Abstract
Despite data linking amphibole asbestos exposure with production of autoantibodies, the role of autoantibodies in subsequent disease is unknown. Residents of Libby, Montana have experienced significant exposure to amphibole asbestos due to the mining of asbestos-contaminated vermiculite near the community over several decades. This population predominantly exhibits pleural disease, and an autoimmune-like disorder that has yet to be well defined. This study sought to determine whether autoantibodies from asbestos-exposed subjects were associated with pleural lesions. Serum samples of subjects from Libby were evaluated for anti-nuclear antibodies (ANA) and mesothelial cell autoantibodies (MCAA) using cell based ELISA. The presence of radiographic abnormalities detected during the time frame of serum collection was determined from screening records. In accord with previous studies, 61.3% (76/124) of the Libby samples were ANA positive, a frequency much higher than expected for a healthy population. The odds of having pleural or interstitial abnormalities in Libby was nearly 3.55 times greater for individuals that tested positive for ANA compared with individuals negative for ANA (p =0.004). MCAA were also detected at a strikingly high frequency (18.5%; 23/124) in samples from Libby. Individuals with MCAA had 4.9 times the risk of having pleural abnormalities compared to MCAA-negative subjects (p=0.044). In conclusion, ANA and MCAA were elevated in a study population that was known to have chronic exposure to asbestos, and these autoantibodies were associated with pleural abnormalities, the predominant finding in the asbestos-exposed population of Libby. Additional research is needed to determine the role these autoantibodies may play in pulmonary disease.
Keywords: Libby MT, amphibole, Autoimmune disease, pleural
1.1 Introduction
Autoimmunity is a large and growing problem not only with known organ-specific and systemic autoimmune diseases, but also with many diseases being identified as having an autoimmune component. The propensity to develop autoimmunity may stem from an intricate relationship among genetics, life history, and exposure to environmental factors. For example, there are several extensive reviews summarizing the link between exposure to silica and autoimmune disease (Brown et al., 2004; Cooper et al., 2008; Hess, 2002). Although less extensively studied, there is evidence to suggest that exposure to asbestos is associated with autoimmune responses including increased serum immunoglobulins, positive autoantibody (ANA) tests, and immune complex deposition (reviewed in Bunderson-Schelvan et al., 2011; Lange et al., 1974; Niagam et al., 1993; Pernis et al., 1965; Pfau et al., 2005; Pfau et al., 2008; Turner-Warwick and Parkes, 1970). Studies regarding the health effects of asbestos exposure are critical and useful on a variety of levels. The United States has mined roughly 30 million tons of asbestos, exposing nearly 27 million people to its harmful effects (Mossman et al., 1990; Nicholson et al., 1982). Exposure to asbestos continues to occur throughout the world via occupational and environmental routes making it a current health concern. Amphibole asbestos and other asbestiform ores are used in many materials in developed and developing countries, creating serious health risks since exposure to such materials is linked to the development of malignant and non-malignant diseases such as lung cancer, pulmonary fibrosis (asbestosis), and increased autoimmune antibody production. In addition, since amphibole asbestos persists in the environment, exposures will continue to occur wherever it has been introduced either occupationally or environmentally (Anderson et al., 2005).
The premise that amphibole asbestos exposure exacerbates autoimmunity is supported by recent studies from the asbestos exposed population in Libby, MT (Noonan et al., 2006; Pfau et al., 2005; Pfau et al., 2008). Due to the mining, transportation, and processing of asbestos-contaminated vermiculite from 1924 to 1990 at the nearby Zonolite mountain, residents of Libby have experienced significant exposure to asbestos (Bandli and Gunter, 2006). The extensive mining at Zonolite once provided approximately 80% of the world’s vermiculite, which happened to be contaminated with naturally occurring asbestos fibers (Bandli and Gunter, 2006). The distinct mixture of asbestos amphibole fibers found in the Libby vermiculite makes it particularly pathogenic. Amphibole asbestos such as that described from the Libby mine is unique in terms of length (the presence of relatively long, greater than 20 μm, fibers) and metallic cations such that it is not readily cleared from the lungs by macrophages and thus more prone to cause disease compared to the more commonly used commercial asbestos, chrysotile (Meeker, et al, 2003; Bernstein et al., 2005; Sanchez et al., 2009; Tomatis et al., 2010).
Libby patients are often observed to have reduced pulmonary function and increased mortality from lung cancer including malignant mesothelioma, or progressive fibrotic disease (McDonald et al., 2004; Whitehouse, 2004). The asbestos-related disease (ARD) in the Libby population is most often distinguished by pleural abnormalities, with a unique subset of patients displaying rapid disease progression (Whitehouse, 2004). In addition, studies have reported an association between Libby amphibole asbestos exposure and increased frequency of positive antinuclear autoantibody (ANA) tests, as well as a possible increased risk of systemic autoimmune disease (SAID), particularly rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (Noonan et al., 2006). It is not known whether a discrete clinical or subclinical autoimmune entity is associated with amphibole asbestos exposure.
