Abstract
One of the central questions that has occupied those disciplines concerned with human development is the nature of continuities and discontinuities from birth to maturity. The amygdala plays a central role in the processing of novelty and emotion in the brain. While there is considerable variability among individuals in the reactivity of the amygdala to novel and emotional stimuli, the origin of these individual differences is not well understood. Four month old infants called high reactive (HR) demonstrate a distinctive pattern of vigorous motor activity and crying to specific unfamiliar visual, auditory, and olfactory stimuli in the laboratory. Low-reactive infants show the complementary pattern. Here we demonstrate that the HR infant phenotype predicts greater amygdalar reactivity to novel faces almost two decades later in adults. A prediction of individual differences in brain function at maturity can be made on the basis of a single behavioural assessment made in the laboratory at four months of age. This is the earliest known human behavioural phenotype that predicts individual differences in patterns of neural activity at maturity. These temperamental differences rooted in infancy may be relevant to understanding individual differences in vulnerability and resilience to clinical psychiatric disorder. Males who were HR infants showed particularly high-levels of reactivity to novel faces in the amygdala that distinguished them as adults from all other sex/temperament subgroups, suggesting that their amygdala is particularly prone to engagement by unfamiliar faces. These findings underline the importance of taking gender into account when studying the developmental neurobiology of human temperament and anxiety disorders. The genetic study of behavioral and biologic intermediate phenotypes (or “endophenotypes”) indexing anxiety-proneness offers an important alternative to examining phenotypes based on clinically-defined disorder. Because the HR phenotype is characterized by specific patterns of reactivity to elemental visual, olfactory, and auditory stimuli, well before complex social behaviors such as shyness or fearful interaction with strangers can be observed, it may be closer to underlying neurobiological mechanisms than behavioral profiles observed later in life. This possibility, together with the fact that environmental factors have less time to impact the four-month phenotype, suggests that this temperamental profile may be a fruitful target for high-risk genetic studies.
Keywords: temperament, infant, individual differences, anxiety, novelty, fear, reactivity, amygdala, face perception, sex differences, longitudinal study, prospective study
Introduction
One of the central questions in developmental psychology over the past 50 years has been the nature of continuities and discontinuities in development from birth to maturity. Over what period of time can continuities of development be detected? The term temperament refers to a biologically based predilection for a distinctive pattern of behaviours, emotions and cognitions first observed in infancy or early childhood. Previous studies have identified two temperamental categories in 2 year olds called behaviourally inhibited and uninhibited, based on direct observation of behaviour in the laboratory.
Inhibited children are timid with unfamiliar people, objects and situations whereas uninhibited children spontaneously approach these same stimuli. We have previously demonstrated that adults who had been categorized in the second year of life as inhibited, compared with those who had been categorized as uninhibited, showed greater amygdalar activation to unfamiliar neutral faces1, suggesting some continuity in neurobiological function. A recent study of 22 year olds using a retrospective self-report questionnaire to measure behavioural inhibition supported this finding2. A longitudinal study that combined laboratory based measures of behavioural inhibition obtained from 2 to 7 years of age found that behaviourally inhibited adolescents showed an abnormally high amygdalar response to a task condition marked by novelty and uncertainty3.
These discoveries in two year olds motivated an effort to detect even earlier evidence of these temperamental imprints in nature. Four month old infants classified as HR display a temperamental profile in the laboratory characterized by vigorous motor activity and crying in response to specific unfamiliar visual, auditory, and olfactory stimuli, whereas low reactive (LR) infants show both low motor activity and low vocal distress to the same experimental stimuli4-6. High-reactive infants are biased to become behaviourally inhibited in the second year of life, whereas low-reactive infants are biased to develop into uninhibited children4-7. Furthermore, an inhibited temperament is a risk factor for the later development of social anxiety disorder8-11 (also known as social phobia), which is also a predictor of subsequent depressive disorder in young adults12, 13.
Because the amygdala plays a central role in the processing of novelty and emotion in the brain, we hypothesized that there might be differences in the reactivity of the amygdala in adults previously classified as HR or LR infants. Using fMRI, we measured amydgala reactivity to faces with neutral expressions in 135 subjects who were enrolled in an 18-year longitudinal study and had been characterized6, 14, 15 as high or low-reactive infants at four months of age (see Table 1). Adults who had been high-reactive and low-reactive infants are also referred to as “high-reactive” and “low-reactive” respectively.
Table 1.
