INTRODUCTION
Inhibitors of the inflammatory cytokine tumor necrosis factor (TNF) have proven to be highly effective in the treatment of various autoimmune conditions, including rheumatoid arthritis (RA). Indeed, according to several international guidelines that were based upon abundant evidence from therapeutic trials and clinical experience, these agents have come to be considered a cornerstone of therapy for RA patients with severe or refractory disease (1, 2) However, in addition to its central role in the immune driven systemic inflammation of RA, TNF is also important to immunologic surveillance. Because of the key role that the immune system plays in host defense, there is concern that therapy with TNF inhibitors (TNFi) might predispose patients to adverse effects related to impaired immunity, including an increased incidence of infections and/or cancer. Despite many years of clinical research and more than a decade since the introduction of TNF inhibitors into the clinic, there is disagreement about the potential association between use of these agents and malignancy.
From a mechanistic standpoint, it could be hypothesized that inhibition of TNF could either enhance or inhibit cancer development (3–6). On the one hand, via mechanisms such as induction of apoptosis or suppressive effects on gene expression, TNF may suppress the development of certain tumors (5). Indeed, the name ‘TNF’, which was coined well before the role of this cytokine in inflammation and in numerous autoimmune diseases was known, reflects the observed inhibitory effects of this cytokine on certain tumors. Therefore, blockade of TNF may enhance the risk of cancer. In addition, TNF serves as a key element of the inflammatory response whose inhibition may increase the risk to various infections. This could potentially place the host at greater risk of cancers driven by chronic infections, particularly viral (7). By these mechanisms, inhibiting TNF might increase the risk of cancer. On the other hand, uncontrolled inflammation itself may also potentiate cancer (3, 4). Also, among the myriad activities of TNF is its profound effect on angiogenesis, which is critical to tumor growth, survival, and metastasis (3, 4). Therefore, potent anti-inflammatory treatments, such as TNFi, could decrease the risk of cancer through suppressing inflammation and reducing angiogenesis. This concept is supported by two lines of data. First, patients with higher levels of systemic inflammation, such as those with RA, are at a greater risk for developing lymphomas (8). Second, treatment with corticosteroids, which possess diverse anti-inflammatory properties, appear to be associated with a lesser risk of lymphoma development (9).
Similar to the basic science suggesting that TNFi may increase or decrease the risk of cancer, randomized controlled trials do not provide definitive evidence about this relationship. Two recent meta-analyses found no clear evidence of increased cancer risk with the use of TNFi. (10, 11). One large meta-analysis that included only the monoclonal antibodies, infliximab and adalimumab, found an increased risk of cancer (12). A meta-analysis focused on etanercept suggested a trend toward an increased risk but the confidence interval spanned one for the primary analysis and secondary analyses did not all suggest increased cancer risk (13). Furthermore, a study of patients enrolled in adalimumab trials for early RA found no significant increase in cancer risk (14). While randomized controlled trials are the gold standard for efficacy, they may not provide the best information regarding a drug’s toxicity, owing to their relatively short duration and strict inclusion criteria that may exclude important at-risk groups (15). Epidemiologic studies of patients in typical care allows for analysis of more relevant subjects with a variety of comorbid conditions using concomitant treatments.
With this background, we undertook a systematic review of epidemiologic studies of the relationship between TNFi and cancer. Prior reviews have examined this risk of cancer in RA (16). Whereas, this review focuses on both the methodologic attributes of studies examining TNFi and cancer, as well as their results. We did not attempt a meta-analysis because of the obvious methodological heterogeneity and opted to present the findings as a systematic review, as suggested by the Cochrane Collaboration (17).
METHODS
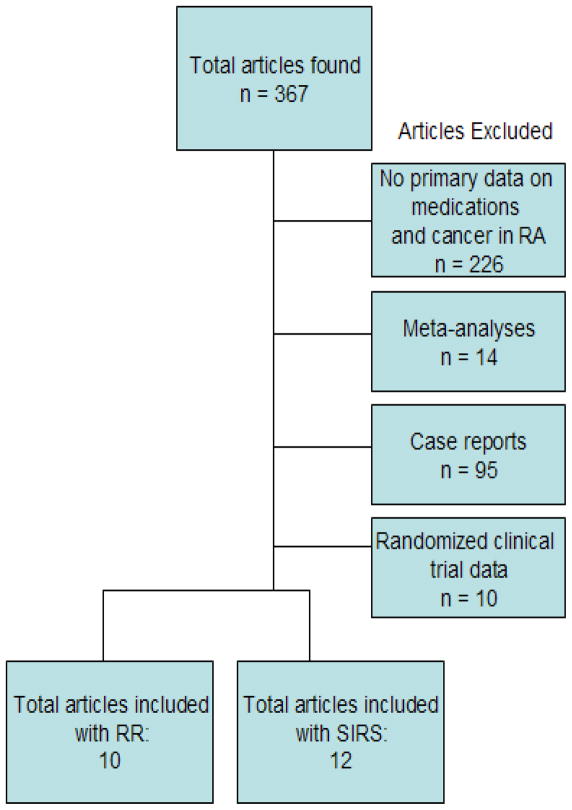
We searched for all English language articles regarding TNFi and cancer on PubMed using the search terms “anti tumor necrosis factor,” or one of each “abatacept,” “entanercept,” “adalimumab,” “anakinra,” “infliximab,” “rituximab,” and “malignancy,” or “cancer,” and “rheumatoid arthritis.” A total of 367 articles were identified found from this search. We excluded articles: without primary data on TNFi and cancer in RA (N = 226); meta-analyses (N=14); case reports (N=95); and randomized clinical trials (N=10). This left 11 articles that calculated relative risks for cancer associated with TNFi included as the primary results in this review (18–27). Twelve articles that included only standardized incidence ratios (versus relative risks) comparing RA patients to the general population were included as secondary results (8, 19, 22–25, 28–34). See Figure for a diagram of the literature search.
