temperature in buffer (Tris 100 mM, NaCl 100 mM and MgCl2 50 mM, pH 9.5) containing NBT and BCIP (Roche). Following signal development, slides were counterstained with nuclear fast red (Vector Laboratories, Burlingame, CA) and mounted in Cytoseal XYL (Richard-Allan Sci. Kalamazoo, MI).
Immunocytochemistry
Immunostaining on the rat normal pancreas, IHoP-siRNA transduced rat pancreas, NOD/wild type mice and human pre- and post-onset type-1 diabetic pancreas tissues was performed following previously described methods.21,22 The following antibodies were used in this procedure: rabbit anti-insulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), goat anti-glucagon (Santa Cruz), rabbit anti-glucagon (Dako, Carpinteria, CA), goat anti-C-peptide (Linco Research Inc., St. Charles, MO), anti-pancreatic polypeptide (Dako), goat anti-somatostatin (Santa Cruz) and rabbit anti-IHoP (prepared by GenScript Corp. Piscataway, NJ). Alexa Fluor 488 or 568 donkey anti-rabbit and Alexa Flour 488 or 568 donkey anti-goat IgG, antigoat (1:500, Invitrogen) were used as secondary antibodies, respectively.
Briefly, the slides were blocked with peroxidase and avidin/biotin (Vector Lab. Burlingame, CA), after which they were incubated with primary antibody for 1 hour, followed by secondary antibody for 30 minutes. Detection was performed using Vector ABC kit (Vector Lab.) and 3,3′-diaminobenzidine tetrahydrochloreide (DAB) reagent (Dako). The test of apoptosis was performed using ApopTag Plus fluorescein in situ apoptosis detection kit (Chemicon, Temecula, CA). DAPI (Vector Lab.).
Statistical Analysis
All data shown represent one of at least three experiments with similar results. Values are expressed as the mean ± standard deviation (S.D.). Statistical differences were determined by Student’s t-test. Values at P<0.05 were considered to be statistically significant.
Results
Detection of IHoP in insulin producing cells and pancreatic islets
We have previously reported the detection of Rattus Norvegicus transketolase and Mus Musculus unnamed protein product23 (gi 26326929; renamed Islet Homeostasis protein; IHoP) in BM-derived IPCs by protein sequence analysis (a protein of approximately 60 kDa).22 When IHoP was detected in the BM-derived IPCs, we focused on determining the function of IHoP within the pancreatic islets.
Confirmation of IHoP expression in undifferentiated BM cells, BM-derived IPCs and isolated rat normal pancreatic islets was accomplished by comparing IHoP gene expression via RT-PCR (Fig. 1a). Also, we tested for DNA contamination in the RNA samples by RNA-PCR, but no bands were detected (bottom panel in Fig. 1a; RNA-PCR). IHoP mRNA was found in BM-derived IPCs and isolated rat pancreatic islets; however, undifferentiated BM cells did not express the IHoP gene. Also, we examined at IHoP expression in isolated pancreatic islet utilizing in-situ hybridization. Figure 1b shows the presence of IHoP mRNA in the cytoplasm of the pancreatic islets using a DIG-labeled IHoP-oligonucleotide probe. IHoP mRNA is clearly visible in a majority of the perimeter cells bordering the islets (Fig. 1b). These results indicate that IHoP expression is limited to a subset of cells within the islet, mostly likely the α-cells, as the β-cells are devoid of IHoP expression.
Figure 1.
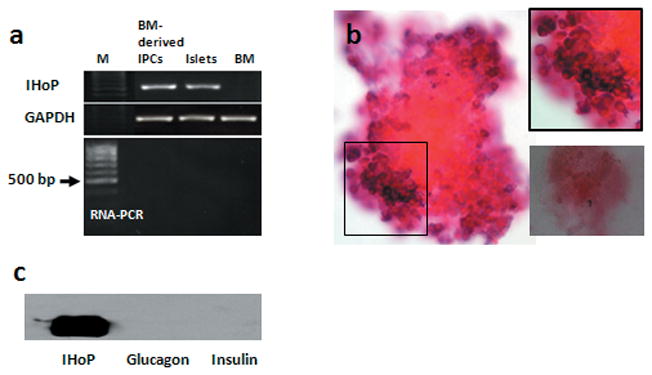
Detection of IHoP in the pancreatic islets. (a) RT-PCR analysis for expression of IHoP (510 bp) in BM-derived IPCs, isolated rat pancreatic islets (islets) and undifferentiated BM cells (BM). GAPDH (580 bp) was used as an internal control. To test for DNA contamination, RNA samples were amplified using IHoP primers without reverse transcription (RNA-PCR). M indicates 100 bp ladder. (b) In situ hybridization of IHoP mRNA in the isolated normal rat pancreatic islets. The islets are positive for IHoP mRNA expression (purple) and counter stained with nuclear fast red. Black box top right is a higher magnification of the origin. The box at the right bottom is a negative control. Original magnification of b is 200X. (c) Western blot analysis to confirm specificity of IHoP antibody for IHoP peptide or pancreatic hormones. IHoP peptide (IHoP; 1 μg), Glucagon (1 μg) and insulin (1 μg) were loaded and transferred to a nylon membrane. Signal was detected by rabbit polyclonal anti-IHoP antibody. Data shown represent one of three experiments with similar results.