Demographics of the study population
| High- Reactive | Low Reactive | Total | |
|---|---|---|---|
| N | 55 | 80 | 135 |
| Gender | |||
| Male | 30 | 42 | 72 |
| Female | 25 | 38 | 63 |
| Age, y, mean (stderr) | 18.19 ± 0.1 | 18.21 ± 0.08 | 18.20 ± 0.07 |
| Handedness, mean (stderr) | 62.4 ± 6.8 | 66.5 ± 5.4 | 64.9 ± 4.2 |
Materials and methods
Infant Assessment and Categorization
The details of the standard 45 minute battery used to assess the infants are described elsewhere6, 16. Initially, the mother looked down at her infant smiling, but not talking, for one minute. The parent then went to a chair behind the infant to be outside the child's field of vision. The examiner then placed a speaker baffle to the right of the infant and turned on a tape recording that played 8 short sentences read by female voices. The speaker baffle was removed and the examiner, standing in back of the infant, presented a set of mobiles composed of one, three or seven colorful toys that moved back and forth in front of the infant's face for 9 twenty-second trials. The examiner then dipped a cotton swab into very dilute butyl alcohol and presented it close to the infant's nostrils for 8 trials (the first and last trials were water rather than alcohol). The speaker baffle was replaced and the infant heard a female voice speaking three nonsense syllables (ma, pa, ga) at three different loudness levels. The examiner then popped a balloon in back of the infant; most were unperturbed by this event. Finally, the mother returned to gaze at her infant for the final minute. Quantitative indices of limb movement and arching of the back, and of crying and fretting to these experimental stimuli were computed from videotapes. About 20% of the infants, called high-reactive, showed a distinctive combination of frequent vigorous motor activity and distress indicated by crying or fretting. These infants repetitively flexed and extended their arms and legs, occassionally assuming a spastic posture for brief periods of time. On some trials high-reactive infants demonstrated a distinctive vigorous arching of their backs away from their padded seat. When motor activity was intense, crying followed the increased movement. In contrast, forty percent of the infants, called low-reactive, showed both low motor activity and low vocal distress to the same experimental stimuli. They occassionally moved an arm or leg but but showed minimal arching behavior and spasticity, and rarely cried or fretted. The decision to define discrete groups based on the combination of motor activity and crying, rather than a continuum of reactivity was supported by a taxonomic analysis of the four month data that implied that the combination of the two variables fit a categorical model better than a continuous one14, 17.
Procedure
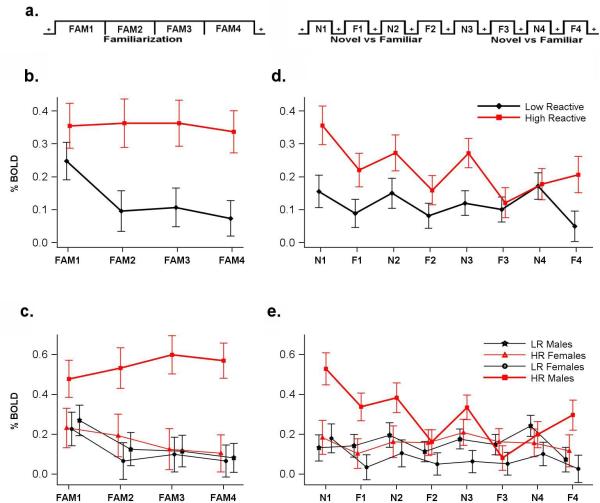
The experimental paradigm (see Figure 1a) consisted of two parts: a familiarization phase and a phase that consisting of alternating blocks of novel and familiar faces. During the 96-sec familiarization phase, six different identities were presented 16 times. During the second phase, alternating blocks of Novel and Familiar faces were presented. Each Novel block consisted of 24 identities unique to that block and never repeated; each familiar block consisted of repeated presentation of the six identities previously presented during the familiarization phase. Faces were drawn from the stimulus set of Gur and colleagues, which was created with careful attention to emotional neutrality18, 19 and were projected onto a screen while subjects lay on the scanner bed. Each face was presented for 500ms with a 500ms interstimulus interval.
Figure 1.
a. Experimental paradigm consisted of two phases During the initial 96-sec familiarization phase, 6 different identities were presented 16 times in pseudorandom order. During the second phase, alternating 24-sec blocks of Novel (Blocks N1-N4) and Familiar (Blocks F1-F4) faces were presented. Each Novel block consisted of 24 different identities completely unique to that block and never repeated; each familiar block consisted of repeated presentation of the same six identities that had been previously presented during the familiarization phase.
b. Amygdala response in HR vs LR (familiarization) Activation in the right amygdala (mean ± s.e.m) during the familiarization phase was greater in high-reactive (HR) compared to low-reactive (LR) subjects. HR subjects did not show a decrease in activation over the familiarization phase, unlike the LR reactive subjects who habituate.
c. Amygdala Response in HR vs LR by Sex (familiarization) HR males showed high right amygdala activation to faces that did not decrease during the familiarization phase. In contrast, females showed no difference in amygdala activation related to their infant phenotype. Amygdala activation was greater in HR males when compared with both LR males, and with females of either infant phenotype.
d. Amygdala Response in HR vs LR (Novel and familiar blocks) with Sexes Combined Right amygdala activation during the alternating novel & familiar blocks phase was significantly greater in HR subjects compared to LR subjects, and greater to the novel faces than to the familiar ones.
e. Amygdala Response in HR vs LR by Sex (Novel and familiar blocks) High-reactive males initially showed higher activation than the other three infant phenotype/gender subgroups but this difference decreases over time (at N1 HR males vs. LR males [t(125)= 3.82, p=.0002], HR males vs. HR females [t(125)= = 2.94, p = .004], HR males vs. LR females [t(125)= = 3.22, p = .002]; whereas by N4 there are no differences between HR males and the other infant phenotype/gender subgroups (infant phenotype × gender × time [F(3, 375) = 3.06, p = .03]).
