Abstract
Objective
Increased circulating cytokine levels are a prominent feature of aging that may contribute to atherosclerosis. However, the role vascular cells play in chronic inflammation induced by aging is not clear. Here, we examined the role of aging on inflammatory responses of vascular cells.
Methods and Results
In an ex vivo culture system, we examined the inflammatory response of aortas from young (2-4 months) and aged (16-18 months) mice under non-stimulatory conditions. We found that basal levels of interleukin (IL)-6 were increased in aged aortas. Aged aortic vascular smooth muscle cells (VSMC) exhibited a higher basal secretion of IL-6 than young VSMC. Gene and protein expression analysis revealed that aged VSMC exhibited upregulation of chemokines (e.g. CCL2), adhesion molecules (e.g., ICAM1), and innate immune receptors (e.g., Toll like receptor [TLR] 4), which all contribute to atherosclerosis. Using VSMC from aged TL4-/- and Myd88-/- mice, we demonstrate that signaling via TLR4 and its signal adaptor, MyD88, are in part responsible for the age-elevated basal IL-6 response.
Conclusion
Aging induces a proinflammatory phenotype in VSMC due in part to increased signaling of TLR4 and MyD88. Our results provide a potential explanation as to why aging leads to chronic inflammation and enhanced atherosclerosis.
Keywords: aging, atherosclerosis, inflammation, mouse, vascular smooth muscle cells
Advanced age is one of the strongest independent risk factors for the development of atherosclerosis and cardiovascular disease.1 One factor associated with both aging and atherosclerosis is inflammation. The inflammatory nature of atherosclerosis is underscored by epidemiological studies in patients with rheumatoid arthritis, systemic lupus or psoriasis. Patients often exhibit low-grade inflammation in the form of elevated circulating cytokines,2-4 including interleukin (IL)-6 levels3,4 and increased risk of atherosclerosis. 5-7 However, the mechanisms by which aging induces increased circulating cytokine levels remain unclear.
Circulating IL-6 is a predictive biomarker for cardiovascular disease. 8, 9 Elevations in circulating cytokines, including IL-6, are a prominent feature of aging and may play a role in the development of atherosclerosis. 10 Typically, elevated circulating IL-6 associated with aging is low-grade (i.e., 2x) compared to IL-6 induction during an acute inflammatory insult (i.e., 20x), such as septic shock. Why aging leads to higher circulating basal level of IL-6 is not clear. The cell types that contribute to the increase in circulating IL-6 have not been identified. Potential candidates include inflammatory cells present within atherosclerotic plaques, vascular cells in disease free arteries, and circulating immune cells11 or adipocytes.12 Animal studies have shown increased production of proinflammatory cytokines such as IL-613, 14 or CCL215, 16 from disease-free arteries with aging. However, these studies neither determined which cell within the vasculature was responsible for the age-enhanced inflammatory response nor found the underlying mechanisms for the phenotype.
In the current study, we investigated the impact of aging on the basal inflammatory responses of arteries and vascular smooth muscle cells (VSMC). We found that in both arteries and VSMC, aging led to an increase in the basal secretion of IL-6. Additionally, we noted that without stimulation, VSMC from aged mice upregulated IL-6 and CCL2 production. Both of these markers have been implicated in atherosclerosis. 17, 18 We also found that aged VSMC expressed higher basal levels of the innate receptor Toll like receptor (TLR) 4 than young VSMC and that signaling via TLR4 and its signal adaptor MyD88 were in part responsible for the age-elevated IL-6 basal secretion. Our results indicate that aged VSMC cells exhibit a pro-atherogenic phenotype under basal, non-stimulatory conditions, which may help to explain why aging leads to elevated circulating cytokine levels and accelerates the development of atherosclerosis.