Autoimmune diseases are characterized by autoantibody profiles that can be used to help predict disease and establish a diagnosis, as well as assess clinical progression. For example, antibodies to dsDNA and histones are indicators for SLE. Similarly, an autoantibody profile characterized by anti-fibroblast antibodies may be predictive of scleroderma (Chizzolini et al., 2002). Determining whether autoantibodies are associated with ARD onset or ARD progression could be critical in terms of disease development toward disability and death and the prediction thereof. ANA test positivity appears to correlate with pulmonary (either pleural or interstitial) disease severity, raising the possibility that an autoimmune process is contributing to disease (Pfau et al., 2005). We hypothesized that autoantibodies may be targeting pulmonary tissues leading to tissue-specific cytotoxicity. We have shown that the Libby serum samples contain autoantibodies that bind to fibroblasts (anti-fibroblast antibodies, AFA), but in that cross-sectional analysis, we did not find a relationship between those antibodies and lung disease in those patients (Pfau et al., 2008). Since the Libby ARD has been reported to present predominantly with pleural abnormalities (Peipins et al., 2003; Whitehouse, 2004), we hypothesized that mesothelial cells of the pleural lining may be potential targets for autoantibodies that could play a role in pleural lesions. The objective of this study was to explore the relationship between Libby amphibole exposure, pulmonary abnormalities, and autoimmunity by analyzing the frequency of pleural or interstitial abnormalities in subjects with or without mesothelial cell autoantibodies (MCAA).
1.2 Materials & Methods
1.2.1 Human Serum Samples and Demographic Data
All serum samples were collected by the Center for Asbestos Related Disease (CARD) in Libby, Montana or by the Center for Environmental Health Sciences in Missoula, Montana under a project approved through the Institutional Review Board (IRB) of the University of Montana, Missoula (Pfau et al., 2005). Follow-up studies on the banked serum samples were given IRB approvals from Idaho State University and the University of Washington School of Medicine. A total of 124 randomly selected Libby serum samples (from a banked set of 318 samples) were tested, of which 65 were from females and 59 were from males. Libby subjects had a mean age of 50 (range = 14–84 years). Twenty-five Missoula samples were also tested, of which 13 were male and 12 were female, with an overall mean age of 45 (range = 19–78 years). This was a reference population with similar demographics and climactic conditions compared to Libby (Pfau et al., 2005), and was used to set the positive/negative cut-off for MCAA, within a healthy population with no known asbestos exposure based on a questionnaire that included questions about potential home or occupational exposure. Blood samples were first collected, from which the serum samples were obtained by standardized methods and stored at −80°C until needed. All Libby samples collected were coded in a database containing sex, age, screening radiograph results, and exposure as determined by a questionnaire previously described in Pfau et al. (2005). Radiographic abnormalities were determined during ATSDR cross-sectional screenings, as described in Peipins et al. (2003). Although a separate project, radiographic data from the closest screening previous to our sample collection were used. Because the Libby pleural disease has been documented to be progressive (Whitehouse, 2004), our estimations of radiographic abnormalities at the time of serum collection are conservative.
Smoking history and asbestos exposures for the Libby subjects included in this study closely represented other descriptions of the Libby cohort (Pfau et al., 2005; Noonan et al., 2006), in which approximately 55–60% report having smoked at some time in their lives, approximately 64% lived or worked in Libby for longer than 5 years, and 30% indicate both occupational and environmental amphibole exposures. These factors were not available at the individual patient level for this study, so they could not be controlled for in our analysis. This may have introduced bias in our results; however, these factors have not been linked to MCAA, so it seems unlikely that their inclusion would have changed the overall conclusions of our study, given the strong associations detected.
1.2.2 Pulmonary Radiographic Rankings
The pulmonary lesions reported in Libby include primarily pleural and some interstitial abnormalities (Peipins et al., 2003). The data were intentionally simplified to a scale, based solely on radiographic evidence of pleural or interstitial abnormalities. The simplified ranking system was established to allow us to address our hypothesis that the autoantibodies (especially MCAA) would be associated with subjects with pleural lesions. The rankings are not intended to represent severity of disease, but rather to simply separate subjects into three groups: a) no evidence of radiographic abnormalities (designated with a zero), b) any diffuse or focal interstitial abnormality but no pleural lesion (designated with a 1), or c) any pleural abnormalities (designated with a 2). Subjects were given a 2 if they exhibited various forms of pleural abnormalities, with or without interstitial lesions. This latter type of abnormality is the most common pulmonary finding in the Libby population and often presents as either pleural thickening or pleural plaque development, both of which scored a 2 in our system.
1.2.3 Detection of Serum Autoantibodies
An indirect immunofluorescence test for autoantibodies to nuclear antigens (ANA assay) was performed on all the sera. Serum samples were diluted 1:40 in a phosphate-buffered saline (PBS) solution. Commercially prepared HEp-2000 Fluorescent ANA-Ro Test slides (ImmunoConcepts Inc., Sacramento, CA) were used for the semi-quantitative detection of antinuclear antibody in the samples. The manufacturer’s protocol was strictly followed for all immunofluorescence testing. Substrate slides were prepared with 25 μl of sample per well and then left to incubate in a moist, covered chamber for 30 minutes at room temperature. Individual wells were then washed with PBS, followed by a 10 minute PBS wash in a slide staining dish. Pre-diluted FITC-conjugated goat anti-human antibody (ImmunoConcepts) was then added to each well (25 μl/well). The slide was again allowed to incubate for 30 minutes in a moist, covered chamber at room temperature and then washed with PBS. Finally the coverslip was mounted and any excess mounting media was cleaned from the slide.