In addition, we designed an antibody for the detection of IHoP which binds specifically to the c-terminus (GenScript Corp.). We first confirmed that the IHoP antibody did not recognize pancreatic hormones such as glucagon and insulin by Western blot analysis (Fig. 1c). The antibody did not recognize glucagon or insulin and only recognized the IHoP peptide (Fig. 1c). These results indicated that IHoP may be a new functional protein as yet undefined within the islets of Langerhans.
Detection of IHoP in Normal Pancreatic Islets
We also examined IHoP protein expression in pancreatic islets using double immunofluorescent and immunohistochemical staining. Normal islets express four types of hormones: glucagon (Fig. 2a), insulin (Fig. 2d), somatostatin (Fig. 2g) and pancreatic polypeptide (Fig. 2j), and IHoP was found to be expressed as well (Fig. 2b, e, h and k). We expected IHoP to co-express with insulin, but immunostaining results on the islet showed that insulin, somatostatin, and pancreatic polypeptide did not co-localize with IHoP (Fig. 2f, i and l). However IHoP (Fig. 2b) was shown to co-localize with the glucagon producing (Fig. 2a) α-cells (Fig. 2c), indicating that IHoP is a new protein expressed by the α-cells of the pancreatic islet of Langerhans.
Figure 2.
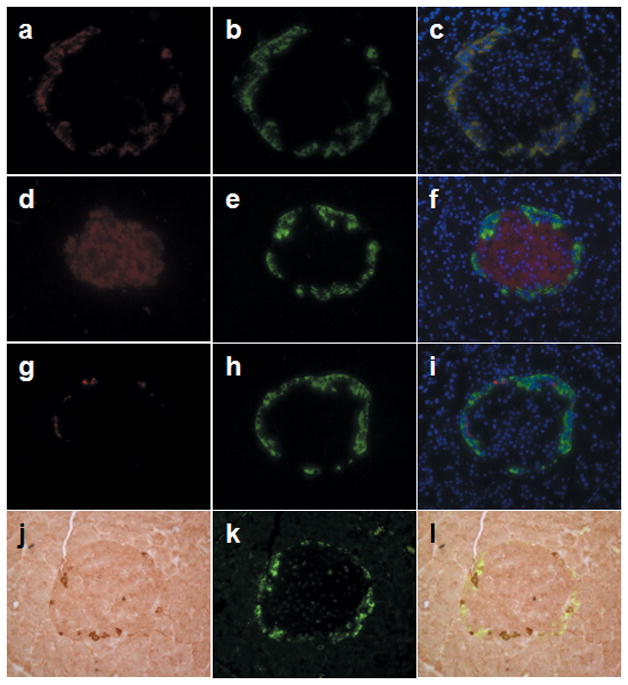
Determination of IHoP in the normal rat pancreatic islets. (a–l) Double-immunofluorescence and immunohistochemical staining for glucagon (red; a), insulin (red; d), somatostatin (red; g) and pancreatic polypeptide (brown; j) with IHoP (green; b, e, h and k) on the normal rat pancreas counter-stained with nuclear DAPI (blue). The image in (c) represents a merged image from (a) and (b), (f) is from (d) and (e), (i) is (g) and (h), (l) is (j) and (k). Yellow signal indicated co-localization of both of proteins in the same cells (c). Original magnification is x400. Data shown represent one of three experiments with similar results.