Method
Animal Procedures
Female mice aged 16-18 months and 10-11 months as well as young (1.5-2 months of age) wild type C57BL/6 mice were purchased from the NIA rodent facility. C57BL/6 Rag-/- mice were purchased from Jackson Laboratory (Bar Harbor, ME). CD11c-MyD88 Tg 19 and LysM-Cre/MyD88-flox mice (all on the C57BL/6 background) were generously provided by Dr. Medzhitov (Yale University). SM22α-Cre/MyD88-flox mice were created by interbreeding SM22α-Cre mice (Jackson Laboratory) with MyD88-flox mice. B6.129/SvJ-MyD88 tm1AKI (denoted as Myd88-/-) mice were a gift from Dr. S. Akira (Osaka University, Osaka, Japan) and were backcrossed 10 times onto the C57BL/6 (H2b) background. C57BL/6 TLR4-/- and Myd88-/- mice were aged to 10-11 months in our colony under pathogen free conditions. Mice were not included in this study if they exhibited signs of illness (reduced feeding, mobility, or grooming, or evidence of skin disease or lymphoadenopathy) before artery procurement. Mice were euthanized by isoflurane administration. The institutional animal care and use committee at Yale University approved the use of animals in this study.
Reagents
Penicillin/streptomycin solution, DMEM (low glucose content), Medium 199 (M199), collagenase II solution were purchased from Invitrogen (San Diego, CA). Fetal Bovine Serum (FBS) was purchased from Sigma-Aldrich (St. Louis, MO). Amphotericin B was obtained from American Analytical (Natick, MA). Elastase was obtained from Worthington Biomedical (Freehold, NJ).
Aorta and Cell Culture
Murine aortas were collected after cardiac puncture with cold PBS. Under a dissection microscope, thoracic aortas were harvested by removing the adipose tissue. Aortas were then cut into half, weighed, and placed in 1ml culture medium (20% FBS M199 containing 100 unit/ml penicillin, 100 μg/ml streptomycin) at 37°C overnight. Medium was free of LPS as measured by E-Toxate assay (Sigma). After 16 hours, culture supernatants were collected and then stored at -20°C for further analysis. VSMC were enzymatically isolated as previously described. 20 Briefly, freshly harvested thoracic aortas were washed in PBS and DMEM (low glucose content) containing 0.25μg/ml amphotericin. After digestion in 1mg/ml collagenase II solution at 37°C for 10 min, adventitia were removed with the aid of a dissecting microscope. The remaining aortas were cut into small pieces and further digested with 2mg/ml collagenase II and 0.5 mg/ml elastase solution for 1 hour at 37°C, with gentle shaking every 10 minutes. The isolated cells were then washed and plated in complete medium (20% FBS DMEM-low glucose containing 100 unit /ml penicillin, 100μg/ml streptomycin and 0.25μg/ml amphotericin). We found that VSMC yields from aged aortas were generally higher than those from young aortas, which may be due to a thickened arterial wall and increased VSMC proliferation associated with aging, as previously described.21, 22 However, the plating efficiency of aged and young VSMC were similar since the RNA yield from 5 ×104 aged and young VSMCs were 3.2 ± 1.1μg and 3.1 ± 0.7 µg, respectively. Studies were performed using passage 3-5 cells.
ELISA
Supernatant levels of IL-6, CCL2, CCL5, CXCL2 and CXCL10 were determined via ELISA (ebioscience, San Diego, CA) according to the manufacturers’ protocol. All cytokine values from aortic culture were adjusted to the weight of tissue.
Quantitative Real-Time PCR Analysis
RNA was isolated from VSMC using an RNeasy Mini Kit (Qiagen, Valencia, CA). First-strand cDNA was reverse transcribed from isolated RNA using Superscript First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Primers and annealing temperatures of each transporter gene are listed in Supplemental Table 1. Real time PCR reactions were performed using DyNAmo HS SYBR Green qPCR kit (New England Biolabs) and CFX96™ Real-Time PCR Detection System and Automation System (Biorad, Hercules, CA). TLR copies were determined using the standard curve method. 23 Data represent average copy number normalized to 18s and all samples were run contemporaneously. For other genes, gene expression was quantified using comparative Ct method with 18s as a reference gene.
Western Analysis
Western analysis was performed as previously described.24 Antibodies against ICAM1 (1:500), VCAM1 (1:500), TLR4 (1:1000) were purchased from Santa Cruz, Biotechnology (Santa Cruz, CA). Anti-β-actin antibody (1:1000, Invitrogen) was used as loading control. All antibodies were prepared in 2% bovine serum albumin.
Statistical Analysis
All data are presented as means ± SEM or SD as indicated in the figure legends. Differences between groups were analyzed using either one-way ANOVA or 2-tailed Student's t test, with Bonferroni's post-hoc test when appropriate. All data were analyzed using GraphPad prism software (San Diego, CA). Differences were considered significant at P< 0.05.