Figure.
This Figure describes the selection of articles from the literature for inclusion in this review.
Each article was read by at least two study investigators using a structured data abstraction form (available upon request) that focused on both the methodology and results. The goal of this structured review was to define important methodologic attributes as well as study results. Aspects of the methodology that were examined included study design (cohort versus case-control), cohort assembly (RA cohort, TNFi cohort, or other), non-TNFi comparator group (non-user versus user of other specific DMARDs), exposure definition (start date and stop date), outcome assessment (cancer diagnosis, cancer registry, self-report of cancer), covariates (which ones and when measured), and co-medications (which ones and when measured). For methodological issues not specified in the prior publications, authors were contacted to obtain additional information. All results reported in the prior publications were assessed. As our primary results, we report here relative risks or odds ratios comparing TNFi users to non-users, focusing on adjusted results. We also include two types of standardized incidence ratios calculated in the prior literature – comparing TNFi users to the general population and comparing subjects with RA to the general population.
RESULTS
Methodologic Considerations
Study design
All of the included studies used a cohort design, as opposed to a case-control or alternative designs.
Cohort assembly
Study cohorts can be assembled in many ways, possibly affecting the generalizability of results. Four of the studies used a Swedish population cohort to look at cancer rates by combining patients with RA diagnosis who were new users of TNFi’s according to the Swedish Biologics Register (ARTIS) (see Table 1). When the study cohort is assembled based on TNFi use such as what is observed in a biologics register, selection of comparator subjects can be problematic in that data may not have been collected on non-TNFi users in a parallel fashion. Enhanced methodology was observed in more recent studies, as an updated version of a previous paper (12) compared TNFi users with TNFi-naïve RA patients (20). Another Swedish study used the South Swedish Arthritis Treatment Group (SSATG) to develop the study cohort, which consists of TNFi starters (22). Three studies used data from the National Data Bank for Rheumatic Diseases (NDB) in the United States (21, 24, 25). The NDB includes RA patients using TNFi’s and other DMARDs. One study compiled insurance database information from the US and Canada, assembling a cohort with diagnoses of RA (23). Most of the studies included only new users of TNFi’s. However, the comparator subjects were not specified as new users, except in one study (23). Three studies did not specify whether they employed new or prevalent TNFi users (21, 24, 25). One study focused on the subset of RA patients with a prior cancer to determine the risk of a subsequent malignancy associated with TNFi (35).
Table 1.
Structured Review of Methodology Employed in Cohort Studies Regarding Cancer Risk Associated with TNF Inhibitors
| Author, Year (reference) | Cohort Assembly; mean age or range | Comparator Group | Exposure Definition | Endpoint Definition | Covariates | Concurrent Medications | |
|---|---|---|---|---|---|---|---|
| Lag period (days) | Once exposed always exposed | Definition and data sources | |||||
| Askling, 2009* (20) | New TNFi users from the Swedish biologics registry (ARTIS); mean 64 years | New users of non-biologic DMARDs | None | Yes | First cancer in national registry, confirmed with chart review in over 50% of subjects, including NMSC | Sociodemographics; time dependent comorbidities; RA characteristics, disease duration | Separate analyses focused on concurrent MTX and other nonbiologic DMARDS |
| Askling, 2009* (18) | New TNFi users from the National RA Cohort linked to Swedish Biologics Register; mean 64 years | TNFi-naive RA patients | None | Yes | Lymphoma diagnosis in national registry | Age, gender, sociodemographics, comorbidities | No |
| Askling, 2005* (19) | New TNFi users from the Swedish biologics registry (ARTIS); range 16–75+ years | TNFi-naïve RA patients | None | Yes | Hematopoietic malignancies in national registry | Age, gender, disease duration | No |
| Bernatsky, 2008 (20) | Subjects with RA found in the Provincial administrative database from Quebec; mean 62 years | Non TNFi-users with RA | None | No | Lung cancer diagnosis from physician billing and hospitalization records | Age, sex andd calendar year matched controls. Concomitant DMARDs and glucocorticoids, number of physician visits, co-morbidities, and extra-articular disease | Yes |
| Bernatsky, 2008 (21) | Subjects with RA found in the Provincial administrative database from Quebec; mean 62 years | Non TNFi-users with RA | None | No | Hematologic malignant neoplasms from physician billing and hospitalization records | Age, sex andd calendar year matched controls. Concomitant DMARDs and glucocorticoids, number of physician visits, co-morbidities, and extra-articular disease | Yes |
| Chakavarty, 2005† (21) | TNFi users in National Data Bank for Rheumatic Diseases (NDB); unclear if new or prevalent users; mean 63 years | TNFi-naïve RA patients | Not specified | Not specified | New NMSC confirmed by interview with patient | Age, gender, sociodemographics, disease duration, RA characteristics, smoking, NMSC prior to enrolling, prednisione, leflunomide, MTX (with and without TNFi) | Yes |
| Geborek, 2005 (22) | New TNFi users in the SSATG register; mean 60 years | TNFi-naïve RA patients in prevalent community based cohort | None | Yes | SSATG linkage to regional cancer registry, including cutaneous cancers | RA characteristics | No |