Effect of IHoP on Cell Proliferation and Insulin Synthesis from INS-1 cells
Our results indicated that IHoP was expressed by α-cells. We examined the role of IHoP in proliferation of β-cells and insulin synthesis. INS-1 cells were cultured in the presence of IHoP or glucagon. Figure 3a shows the effect of IHoP on INS-1 cell proliferation as determined by MTT assay. When cultured with 10% FBS supplemented medium, INS-1 cell proliferation was significantly activated. When cultured with IHoP in 0.5% BSA medium, INS-1 cell proliferation was significantly increased as compared to either 0.5% BSA medium alone or with glucagon alone (Fig. 3a). Furthermore, we tested the effect of glucose challenge in combination with glucagon or IHoP treatment on insulin synthesis. The glucose challenge with glucagon and IHoP treated groups demonstrated slightly enhanced secretion of insulin into the media as compared to 25 mM glucose medium alone (Fig. 3b). However the data was not significant. These results indicate that IHoP did not have a direct effect on insulin secretion; though, the peptide does appear to stimulate cellular proliferation.
Figure 3.
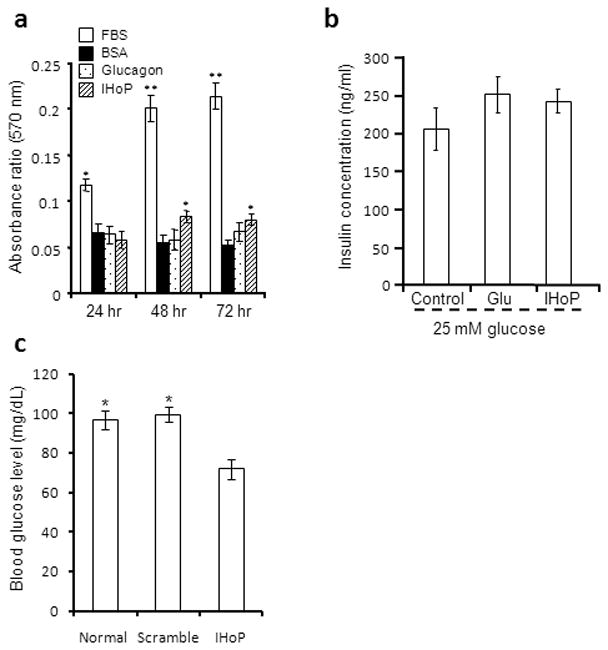
Physiological test of IHoP function in insulin-producing INS-1 cells. (a) Effect of IHoP on INS-1 cell proliferation using MTT assay. The INS-1 cells were cultured with 10% FBS supplemented INS-1 culture medium (FBS; positive control), serum free INS-1 culture medium containing 0.5% BSA (BSA; negative control), 0.5% BSA with glucagon (1 μM) or IHoP (1 μM). The MTT assay was performed on cells cultured for 24, 48 and 72 hours. The data represents the mean ± S.D. of five independent experiments. *p<0.05 and **p<0.01. (b) Determination of insulin secretion into media following treatment with glucagon and IHoP. ELISA analysis of insulin secretions measured following collection of cell culture-conditioned media. INS-1 cells cultured in high-glucose medium, with glucagon (1 μM) or IHoP (1 μM) for 2 hours. Data represent the mean ± S.D. of four independent experiments. (c) Blood glucose level following treatment with IHoP-siRNA. Rats received 50 μg of scramble-siRNA (Scramble) or IHoP-siRNA (IHoP) each, and non-treated (Normal). The data represents the mean ± S.D. of blood glucose levels. *p<0.05.
Detection of apoptosis signal in the IHoP-siRNA injected Pancreatic Islets
IHoP-siRNA was injected twice at two-week intervals, and then pancreatic tissue was harvested two weeks later. We tested blood glucose levels in the animals receiving injections before and after sacrifice (Fig. 3c). Before injection of siRNA, the blood glucose levels shown were within the normal range, around 81–107 mg/dl (97.5 ± 3.3 mg/dL). After siRNA treatments, IHoP-siRNA injected rats demonstrated significantly lower blood glucose levels 72 ± 4.9 mg/dL (63–85 mg/dL), compared to either rats receiving scramble-siRNA and control group showed no change in blood glucose levels 99.5 ± 4..66 mg/dL (82–111 mg/dL) and 96.7 ± 3.85 mg/dL (81–117 mg/dL). These data indicate that IHoP might play a role in the regulation of blood glucose levels.