Results
Aging increases IL-6 secretion by murine aortas without stimulation
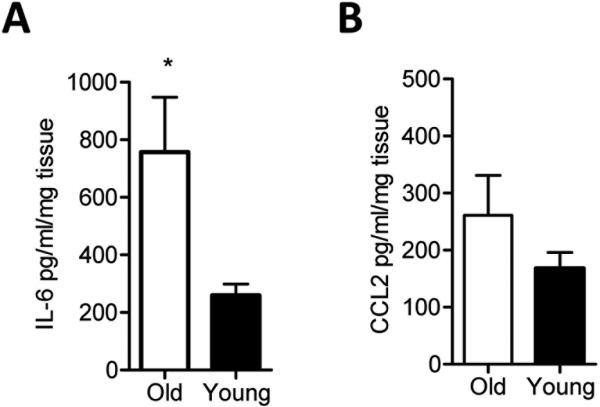
When we measured cytokines from the culture supernatants of isolated murine aortas of aged and young mice, we found that the proinflammatory cytokine IL-6 but not CCL2 levels were increased in unstimulated aortas from aged mice compared to those of young mice (Figure 1A-B). We did not detect IL-1β, IL-10, IL-17, TNF-α, or IFN-γ cytokines in the supernatants of young or old aortas using ELISA (data not shown).
Figure 1. Basal IL-6 cytokine production is increased in aged aortas than young aortas.
Thoracic aortas from young or aged mice were harvested and cultured in media devoid of LPS for 16 hours. IL-6 (A) and CCL2 (B) levels were measured by ELISA. The data shown are representative of three independent experiments. Results are presented as means ± SEM, n at least 5 mice / group / experiment. Differences between groups were analyzed using Student's t-test. *, P<0.05. O: old 16-18 month; Y: young 6-8 weeks.
MyD88 signaling within vascular smooth muscle cells is critical to the TLR-induced IL-6 response by young aortas
Innate immune activation via TLRs leads to IL-6 production. Since MyD88 is a signal adaptor downstream of all the TLRs except TLR3, we examined the role of MyD88 in the basal production of IL-6 in young aortas. We found that aortas harvested from young Myd88-/- mice exhibited a reduced IL-6 response in comparison to wild type aortas young wild type aortas (Figure 2A), demonstrating that MyD88 signaling contributed to the basal secretion of IL-6 of young aortas.
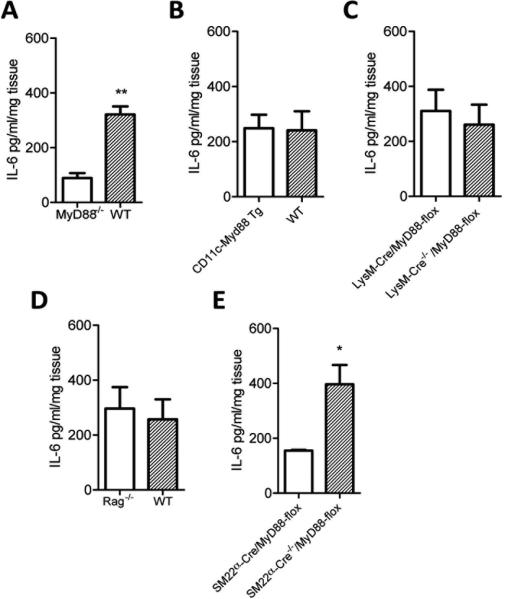
Figure 2. Vascular smooth muscle cells are major contributors to the basal IL-6 response of young murine aortas.
Aortas from genetically altered young mice (6-8 weeks of age) were used to identify the MyD88 expressing cells that contribute to the basal IL-6 response. Aortas were harvested from young wild type and Myd88-/- mice and IL-6 measured in the culture supernatants (A). CD11c-Myd88 Tg mice are Myd88-/- except in CD11c+ cells and were compared to wild type controls (B). The aortas of LysMCre/MyD88-flox mice, which are Myd88-/- in Lysm+ cells, were compared to their LysM-Cre-/-/MyD88-flox Cre negative control (C). Rag-/- mice lack mature T cells and B cells and aortas of these mice were compared to wild type controls (D). SM22α-Cre/MyD88-flox are MyD88 deficient in smooth muscle cells and were compared with their Cre negative control (E). IL-6 levels were measured by ELISA. Differences between groups were analyzed using Student's t-test, n = at least 4 mice/group/experiment. *, P<0.05; **, P<0.01. The data shown are representative of three independent experiments.