| Setoguchi, 2007 (23) | New biologic DMARD users from Medicare or British Columbia; mean 73 years | New MTX users in an RA cohort | 180 days | Yes | Diagnosis and procedure codes in healthcare claims database, including melanoma | Age, gender, RA characteristics | Yes |
| Wolfe, 2007† (25) | TNFi users from the NDB; unclear if new or prevalent‡; mean 59 years | Non TNFi treated patients in NDB | None | Yes | Lymphoma based on hospital and/or physician reports or death certificates | Age, gender, sociodemographics, comorbidities, smoking, RA characteristics, prednisone, and number of prior DMARDs | Yes |
| Wolfe, 2007† (24) | TNFi users from NDB; unclear if new or prevalent‡; mean 59 years | Non TNFi treated patients in NDB | 180 days | Yes | Patient questionnaire, interview, and medical record review, including melanoma and NMSC | Age, gender, sociodemographics, comorbidities, smoking, RA characteristics, prednisone | Yes |
| Dixon, 2010 (35) | TNFi users from the BSRBR; mean 64 years | Non TNFi treated patients in BSRBR | None | Yes | Linkage to national cancer registry, excluding NMSC and CIS | Age, sex, RA characteristics, disease duration, year of entry, and smoking status | Yes |
Abbreviations: MTX, methotrexate; RA, rheumatoid arthritis; TNFi, tumor necrosis factor inhibitor; NMSC, non-melanoma skin cancer; CIS, carcinoma in situ; NDB, National Data Bank for Rheumatic Diseases; BSRBR, British Society for Rheumatology Biologics Register
These studies all used data from the same population, although the specific cohort design varied.
These studies all used data from the National Data Bank for Rheumatic Diseases (NDB)
While Wolfe’s studies are performed in a cohort setting, analysis was done as if they were nested case-control studies. Analysis for these studies was done nested case-control studies.
Bernatsky’s studies were both nested case-control studies.
Comparator Group
The choice of a comparator (reference) group is critical. Comparators should be using alternative DMARDs (versus no DMARD or glucocorticoids) to better match the groups on the need for treatment. In addition, it facilitates interpretation if the comparator group is somewhat homogeneous, i.e., using one treatment or a few. Among the 11 studies included, the comparator groups were patients who had not received TNFi’s, i.e., TNFi naïve, but in most analyses there was no specific reference exposure (see Table 1). For the Swedish studies using a biologics register, a group of RA patients not in the biologics register were chosen as comparators (18–20). Since these comparator subjects are derived from a slightly different source population, this may introduce bias. However, the authors clarified that virtually all patients on anti-TNF were also (until censoring) members of the comparator RA cohort which in turn is reflective of the source population of all RA patients in the country. Most of the studies did not clearly specify which DMARDs were being used by the comparator group (18–22, 24, 25). Most studies compared methotrexate (MTX) users with RA to biologic DMARD users in an insurance database study (23).
Exposure Definition
The most difficult aspect of a drug safety study is the assessment of exposures and the risk-exposure window. The risk-exposure window refers to the time between the initiation and discontinuation of drug and the assessment of outcomes. We can consider two separate aspects of this window -- first, whether exposed subjects are starting drug or continuing drug. It is optimal to compare groups of subjects starting drug, the so-called new user design (36). If the risk of a toxicity such as cancer varies with exposure time, comparing new users to subjects who may have already used a drug for years can produce a biased result. The new user design limits the potential bias from differential “unmasking” of latent cancers with the initiation of a new DMARD. The second issue pertains to the lag time between the start of drug and the start of outcome assessment and the extension period after drug has discontinued. Depending on the proposed biologic relationship between the drug and outcome, the lag time might be 1 day or longer. Some have questioned whether cancers diagnosed soon after the start of a TNFi can possibly be related to the drug. Thus, one might consider a lag period of 90 or 180 days before cancers are considered related to the TNFi. In addition, once the TNFi has been discontinued, one could plausibly pursue three different options for assessing outcomes: 1) stop outcome assessment immediately; 2) stop outcome assessment after several half-lives; or 3) continue assessing outcomes ad infinitum.
The studies included in this review were inconsistent in defining exposures. Most gave little detail about the issues discussed above, with only two of 11 studies describing the lag period as 180 days (23, 24). In the Swedish studies, no lag period was employed. However, in at least one study, specific time periods of exposure were examined (20). All of the studies that specified an extension period considered TNFi exposure permanent -- once a patient was exposed to a TNFi, they were always exposed, and therefore would be kept in the exposed group until the study follow-up ended (18–20, 22–25).
Outcome Assessment
All of the Swedish studies used the endpoint definition of cancer based on a national registry of malignancy (18–20, 22). One of these articles confirmed the diagnosis with a chart review (20). Endpoint definition varied among the remaining four studies: one looked for skin cancer diagnoses based on interviews with the patient (21), one employed a tested methodology of searching for diagnosis and procedure codes in a healthcare claims database (23), another confirmed a patient questionnaire with an interview and medical records review (24), and the last examined hospital and/or physician records or death certificates (25).