Positive and negative quality controls provided by ImmnuoConcepts were included on each slide. The staining pattern and relative fluorescence intensities were compared with the manufacturer’s positive and negative controls using a Zeiss fluorescence microscope at 400 × magnification. Staining patterns were noted and samples were recorded as positive (1+ to 4+ based the intensity of the fluorescence) or negative (0). Subsequently categories 3 and 4 were combined because of the limited number of individuals with the highest score. Results were also classified as positive or negative for some of the statistical analyses. Any information regarding the source (Libby or Missoula) of samples or the radiographic data was blinded to the two independent readers. In addition to the scoring, the staining pattern was determined for all samples. The same microscope and settings were used to read all slides.
1.2.4 Cell Based ELISA for Mesothelial cell autoantibody (MCAA)
A cell based ELISA was performed to test sera for the presence of anti-mesothelial antibody in both the Libby and Missoula samples. 100 μl per well of MET-5A human mesothelial cells (ATCC, Manassas, VA) at a density of 5 × 105 cells/ml were plated in RPMI (CellGro Mediatec, Manassas, VA) containing 10% fetal calf serum and antibiotics in a 96-well culture plate (Greiner Cellstar). The cells were allowed to adhere and grow to confluence overnight at 37°C in a 5% CO2 incubator. After the overnight incubation the cells were washed carefully one time with phosphate-buffered saline solution (PBS) and fixed by adding 100 μl of 1% paraformaldehyde per well for one hour at room temperature. Following fixation the cells were washed one time with PBS and then blocked for one hour at room temperature with 200 μl per well of PBS containing 3% bovine serum albumin (BSA). Serum samples were then prepared as the primary antibody at a 1:100 dilution in 3% BSA/PBS and 100 μl of each sample was added to the appropriate well in quadruplicate. The plate was allowed to develop with the primary antibody for two hours at room temperature, during which the plate was gently rocked by hand. Then the cells were gently washed three times with 0.5% Tween-20 in PBS. The secondary antibody (HRP-conjugated goat anti-human IgG (Jackson ImmunoResearch, West Grove, PA) was then applied at a dilution of 1:1000 in 3% BSA/PBS and again allowed to incubate for one hour at room temperature. After gently washing the plate three times with PBS, 100 μl of HRP-TMB substrate (Thermo Scientific 1-Step Ultra TMB-ELISA) was added to the wells and allowed to develop color. The reaction was stopped with 50 μl of 2N HCl and the plates were analyzed on a microtiter plate reader (BioTek Instruments, Winooski, VT) at 450nm. Non-specific secondary antibody binding was corrected for on a plate to plate basis by subtracting the mean optical density (OD) for the secondary antibody-only wells from the mean OD of each sample.
Twenty-five individuals from Missoula, with no known exposure to asbestos, were used to determine a cut-off for the presence of anti-mesothelial cell antibodies. The mean OD reading from these samples was calculated and 3 times the standard deviation of these samples was added to this value to establish the positive cut-off value for the presence of MCAAs. Any samples with an OD value greater than this value were considered positive.
1.2.5 Immunocytochemistry for Mesothelial cell autoantibody (MCAA)
Fluorescence microscopy was performed to determine the location of MCAA binding to mesothelial cells. Met-5A cells were seeded on a 4-well, culture treated chamber slide (Nalge Nunc International) at a density of 5×104 per well in 500 μl RPMI. Following an overnight incubation, cells were treated as described for the cell-based ELISA, with the following exceptions. Primary antibody consisted of serum previously identified at MCAA-positive, or matched serum cleared of antibody (IgG isotype) using protein G agarose beads (Thermo Scientific). Secondary antibody was FITC conjugated rabbit anti-human IgG (Southern Biotech). Cells were visualized on a Leica DMR fluorescence microscope and images acquired using Spot software version 4.0.9.
1.2.6 Statistical Analysis
The prevalence of ANA and MCAA was determined by dividing the number of positive samples by the total number of individuals tested in Libby MT. The same descriptive statistic was done for the samples collected from Missoula MT. The odds of pleural and interstitial lesions were compared for individuals with ANA and MCAA separately using a binary logistic regression. The prevalence of autoantibodies in individuals with these types of pulmonary abnormalities was compared to the prevalence of autoantibodies in individuals without any radiographic evidence of pulmonary abnormalities from Libby. The initial models controlled for age and sex; however, these parameters were never significant (P value always greater than 0.05) and therefore removed from the final models. We also examined whether there was an increasing occurrence of pulmonary (either pleural or interstitial) lesions with an increasing ANA score (0–3) using a Cochran-Armitage test of trend in SAS (SAS 2008, release 9.2). Statistical significance was established as a p-value less than 0.05. All statistical analyses were performed with the use of Minitab 15 software or SAS.
1.3 Results
1.3.1 Frequency of Antinuclear Antibodies
A total of 124 Libby serum samples were tested in this study, of which 76 (61.3%, CI = 52.1% to 69.9%) were positive for ANA, scoring 1–4 on a scale of 0–4 where a score of zero was negative for antibody presence. This number was consistent with results previously published on this population (Pfau et al., 2005). Normal populations will screen positive for ANA at a 1:40 dilution approximately 20–30% of the time (Tan et al., 1997). The homogenous staining pattern (47% of positive samples) was the most commonly seen pattern in the Libby population. This suggests anti-nuclear antibodies targeting various chromatin components were present in the patients’ sera. The Scl-70 and nucleolar patterns were also commonly seen (22% and 16%, respectively). The speckled pattern was seen in 9% of the positive samples. Statistical analysis revealed no relationship between ANA pattern and either pleural or interstitial radiographic abnormalities.
