Abstract
In this article we review recent research on diffusion tensor imaging (DTI) of white matter (WM) integrity and the implications for age-related differences in cognition. Neurobiological mechanisms defined from DTI analyses suggest that a primary dimension of age-related decline in WM is a decline in the structural integrity of myelin, particularly in brain regions that myelinate later developmentally. Research integrating behavioral measures with DTI indicates that WM integrity supports the communication among cortical networks, particularly those involving executive function, perceptual speed, and memory (i.e., fluid cognition). In the absence of significant disease, age shares a substantial portion of the variance associated with the relation between WM integrity and fluid cognition. Current data are consistent with one model in which age-related decline in WM integrity contributes to a decreased efficiency of communication among networks for fluid cognitive abilities. Neurocognitive disorders for which older adults are at risk, such as depression, further modulate the relation between WM and cognition, in ways that are not as yet entirely clear. Developments in DTI technology are providing new insight into both the neurobiological mechanisms of aging WM and the potential contribution of DTI to understanding functional measures of brain activity.
Keywords: Magnetic resonance imaging, Brain, Behavior, Adult development, Neuroaxonal damage
1. Introduction
The recent decade has seen a rapidly emerging body of scientific investigation in magnetic resonance imaging (MRI) of the brain and cognition, including the changes associated with human aging. The majority of cognitive and behavioral investigations have focused on either structural MRI measures of brain volume and pathology [1–5] or functional MRI (fMRI) measures of the blood-oxygen-level-dependent (BOLD) changes in cortical gray matter (GM) [6–8]. Research with another form of MRI, diffusion tensor imaging (DTI), has provided a new and complementary perspective on age-related differences in brain structure and function, by allowing an in vivo characterization of the microstructural properties of white matter (WM) [9–11]. Measures from DTI exhibit increased sensitivity to age-related decline, relative to volumetric measures [12–14], and DTI is beginning to show promise for the detection of brain disorders such as Alzheimer’s disease and related forms of dementia (see Section 4, below, and Gold, this issue).
The success of this clinical translation, however, rests on the foundation provided by DTI to studies of aging and WM in the absence of significant disease. Measures from DTI are promising in this regard because they comprise several indices with a sufficient range of values to permit detailed analyses of normative data. In addition, independently of clinical translation, the empirical measures from DTI can be combined with behavioral measures of cognitive performance, to further advance understanding of structure-function relations [15]. Research with healthy adults has enabled the development of larger sample sizes and increased statistical power, yielding more reliable estimates of the relations between WM integrity and cognitive performance, and of age-related effects [16–18].
Most frequently, DTI research on aging is conducted with different groups of individuals at one point in time and thus yields information regarding age-related differences (i.e., cross-sectional differences). The vast majority of the research we consider here is cross-sectional, and thus the estimated effects of age may be influenced by a variety of individual differences between the age groups (cohort differences). Measurement of the same individuals over time is necessary to assess age-related changes within individuals (i.e., longitudinal changes). Historically, longitudinal MRI studies have focused on measures of brain volume and pathology, such as WM lesions [19–22], but longitudinal studies conducted with DTI are beginning to emerge [23–25]. Our goal in this article is to review the application of DTI for understanding the changes in both WM integrity and cognitive performance associated with aging.
2. Microstructural properties of WM integrity
2.1 Measurement of diffusivity in white matter
Measures from DTI allow inferences about WM microstructure in vivo by quantifying the directionality and rate of diffusion of water within tissue. At the level of the basic unit of the MRI image (i.e., voxel), this information is represented mathematically as a diffusion ellipsoid. Diffusion along the major axis or eigenvector (λ1) of the ellipsoid is termed axial diffusivity (AD), whereas the average of the second and third minor axes (λ2, λ3), termed radial diffusivity (RD), reflects diffusivity perpendicular to major axis of the tensor [26–29].
The most frequently used DTI summary measures are fractional anisotropy (FA) and mean diffusivity (MD). Fractional anisotropy represents the fraction of the tensor that can be assigned to anisotropic (directional) diffusion [26, 30–31]. Values for FA range between 0 and 1, with higher values reflecting increased directionality of diffusion, independently of the rate of diffusion. The directionality of diffusion depends on the density of physical obstructions such as membranes and the distribution of water molecules between different cellular compartments. Thus, FA is typically higher in WM, in which diffusion is restricted by the myelin sheaths of axons, particularly in compact tracts with uniform fiber alignment, such as the corpus callosum, whereas diffusion in GM is less bounded and more isotropic. MD is a mean of all three axes of the diffusion ellipsoid and reflects the rate of water diffusion within a voxel, independently of the directionality.