Functional Magnetic Resonance Imaging
Data acquisition
Each subject underwent two 3D MPRAGE structural scans on a 3T Siemens TrioTim scanner (128 sagital slices; 1.3×1.3×1 mm; anterior to posterior phase encoding; repetition time= 2530 ms; echo time = 3.39 ms; flip angle 7°, bandwidth 190 Hz/Px). A gradient echo T2*-weighted sequence was used to acquire functional images (blood oxygenation level dependent or BOLD20 with a 12-channel gradient head coil (45 coronal slices oriented perpendicular to the anterior commissure –posterior commissure line; 3.1x3.1x4.0 mm; interleaved excitation order, and foot-to-head phase encoding; repetition time = 3000ms; echo time = 40 ms; flip angle 90°).
Imaging Data analysis
Functional and structural MRI data were analyzed using Freesurfer and FS-fast (available at http://surfer.nmr.mgh.harvard.edu), utilizing previously described techniques1, 21. The two 3D MPRAGE structural scans from each subject were averaged, after motion correction, to create a single high signal-to-noise average volume. Functional data were motion corrected using AFNI (http://afni.nimh.nih.gov/afni/index.shtml)22, 23 and spatially smoothed (fwhm=5mm) using a 3D Gaussian filter (www.fmrib.ox.ac.uk/fsl). The spatially smoothed, normalized, motion-corrected functional images were aligned to a 3D structural image created by motion correcting and averaging the high-resolution 3D sagittal images. As part of the alignment procedure, the raw functional data from each subject were visualized over the high-resolution 3D anatomical from that individual to ensure that the BOLD signal in the amygdala was not obscured by susceptibility artifact. Individual subject functional data were spatially normalized using an optimal linear transformation method24 that maximizes the likelihood that anatomic structures of individual subjects will overlap with each other across subjects. It is based on a previously described group atlas that retains the most common anatomic features in the majority of subjects25-28. Talairach transformation using the Montreal Neurological Institute automated registration algorithm was also performed for comparison (available at ftp://ftp.bic.mni.mcgill.ca/pub/mni_autoreg)29, but a better registration between anatomical structures and the coordinates in the Talairach atlas30 was obtained with the optimal linear transformation method24. To facilitate comparisons across studies, we report Talairach coordinates based on registration of the images from the optimal linear transformation with the Talairach atlas30. After spatial normalization, functional data were averaged for each subject and then across subjects. Paradigm files were constructed to allow the separate averaging of the images acquired during fixation blocks, the novel face presentations, and the familiar faces. A group statistical map was computed using a random-effects model for the contrast novel and familiar faces (i.e. collapsed across condition) vs. the fixation cross (software available at http://surfer.nmr.mgh.harvard.edu/docs/index.html). For this group average we examined the responses to faces collapsed across all subjects. This analytic strategy assesses the role of temperament in a manner that was unbiased with respect to between group differences, and avoids circularity in the data analysis1, 21. A functionally constrained ROI was used because different regions within the amygdala, possibly representing subnuclei respond differently to facial stimuli31-34. For example, Leonard33 found no responses to faces in the lateral nucleus of the amygdala in primates. Therefore, a ROI based on anatomical constraints alone might include portions of the amygdala that do not respond similarly to faces. A 43 voxel ROI were identified in the right amygdala, our a priori region of interest, with a statistical threshold of 10-10. Sixty-one of the 135 subjects had no voxels in the left amygdala exceeding this high threshold. However, at a reduced threshold of 10-6, a 41 voxel region was identified in left amygdala. Labels derived from the coordinates of these ROI's were used to extract percent BOLD signal change from baseline (the fixation cross) during Novel or Familiar face presentations in the functional data of each subject (“ROI analysis”) (software at http://surfer.nmr.mgh.harvard.edu/docs/index.html). A repeated-measures general linear model (PROC GLM with LSMEANS/tdiff) (SAS v9.2), with infant phenotype (high-reactive, low-reactive) and gender (female, male) as the between-group factors and time block (familiarization 1,2,3,4) as within-group factors, was performed on functional data from the familiarization phase of the protocol (Figure 1). A second repeated-measures GLM with infant phenotype (high-reactive, low-reactive) and gender (female, male) as the between-group factors and face type (novel, familiar), and time block (1,2,3,4) as within- group factors, was performed on functional data from the second phase of the protocol in which alternating Novel (Blocks N1-N4) and Familiar (Blocks F1-F4) faces were presented. Covariance matrices for the time blocks in each GLM were tested for type-H structure35 with a sphericity test36; if the assumption of H-type covariance was not met, the more conservative p value based on the Greenhouse-Geisser Epsilon adjustment was reported37. All t-tests were two-tailed. A repeated-measures ANOVA with left and right hemisphere as an additional between-group factor was used to test for laterality effects.