1.3.2 Presence of Antibodies to Mesothelial Cells
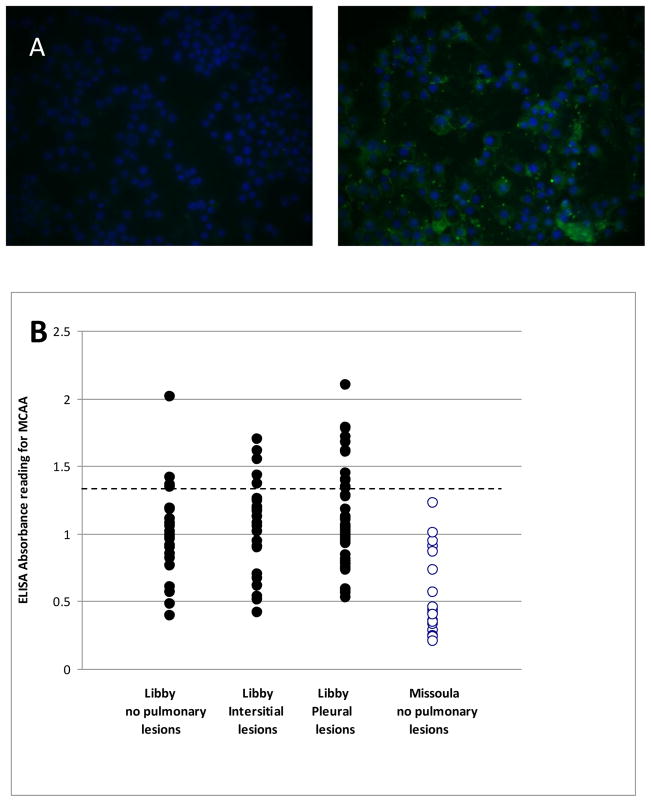
Individuals from Libby MT not only possess anti-nuclear antibodies as revealed by the ANA test, but they also had autoantibodies against non-nuclear antigens of mesothelial cells (Figure 1a). There was no serum staining of the nuclei, and the peripheral staining indicates that at least most of the staining was at the surface of the cells. A total of 149 serum samples were tested in this manner (124 Libby samples and the 25 Missoula samples). Results of the cell-based ELISA with the MET-5A cells revealed a mean absorbance for the Libby samples of 1.096 (SEM = 0.0311). In contrast, the mean absorbance for the Missoula samples was 0.5373 (SEM = 0.056). Using the mean for the Missoula samples plus three standard deviations as a positive cut-off, 23 samples (18.5%) from Libby MT were positive for MCAA (Figure 1b).
Figure 1.
Anti-Mesothelial Cell antibodies. A. Visualization of surface binding of human serum autoantibodies. Met-5A cells grown on chamber slides were plated, fixed and stained exactly as for the cell-based ELISA, but the secondary antibody was anti-Mouse FITC and nuclei were counterstained with DAPI. Image on the left is stained with serum cleared of IgG using Protein G; image on the right is stained with uncleared serum from a subject from Libby. B. Optical density scores for mesothelial cell autoantibodies (MCAA) in Libby and Missoula serum samples. Cell-based ELISA analysis of Libby and Missoula serum samples revealed a significant difference between the average absorbance levels for MCAA in Missoula and Libby MT populations (p<0.001). Positive samples were defined as any having absorbance readings higher than three standard deviations above the mean value of the Missoula samples (Abs >1.38, dotted line). The 23 Libby samples were determined to be MCAA positive based on this definition.
1.3.3 Pulmonary Radiographic Abnormalities and ANA
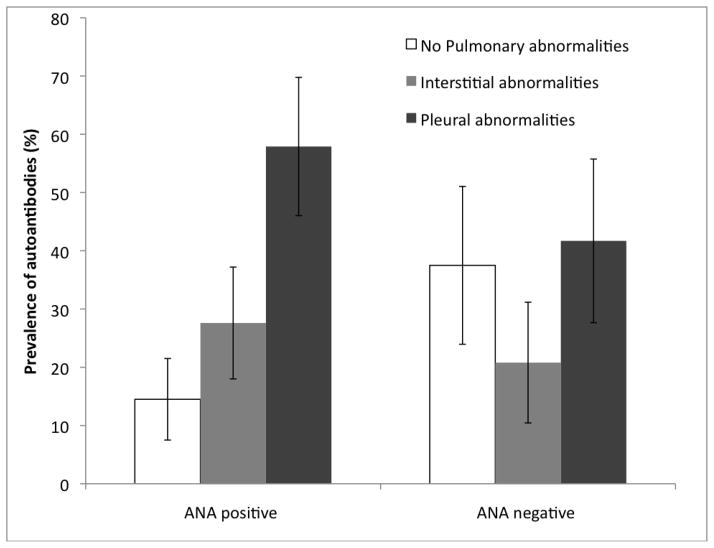
Pleural abnormalities were seen in 52% of the Libby subjects included in this project, while another 25% had interstitial abnormalities. The remaining 29 individuals from Libby did not have detectable pulmonary lesions (neither pleural nor interstitial). Nearly 58% (44/76) of the Libby subjects who were ANA positive (receiving an ANA score of 1–4) presented with pleural abnormalities, while almost 28% (21/76) of ANA positive subjects had interstitial abnormalities (Figure 2). Only about 14% (11/76) of the ANA positive subjects had no radiographic findings (Figure 2). Of the individuals without ANAs (n=48), 42% (20/48) had pleural abnormalities, 21% (10/48) had interstitial abnormalities and 37% (18/48) had normal pulmonary radiographs (Figure 2).