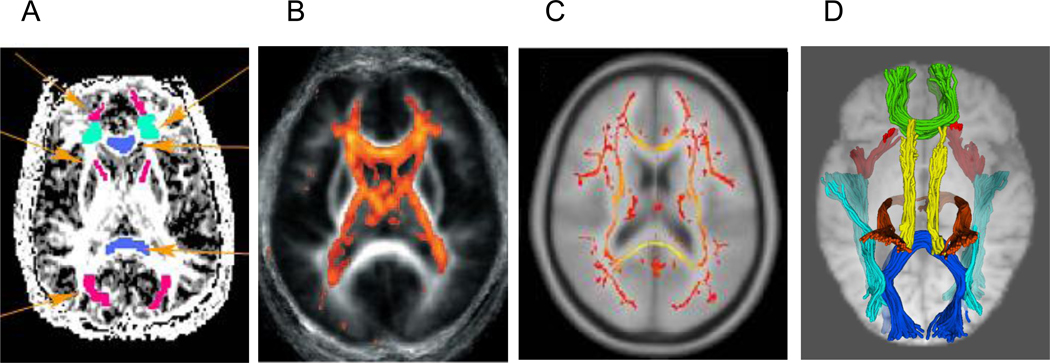
Researchers have used several different methods of post-processing for DTI data and presenting the results [9–11, 32]; and Figure 1 illustrates four approaches. In region of interest (ROI) analysis (panel A), data are extracted from regions that are placed directly on the DTI image, typically in native (non-normalized) space, for each individual. Voxel-based morphometry (VBM; panel B) and tract-based spatial statistics (TBSS; panel C) both rely on group-normalized data to estimate either the tissue composition of individual voxels (VBM) or the “skeleton” of WM tracts (TBSS). Fiber tracking of WM pathways (tractography; panel D) is typically conducted in native space, although information from normalization may be incorporated into the procedure.
Figure 1.
Methods for representing diffusion tensor imaging (DTI) data. A = regions of interest (in color) placed directly on DTI image; B = voxel-based morphometry (VBM); C = mean “skeleton” of white matter tracts from tract-based spatial statistics (TBSS); D = fiber tracking of white matter pathways. Image courtesy of Simon Davis, University of Cambridge.
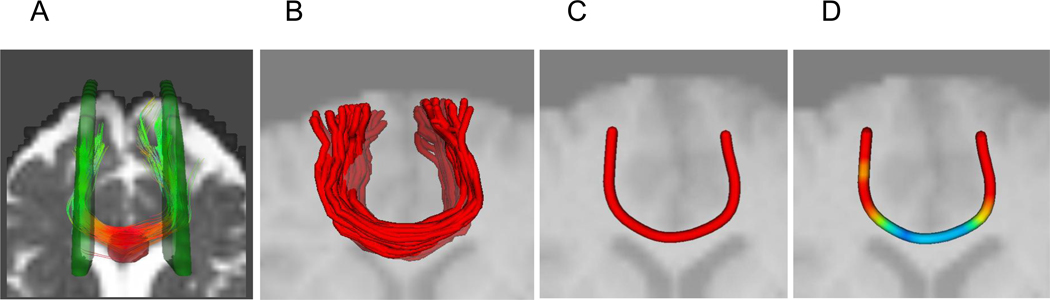
Fiber tracking uses seed and target ROIs to estimate the location of sets of spatially coherent WM fibers, and tractography is emerging as a widely used procedure for DTI analysis. Individual fiber tracts can be color-coded in terms of tract identity, as in Figure 1, but alternatively, color is often used to represent either the diffusion directionality or a related property of individual voxels within a tract. Figure 2 is an example of WM tractography in which seed and target ROIs (panel A) are placed on a DTI image as the basis for estimation of a WM pathway, in this case the genu of the corpus callosum (panel B). The estimated fiber tract can be simplified by representing a “tube” of the mean values of the fibers within a tract (panel C). Color-coding the diffusion property of individual voxels (typically FA) can illustrate variation in WM integrity within the tract. In the case of the genu, for example, FA is higher in the center (blue-green values), where the fibers are most compact and directionally coherent.