Because TLRs are expressed in a variety of immune cells including macrophage, dendritic cells (DCs), B and T cells, which reside in disease free arteries25, we next employed a series of genetically manipulated mice to identify the MyD88 expressing cells responsible for the basal IL-6 response of young aortas. We first assessed the basal IL-6 response of young mice in which the only MyD88 competent cells are CD11c+ (a marker expressed on certain DCs and macrophages). Aortas from these CD11c-MyD88 Tg mice exhibited a similar basal IL-6 response as wild type aortas (Figure 2B), indicating that MyD88 signaling within CD11c+ cells was not sufficient for the basal IL-6 response.
We next harvested aortas from LysM-Cre/MyD88-flox mice in which macrophages are selectively MyD88-deficient 26 to determine if macrophages were required for the basal IL-6 response. We found that aortas from these LysM-Cre/MyD88-flox mice exhibited a similar basal IL-6 response as their wild type controls (Figure 2C), indicating that MyD88 signaling within macrophages is not required for the basal IL-6 response. Moreover, we found that T cells and B cells were not critical for the basal IL-6 response, as aortas from Rag-/- mice, which lack T cells and B cells, produce basal levels of IL-6 similar to those of young wild type aortas (Figure 2D).
Given that VSMC express TLRs 27 and that immune cells were not responsible for the basal IL-6 response of young aortas, we next examined whether MyD88 expression within VSMC is required for the basal IL-6 secretion of aortas. Hence, we harvested aortas in which MyD88 signaling was selectively deficient in smooth muscle cells (i.e., SM22α-Cre/MyD88-flox mice). We found that the basal IL-6 response was reduced in the aortas of young SM22α-Cre/MyD88-flox mice compared to their Cre negative controls (Figure 2E), demonstrating that VSMC are major contributors for the basal IL-6 response.
Aging increases IL-6 and CCL2 production by VSMC under non-stimulatory conditions
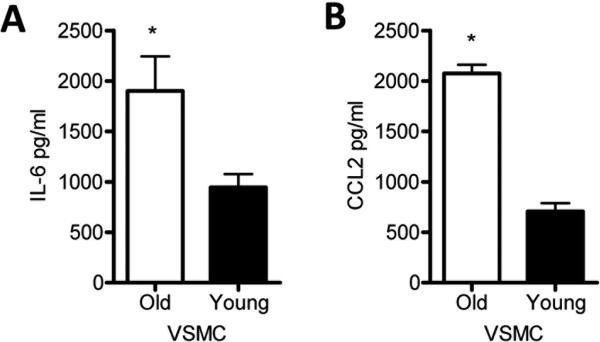
Our results demonstrate that MyD88 signaling within VSMC is important for the basal IL-6 secretion of young aortas. Hence, a potential explanation as to why aortas from aged mice exhibited a higher basal IL-6 response than young aortas is qualitative differences in the intrinsic function of VSMC with aging. Thus, we propagated VSMC from young and aged aortas and measured IL-6 and CCL2 levels under nonstimulatory conditions. Similar to the response of aortas, we found that aged VSMC exhibited an increased basal secretion of IL-6 and also an increased basal production of CCL2 in culture supernatants relative to VSMC from young mice (Figure 3). These results indicate that aged VSMC exhibit increased basal secretion of IL-6 and CCL2.
Figure 3. IL-6 and CCL2 levels were increased in aged VSMC than young VSMC without stimulation.
VSMC were isolated from thoracic aortas of young or aged mice as described in Methods, and equal number of cells from young and aged mice were cultured in fresh medium for 16 hours. Basal IL-6 (A) and CCL2 (B) levels measured in culture supernatants were measured by ELISA. The data shown are representative of three independent experiments. Results are presented as means ± SEM, n at least 4 mice/group/experiment as a source of cells. Differences between groups were analyzed using Student's t-test. *, P<0.05. O, old 16-18 month; Y, young 6-8 weeks.