In addition to insulin secretion, we determined both glucagon and IHoP expression in the IHoP-siRNA treated pancreas via immunohistochemistry. Normal pancreas expressed both glucagon (Fig. 4a) and IHoP (Fig. 4b). Treatment with scrambled-siRNA showed no effect on IHoP expression (Fig. 4c), nor any significant impact on other hormones such as insulin, glucagon, somatostatin and pancreatic polypeptide expression (data not shown). However, IHoP-siRNA treated rat pancreas showed a dramatic loss of IHoP expression (Fig. 4e), as well as loss of glucagon expression (Fig. 4d). IHoP-siRNA treatment did not affect the expression of insulin (Fig. 4f), somatostatin (Fig. 4g) or pancreatic polypepteide (Fig. 4h). We next sought to determine if the loss of both IHoP and glucagon would affect the islet. We first examined apoptosis in both siRNA and scrambled injected rats. We saw a significant amount of apoptosis occurring in the islet, mainly within the insulin producing β– cells, but all islet cell types were effected to some degree (Fig. 4i), an atypical result that was not seen in the scrambled-siRNA injected islets (Fig. 4j). Together, these results indicate that IHoP appears mediate to suppression of glucagon synthesis from the α-cells in the pancreatic islets, as well as mediating activation of apoptosis signal in islets.
Figure 4.
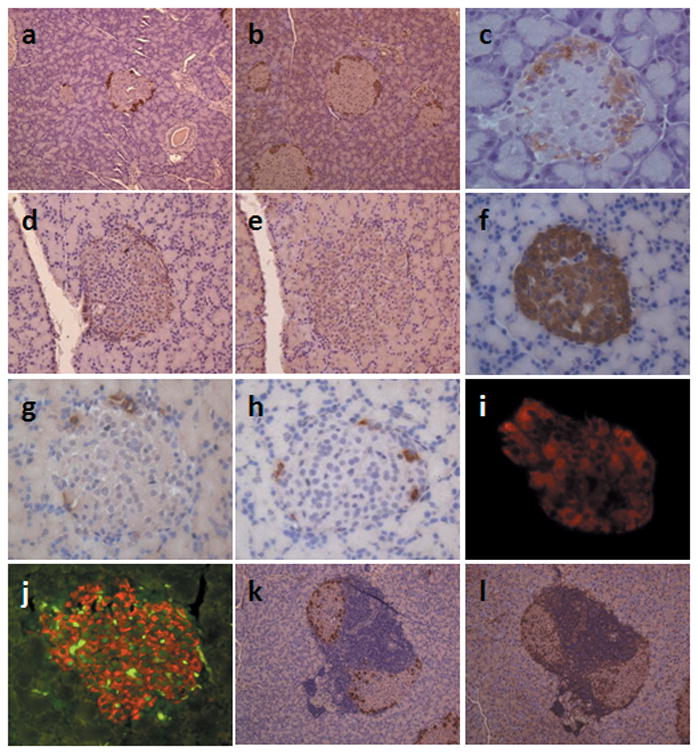
Detection of apoptosis signal in the islets of IHoP knockdown rats and pattern of IHoP in NOD/wild type mice pancreatic islets. (a–e) Normal rat islets expressed glucagon (a; brown) and IHoP (b; brown). Islets transduced with scrambled-siRNA were positive for IHoP (c; brown). However, after transduction with IHoP-siRNA, IHoP was successfully inhibited (e; brown), also glucagon was suppressed (d; brown) in the islets. Original magnification of (a) to (e) is 200X. (f–h) IHoP knockdown did not affect expression of other islet specific hormones, such as insulin (f; brown), somatostatin (g; brown) and pancreatic polypeptide (h; brown). Original magnification of (f) to (h) is 400X. (i–j) Detection of apoptosis in the IHoP-siRNA treated pancreatic islet. Scrambled siRNA injected rat islets were stained by insulin (i; red) and did not express an apoptotic signal (green in nuclei) in β-cell of islet (insulin; red); or acinar area. However, suppression of IHoP led to detection of apoptosis (j; green) in islet. This was true for insulin-positive cells (j; red) as well as the other islet cells. (i) and (j) used dual-filters for detection of rear green signal in the nuclei. Original magnification of (i) and (j) is 200X. (k–l) The NOD diabetes phonotype mice islets were infiltrated by T-cells, and the islets were shown to express glucagon (k; brown). IHoP was expressed in the islet and in T-cell-rich areas (l; brown), while infiltrating T-cells within the islets stained IHoP. Original magnification of (k) and (l) is 200X. Data shown represent one of three experiments with similar results.
We also examined expression of both glucagon and IHoP on the pancreatic tissues from NOD diabetes phenotyp mice (Fig. 4k and l). In NOD/wild type mice, the pancreatic islets expressed glucagon (Fig. 4k) and IHoP (Fig. 5l), also infiltrated T-cells were stained by IHoP (Fig. 4l). These results indicate that IHoP may control of glucagon synthesis and apoptosis in the pancreatic islets.
Figure 5.