Figure 2.
Fiber tracking methodology. A = seed and target regions placed on DTI raw image; B = estimated fibers of the genu of the corpus callosum passing through the seed and target regions; C = “tube” of averaged fibers within the genu; D = voxels within averaged fibers color coded by age group differences in fractional anisotropy (FA), with the magnitude of age-related decline in FA scaled from lower (blue) to higher (red) values [54]. Adapted with permission from Neuroimage, Volume 46/Issue 2, S. W. Davis, N. A. Dennis, N. G. Buchler, L. E. White, D. J Madden, & R. Cabeza, Assessing the effects of age on long white matter tracts using diffusion tensor tractography, pp. 530–541, Copyright 2009, with permission from Elsevier.
The parameters of the ellipsoid reflect distinct aspects of WM microstructure. Animal models of nerve injury suggest that different types of WM injury lead to differential effects in AD and RD. S. K. Song, Sun, and colleagues have reported that axonal damage without associated myelin injury led to a decrease in AD without accompanying change in RD up to three days after injury, whereas at 14 days following injury RD increased significantly, consistent with myelin damage and secondary (Wallerian) degeneration [33–36]. The interpretation of the neurobiological mechanisms of AD and RD, however, is complicated by many variables (Section 2.4), for example, the local architecture of WM tracts with crossing fibers, in which case the major axis of the tensor model may not be parallel to myelinated axons [37–38]. Assaf and Pasternak [39] suggested that conjoint analysis of all measures derived from the diffusion tensor should yield a more comprehensive picture of different elements of WM microstructure. By analyzing FA and MD conjointly with RD and AD, it is possible to distinguish between diffusivity patterns with different neurobiological foundations. In this approach, for example, Wallerian degeneration is characterized by decreases in both FA and AD, combined with an RD increase and no net difference in MD [36–37, 40–42].
2.2 Adult age differences in WM diffusivity: Neurobiological mechanisms
Across many DTI investigations of aging, a general trend of the findings is a decrease in FA and an increase with measures of diffusivity, with increasing adult age, suggesting an age-related decline in the composition and integrity of WM [16–18]. These DTI findings are validated by neurobiological data. Post-mortem histological studies show that advanced age in the absence of neurological disease is linked to alterations of virtually all the WM components. Some fraction of axons degenerate and swell, myelin becomes less compact, degenerates, or forms redundant wraps; glial cells accumulate cellular debris, form glial scars, increase in number or show activated morphology [43–47]. At the technical level, as noted previously, the presence of crossing fibers within a voxel, as well as estimation error in the primary eigenvector in the tensor model [37–38], lead to difficulties in directly correlating specific neurobiological mechanism with individual DTI parameters.
Variability in the pattern of age-related differences in DTI measures, across brain regions, also complicate direct interpretation of the DTI data but do help to provide a link to neurobiology. The first series of DTI studies of age-related effects, using an ROI approach, reported consistently that aging was associated with decreased FA in the genu of the corpus callosum and associated frontal pericallosal regions, whereas age-related decline was less pronounced (though detectable) in the splenium of the corpus callosum and parietal WM [48–50]. This anterior-posterior gradient was replicated in subsequent investigations [51–58].
Davis et al. [54] have demonstrated that this gradient is not isomorphic with the concept of age-related decline in frontal lobe functioning [59–61]. Specifically, tracts traversing the frontal lobe in an anterior-posterior direction (e.g., the uncinate fasciculus and cingulum) showed a monotonic decrease in the age-related decline in FA in an anterior-posterior direction, and these age differences in FA did not change abruptly the boundary of the frontal lobe. This pattern of age-related difference in WM integrity is in line with mylodegeneration as a potential neurobiological mechanism for regional variation in age-related differences in FA. That is, those brain regions that are the latest to myelinate fully, during development, may be those most vulnerable to the negative effects of adult aging. These include frontal regions but also more posterior sites (e.g., occipitotemporal boundary). The assumption is that oligodendrocytes of associative WM pathways are the most metabolically active cells and thus correspondingly vulnerable to the accumulation of metabolic damage. The anterior-posterior gradient is not an entirely comprehensive framework, however, and Sullivan and colleagues have recently proposed that age-related decline in FA can also be characterized by a superior-inferior gradient, particularly with regard to fibers tracts in the internal capsule (e.g., centrum semiovale and corticospinal tract) [62–64]. Both the anterior-posterior and superior-inferior gradient theories share the assumption that damage within white matter pathways decreases the efficiency of communication among the widely distributed neural systems comprising cognition, thus leading to a disconnection of cognitive networks [4, 65–70].