Figure 2.
Prevalence of pulmonary radiographic abnormalities (lesions) including 95% confidence intervals (indicated with bars) among individuals from Libby MT with and without autoantibodies to nuclear antigens (ANA). There were 76 ANA positive subjects, and 48 ANA negative subjects analyzed.
These data were further analyzed by logistic regression analysis. On average it was determined that the odds of having pulmonary abnormalities (either pleural or interstitial) was 3.55 (95% confidence interval 1.49 – 8.43) times greater for individuals with positive ANA tests compared with individuals without positive ANA tests (p=0.004) (Table 1). When examined separately, the odds ratio for pleural abnormalities comparing ANA positive to ANA negative subjects was 3.60 (95% CI: 1.44–9.01), and for interstitial abnormalities the odds ratio was 3.44(95% CI:1.19–9.95) (Table 1). In addition, on average, a higher ANA score was associated with a higher risk of radiographic abnormalities (Cochran-Armitage Trend X2 (df=1) = 7.86; p = 0.005).
Table 1.
Odds ratios and p-values associated with the logistic regression analyses used to compare the radiographic abnormalities of individuals with anti-nuclear antibodies (ANA) and anti-mesothelial antibodies (AMA).
| Antibody status | Pleural abnormalities | Interstitial abnormalities | Any pulmonary abnormalities (Either pleural or interstitial) | |||
|---|---|---|---|---|---|---|
| OR (95% confidence interval) | p valuea | OR (95% confidence interval) | p valueb | OR (95% confidence interval) | p value | |
| ANA +vec | 3.6 (1.44, 9.01) | 0.006 | 3.44 (1.19, 9.95) | 0.023 | 3.55 (1.49, 8.43) | 0.004 |
| AMA+ve | 4.88 (1.05, 22.77) | 0.044 | 2.0 (0.34,11.85) | 0.445 | 3.83 (0.84, 17.44) | 0.082 |
Comparisons were between individuals with pleural abnormalities and with no radiographic evidence of either interstitial or pleural abnormalities. The reference categories were individuals with no detectable autoantibodies. Age and sex were initially included in the model, however they were never significant; therefore they were excluded from the final models.
Comparisons were between individuals with interstitial abnormalities and with no radiographic evidence of either interstitial or pleural abnormalities. The reference categories were individuals with no detectable autoantibodies. Age and sex were initially included in the model, however they were never significant; therefore they were excluded from the final models.
+ve = positive, −ve = negative
1.3.4 Pulmonary Radiographic Abnormalities and MCAA
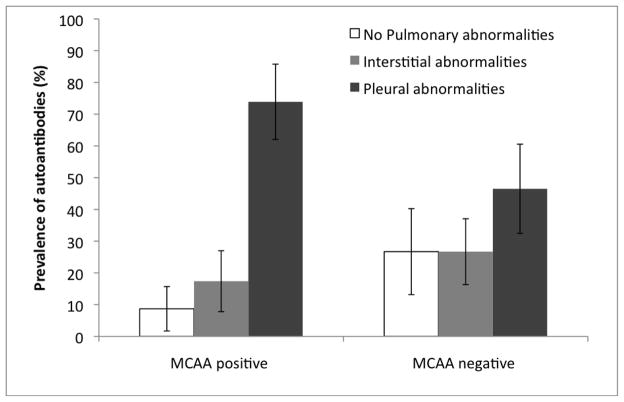
The relationship between radiographic status and MCAA positivity was also investigated. It was found that 17 of the 23 (74%) samples that were determined to be MCAA positive had pleural abnormalities (Figure 3). Four of the 23 (17%) MCAA positive samples had interstitial lesions while only two (9%) were free of pulmonary findings (Figure 3). In contrast, 27 of the 101 (27%) individuals that were negative for MCAAs had no abnormalities, 47 (47%) had pleural abnormalities, and 27 (27%) had interstitial abnormalities (Figure 3). Statistical analysis revealed that on average the odds of having pleural abnormalities was 4.88 (95% CI: 1.05–22.77) times greater for individuals with positive MCAA compared to subjects without positive MCAA levels (p=0.044) (Table 1). Importantly, there was no significant relationship between MCAA positivity and interstitial abnormalities (p=0.445).
Figure 3.
Prevalence of pulmonary radiographic abnormalities (lesions) including 95% confidence intervals (indicated with bars) among mesothelial cell autoantibodies (MCAA) positive and negative individuals from Libby. There were 23 MCAA positive and 101 MCAA negative samples.