Results
A 43-voxel region of interest (ROI) in the right amygdala was identified with a statistical threshold of 10-10. The peak voxel was located at 20, -11, -13 (p < 10-22), with the centroid located at 23, -8, -18 (p < 10-12). Amygdala reactivity to the faces during the familiarization phase of the protocol (Figure 1b) was significantly greater in adult subjects with the high-reactive infant (HR) phenotype (.35 ± .06, mean ± s.e.m) than the low-reactive (LR) phenotype (.13 ± .05) [F(1, 125) = 7.68, p=.006]). There was also a significant interaction between infant phenotype and time [F(1,125) = 2.70, p = .05]; adults who had been HR infants did not show a decrease in activation over the familiarization phase, unlike the LR group (Figure 1b).
During the familiarization phase, amygdalar reactivity was also greater in males (.35 ± .05) than females (.14 ± .06) [F(1,125) = 6.62, p = .01]. Furthermore, there was a significant interaction between infant phenotype and gender [F(1,125) = 4.65, p = .03] in activity recorded from the right amygdala. Adult males who had been HR infants showed a high amygdalar response to faces that does not habituate during the familiarization period (Figure 1c). The mean bold signal was greater in HR males (.55 ± 0.08), compared both with LR males (.15 ± 0.07) [t(125 ) = 3.63, p=.0004] and with females of either infant phenotype [HR males (.55 ± 0.08) vs. HR females (.16 ± .09) [t(125) = 3.09, p = .003]; HR males (.55 ± 0.08) vs. LR females (.11 ± .08) [t(125) = 3.76, p = .0003]. In contrast, adult females showed no difference in amygdala activation related to their infant phenotype (HR females (.16 ± .09) vs. LR females (.11 ± .08) [t(125) = 0.42, p = 0.68). Furthermore, LR females (.11 ± .08) did not differ from the LR males (.15 ± 0.07) [t(125) = -0.32 , p = 0.75].
During the second phase of the paradigm that presented alternating blocks of Novel and Familiar faces (Figure 1d), right amygdala reactivity to faces was significantly greater in the subjects with the HR infant phenotype (.22 ± .03), than the LR phenotype (.12 ± .03) [F(1,125) = 7.71, p=.006]), and greater to novel faces (.21 ± .01 than to familiar ones (.13 ± .01) [F(1,125) = 22.34, p < .0001. Turning to the role of gender (Figure 1e), amygdalar reactivity was greater in males (.22 ± .03) than females (.12 ± .03) [F(1,125) = 7.22, p = .008 ] as was observed during the familiarization part of the experiment. Furthermore, there was a significant 3-way interaction in amygdala reactivity between infant phenotype, gender and time (Figure 1e) [F(3, 375) = 3.06, p = .03].
The pattern of amygdalar responses to novel faces in the subgroups defined by infant phenotype and gender was similar to that observed during the familiarization part of the protocol (see Figure 2). Mean reactivity to novel faces was greater in HR males compared both with LR males, and with females of either infant phenotype. Once again, adult females showed no difference in amygdala activation to novel faces related to their behavioural phenotype in infancy, nor did LR females differ from LR males. Reactivity to familiar faces did not distinguish HR from LR subjects within either gender, although there was a trend for HR males to show greater reactivity to familiar faces compared with the LR males (0.22±.04 vs. 0.12±.03; [t(125)= 1.90, p=.06].
Figure 2.
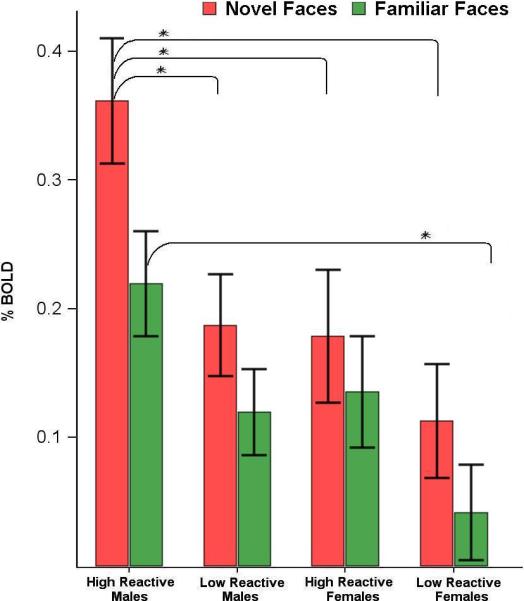
Amygdala response in infant phenotype/sex subgroups to novel and familiar faces (mean ± s.e.m, * indicates significant pair-wise contrast p<.05) The mean reactivity to novel faces was greater than to the familiar faces for all sex/temperament subgroups. Activation to novel faces was greater in HR males (.36 ± .05), compared with both LR males (.19 ± .04) [t(125 ) = 2.77, p=.006], and with females of either infant phenotype [HR males (.36 ± .05) vs. HR females (.18 ± .05) [ t(125) = 2.58, p = .01]; HR males (.36 ± .05) vs. LR females (.11 ± .04) [t(125) = 3.78, p = .0002]. HR (.18 ± .05) and LR (.11 ± .04) females did not differ in activation to novel faces.