Covariates
All studies attempted to adjust for confounders in the statistical models, but studies varied widely in how this was carried out. Another source of bias is confounding by indication. In other words, the indication for a given drug may be disease severity, which may not be measured completely, or at all, in some datasets. In the case of RA, severe RA may predispose towards the use of TNFi’s and may also be associated with cancer. However, the study databases included inconsistent information about RA and severity markers. One of the more recent Swedish studies included RA characteristics and disease duration(20). All three studies from the NDB adjusted extensively for confounding, including measures such as smoking and RA characteristics (21, 24, 25). The study from the health insurance database used a time-varying propensity score to attempt to adjust for confounders, however there were no RA-specific measures included (23).
Concurrent Medications
The use of multiple concurrent medications is the rule in RA. All DMARDs, as well as glucocorticoids and non-steroidal anti-inflammatory drugs, may have the potential to increase or decrease the risk of cancer. Thus, it is critical for investigators to clearly describe concurrent use of these medications. There are at least three ways to describe concurrent medication use in regression analyses. Each medication can enter the regression as a separate exposure. While accurate, this method makes interpretation of risk difficult in subjects who are exposed to multiple drugs. Explicit combinations of medications can be considered, so that a separate category for TNFi plus methotrexate users is entered into the regression. Finally, explicit hierarchies of medication “potency” can be described such that users of multiple DMARDs who concurrently use a TNFi would be included in the TNFi category. This is convenient but makes many assumptions.
Several of the studies did not describe the concurrent medication use (18, 19, 22). The studies from the NDB described included concurrent medications, but did so inconsistently (21, 24, 25). The study using health insurance information explicitly described the concurrent medications using a hierarchical approach (23).
Prior medication use is also important to consider, but we did not find studies that include prior medication as a covariate.
Association Between TNFi and Cancer
While methodology varied widely for these studies, the relative risks of cancer among TNFi users were similar and showed no increased risk of malignancy (see Table 2). Most studies found a numerically increased risk for lymphoproliferative cancers (18, 19, 22, 23) and non-melanoma skin cancers (NMSC) (21, 24). Increased risk estimates for lymphoma ranged from 1.1 (95% CI 0.6–2.1) (19) to 4.9 (95% CI 0.9–26.2) (22), with 4.9 being an outlier and more values falling near the estimate of 1.1. However, 2 studies indicated no additional risk of lymphoma among TNFi users (24, 25). One study showed a non-significant increase in risk of NMSC in TNFi users, 1.24 (95% CI 0.97–1.58) (21). When the combination of TNFi and MTX was considered, the relative risk was significantly elevated 1.97 (95% CI 1.51–2.58). Another study examining NMSC found an increased risk for TNFi users with an odds ratio of 1.5 (95% CI 1.2–1.8) (24).
Table 2.
Structured Review of Results of Cohort Studies Regarding Cancer Risk Associated with TNF Inhibitors
| Author, Year (reference) | TNFi-Treated | Reference Exposure | Results‡ (95% Confidence Interval) | ||
|---|---|---|---|---|---|
| N events | Person-years | N events | Person-years | ||
| Askling, 2009 (20) | 240 | 25,693 (25, 698 for biologics naïve comparison) | biologics naïve: 4,244 MTX: 260 nbDMARDs: 64 |
330,498 23,558 7,034 |
RR: 1.0 (0.87–1.17) RR: 0.99 (0.79–1.24) RR: 0.97 (0.69–1.36) |
| Askling, 2009 (18) | 26 | 26,981 | National RA TNFi naïve: 336 | 365,026 | Lymphoma RR: 1.35 (0.82 to 2.11) |
| Askling, 2005 (19) | 9 | 9,715 | 319 | 297,102 | Lymphoma RR: 1.1 (0.6–2.1) |
| Bernatsky, 2008 (26) | 2 | NA | 19 | NA | Lung Cancer RR: 0.84 (0.19–3.73) |
| Bernatsky, 2008 (27) | 3 | NA | 12 | NA | Hematologic malignancy RR: 1.92 (0.49–7.50) |
| Chakavarty, 2005 (21) | Not specified | 40,125 | Not specified | Not specified | NMSC HR: TNFi: 1.24 (0.97–1.58, p = 0.89) TNFi and MTX: 1.97 (1.51–2.58, p = 0.001) |
| Geborek, 2005 (22) | 16 tumors, 5 lymphomas | 1,603 | TNFi naïve: 69 tumors, 2 lymphomas | 3,948 | Lymphoma HR: 4.9 (0.9–26.2, p =0.07) |
| Setoguchi, 2006 (23) | *88 hematologic, 11 solid | 2,940 | MTX: 88 hematologic, 558 solid | 30,300 | HR: 0.98 (0.75–1.3) Lymphoproliferative HR: 1.11 (0.51–2.37) Hematologic malignancy HR: 1.37 (0.71–2.65) Solid tumor HR: 0.91 (0.65–1.26) |
| Wolfe, 2007 (25) | TNFi: 95 Biologic and MTX: Not specified |
10,364† 10,364† |
TNFi naïve: Not specified MTX only: Not specified |
Not specified Not specified |
Lymphoma OR: 1.0 (0.6–1.8, p = 0.875) OR: 1.1 (0.6–2.0, p =0.710) |
| Wolfe, 2007 (24) |
* All: 231 Leukemia: 13 Lymphoma: 23 Melanoma: 23 NMSC: 308 |
13,001† | TNFi naïve: All: 306 Leukemia: 11 Lymphoma: 22 Melanoma: 9 NMSC: 315 |
Not specified | OR: 1.0 (0.8–1.2) Leukemia OR: 1.2 (0.5–3.1) Lymphoma OR:1.0 (0.5–2.0) Melanoma OR:2.3 (0.9–5.4) NMSC OR: 1.5 (1.2–1.8) |
| Dixon, 2010 (35) | TNFi: 13 | 515 | DMARDs only: 9 | 235 | RR: 0.45 (0.09–2.17) |
Also included anakinra (n= 11 for Setoguchi and n = 6 for overall cancers for Wolfe)
Reports persons not person-years for entire cohort analyzed. Persons by comparison group or by person-years were not available.