2.3 Axonal versus myelin degeneration
Analyses of the magnitude of age-related differences in AD and RD suggest that age-related RD differences(usually increases) are more prominent than AD differences [54–55, 71–72], which has been interpreted as support for a predominantly myelin-specific effect in aging. Age-related decreases as well as increases in AD, however, have been reported [12, 63–64], and thus differential age-related effects in axonal and myelin-specific variables are not clear. Two recent studies, by Bennett et al. [51] and Burzynska et al. [53] provided a more complete account by examining systematically the age-related differences in the diffusivity components, in voxelwise analyses. Both of the studies used TBSS to analyze FA, AD, and RD concurrently, but Burzynska et al. also included MD as a variable.
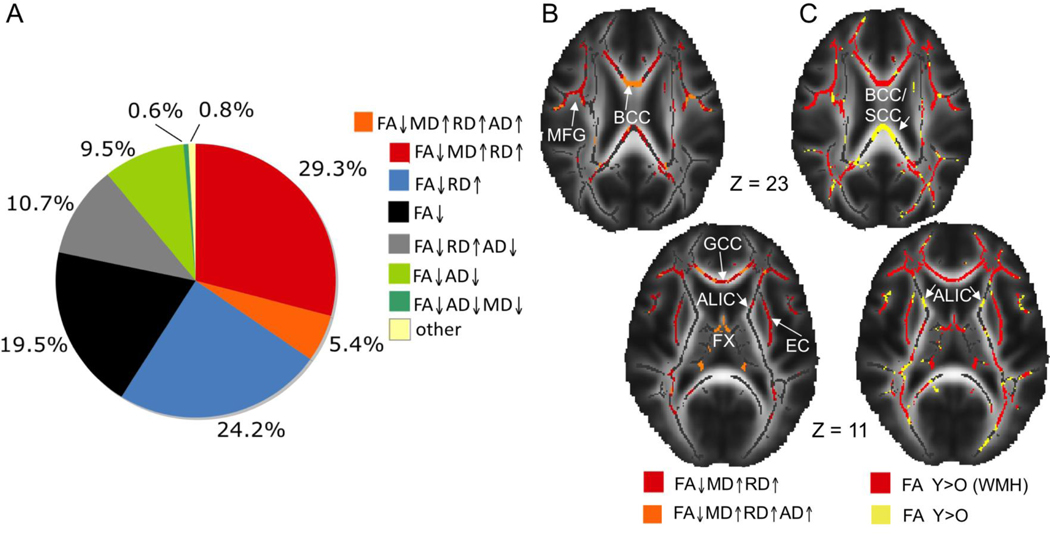
From the whole-brain analyses of the diffusivity components, Bennett et al. [51] reported three main patterns of age-related difference, and Burzynska et al. [53] obtained five distinct patterns. The two studies agree that the most spatially prominent pattern was a decrease in FA for older adults, relative to younger adults, accompanied by an increase in RD. In a subset of regions (e.g., genu of the corpus callosum), this pattern also included an age-related increase in MD and AD. Figure 3 (panels A and B) illustrates this result in the Burzynska et al. data. Table 1 provides a direct comparison of the two data sets as well as proposed neurobiological mechanisms for different patterns.
Figure 3.