Aged non-stimulated VSMC display pro-atherogenic features and increased TLR4 expression
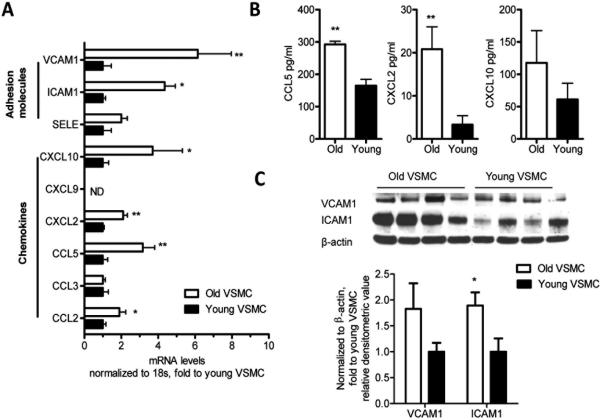
Chemokines such as CCL2, CCL5, and CXCL10 and adhesion molecules such as the vascular cell adhesion molecule 1 (VCAM1) and the intracellular adhesion molecule 1 (ICAM1) are important for immune cell migration from the circulation to the arterial wall, and contribute to the development of atherosclerosis. 28 We found that aged VSMC exhibited gene upregulation of CCL2, CCL5, CXCL2, and CXCL10, but not of CCL3 compared to young VSMC (Figure 4A). We also determined that CCL2 (Figure 3), CCL5, CXCL2 and CXCL10 (Figure 4B) protein levels were elevated with aging, whereby the increases in CCL2 (Figure 3) CCL5 and CXCL2 protein levels reached statistical significance (Figure 4B). ICAM1 and VCAM1 gene expression were also increased in non-stimulated aged VSMC in comparison to young VSMC (Figure 4A). In addition, ICAM1 and VCAM1 protein levels were augmented in aged VSMC in comparison to young VSMC (Figure 4C). The increase in ICAM1 was statistically significant (Figure 4C).
Figure 4. Pro-atherogenic chemokines and adhesion molecules are upregulated in non stimulated VSMC with aging.
VSMC were isolated from murine thoracic aortas as described in Methods. Non-stimulated VSMC were collected, and chemokines and adhesion molecules were measured by qRT-PCR (A). Results are presented as means ± SEM. Data shown represent one experiment with n =12 mice as a source of cells / group. CCL5, CXCL2, and CXCL10 were measured in the supernatants of cultured, non-stimulated VSMC by ELISA (B). Western blot analysis measuring ICAM1 and VCAM1 protein levels (C). Differences between young and aged VSMC were analyzed using Student's t-test. *, P<0.05; **P<0.01. In (C) the values are normalized to β-actin expression and represent fold change to young VSMC value. Data shown in (B) and (C) are representative of two independent experiments, with n = at least 4 mice / group / experiment as a source of cells. All comparisons between young and aged groups were paired and performed contemporaneously.
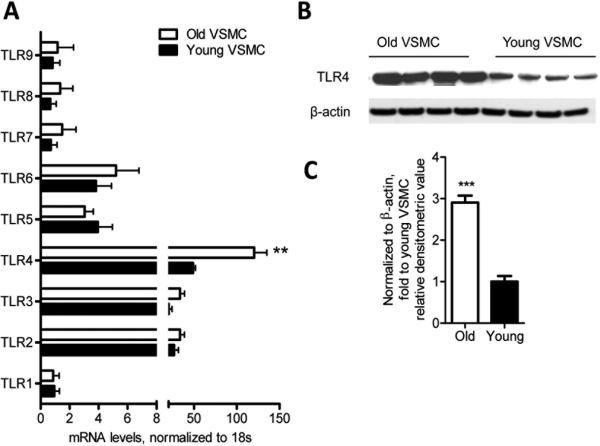
As TLRs have also been implicated in the development of atherosclerosis 29-31, we examined the TLR gene expression profiles of young and aged VSMC under non-stimulatory conditions. In both aged and young VSMC, the most highly expressed among all nine measured TLRs were the TLR2, TLR3, and TLR4 genes. TLR4 mRNA expression and protein levels were significantly higher in aged VSMC than in young VSMC (Figure 5A-C). Together, these results indicate that aging upregulates several chemokines, adhesion molecules, and innate immune receptors that have been implicated in atherosclerotic disease pathogenesis.
Figure 5. VSMC express increased TLR4 gene expression and protein levels with aging.