1.4 Discussion
Autoimmunity is, and will continue to be, a major cause of disease and disability throughout the world. Although the malignant form of ARD, mesothelioma, receives considerable attention, there is growing concern about the autoimmune element of ARD, at least among the Libby MT population. This study supports previous preliminary studies that reported a high frequency of ANA in a small set of samples from this population (Pfau et al., 2005; Pfau et al., 2008) by demonstrating that over 61% of the new samples tested had anti-nuclear antibodies. These autoantibodies are usually present in less than 30% of the population at the 1:40 serum screening dilution. ANA are associated with systemic autoimmune diseases although their contribution to actual disease is not always clear. If the presence of these autoantibodies precedes systemic autoimmune diseases, our results suggest the Libby population may be more prone to develop these diseases given their high levels of ANA. Further, the staining patterns most commonly seen in this population (homogeneous and nucleolar) are associated with diseases such as SLE and systemic sclerosis. Higher ANA scores were more likely to have radiographic abnormalities than low ANA scores, consistent with our previous study (Pfau et al., 2005).
Although it remains uncertain why the population of Libby MT has such a high prevalence of autoantibodies, it is possible that asbestos exposure may have triggered their production. Asbestos exposure has been associated with the production of ANA (Reviewed in Bunderson et al., 2011), possibly through a mechanism similar to that hypothesized for silica which includes release of antigen from dying cells (Cooper et al., 2008). Amphibole fibers are biopersistent and cytotoxic, possibly resulting in repeated exposure of the immune system to cellular debris. Fibers reaching the pleural cavity would cause damage to the mesothelial cells that line the cavity: over time, mesothelial debris could lead not only to production of ANA, but also to autoantibodies to mesothelial surface proteins. This mechanism, in which apoptotic debris provides the antigens for autoimmune responses, has been proposed for production of autoantibodies following exposure to silica or asbestos (Brown et al., 2004; Blake et al., 2008), and in diseases such as SLE and SSc (Muñoz et al., 2010; Kahaleh, 2008). The data reported here are admittedly indirect evidence that asbestos caused these autoantibodies, and also that they are now contributing to the pleural disease in these patients. The population of Libby was exposed for over 60 years to various levels of amphibole asbestos and, as expected, now has unusually high levels of ARD (Peipins et al., 2003). In addition, they also have higher levels of systemic autoimmune diseases than would be expected in an unexposed population, which also implicates asbestos exposure as the trigger (Noonan et al., 2006). Therefore, this study challenges us to explore the mechanisms in future studies, based on the hypotheses mentioned above.
The possible role of autoantibodies to epithelial cells and fibroblasts in vascular and fibrotic disorders has received attention as evidence of their role in the pathogenicity has grown (Chizzolini et al., 2002; Henault et al., 2006). Since pleural abnormalities seem to account for a large percentage of the ARD of the Libby subjects, we investigated the possible presence of autoantibodies to mesothelial cells, the epithelial cells lining the pleural cavity. Our results show that 18.5% of the Libby population had measurable mesothelial cell autoantibodies (MCAA) by our ELISA technique. Such a tissue-specific autoantibody could target the lung pleura, causing plaque formation and pleural thickening through complement activation, FcR-mediated cytotoxic pathways, or through cell signaling that activates the cells (Arnett, 2006). Given this mechanism, we hypothesize that, after damage is initiated by amphibole fibers and self-antigen is repeatedly released, the resulting autoantibodies could potentially promote ARD and its progression in the Libby population. Our results show that both types of autoantibodies (ANA and MCAA) are associated with pleural abnormalities (Table 1). Individuals with either of these antibodies were on average between 3 and 5 times more likely to have pleural abnormalities.
Being ANA positive was also associated with interstitial abnormalities, resulting in a very strong association between having antinuclear autoantibodies and any radiographic abnormalities. Importantly, however, being MCAA positive did not increase the odds that a subject would have interstitial abnormalities (Table 1), highlighting the fact that these MCAA are specific to subjects with pleural changes. This is important because pleural abnormalities are the most common radiographic findings among the population being studied, both in our study and as reported by others (Whitehouse, 2004). Although this study does not provide evidence of the chronology of events, previously published evidence supports the appearance of autoantibodies prior to the onset of clinically overt disease (Hess, 2002; Turner-Warwick and Parkes, 1970), and seroconversion to ANA positive has been reported to correlate with increased pulmonary disease severity (Tamura et al., 1993; Tamura et al., 1996). Definitive identification of an autoantibody contributing to ARD in this population could be a potential breakthrough in managing their disease. Further mechanistic exploration of the cellular function effects of MCAA binding to mesothelial cells is in progress.
This study was performed entirely on banked samples for which the ARD status for each patient had been entered solely from early screening. It is therefore important to note that this introduces caveats to the interpretation of the results. First, assessment of radiographic findings is challenging, and mis-interpretation is always a risk. However, this study did not attempt any assessment of severity: only the presence or absence of apparent radiographic lesions was used. With this initial assessment providing the rationale, larger and prospective studies will be designed in which further radiographic details can be considered. It will be important to follow the subjects who were positive for MCAA but did not have pleural abnormalities, to determine whether lesions and disease developed over time. These planned studies will also be able to look at possible factors such as smoking and specific exposure histories that were not included here.