In the left amygdala, 61 of the 135 subjects had no voxels at the p< 10-10 threshold used to define the right amygdala ROI. At a less stringent threshold of 10-6, however, a 41-voxel ROI could be identified in left amygdala. The peak voxel was located at -20, -10, -15 (p < 10-13), with the centroid located at –22, -9, -20 (p < 10-11). In general, the pattern of results with respect to temperament in both phases of the paradigm was similar to those observed on the right, although less robust (see Supplementary Information for details). Amydgalar activation to novelty was greatest in the high reactive males compared both with LR males, and with HR or LR females. In the left as on the right, adult females did not differ in activation to novelty based on whether they were HR or LR infants, and LR females did not differ from LR males. In the left amygdala, as on the right, neither gender showed significant differential reactivity to familiar faces related to temperament in infancy.
Discussion
Adults who had been high-reactive (HR) infants showed greater amygdalar reactivity and delayed habituation to neutral faces when compared to adults who had been low-reactive infants. These results demonstrate that individual differences in reactivity of the amygdala to faces at maturity can be predicted solely on the basis of an infant behavioural phenotype identified at 4 months of age. These data imply that continuities exist at the level of neurobiology over 18 years of development between early infancy and adulthood. This is the earliest known human behavioural phenotype that predicts individual differences in patterns of neural activity at maturity.
The amygdala plays a central role during the assessment of novelty21, 38, ambiguity39, and threat40, and in forming associations about biologically relevant phenomena, including potentially danger-related stimuli40, 41 -- functions that are all salient to the clinical manifestations of anxiety disorders. Genes confer susceptibility to anxiety proneness that cuts across clinical diagnostic labels and categories42-49. High reactivity, observable early in infancy before the accumulated influence of environmental factors complicates the detection of genetic influences on anxiety disorders, is a promising intermediate phenotype in the effort to unravel the phenotypic and genetic complexity of anxiety disorders.
We previously demonstrated that adults who had been categorized in the second year of life as inhibited, compared with those were uninhibited, showed greater amygdalar activation to unfamiliar neutral faces1. That classification at two years was based on behaviors such as clinging to, or remaining proximal to, the mother and the reluctance to approach or actual retreat from unfamiliar events or people, for instance a woman in an unusual costume or an unfamiliar child of the same sex and age in the same playroom with both mothers present. In contrast, the assessment of high-reactivity and low-reactivity, the temperaments that are the focus of the present report, is made before complex social behaviors such as shyness or avoidance of interaction with strangers is observable. The classification at four months of age is based on elemental behavioural responses of infancy, namely crying, arching of the back, and thrashing of the arms and limbs to relatively simple sensory probes in multiple modalities (see Methods). These more elemental, but still complex behaviours may be more conducive to analysis at the level of neural circuitry and genetics. These phenotypes of early infancy have had less time to be influenced by parental and other environmental factors than phenotypes based on behaviour observed later in development and hence may be closer to underlying biological mechanisms under genetic control. The genetic and neurobiological dissection of behavioral and biologic intermediate phenotypes (or “endophenotypes”) indexing anxiety-proneness is an important alternative strategy to examining the discrete, clinically-defined anxiety disorders enumerated in the DSM. Temperament, once principally a construct of developmental psychology, now has a second act, with a role as an intermediate phenotype. Such an approach is in accord with the perspective of the NIMH Research Domain Criteria project50, 51.
What details of amygdala circuitry and connectivity could explain the fMRI findings in this report and the associated behavioural and physiological profile of these temperaments? Animal studies of the amygdala have revealed an intricate internal architecture of discrete nuclei, with complex patterns of intranuclear and internuclear connectivity, in addition to widespread extrinsic connections41, 52. Although a gap exists between the level of detail revealed by animal studies and the ability to observe this architecture in humans with the tools currently available for functional neuroimaging, findings from animal studies are heuristic for hypothesis generation. The basolateral complex of the amydgala (BLA), especially the lateral nucleus (LA) is the major gateway for both somatosensory and gustatory/visceral input to the amydgala. Somatosensory inputs to the amygdala arise primarily from association areas of the cortex and multisensory components from the posterior thalamic complex. The assessment of high-reactivity and low-reactivity at 4 months of age is based on the infants behavioural response to probes in multiple sensory modalities. This sensory information travels medially within BLA from the LA to the basal nuclei. Projections from the BLA to the ventral striatum (accumbens) and medial caudate are thought to mediate voluntary instrumental behavior related to emotional events. The basal nuclei of the amygdala have major extrinsic connections with the medial prefrontal, orbitofrontal, and rostral anterior cingulate cortex, and with the medial temporal lobe memory system that allow the basal nuclei to integrate contextual and motivational factors with the sensory information from LA. Temperamental based differences in the excitability of the basal nuclei and its cortical afferents and efferents might mediate the overgeneralization from dangerous to safe contexts, maintenance of avoidant behaviors, and the behavioral as well as cognitive perseveration seen when high reactive infants grow into inhibited children.