TLR gene expression profiles were evaluated in aged and young non-stimulated VSMC by qRT-PCR. TLR4 expression was significantly higher in aged VSMC than young VSMC (A). Western blot analysis showing that aged VSMC exhibit higher TLR4 protein levels than young VSMC (B-C). Results are presented as means ± SEM and in (A) data shown represent one experiment with n = 15 mice as a source of cells / group. In (C) the values are normalized to β-actin expression and represent fold change relative to young VSMC value. In (B) and (C), data shown are representative of two independent experiments, with n = 4 mice / group / experiment as a source of cells. Differences between young and aged VSMC were analyzed using Student's t-test. **, P<0.01, ***, P<0.0001. All comparisons between young and aged groups were paired and performed contemporaneously.
Basal IL-6 production is reduced in aged VSMC and aortas deficient in MyD88
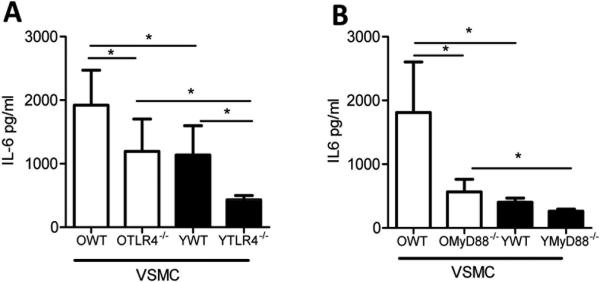
MyD88 is a key intracellular adaptor protein involved in TLR signaling. 32 To examine whether the increased TLR4 expression on VSMC is linked to the elevated basal secretion of IL-6, we isolated VSMC from either aged (i.e., 10-11 months of age) TL4-/- or Myd88-/- mice and from their wild type counterparts, and measured IL-6 production under non-stimulatory conditions. Similar to what we noted in VSMC from mice 16-18 months of age (Figure 3), we found that VSMC from mice aged 10-11 months exhibited higher basal secretion of IL-6 than young counterparts (Figure 6A-B). We found that IL-6 production in aged TL4-/- VSMC was significantly reduced compared to that found in aged wild type VSMC (Figure 6A). Similar results were noted in aged Myd88-/- VSMC compared to that found in aged wild type counterparts (Figure 6B). Neither TLR4 nor MyD88 deficiency entirely abolished the effects of aging on basal IL-6 production as aged TL4-/- and Myd88-/- VSMC still produced higher basal IL-6 levels than the young TL4-/- and Myd88-/- VSMC (Figure 6A-B). These results indicate that TLR4 and MyD88 signaling are an important mechanism by which aging leads to increased basal IL-6 production by VSMC.
Figure 6. Elevated IL-6 secretion by aged VSMC and aortas is in part TLR4 and MyD88 dependent.
Aortas were harvested from aged (10-11 month of age) or young (6-8 weeks of age) WT, TLR4-/- or Myd88-/- mice, and VSMC were isolated from these aortas. VSMC were cultured in fresh medium for 16 hours without stimulation, and IL-6 was measured in the supernatants by ELISA. Aged TLR4-/- (denoted as OTLR4-/-) (A) Myd88-/- (denoted as OMyD88-/-) (B) VSMC exhibited a significant reduction in IL-6 production in comparison with the aged WT counterparts. This was also evident in the young VSMC. Results are presented as means ± SD, n = 3 mice / group for both panels A and B. Differences between groups were analyzed using one-way ANOVA with Bonferroni's post-hoc test. *, P<0.05. O:old 10-11 month; Y: young 6-8 weeks.
Discussion
In this study, we demonstrated that disease-free aortas from aged mice exhibit increased basal IL-6 production compared to young aortas. We also found that VSMC, which are known to produce inflammatory mediators33, exhibit elevated IL-6 and CCL2 basal production with aging. Increased signaling via TLR4 and MyD88, a signal adaptor downstream of most TLRs, was in part responsible for this elevated IL-6 VSMC response. As MyD88 signaling within VSMC was critical for the basal IL-6 in young aortas, it is possible that qualitative changes occur in aged aortas and that, as a consequence, other cells contribute to the IL-6 response with aging. Further studies with aged mutant mice are required to determine what cells within aged aortas are responsible for the basal IL-6 response.