Another caveat is that ANA testing is subject to sensitivity and specificity issues. The fact that both independent readers were blinded to the sample identifications helps remove bias. Perhaps a more important issue is that it will be extremely difficult to establish a causal relationship between ANA and disease progression. The relationship reported here between ANA and any radiographic abnormalities could simply be due to exposure issues, in which higher/longer asbestos exposures may lead to both ANA and worsened disease progression, but that these latter effects are not causally related to each other. The MCAA assay has less subjectivity than ANA testing, and the data reported here suggest that MCAA are specifically associated with only one type of lesion in Libby, making it less likely (although still possible) that this is purely an effect of exposure amount or duration. Lastly, the Libby amphibole appears to be a unique combination of fibers, with a disease presentation that has been, and continues to be, re-evaluated (Bandli and Gunter, 2006; Peipins et al., 2003; Whitehouse, 2004). Studies are currently underway in our laboratory to explore autoimmune outcomes in different asbestos-exposed groups.
In conclusion, these data suggest a relationship between autoantibodies and pleural abnormalities in Libby amphibole exposed subjects. Understanding the autoimmune component to the pathologic outcomes of amphibole exposure is a critical public health issue due to its implications in screening, diagnosis, prognosis, and therapeutic approaches. As other studies have implicated, amphibole asbestos exposure appears to drive autoimmunity, and the autoantibodies may be associated with specific pathologic outcomes. Further research is needed to identify not only the mechanism leading to MCAA, but also to demonstrate how the autoantibodies lead to damage that exacerbates pleural manifestations of asbestos exposure.
Highlights.
This study sought to determine whether autoantibodies from asbestos-exposed subjects from Libby MT were associated with pleural lesions.
Only anti-mesothelial cell autoantibodies were associated with pleural disease specifically.
Therefore, autoantibodies may play a role in the pleural abnormalities that occur after asbestos exposure.
Additional research is needed to establish the mechanistic role these autoantibodies play in pulmonary disease.
Acknowledgments
The authors gratefully acknowledge Dr. Teri Peterson (Idaho State University) for consultation on the statistical analyses, and the contributions of Dr. Brad Black, Center for Asbestos Related Diseases, Libby MT and the CARD Staff, as well as the Center of Environmental Health Sciences at The University of Montana.
Grants
The work was funded by a fellowship from the University of Washington’s School of Medicine Medical Student Research Training Program (MSRTP) to LSM, The University of Montana COBRE grant (RR017670), the Idaho State University INBRE grant (RR016454), and ES011676 to EAP.
Abbreviations
- MCAA
Mesothelial Cell Autoantibodies
- ANA
Anti-Nuclear Antibodies
- ARD
Asbestos Related Diseases
- CARD
Center for Asbestos Related Disease
- OD
Optical Density
- RA
Rheumatoid Arthritis
- SAID
Systemic Autoimmune Disease
- SLE
Systemic Lupus Erythematosus
Footnotes
Conflicts of Interest
The Authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson BA, Dearwent SM, Durant JT, Dyken JJ, Freed JA, Moore SM, Wheeler JS. Exposure pathway evaluations for sites that processed asbestos-contaminated vermiculite. Int J Hyg Environ Health. 2005;208:55–65. doi: 10.1016/j.ijheh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Arnett FC. Is scleroderma an autoantibody mediated disease? Curr Opin Rheumatol. 2006;18:579–581. doi: 10.1097/01.bor.0000245726.33006.c3. [DOI] [PubMed] [Google Scholar]
- Bandli BR, Gunter ME. A review of scientific literature examining the mining history, geology, mineralogy, and amphibole asbestos health effects of Rainy creek igneous complex, Libby, Montana, USA. Inhal Toxicol. 2006;18:949–962. doi: 10.1080/08958370600834982. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Chevalier J, Smith P. Comparison of Calidreia chrysotile asbestos to pure tremolite: final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal Toxicol. 2005;17:427–449. doi: 10.1080/08958370591002012. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Wetzel SA, Pfau JC. Autoantibodies from mice exposed to Libby amphibole asbestos bind SSA/Ro52-enriched apoptotic blebs of murine macrophages. Toxicology. 2008;246:172–179. doi: 10.1016/j.tox.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Pfau JC, Pershouse MA, Holian A. Silica, Apoptosis, and Autoimmunity. J Immunotoxicol. 2004;1:177–187. doi: 10.1080/15476910490911922. [DOI] [PubMed] [Google Scholar]