Results are for all cancers unless specified. The cancers reported in the Results column were chosen based on several criteria: the main outcome of a given study; the risk of cancer overall; and the risk of solid tumors, lymphoma, leukemia, or skin cancer.
Abbreviations: RA, rheumatoid arthritis; MTX, methotrexate; TNFi, tumor necrosis factor inhibitor; RR, relative risk; SIR, standardized incidence ratio; RR, relative risk; HR, hazard ratio; OR, odds ratio; NMSC, non-melanoma skin cancer; NA, not applicable in a case-control study.
The most recent Swedish study (20) which also included the largest number of person-years, showed no increased risk of overall cancer when compared with TNFi naïve 1.00 (95% CI 0.87–1.17), MTX users 0.99 (95% CI 0.79–1.24), and nonbiologic DMARD users 0.97 (95% CI 0.69–1.36) (20). In this study, no clear pattern emerged between the number of patient years or the number of events and the overall risk estimate for malignancy, nor did any particular use of methodology tend to affect the risk estimate. The one study that examined recurrent cancers found no increased risk associated with TNFi use (35).
Standardized Incidence Ratios
As a secondary analysis, we examined the studies calculating SIRs (see Table 3), including both studies that calculated the SIR among patients using TNFi and those with RA not necessarily using a TNFi. Several consistent patterns emerge from theses data. The SIR for all cancers among TNFi users and RA in general is not increased. However, the SIR for lymphoma and hematologic malignancies is increased. For lymphoma, the SIR ranged from 1.8 (95% CI 1.5–2.2) to 6.0 (95% CI 1.6–15) (25, 33) among TNFi users and 1.7 (95% CI 1.3–2.2) to 5.4 (95% CI 1.1–15.7) (24, 28) among the general RA population. For hematologic malignancies, the SIR ranged from 2.0 (95% CI 0.2–7.3) to 4.1 (95% CI 1.3–9.5) (19, 33) among TNFi users and 1.7 (95% CI 1.2–2.4) to 8.8 (95% CI 2.4–22.6) (24, 28) among the general RA population.
Table 3.
Standardized Incidence Ratios from Studies Regarding Cancer Risk Associated with Rheumatoid Arthritis or Inflammatory Polyarthritis
| Article, year (reference) | N cancer events among subjects on TNF inhibitor | Population; mean age or range | Cancer? Exclusions | Stratifying variables | Standardized incidence ratios (95% CI) |
|---|---|---|---|---|---|
|
TNF Inhibitor Only
| |||||
| Askling, 2005 (29) | 67 solid cancers | Sweden; range 16–75+ years | Cancers occurring during first 365 days after entry into cohort are excluded | Age, sex, calendar period | Solid cancers: 0.9 (0.7–1.2) |
|
| |||||
| Askling, 2005 (19) | 9 lymphomas 2 leukemias |
Sweden; range 16–75+ years | Cancers occurring during first 365 days after entry into cohort are excluded | Age, sex, calendar period | Lymphoma: 2.9 (1.3 – 5.6) Leukemia: 2.0 (0.2 – 7.3) |
|
| |||||
| Geborek, 2005 (22) | 16 all cancers 5 lymphomas |
Sweden; mean 60 years | Previous malignancy | Age, sex, calendar period | All cancers: 1.1 (0.6 – 1.8) Lymphoma: 11.5 (3.7 – 26.9) |
|
| |||||
| Mariette, 2010* (32) | 27 lymphomas | France; mean 63 years† | None specified | None specified | Lymphoma: 2.3 (1.6–3.3) |
|
| |||||
| Pallavicini, 2010 (33) | 18 all cancers | Italian; mean 56 years | None specified | Age, sex | All cancers: 0.9 (0.6 – 1.5) |
| Milan | 4 lymphomas 5 heamatologic |
Lymphoma: 6.0 (1.6 – 15) Hematologic: 4.1 (1.3 – 9.5) |
|||
|
|
|
||||
| Varese | 18 all cancers 4 lymphomas 5 heamatologic |
All cancers: 1.1 (0.6 –1.7) Lymphoma: 5.0 (1.3 – 12) Hematologic: 4.1 (1.3 – 9.5) |
|||
|
| |||||
| Wolfe, 2004 (34) | 14 lymphomas | U.S.; mean 68 years | Lymphoma prior to enrollment in database | Age, sex | Lymphoma: 2.9 (1.7 – 4.9) |
|
| |||||
|
All RA Patients
| |||||
| Abasolo, 2008 (28) | 25 all cancers 3 non-Hodgkin’s lympomas 4 hematologic |
Spain; mean 61 years | None specified | Age, sex | All cancers: 1.23 (0.78 – 1.85) Lymphoma: 5.4 (1.1 – 15.7) Hematologic: 8.8 (2.4 – 22.6) |
|
| |||||
| Ekstrom, 2003 (8) | 8,898 all cancers 535 lymphomas |
Sweden; range 16–60+years | Diagnosis of another rheumatic disease, lost to follow-up, data irregularities, and death | Age, sex, calendar period | All cancers: 1.07 (1.05 – 1.09) Lymphoma: 2.00 (1.83 – 2.17) |
|
| |||||
| Franklin, 2006‡ (30) | 11 lymphomas | United Kingdom; mean 56 years | None specified | Age, sex, calendar period | Lymphoma: 2.4 (1.2 – 4.2) |
|
| |||||
| Setoguchi, 2006 (23) | 58 non-Hodgkin’s lymphoma | U.S.; mean 73 years | Cancer diagnosis or HIV | Age, sex | Lymphoma: 2.2 (1.7 – 2.9) |
|
| |||||
| Wolfe, 2007 (25) | 95 lymphomas | U.S.; mean 59 years | Lymphomas prior to enrollment in database | Age, sex | Lymphoma: 1.8 (1.5 – 2.2) |
|
| |||||
| Wolfe, 2007 (24) | 543 all cancers 45 lymphomas 24 leukemias |
U.S.; mean 59 years | All pre 6 month data excluded | Age, sex | All cancer: 1.0 (1.0 – 1.1) Lymphoma: 1.7 (1.3 – 2.2) Leukemia: 1.7 (1.2 – 2.4) |
Lymphoma includes Hodgkin’s and non-Hodgkin’s lymphoma, except where specified. Hematologic malignancy refers to leukemias primarily.