Prior work has indicated that VSMC plays a role in arterial wall remodeling with aging. 34 For instance, aging increases proliferation and migration of VSMC and accelerates replicative senescence of these cells. 35, 36 Prior work has also shown that VSMC isolated from rat aortas exhibit increased CCL2 expression. 15 All of these properties of VSMCs have been associated with atherosclerosis. 37, 38 We found that under non-stimulatory conditions, aged VSMC exhibited increased levels of several chemokines (e.g., CCL2) and adhesion molecules (ICAM1) compared to young VSMC. These chemokines and adhesions molecules have been previously shown to contribute to atherosclerosis disease pathogenesis.18,39,40 Hence, aged VSMC display a phenotype that may predispose or enhance the progression of atherosclerosis. As hypomethylation of genomic DNA increases with aging, 41 and similar epigenetic changes are associated with atherosclerosis, 42 it is possible that hypomethylation of chemokine, adhesion molecule and the IL-6 gene loci may explain the findings of our study. Further mechanistic studies will be required to confirm this hypothesis and to determine whether the aged “atherosclerotic prone” phenotype of VSMC is related to atherosclerosis development. Such studies may require aging atherosclerotic mice (e.g., Ldlr-/-) that are specifically deleted in one or more of these chemokines or adhesion molecules in VSMC.
Our findings further indicate that TLR4 and its adaptor protein MyD88 were in part responsible for the increased basal IL-6 production by aged VSMC. MyD88 is downstream of multiple TLRs and the IL-1β and IL-18 receptors. 43 This may explain why the differences between aged Myd88-/- VSMC and wild type VSMC were larger than the differences between aged TLR4-/- VSMC and wild type cells. Clearly, other receptors besides TLR4 contribute to the increased basal IL-6 response of VSMC with aging, an issue that warrants future investigation.
It is not clear why TLR4 expression on VSMC increases with aging. TLRs are not only activated by microbial motifs but also by endogenous ligands, such as hyaluronan and oxidized LDL. 32 The increased TLR4 expression and the IL-6 production may reflect chronic sensing of innate ligands over time. Hence, in aged mice chronic sensing of innate ligands, either derived from pathogens or endogenously derived, may alter the basal state of VSMC compared to VSMC in young mice.
TLRs are involved in atherosclerosis development as MyD88 deficiency—as well as TLR4 or TLR2 deficiency— results in reduced lesion size in experimental murine models of atherosclerosis.29-31 Although these studies indicate the possible involvement of TLR and MyD88 signaling from non-bone marrow derived cells in the development of atherosclerosis, the specific role of MyD88 signaling within VSMC will require additional studies with conditional mutant atherosclerotic prone mice in which MyD88 is deleted within VSMC.
In conclusion, our study provides evidence that with aging, VSMC may contribute to the heightened basal IL-6 response. Signaling via a TLR4 and its adaptor protein MyD88 are in part responsible for the increased basal IL-6 production by aged VSMC. Taken together, our results provide a possible explanation as to why circulating IL-6 levels increase with aging. Furthermore, our study suggests that the altered inflammatory profile of VSMC with aging maybe atherosclerotic prone, an issue that will require future mechanistic examination.
Supplementary Material
Acknowledgments
Source of funding:
Work supported by NIH grants AG028082 and AG033049 and in part by AI064660, and American Heart Association Established Investigator Award to DRG.