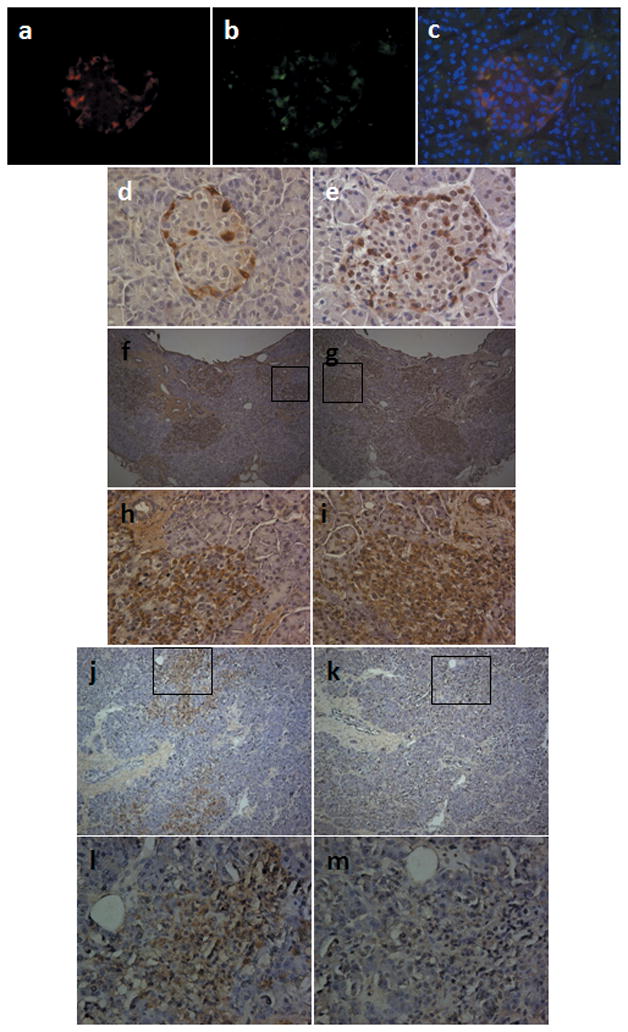
Detection of IHoP in the pancreatic islets of pre- and post-onset type-1 diabetic patients. (a–f) Determination of IHoP in the human pancreatic tissues. Double-immunofluorescence staining for glucagon (a; red) with IHoP (b; green) in normal human pancreatic tissue counter-stained with nuclear DAPI (blue). (c) Merged image from (a) and (b) (c; yellow). Immunohistochemistry for glucagon (d; brown) and IHoP (e; brown) in the normal human pancreas. Original magnification is 400X. (f–m) Detection of glucagon and IHoP in the pancreatic islets of pre- and post-onset type-1 diabetic patients. Glucagon (f and h; brown) and IHoP (g and i; brown) were expressed in the pre-onset type-1 diabetic patient pancreatic islets. The post-onset type-1 diabetic pancreatic islets were positive for glucagon (j and l; brown), however IHoP was not detected in the islets (k and m). Original magnification of (f), (g), (j) and (k) is 100X, and (h), (i), (l) and (m) is 400X. The boxed areas in (f), (g), (i), (j) and (k) are shown in higher magnification in (h), (i), (l) and (m). Data shown represent one of three experiments with similar results.
Detection of IHoP in the Pancreatic Islet from Pre- and Post-Onset Type-1 Diabetic Patients
Upon evaluation of IHoP expression in normal human pancreatic islets, double immunofluorescence staining showed co-localization of glucagon (Fig. 5a) and IHoP (Fig. 5b) on the α-cells within the islet (Fig. 5c). We also determined expression of glucagon and IHoP in the pre- and post-onset diabetic patient’s pancreatic tissues. Immunohistochemical staining of normal human pancreatic islets for glucagon (Fig. 5d) and IHoP (Fig. 5e) localized these proteins to the islet. Furthermore, the islets from pre-type-1 diabetic patients demonstrated stronger expression of glucagon (Fig. 5f and h), along with IHoP (Fig. 5g and i) as compared to normal islets. In the islets of post-onset-type-1 diabetic patients, glucagon was detected in the islets (Fig. 5j and l), but there was no expression of IHoP (Fig. 5k and m), suggesting that the absence of IHoP may be contributing, to the pathophysiological effects seen in these tissues. Moreover these results indicate that IHoP may represent a new target for treatment of diabetes mellitus.