The basal nuclei project to the central nucleus of the amygdala (CEA), which is the main output structure for projections to the hypothalamus, periaquductal grey (PAG), pons and medulla. These targets of the CEA mediate many of the characteristic physiological and behavioural responses of high reactive infants and inhibited children: vigorous distress vocalizations and defensive arching of the back (PAG), elevated plasma cortisol (via the paraventricular nucleus of the hypothalamus that controls ACTH release), dilation of the pupils, increased heart rate, decreased heart rate variability, and increased blood pressure (by activation of the medulla and sympathetic chain via both direct projections to the medulla, as well as indirectly through projections to the lateral hypothalamus). Increased excitability of the CEA, whether intrinsic to the CEA or extrinsic in origin, could therefore explain the behavioural and physiological profile of the high reactive/inhibited phenotype. The amygdala has an elegant mechanism for regulating the level of output from the CEA. The intercalated cells (ITC) are clusters of inhibitory GABAergic cells that lie in the narrow margins between the between the nuclei of the BLA, and between BLA and the CEA. These cells can gate (or fail to gate) the neuronal traffic arising from sensory inputs to the BLA and thereby modulate CEA output53. In addition to the direct excitatory projections from the BLA to CEA discussed above, some of these excitatory projections from the BLA that carry traffic towards the CEA terminate on the ITC, which in turn can inhibit outflow from the CEA. A deficiency of GABAergic activity within the ITC or a decrease in the number of functioning clusters of ITC, with resultant failure of feed-forward inhibition of the CEA, could generate the cascade of physiological events observed in high reactive/inhibited temperament. There is also “top-down”cortical control of the ITC via an especially dense unidirectional excitatory projection from posterior orbitofrontal cortex, allowing indirect modulation of CEA output54-56. Therefore, functional or anatomically based variations in this circuit could also contribute to the temperamental profile observed. Although current imaging tools do not allow resolution at the level of distinct nuclei and the ITC, the amygdala activations in this report map to central and medial portions of the ventral amygdala with some extension more laterally, potentially implicating regions containing the basal and accessory basal nuclei and their associated ITC cluster. Such a preliminary conclusion must be further qualified in light of the limited temporal resolution of fMRI. The effect of infant temperament on adult amygdala reactivity was observed bilaterally. This is consistent with the fact that HR infants are biased to become behaviourally inhibited in the second year of life, and that neuroimaging studies of inhibited temperament have not generally found significant lateralization of effects in the amygdala1-3 related to temperament.
Pavlovian fear conditioning is an influential animal model that has been extended to the investigation of the neurobiology of human anxiety disorder57, 58. Differences in fear acquisition, fear extinction, and extinction retention have been described both between anxiety disordered patients59-67 especially PTSD and normal controls, as well as between individuals in normal populations58, 68-70. Studies have identified some correlates of these differences including cortical thickness71, 72, amygdala volume72, and trait anxiety73-75. Future neuroimaging studies should directly examine the relationship between infant temperament and individual differences in the functioning adult circuitry sub-serving fear acquisition, extinction and extinction-retention. Differences between the sexes have been described in both classical conditioning, including fear conditioning, and operant conditioning76. In the present study, gender appears to play an important role in predicting the impact of infant temperament on amygdalar reactivity at adulthood. Male adults who were high-reactive infants showed particularly high-levels of reactivity to novel faces in the amygdala that distinguished them from all other sex/temperament subgroups, suggesting that their amygdala is particularly prone to engagement by unfamiliar faces.
The sex differences reported here could involve the interplay of genes, hormones (both in utero during early brain development, infancy, childhood, puberty, and adulthood), environmental and social factors, brain structure and function, and epigenetic interactions that modulate gene function and expression.76-81. The high levels of amydalar activation in the high-reactive males might be related to the fact that males generally show greater conditioned responses in fear conditioning paradigms than females, a finding that has been attributed to the effect of estrogen76, 82-84. Animal studies have demonstrated that these differences in conditioning can be manipulated by both ovariectomy and the subsequent replacement of estrogen85. In human females changes in reactivity of the amygdala across phases of the menstrual cycle86, 87 have been detected with fMRI. Our data did not show any evidence of a subgroup of females defined by menstrual phase in which temperament showed the effect on amygdala reactivity observed in males. Animal models do not suggest a role for testosterone levels in the higher levels of fear conditioning seen in adults88. However, the profound effects of testosterone on brain development in utero could play a role in the present findings.