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collaboration Asia Pacific Cohort Studies Collaboration The impact of cardiovascular risk factors on the age-related excess risk of coronary heart disease. Int J Epidemiol. 2006;35:1025–1033. doi: 10.1093/ije/dyl058. [DOI] [PubMed] [Google Scholar]
- 2.Robak T, Gladalska A, Stepien H, Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators Inflamm. 1998;7:347–353. doi: 10.1080/09629359890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robak E, Sysa-Jedrzejowska A, Stepien H, Robak T. Circulating interleukin-6 type cytokines in patients with systemic lupus erythematosus. Eur Cytokine Netw. 1997;8:281–286. [PubMed] [Google Scholar]
- 4.Deeva I, Mariani S, De Luca C, Pacifico V, Leoni L, Raskovic D, Kharaeva Z, Korkina L, Pastore S. Wide-spectrum profile of inflammatory mediators in the plasma and scales of patients with psoriatic disease. Cytokine. 2010;49:163–170. doi: 10.1016/j.cyto.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 5.McMahon M, Hahn BH, Skaggs BJ. Systemic lupus erythematosus and cardiovascular disease: Prediction and potential for therapeutic intervention. Expert Rev Clin Immunol. 2011;7:227–241. doi: 10.1586/eci.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121:S9–14. doi: 10.1016/j.amjmed.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: Cohort study using the general practice research database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodondi N, Marques-Vidal P, Butler J, Sutton-Tyrrell K, Cornuz J, Satterfield S, Harris T, Bauer DC, Ferrucci L, Vittinghoff E, Newman AB. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am J Epidemiol. 2010;171:540–549. doi: 10.1093/aje/kwp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and c-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 10.Davi G, Falco A. Oxidant stress, inflammation and atherogenesis. Lupus. 2005;14:760–764. doi: 10.1191/0961203305lu2216oa. [DOI] [PubMed] [Google Scholar]
- 11.Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: Effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:M20–26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 12.Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: Adipose tissue as a major source of il-6. J Gerontol A Biol Sci Med Sci. 2009;64:723–730. doi: 10.1093/gerona/glp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. AM J Physiol. 1995;268:H2288–2293. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- 14.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. Faseb J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 15.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic mcp-1 and its receptor ccr2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Spinetti G, Monticone RE, Zhang J, Wu J, Jiang L, Khazan B, Telljohann R, Lakatta EG. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS One. 2011;6:e16653. doi: 10.1371/journal.pone.0016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 19.Pasare C, Medzhitov R. Control of B cell responses by toll-like receptors. Nature. 2005;17:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 20.Deng J, Ma-Krupa W, Gewirtz AT, Younge BR, Goronzy JJ, Weyand CM. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakatta EG. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part iii: Cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Fukagawa NK. Age-related changes in redox signaling and vsmc function. Antioxidants & redox signaling. 2009 doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhanasekaran S, Doherty TM, Kenneth J. Comparison of different standards for real-time pcr-based absolute quantification. J Immunol Methods. 2010;354:34–39. doi: 10.1016/j.jim.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Elias V, Loban A, Scrimgeour AG, Ho E. Marginal zinc deficiency increases oxidative DNA damage in the prostate after chronic exercise. Free Radic Biol Med. 2010;48:82–88. doi: 10.1016/j.freeradbiomed.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou B, Benson A, Kuzmich L, DeFranco AL, Yarovinsky F. Critical coordination of innate immune defense against toxoplasma gondii by dendritic cells responding via their toll-like receptors. Proc Natl Acad Sci U S A. 2011;108:278–283. doi: 10.1073/pnas.1011549108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Coriolan D, Schultz K, Golenbock DT, Beasley D. Toll-like receptor 2 mediates persistent chemokine release by chlamydia pneumoniae-infected vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:2308–2314. doi: 10.1161/01.ATV.0000187468.00675.a3. [DOI] [PubMed] [Google Scholar]
- 28.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 29.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein e. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in myd88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 32.Shirali A, Goldstein D. Tracking the toll of kidney disease. J Am Soc Nephrol. 2008;19:1444–1450. doi: 10.1681/ASN.2008010123. [DOI] [PubMed] [Google Scholar]
- 33.Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, Davies AH, Flavell RA, Feldmann M, Monaco C. Unexpected protective role for toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci U S A. 2011;108:2372–2377. doi: 10.1073/pnas.1018515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Fukagawa NK. Age-related changes in redox signaling and vsmc function. Antioxidants & redox signaling. 2010;12:641–655. doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic mmp-2 activity and angiotensin ii in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Monticone RE, Lakatta EG. Arterial aging: A journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiechl S, Willeit J. The natural course of atherosclerosis. Part ii: Vascular remodeling. Bruneck study group. Arterioscler Thromb Vasc Biol. 1999;19:1491–1498. doi: 10.1161/01.atv.19.6.1491. [DOI] [PubMed] [Google Scholar]
- 38.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 39.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of rantes receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 40.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine cxcl10 promotes atherogenesis by modulating the local balance of effector and regulatory t cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Wylie RC, Andrews LG, Tollefsbol TO. Aging, cancer and nutrition: The DNA methylation connection. Mech Ageing Dev. 2003;124:989–998. doi: 10.1016/j.mad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Turunen MP, Aavik E. Ylä-Herttuala S. Epigenetics and atherosclerosis. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790:886–891. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Taro Kawai SA. Toll-like receptor and rig-1-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.