We include only the cases and SIR for the rheumatoid arthritis patients.
Mean age at cancer diagnosis
Inflammatory polyarthritis cohort and not only rheumatoid arthritis.
DISCUSSION
While treatment with TNFi has become an important part of the therapeutic armamentarium for several autoimmune diseases, the potential risk of cancer associated with TNFi remains a major concern for patients, providers, and regulators. Randomized controlled trials have convincingly demonstrated the clinical benefits of these drugs. However, because of their relatively small size, short follow-up and restricted patient populations, clinical trials have limited ability to assess toxicity, particularly uncommon risks such as cancer. Observational studies have several potential advantages – potential for very large populations and longer follow-up, heterogeneous subject population, and good data about comorbid conditions. For example, observational studies allow for examination of the risk of recurrent cancers among TNFi users (37), while RCTs would exclude such subjects. However, non-experimental study designs present many challenges. Recognizing the inherent challenges and limitations of observational studies regarding all exposures (TNFi or any other DMARD), our review finds a consistent lack of association between TNFi and cancer across the 11 papers included.
As others have mentioned (37), the methodologic challenges of observational studies include assessment of outcomes, characterization of TNFi exposure, comparison with an appropriate reference exposure, handling of concomitant treatments, and adjustment for potential confounders (see Table 4). Outcomes were assessed in different ways across the reviewed studies (see Table 2). While an accurate and complete assessment of cancer is the goal, most critical is the consistent assessment of outcomes across all subjects, independent of exposure. Exposure to a TNFi can begin with the first dosage or after some lag period. Exposure can end with the last dosage, after some number of half-lives beyond last dosage, or continue indefinitely. For example, to understand the risk of chronic viral infections (and possible related cancers), a long follow-up would be imperative. There is no known correct answer to these exposure questions and thus it is preferred to test these assumptions in secondary analyses. The reference exposure should be an active treatment used in similar circumstances as a TNFi. It should not be simply the absence of a TNFi, as this comparator is heterogeneous and poorly defined.
Table 4.
Key Methodologic Issues for Observational Studies of TNF Inhibitors and Cancer Risk
| Methodologic Issue | Considerations |
|---|---|
| Outcome assessment | Must be consistent across subjects without respect to exposure; use of an external cancer “registry” linked to the study database is ideal; subject report is of unknown accuracy |
| TNF inhibitor exposure definition | Exposure should begin with the first dosage or after some defined “lag period”; exposure can end with the last dosage, extend until after some number of half-lives beyond last dosage, or continue indefinitely after the last dosage; secondary analyses can help test the above assumptions |
| Reference group selection | Reference exposure should be active treatment used in similar circumstances as a TNFi (versus the absence of a TNFi); reference exposure and the TNFi should both be initiators versus ongoing use |
| Concomitant treatment inclusion | Other immunosuppressive treatments must be included as covariates or exposures of interest |
| Confounder adjustment | Other comorbid conditions and proxies for rheumatic disease severity should be included as covariates |
While we recognize that restricting the comparison group may limit the ability to generalize, the use of a fixed reference group, such as MTX, allows for easier interpretation and is the standard in most pharmacoepidemiology studies. The “non-use” reference groups (i.e., everyone not using at TNFi) means that each of the different exposures of interest is being compared to a different reference group; without a fixed comparator, determining the relative safety of a different DMARDs becomes impossible. It is true that the risk of cancer is not known with some active comparators. However, active comparators provide at least several key strengths: 1) they provide the most clinically relevant comparisons (i.e., clinicians are not deciding between no treatment versus a TNFi); 2) they increase the probability that patients in the two exposures are similar with respect to unmeasured confounders, such as disease severity; and 3) they allow for greater confidence that the diagnosis of rheumatoid arthritis is correct (a patient with diagnoses of RA without a DMARD has a lower probability of actually having RA versus a patient also using a DMARD). Moreover, the reference exposure and the TNFi should both be initiators versus ongoing use (36).