Further studies will be required to elucidate the origin and meaning of the sex differences we observed. Nonetheless, these findings underline the importance of taking gender into account when studying the developmental neurobiology of human temperament and anxiety disorders. These data cannot resolve the question of whether these differences exist in infancy or whether they represent different trajectories and outcomes at adulthood; a definitive answer to this question will require technological advances to enable the functional neuroimaging of the amygdala in awake and active human infants.
Supplementary Material
Acknowledgements
The authors thank the families and children who have stayed with the study over 18 years. This study was supported by the National Institutes of Mental Health R01MH071467 and R01MH074848 (C.E.S). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health.
Footnotes
Author contributions
C.E.S. designed the study, directed data collection and analyses, conducted analyses, and wrote the paper. P.S.K conducted analyses. D.N.G. wrote analytic software and advised on its use. J.K. and N.C.S. characterized the temperament of the subjects at 4 months of age. R.B.B. served as a study coordinator and collected MRI data.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 2.Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neurosci. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Dev. 1996;67(2):523–540. [PubMed] [Google Scholar]
- 5.Kagan J, Snidman N, Arcus D. Childhood derivatives of high and low reactivity in infancy. Child Dev. 1998;69(6):1483–1493. [PubMed] [Google Scholar]
- 6.Kagan J. Galen's Prophecy. Basic Books; New York: 1994. [Google Scholar]
- 7.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240(4849):167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz C, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158(10):1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- 10.Hayward C, Killen JD, Kraemer HC, Taylor CB. Linking self-reported childhood behavioral inhibition to adolescent social phobia. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1308–1316. doi: 10.1097/00004583-199812000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Mick MA, Telch MJ. Social anxiety and history of behavioral inhibition in young adults. J Anxiety Disord. 1998;12(1):1–20. doi: 10.1016/s0887-6185(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 12.Stein MB, Fuetsch M, Muller N, Hofler M, Lieb R, Wittchen HU. Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Arch Gen Psychiatry. 2001;58(3):251–256. doi: 10.1001/archpsyc.58.3.251. [DOI] [PubMed] [Google Scholar]
- 13.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Kagan J, Snidman N. The Long Shadow of Temperament. Belknap Press; Cambridge, MA: 2004. [Google Scholar]
- 15.Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monogr Soc Res Child Dev. 2007;72(2):1–75. vii. doi: 10.1111/j.1540-5834.2007.00436.x. discussion 76-91. [DOI] [PubMed] [Google Scholar]
- 16.Kagan J, Snidman N, McManis M, Woodward S. Temperamental contributions to the affect family of anxiety. Psychiatr Clin North Am. 2001;24(4):677–688. doi: 10.1016/s0193-953x(05)70257-4. [DOI] [PubMed] [Google Scholar]
- 17.Woodward SA, Lenzenweger MF, Kagan J, Snidman N, Arcus D. Taxonic structure of infant reactivity: evidence from a taxometric perspective. Psychol Sci. 2000;11(4):296–301. doi: 10.1111/1467-9280.00259. [DOI] [PubMed] [Google Scholar]
- 18.Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Res. 1992;42(3):231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- 19.Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42(3):241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- 20.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, et al. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol Psychiatry. 2003;53(10):854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- 22.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 23.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42(6):1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 25.Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4(4):344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy DN, Filipek PA, Caviness VR. Anatomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Trans Med Imaging. 1989;8(1):1–7. doi: 10.1109/42.20356. [DOI] [PubMed] [Google Scholar]
- 27.Rademacher J, Caviness VS, Jr., Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3(4):313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- 28.Caviness VS, Meyer J, Makris N, Kennedy DN. MRI-based topographic parcellation of human neocortex: an anatomically specified method with estimate fo reliability. J Cog Neurosci. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- 29.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. 3-D Proportional System: an Approach to Cerebral Imaging. Thieme Medical Publishers, Inc.; New York: 1998. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 31.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- 33.Leonard CM, Rolls ET, Wilson FA, Baylis GC. Neurons in the amygdala of the monkey with responses selective for faces. Behav Brain Res. 1985;15(2):159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- 34.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 35.Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurements designs have exact F-distributions. J Am Stat Assoc. 1970;65:1582–1589. [Google Scholar]
- 36.Anderson TW. An Introduction to Multivariate Statistical Analysis. John Wiley & Sons; New York: 1958. [Google Scholar]
- 37.Greenhouse SWG, S On methods in the analysis of profile data. Psychometrika. 1959;32:95–112. [Google Scholar]
- 38.Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49(3):2871–2878. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis M, Whalen PJ. The amygdala: vigilance and emotion. MolPsychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 40.Ledoux JE. Emotion circuits in the brain. AnnuRevNeurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 41.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90(2):419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of phobias in women: the interrelationship of agoraphobia, social phobia, situational phobia, and simple phobia. Archives of General Psychiatry. 1992;49:273–281. doi: 10.1001/archpsyc.1992.01820040025003. [DOI] [PubMed] [Google Scholar]