Discussion
Our findings suggest that IHoP may be a new functional protein in the pancreatic islet, functioning in a role of hormone synthesis for islet homeostasis. Our previous report indicated that BM-derived IPCs cluster expressed the four major proteins of glucagon, insulin somatostatin and pancreatic polypeptide, found in the pancreatic Langerhans islet.21 We expected that IHoP expressed on the insulin-producing β-cells, however this protein co-localized with glucagon synthesizing α-cell. These results indicated that the IHoP is a new marker of α-cell and pancreatic islet.
The data presented here demonstrate that the control of glucagon synthesis in the α-cell of the pancreatic islet is regulated by IHoP. In vitro data indicate that IHoP has an effect on proliferation of insulin producing INS-1 cells. Furthermore, treatment of normal rats with IHoP-siRNA, resulted in suppression of glucagon synthesis, and subsequent loss of regulation of insulin synthesis from β-cells. Finally, IHoP suppression led to a break in homeostasis and induction of apoptosis in the pancreatic islet. In NOD/wild type mice, it was found that IHoP and glucagon were overexpressed in the pancreatic islet. Additionally, infiltrating T-cell expressed IHoP, but not glucagon. A similar expression pattern was seen in the human pre-type-1 diabetic islet. However, although the islets of post-onset-type-1-diabetics were positive for glucagon, there was no expression of IHoP. This may suggest that IHoP plays a critical role in the regulation of the islet homeostasis via mediating glucagon synthesis by α-cells.
Recent reports have identified several factors that directly regulate α-cell secretion of glucagon. For example, insulin is a potent inhibitor of islet glucagon release, and somatostatin and GLP-1 also inhibit glucagon secretion.29,30 Glucose also suppresses glucagon secretion, but may do so indirectly through insulin or GABA.31 During glucagon synthesis from the α-cells, proglucagon is controlled by cell-specific expression of prohormone convertase (PC) enzymes. An essential role for PC2 in the processing of islet proglucagon is revealed by studies of the PC2 knockout mouse. This mouse has a mild to low level of blood glucose, and elevated proinsulin, and exhibits a major defect in the processing of proglucagon, secreted by a typical secretory granule in the α-cell, to mature pancreatic glucagon.32,33 Similarly, our data indicates that following IHoP knockdown, rat pancreatic islets showed suppression of glucagon synthesis from pancreatic α-cells resulted in an induction of insulin secretion by β-cells, resulting in decreased blood glucose level. These results indicate that IHoP may directly control glucagon synthesis by α-cells within the pancreatic islets.
Utilizing siRNA technology, the resulting data indicated that IHoP-siRNA suppressed both IHoP and glucagon (directly or indirectly) in the pancreatic islets. The β-cells will continuously produce and secrete insulin (Fig. 3c), leading to uncontrolled insulin secretion in these cells and subsequent apoptosis (Fig. 4). Type 1-diabetes is considered to be a chronic autoimmune disease in which insulin-producing β-cells are gradually destroyed by autoreactive T-cells. The autoimmune disease associated with type-1 diabetes is mediated through the major histocompatibility complex (MHC) class I molecules that is required for the negative selection of autoreactive T-cells.34,35 Also Fas receptor activation has been demonstrated in pancreatic islet cells during the onset of type-1 diabetes.36 However, contradictory evidence has been published suggesting that apoptosis is not a major mechanism of β-cell destruction in type-1 diabetes.37 Our data certainly favors the notion that apoptosis plays a role in type-1 diabetes, and that IHoP expression influences (directly or in directly) this process.
The following treatments of IHoP-siRNA, blood glucose levels were decreased. This is likely a result of the concurrent suppression of glucagon synthesis (Fig. 3c). It is possible that suppression of IHoP may lead to a inhibition of glucagon synthesis and subsequently increase β-cell function and proliferation. Generally, approximately 15–20% of cells in the islet expressed glucagon.1 However, in the pre-type-1 diabetic pancreatic islets, glucagon seemed to be expressed in a majority of the cells comprising the islet (Fig. 5f),8 and IHoP appeared to follow a similar pattern (Fig. 5g). However the post-onset-type-1 diabetic pancreatic islets showed a loss of IHoP but remained positive for glucagon. The loss of IHoP with continued glucagon output may be lead to activation of apoptosis in the islets, which then leads to decreased insulin synthesis and secretion. This may promote T-cell infiltration and removal of the abnormally functioning β-cells (Fig. 4k and l). These data indicate that IHoP may function to regulate glucagon synthesis and maintain a balance in the secretion of both glucagon and insulin from islets. Take together, up- or down regulation of IHoP in the pancreatic islets appears to play an important role in maintaining islets homeostasis. However, the question still remains as to how the balance between IHoP and glucagon becomes dysregulated, and which factor(s) control the interaction of these hormones.