Concomitant immunosuppressive treatments may also be associated with cancer. Since combination therapy is so common in rheumatic disease, analyses of TNFi must include such treatments as covariates or as part of explicit exposure combination treatment categories. Without inclusion of these exposures there is the obvious potential for confounding. In addition, other confounders should be considered in adjusted models or matched on using propensity scores (38, 39). Disease severity presents the most difficulty as a confounder, since it likely relates to treatment choice and may associate with cancer. Clinical cohorts may have disease severity information but many cohorts only have sparse disease-specific data, and may not have it beyond the start of follow-up. If increased RA disease severity is associated with cancer risk and the use of TNFi is also associated with increased disease severity, then confounding would falsely elevate the RR of cancer found with TNFi. However, it is also possible that TNFi treatment is not used in patients with other comorbid conditions. Thus, in observational studies it may be difficult to predict the direction of bias by unmeasured covariates. It might also be anticipated, therefore, that treatment with TNFi should ultimately decrease the incidence of certain types of cancers. As treatment paradigms for RA become more aggressive, with novel therapies and tailored treatment regimens, the disease activity of RA should decrease. As noted, treatment with corticosteroids, which possess diverse anti-inflammatory properties, appeared to be associated with a lesser risk of lymphoma development (9). Over time, if a similar decrease is not observed with TNF inhibitors, perhaps factors other than just disease activity may be at play.
It is interesting to conjecture why most observational studies observe no increase cancer risk with TNFi’s (10, 11), but the meta-analyses of infliximab, adalimumab, and etanercept trials found these agents to be associated with cancer risk (12). This meta-analysis has been criticized for not using person-level data (40). The use of an average follow-up period for each study may have introduced bias away from the null. This would have occurred because the follow-up in the TNFi arms was longer, allowing for greater ascertainment of cancers. It is unclear that this bias would have been large enough to explain the elevation in risk observed. It may also be suggested that had the patients entering those clinical trials had more thorough screening, some of the observed cancers may have been picked up before study entry, thus decreasing the observed incidence (23). It has been shown that the risk of serious adverse effects with a given agent is not constant across autoimmune diseases, but tends to be highest in patients with established RA and Crohn’s disease, and lower in patients with early RA, psoriatic arthritis, and ankylosing spondylitis (14, 41) Some of this may be explained by factors such as comorbidity, age, concomitant medications and systemic inflammation. However, it raises caution as regards to extrapolating risks across distinct diseases.
This review has several important limitations. First, several of the studies reviewed include overlapping data. Thus, not each set of results comes from unique data. Second, some of the methodologic attributes that we list as “not specified” in Table 2 may have been specified by the author in their study protocol. However, we were not able to find this information in the respective manuscript. We also did contact all authors of studies where some information appeared to be “not specified” asking for clarification; their responses were included in Table 2.
In conclusion, we found 11 studies that met our inclusion criteria for epidemiologic analyses of cancer risk in TNFi users and 12 studies that calculated SIRs. Methodologies varied greatly across studies and many provided limited explanation of important methodologic attributes and most studies were of relatively short duration. Authors and editors should be urged to follow guidelines for conducting and reporting observational studies (37). With these caveats in mind, it is interesting to note that these studies found little if no cancer risk associated with TNFi’s. Comparison across studies would be greatly facilitated if methodologies were harmonized. The suggestions we provide in Table 3 may be a starting place for such conversations. Long-term follow-up of TNFi users may give different results because many cancers develop very slowly. The risk of cancer associated with new biologics should be examined as experience with these drugs accumulates.
Acknowledgments
Support: Abbott Immunology provided a research grant to Brigham and Women’s Hospital.
Footnotes
Disclosures: Dr. Solomon receives other salary support from NIH, AHRQ, Arthritis Foundation, and Amgen. He ran a clinical research course in the past three years sponsored by Bristol-Myers Squibb. Dr. Arthur Kavanaugh has received research grants from Abbott, Amgen, Centocor, and UCB.