- 43.Nestadt G, Samuels J, Riddle MA, Liang KY, Bienvenu OJ, Hoehn-Saric R, et al. The relationship between obsessive-compulsive disorder and anxiety and affective disorders: results from the Johns Hopkins OCD Family Study. Psychol Med. 2001;31(3):481–487. doi: 10.1017/s0033291701003579. [DOI] [PubMed] [Google Scholar]
- 44.Smoller JW, Tsuang MT. Panic and phobic anxiety: defining phenotypes for genetic studies. Am J Psychiatry. 1998;155(9):1152–1162. doi: 10.1176/ajp.155.9.1152. [DOI] [PubMed] [Google Scholar]
- 45.Hudson JI, Mangweth B, Pope HG, Jr., De Col C, Hausmann A, Gutweniger S, et al. Family study of affective spectrum disorder. Arch Gen Psychiatry. 2003;60(2):170–177. doi: 10.1001/archpsyc.60.2.170. [DOI] [PubMed] [Google Scholar]
- 46.Kendler KS, Walter EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Arch Gen Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 47.Scherrer JF, True WR, Xian H, Lyons MJ, Eisen SA, Goldberg J, et al. Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. J Affect Disord. 2000;57(1-3):25–35. doi: 10.1016/s0165-0327(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 48.Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, et al. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103(2-3):133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 49.Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Dev. 2002;73(5):1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- 50.Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 52.McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55(3):257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 53.Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19(23):10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbas H. Specialized elements of orbitofrontal cortex in primates. Ann N Y Acad Sci. 2007;1121:10–32. doi: 10.1196/annals.1401.015. [DOI] [PubMed] [Google Scholar]
- 55.Barbas H, Zikopoulos B. Sequential and parallel circuits for emotional processing in primate orbitofrontal cortex. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford University Press; New York: 2006. pp. 57–91. [Google Scholar]
- 56.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 57.Kim Jj, Fau - Jung MW, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. (0149-7634 (Print)) [DOI] [PMC free article] [PubMed]
- 58.Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol(Amst) 2008;127(3):567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, et al. Altered Processing of Contextual Information during Fear Extinction in PTSD: An fMRI Study. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and Acquired Origin of Reduced Recall for Fear Extinction in PTSD: Results of a Twin Study. JPsychiatrRes. 2008 doi: 10.1016/j.jpsychires.2008.01.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. BehavRes Ther. 2007 doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 63.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109(2):290–298. [PubMed] [Google Scholar]
- 64.Peri T, Ben Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. BiolPsychiatry. 2000;47(6):512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 65.Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in panic disorder: Enhanced resistance to extinction. J Abnorm Psychol. 2007;116(3):612–617. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- 66.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. BiolPsychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. PsycholMed. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Maren S, Quirk GJ. Neuronal signalling of fear memory. NatRevNeurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 70.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 71.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102(30):10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartley CA, Fischl B, Phelps EA. Brain Structure Correlates of Individual Differences in the Acquisition and Inhibition of Conditioned Fear. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrett J, Armony JL. Influence of trait anxiety on brain activity during the acquisition and extinction of aversive conditioning. Psychol Med. 2008:1–11. doi: 10.1017/S0033291708003516. [DOI] [PubMed] [Google Scholar]
- 74.Sehlmeyer C, Dannlowski U, Schoning S, Kugel H, Pyka M, Pfleiderer B, et al. Neural correlates of trait anxiety in fear extinction. Psychol Med. 2010:1–10. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- 75.Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69(3):563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97(2):229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Vries GJ, Villalba C. Brain sexual dimorphism and sex differences in parental and other social behaviors. Ann N Y Acad Sci. 1997;807:273–286. doi: 10.1111/j.1749-6632.1997.tb51926.x. [DOI] [PubMed] [Google Scholar]
- 78.McCarthy MM, deVries GJ, Forger NG. Sexual differentiation of the brain: mode, mechanisms, and meaning: In:Hormones, brain and behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain, and behavior. 2 edn Vol. 3. Academic; San Diego: 2009. [Google Scholar]
- 79.de Vries GJ, Sodersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55(5):589–596. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92(2):205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- 81.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145(3):1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 82.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Molen GM, Merckelbach H, van den Hout MA. The possible relation of the menstrual cycle to susceptibility to fear acquisition. JBehavTherExpPsychiatry. 1988;19(2):127–133. doi: 10.1016/0005-7916(88)90026-2. [DOI] [PubMed] [Google Scholar]
- 84.Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29(7):883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1). Brain Res. 2001;888(2):356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- 86.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2009;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anagnostaras SG, Maren S, DeCola JP, Lane NI, Gale GD, Schlinger BA, et al. Testicular hormones do not regulate sexually dimorphic Pavlovian fear conditioning or perforant-path long-term potentiation in adult male rats. BehavBrain Res. 1998;92(1):1–9. doi: 10.1016/s0166-4328(97)00115-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.