We demonstrated that IHoP protein co-localizes with glucagon secreting α-cells in normal pancreas. Gene knockdown by siRNA technology has proven to be a reliable method for the determination of gene functionality; however it does not completely explain the mechanisms by which the gene acts in vivo. To this end, upon suppression of IHoP expression via induction of siRNA, the islet loses IHoP expression and glucagon suppression. Recent studies demonstrate of processing of glucagon release, the proglucagon in the α-cell remains under active investigation, current evidence supports an important role for PC2 in the process.38–40 However whether or not PC2 directly cleaves proglucagon to glucagon remains unclear. Our results indicate that IHoP positively regulates glucagon synthesis, and controls insulin secretion from β-cells. This suggests that IHoP may possess therapeutic potential as a counter-regulator of the hormones responsible for maintenance of blood glucose concentration.
This manuscript focused on the expression of IHoP within the pancreatic islets in relation to type-1 diabetes. In Figure 6, a schematic representation of our findings on the function of IHoP in the pancreatic islet is presented. IHoP functions both to maintain homeostasis via the control of glucagon expression, as well as by regulating apoptosis in the islet. Further research will be required to fully characterize the IHoP protein, establish its receptor, and determine if IHoP is expressed by cell types other than α-cells. Moreover, the relationship between IHoP, insulin, and glucagon synthesis will need to be further investigated to elucidate the function of this protein in the pathogenesis of diabetes Mellitus, and other diseases.
Figure 6.
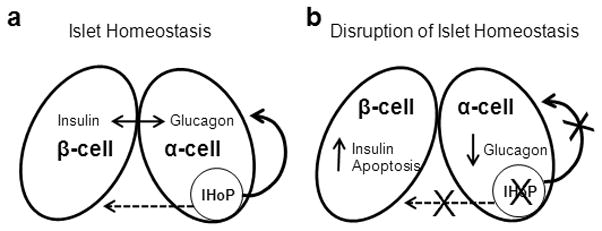
Schematic diagram of possible IHoP function within the pancreatic islet. (a) Normal islet homeostasis. The dashed arrow is indicative of the effects of IHoP on β-cells. Whether these effects are direct or indirect has yet to be determined. (b) Inhibition of IHoP in islet results in several changes in the islet, including apoptosis in β-cells as well as a decrease in glucagon secretion in α-cells.
In conclusion we have found a new functional protein (IHoP), and have demonstrated that this protein co-localizes with glucagon expressing α-cells in the pancreatic islets. The role of IHoP in the islet appears to involve the regulation of hormone secretion as well as activation of apoptosis within the islets. However, the mechanism(s) by which IHoP regulates these processes (i.e. directly or indirectly) are still unclear and will require further studies. Our results suggest that IHoP could be a powerful tool for the study of pancreatic islet homeostasis, as well as offering a new potential target for the treatment of type-1 diabetes.
Acknowledgments
The authors would like to thank Dr. Roberto Gianani (University of Colorado Health Science Center, Denver, CO) for donated slides of human type-1 diabetic patients, as well as Dr. Si-Hyun Bae for the helped preparation of this manuscript from the Department of Internal Medicine (The Catholic University of Korea, Seoul, Korea). The rat INS-1 cell line was a generous gift from Dr. Christopher B. Newgard (Duke University, Durham, NC). We also thank Marda Jorgensen for her expertise and assistance with immunohistochemistry. SHO and BEP are inventor/coinventor of a patent(s) related to this technology and may benefit from royalties paid to the University of Florida related to its commercialization. This work was supported by National Institute of Health grants DK60015 and DK58614 awarded to BEP.
Abbreviations
- IHoP
Islet Homeostasis Protein
- BM
Bone Marrow
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Contributor Information
Seh-Hoon Oh, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, North Carolina.
Houda Darwiche, Sciences for Health Programs, Santa Fe College, Gainesville, Florida.
Jae-Hyoung Cho, Department of Internal Medicine, Kangnam St. Mary Hospital, The Catholic University of Korea, Seoul, South Korea.
Thomas Shupe, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, North Carolina.
Bryon E. Petersen, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, North Carolina.