References
- 1.Furst DE, Keystone EC, Fleischmann R, Mease P, Breedveld FC, Smolen JS, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases. Ann Rheum Dis. 2009;69 (Suppl 1):i2–29. doi: 10.1136/ard.2009.123885. [DOI] [PubMed] [Google Scholar]
- 2.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14(2):64–9. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Strangfeld A, Hierse F, Rau R, Burmester GR, Krummel-Lorenz B, Demary W, et al. Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res Ther. 2010;12(1):R5. doi: 10.1186/ar2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorley-Lawson DA. EBV the prototypical human tumor virus--just how bad is it? J Allergy Clin Immunol. 2005;116(2):251–61. doi: 10.1016/j.jaci.2005.05.038. quiz 262. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrom K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48(4):963–70. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 9.Hellgren K, Iliadou A, Rosenquist R, Feltelius N, Backlin C, Enblad G, et al. Rheumatoid arthritis, treatment with corticosteroids and risk of malignant lymphomas: results from a case-control study. Ann Rheum Dis. 2010;69(4):654–9. doi: 10.1136/ard.2008.096925. [DOI] [PubMed] [Google Scholar]
- 10.Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2010;20(2):119–30. doi: 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 11.Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68(7):1136–45. doi: 10.1136/ard.2008.091025. [DOI] [PubMed] [Google Scholar]
- 12.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 13.Bongartz T, Warren FC, Mines D, Matteson EL, Abrams KR, Sutton AJ. Etanercept therapy in rheumatoid arthritis and the risk of malignancies: a systematic review and individual patient data meta-analysis of randomised controlled trials. Ann Rheum Dis. 2009;68(7):1177–83. doi: 10.1136/ard.2008.094904. [DOI] [PubMed] [Google Scholar]
- 14.Burmester GR, Mease P, Dijkmans BA, Gordon K, Lovell D, Panaccione R, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;68(12):1863–9. doi: 10.1136/ard.2008.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10(2):R45. doi: 10.1186/ar2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green PEaS. Cochrane Collaboration open learning material for reviewers. 1.1. 2002. [Google Scholar]
- 18.Askling J, Baecklund E, Granath F, Geborek P, Fored M, Backlin C, et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish Biologics Register. Ann Rheum Dis. 2009;68(5):648–53. doi: 10.1136/ard.2007.085852. [DOI] [PubMed] [Google Scholar]
- 19.Askling J, Fored CM, Baecklund E, Brandt L, Backlin C, Ekbom A, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64(10):1414–20. doi: 10.1136/ard.2004.033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askling J, van Vollenhoven RF, Granath F, Raaschou P, Fored CM, Baecklund E, et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: does the risk change with the time since start of treatment? Arthritis Rheum. 2009;60(11):3180–9. doi: 10.1002/art.24941. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32(11):2130–5. [PubMed] [Google Scholar]
- 22.Geborek P, Bladstrom A, Turesson C, Gulfe A, Petersson IF, Saxne T, et al. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis. 2005;64(5):699–703. doi: 10.1136/ard.2004.030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setoguchi S, Solomon DH, Weinblatt ME, Katz JN, Avorn J, Glynn RJ, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2757–64. doi: 10.1002/art.22056. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–95. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56(5):1433–9. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 26.Bernatsky S, Clarke A, Suissa S. Lung cancer after exposure to disease modifying anti-rheumatic drugs. Lung Cancer. 2008;59(2):266–9. doi: 10.1016/j.lungcan.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Bernatsky S, Clarke AE, Suissa S. Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis. Arch Intern Med. 2008;168(4):378–81. doi: 10.1001/archinternmed.2007.107. [DOI] [PubMed] [Google Scholar]
- 28.Abasolo L, Judez E, Descalzo MA, Gonzalez-Alvaro I, Jover JA, Carmona L. Cancer in rheumatoid arthritis: occurrence, mortality, and associated factors in a South European population. Semin Arthritis Rheum. 2008;37(6):388–97. doi: 10.1016/j.semarthrit.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Feltelius N, et al. Risks of solid cancers in patients with rheumatoid arthritis and after treatment with tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64(10):1421–6. doi: 10.1136/ard.2004.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis. Ann Rheum Dis. 2006;65(5):617–22. doi: 10.1136/ard.2005.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J. Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood. 2002;99(11):3909–15. doi: 10.1182/blood.v99.11.3909. [DOI] [PubMed] [Google Scholar]
- 32.Mariette X, Tubach F, Bagheri H, Bardet M, Berthelot JM, Gaudin P, et al. Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Ann Rheum Dis. 2010;69(2):400–8. doi: 10.1136/ard.2009.117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallavicini FB, Caporali R, Sarzi-Puttini P, Atzeni F, Bazzani C, Gorla R, et al. Tumour necrosis factor antagonist therapy and cancer development: analysis of the LORHEN registry. Autoimmun Rev. 2010;9(3):175–80. doi: 10.1016/j.autrev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50(6):1740–51. doi: 10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 35.Dixon WG, Watson KD, Lunt M, Mercer LK, Hyrich KL, Symmons DP. Influence of anti-tumor necrosis factor therapy on cancer incidence in patients with rheumatoid arthritis who have had a prior malignancy: results from the British Society for Rheumatology Biologics Register. Arthritis Care Res (Hoboken) 2010;62(6):755–63. doi: 10.1002/acr.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 37.Dixon WG, Carmona L, Finckh A, Hetland ML, Kvien TK, Landewe R, et al. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann Rheum Dis. 2010;69(9):1596–602. doi: 10.1136/ard.2009.125526. [DOI] [PubMed] [Google Scholar]
- 38.Schneeweiss S, Patrick AR, Sturmer T, Brookhart MA, Avorn J, Maclure M, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 Supl 2):S131–42. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161(9):891–8. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon W, Silman A. Is there an association between anti-TNF monoclonal antibody therapy in rheumatoid arthritis and risk of malignancy and serious infection? Commentary on the meta-analysis by Bongartz et al. Arthritis Res Ther. 2006;8(5):111. doi: 10.1186/ar2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson GM, Nakada MT, DeWitte M. Current Opinion in Pharmacology. Elsevier; 2004. Tumor necrosis factor- in the pathogenesis and treatment of cancer; pp. 314–320. [DOI] [PubMed] [Google Scholar]