- Bunderson-Schelvan M, Pfau JC, Crouch R, Holian A. Non-pulmonary outcomes of Asbestos Exposure. J Toxicol Env Health. 2011;14(1):122–152. doi: 10.1080/10937404.2011.556048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzolini C, Raschi E, Rezzonico R, Testoni C, Mallone R, Gabrielli A, Facchini A, Del Papa N, Borghi MO, Dayer JM, Meroni PL. Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum. 2002;46:1602–1613. doi: 10.1002/art.10361. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Gilbert KM, Greidinger EL, James JA, Pfau JC, Reinlib L, Rishardson BC, Rose NR. Recent advances and opportunities in research on Lupus: Environmental Influences and mechanisms of disease. Environ Health Persp. 2008;116:695–702. doi: 10.1289/ehp.11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henault J, Robitaille G, Senecal JL, Raymond Y. DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti-topoisomerae I autoantibodies to the cell surface of fibroblasts in patients with systemic sclerosis. Arthritis Rheum. 2006;50:3265–3274. doi: 10.1002/art.21646. [DOI] [PubMed] [Google Scholar]
- Hess EV. Environmental chemicals and autoimmune disease: cause and effect. Toxicology. 2002;181–182:65–70. doi: 10.1016/s0300-483x(02)00256-1. [DOI] [PubMed] [Google Scholar]
- Kahaleh B. The microvascular endothelium in scleroderma. Reumatology (Oxford) 2008;47(Suppl 5):14–15. doi: 10.1093/rheumatology/ken279. [DOI] [PubMed] [Google Scholar]
- Lange A, Smolik R, Zatonski W, Szymanska J. Autoantibodies and serum immunoglobulin levels in asbestos workers. Int Arch Arbeitsmed. 1974;32:313–325. doi: 10.1007/BF02178970. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Harris J, Armstrong B. Mortality in a cohort of vermiculite miners exposed to fibrous amphibole in Libby, Montana. Occup Environ Med. 2004;61:363–366. doi: 10.1136/oem.2003.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker GP, Bern AM, Brownfield IK, Lowers HA, Sutley SJ, Hoefen TM, Vance JS. The composition and morphology of amphibole from the Rainy Creek Complex, near Libby, Montana. Am Mineral. 2003;88:1955–1969. [Google Scholar]
- Mossman BT, Bignon J, Corn M, Seaton A, Gee JB. Asbestos: scientific developments and implications for public policy. Science. 1990;247:294–301. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- Nicholson WJ, Perkel G, Selikoff IJ. Occupational exposure to asbestos: population at risk and projected mortality—1880–2030. Am J Ind Med. 1982;3:259–311. doi: 10.1002/ajim.4700030305. [DOI] [PubMed] [Google Scholar]
- Nigam SK, Suthar AM, Patel MM, Karnik AB, Dave SK, Kashyap SK, Venkaiah K. Humoral immunological profile of workers exposed to asbestos in asbestos mines. Indian J Med Res. 1993;98:274–277. [PubMed] [Google Scholar]
- Noonan CW, Pfau JC, Larson TC, Spence MR. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environ Health Persp. 2006;114:1243–1247. doi: 10.1289/ehp.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipins LA, Lewin M, Campolucci S, Lybarger JA, Miller A, Middleton D, Weis C, Spence M, Black B, Kapil V. Radiographic Abnormalities and Exposure to Asbestos-Contaminated Vermiculite in the Community of Libby, Montana, USA. Environ Health Persp. 2003;111:1753–1759. doi: 10.1289/ehp.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B, Vigliani EC, Selikoff IJ. Rheumatoid factor in serum of individuals exposed to asbestos. Ann NY Acad Sci. 1965;132:112–120. doi: 10.1111/j.1749-6632.1965.tb41094.x. [DOI] [PubMed] [Google Scholar]
- Pfau JC, Blake DJ, Fritzler MJ. Autoantibody Profile of an Asbestos-Exposed Population. In: Columbus F, editor. Autoimmunity: Role, Regulation and Disorders. NOVA Science Publications; Hauppauge NY: 2008. pp. 245–268. [Google Scholar]
- Pfau JC, Sentissi JJ, Weller G, Putnam EA. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environ Health Persp. 2005;113:25–30. doi: 10.1289/ehp.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez VC, Pietruska JR, Miselis NR, Hurt RH, Kane AB. Biopersistence and potential adverse health impacts of fibrous nanomatériaux : what have we learned from asbestos ? Rev Nanomed Nanobiotechnol. 2009;1:511–529. doi: 10.1002/wnan.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Liang D, Tokuyama T, Yoneda T, Kasuga H, Narita N, Sada K, Miyazaki R, Okada S. Study on the relationship between appearance of autoantibodies and chest X-ray findings of asbestos plant employees. Sangyo Igaku. 1993;35:406–412. doi: 10.1539/joh1959.35.406. [DOI] [PubMed] [Google Scholar]
- Tamura M, Tokuyama T, Kasuga H, Yoneda T, Miyazaki R, Narita N. Study on correlation between chest X-P course findings and change in antinuclear antibody in asbestos plant employees. Sangyo Eiseigaku Zasshi. 1996;38:138–141. [PubMed] [Google Scholar]
- Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, Gordaon T, Hardin JA, Kalden JR, Lahitia RG, Maini RN, McDougal JS, Rothfield NF, Smeenk RJ, Takasaki Y, Wiik A, Wilson MR, Koziol JA. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- Tomatis M, Turci F, Ceschino R, Riganti C, Gazzano E, Martra B, Ghigo D, Fubini B. High aspect ratio materials: role of surface chemistry vs. length in the historical “long and short amosite asbestos fibers”. Inhal Toxicol. 2010;22:984–998. doi: 10.3109/08958378.2010.504243. [DOI] [PubMed] [Google Scholar]
- Turner-Warwick M, Parkes WR. Circulating rheumatoid and antinuclear factors in asbestos workers. Br Med J. 1970;3:492–495. doi: 10.1136/bmj.3.5721.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AC. Asbestos-related pleural disease due to tremolite associated with progressive loss of lung function: serial observations in 123 miners, family members, and residents of Libby, Montana. Am J Ind Med. 2004;46:219–225. doi: 10.1002/ajim.20053. [DOI] [PubMed] [Google Scholar]