References
- 1.Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186:629–637. [PMC free article] [PubMed] [Google Scholar]
- 2.Dunbar JC, Walsh MF. Glucagon and insulin secretion by dispersed islet cells: possible paracrine relationships. Horm Res. 1982;16:257–267. doi: 10.1159/000179510. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto WY, Kawazu S, Ikeuchi M, et al. In vitro paracrine regulation of islet B-cell function by A and D cells. Life Sci. 1983;32:1873–1878. doi: 10.1016/0024-3205(83)90066-8. [DOI] [PubMed] [Google Scholar]
- 4.Stephen LA, Kathy B, Barb S, et al. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spec. 2004;17:183–190. [Google Scholar]
- 5.Webb GC, Akbar MS, Zhao C, et al. Glucagon replacement via micro-osmotic pump corrects hypoglycemia and alpha-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes. 2002;51:398–405. doi: 10.2337/diabetes.51.2.398. [DOI] [PubMed] [Google Scholar]
- 6.Gerich JE, Langlois M, Noacco C, et al. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 7.Bolli G, de Feo P, Compagnucci P, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus: interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983;32:134–141. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- 8.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 9.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28:574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- 10.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial-Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 11.Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Com. 2005;19:238–246. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Raz I, Elias D, Avron A, et al. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 13.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 14.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 15.Vincenti F, Kirkman R, Light S, et al. Interleukin-2–receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med. 1998;338:161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 16.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevens and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;4:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 19.Ramiya VK, Maraist M, Arfors KE, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 20.Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 21.Oh SH, Muzzonigro TM, Bae SH, et al. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84:607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 22.Oh SH, Witek RP, Bae SH, et al. Detection of transketolase in bone marrow-derived insulin producing cells: Benfotiamine enhances insulin synthesis and glucose metabolism. Stem Cell & Dev. 2008;18:37–45. doi: 10.1089/scd.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The RIKEN Genome Exploration Research Group Phase II Team and FANTOM Consortium. Functional annotation of a full-length mouse cDNA collection. Nature. 2001;409:685–690. doi: 10.1038/35055500. [DOI] [PubMed] [Google Scholar]
- 24.Gotoh M, Maki T, Kiyoizumi T, et al. An improved method for isolationof mouse pancreatic islets. Transplantation. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 26.Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A. 2001;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Shi ZQ, Rastogi KS, Lekas M, et al. Glucagon response to hypoglycemia is improved by insulin-independent restoration of normoglycemia in diabetic rats. Endocrinology. 1996;137:3193–3199. doi: 10.1210/endo.137.8.8754739. [DOI] [PubMed] [Google Scholar]
- 30.Dumonteil E, Magnan C, Ritz-Laser B, et al. Insulin, but not glucose lowering corrects the hyperglucagonemia and increased proglucagon messenger ribonucleic acid levels observed in insulinopenic diabetes. Endocrinology. 1988;139:4540–4546. doi: 10.1210/endo.139.11.6294. [DOI] [PubMed] [Google Scholar]
- 31.Rorsman P, Berggren PO, Bokvist K, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 32.Furuta M, Carroll R, Martin S, et al. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem. 1988;273:3431–3437. doi: 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- 33.Furuta M, Zhou A, Webb G, et al. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J Biol Chem. 2001;276:27197–27202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- 34.Faustman D, Li XP, Lin HY, et al. Linkage of faulty major histocompatibility complex class I to autoimmune diabetes. Science. 1991;254:1756–1761. doi: 10.1126/science.1763324. [DOI] [PubMed] [Google Scholar]
- 35.Yan G, Fu Y, Faustman DL. Reduced expression of Tap1 and Lmp2 antigen-processing genes in the nonobese diabetic (NOD) mouse due to a mutation in their shared bidirectional promoter. J Immunol. 1997;159:3068–3080. [PubMed] [Google Scholar]
- 36.Chervonsky AV, Wang Y, Wong FS, et al. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 37.Kang SM, Schneider DB, Lin Z, et al. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 38.Rouille Y, Westermark G, Martin SK, et al. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proc Natl Acad Sci U S A. 1994;91:3242–3246. doi: 10.1073/pnas.91.8.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenberg ME, Eilertson CD, Klein K, et al. Processing of mouse proglucagone by recombinant prohormone convertase 1 and immunopurified prohormone convertase 2 in vitro. J Biol Chem. 1995;270:10136–10146. doi: 10.1074/jbc.270.17.10136. [DOI] [PubMed] [Google Scholar]
- 40.Rothenberg ME, Eilertson CD, Klein K, et al. Evidence for redundancy in propeptide/prohormone convertase activities in processing proglucagon: an antisense study. Mol Endocrinol. 1996;10:331–341. doi: 10.1210/mend.10.4.8721979. [DOI] [PubMed] [Google Scholar]