A = The spatial extent of five patterns of age-related difference in diffusivity, relative to the total volume of age-related decrease in FA values across regions (55,973 mm2), from Burzynska et al. [53]; B = spatial localization of the main diffusivity pattern: age-related decrease in FA, increase in RD and MD (in red) and additionally an increase in AD (in orange); C = WMH volume accounts for age-related decrease only in a few WM regions. Age-related FA decrease (in yellow) is contrasted with age-related FA decrease after accounting for WMH volume (in red). FA = fractional anisotropy; RD = radial diffusivity; AD = axial diffusivity; MD = mean diffusivity; WMH = white matter Hyperintensity; ALIC = anterior limb of the internal capsule; BCC = body of the corpus callosum; EC = external capsule; FX = fornix; GCC = genu of the corpus callosum; MFG = middle frontal gyrus; SCC = splenium of the corpus callosum. Adapted with permission from Neuroimage, Volume 49/Issue 3, A.Z. Burzynska, C. Preuschhof, L. Backman, L. Nyberg, S. C. Li, U. Lindenberger, & H. R. Heekeren, Age-related differences in white matter microstructure: region-specific patterns of diffusivity, pp. 2104–2112, Copyright 2010, with permission from Elsevier.
Table 1.
Patterns of adult age differences in white matter diffusivity properties
| Anatomical location | Pattern for older adults, relative to younger adults |
Biological interpretation |
|---|---|---|
| Genu of the corpus callosum | FA ↓ RD↑ AD↑ (MD↑)* | Loss/disruption of both axons and myelin |
| Body of the corpus callosum | (FA ↓ RD↑ AD↑ MD↑)* | |
| External capsule | FA ↓ RD↑ AD↑ (MD↑)* | |
| Anterior dorsal cingulum | (FA ↓ RD↑ AD↑ MD↑)* | |
| Fornix | FA ↓ RD↑ AD↑ (MD↑)* | Loss/disruption of both axons and myelin; partial volume with CSF |
| Anterior limb of internal capsule | (FA↓ RD↑ MD↑)* | Predominant myelin disruption or loss |
| Middle frontal gyrus (SLF) | FA ↓ RD↑ (AD↑ MD↑)* | |
| WM of the straight gyrus | FA↓ RD↑ | |
| Forceps major | FA↓ RD↑ | |
| Sagittal stratum | FA↓ RD↑ (MD↑)* | |
| Posterior dorsal cingulum | (FA ↓ RD↑)* | |
| Superior corona radiata | FA↓ RD↑ AD↓ | Fiber loss with glial infiltration |
| Forceps minor | FA↓ RD↑ AD↓ | |
| Retrolenticular internal capsule | FA↓ RD↑ AD↓ | |
| Parahippocampal WM | FA↓ RD↑ (AD↓)* | |
| Posterior limb of internal capsule | (FA↓ AD↓ MD↓)* | Gliosis or early axonal injury/Less coherent tract organization |
| Cerebral peduncles | FA↓ AD↓ | |
Note. The patterns of age differences (second column) are expressed as the direction of significant diffusivity effects, for older adults, relative to younger adults, reported by Bennett et al. [51] and Burzynska et al. [53]. FA = fractional anisotropy; AD = axial diffusivity; RD = radial diffusivity; MD = mean diffusivity; CSF = cerebrospinal fluid; SLF = superior longitudinal fasciculus; WM = white matter; ↓ = decrease; ↑ = increase.
When age-related increases in both RD and AD are present, it is likely that pathology of both axon fibers and the surrounding myelin sheaths is involved. Cellular debris clearance by phagocytic cells limits the restriction of water diffusion and contributes to an increase in isotropic diffusivity. In some regions (e.g., fornix, in Table 1), severe fiber degeneration may lead to decreased tract volume, and, as a result, to a partial volume effect with the surrounding cerebrospinal fluid (CSF), which magnifies the effect of the increase in the isotropic diffusivity. This pattern of diffusivity differences likely reflects higher extracellular volume fraction and lower membrane density as a result of both myelin disruption and axon loss, a smaller volume fraction of axons, an increase in axonal spacing [31], as well as a reduction in extracellular tortuosity [73]. A more specific disruption of myelin is implied when an increase in RD occurs without an accompanying increase in AD (e.g., forceps major, dorsal cingulum, in Table 1). Age-related increase in RD is a signature of demyelination is consistent with animal models, for example in the loss of myelin due to ischemia [33, 35]. In human post-mortem data, from multiple sclerosis patients, RD correlated with demyelination severity [74–75].
Bennett et al. [51] and Burzynska et al. [53] both reported that some WM regions in older adults (e.g., superior corona radiata, in Table 1) were characterized by a pattern in which the decreased FA and increased RD were accompanied by a decreased AD, resulting in no net difference in MD. This type of diffusivity pattern has been observed in secondary (Wallerian) degeneration, which is the degeneration, over time, of axon fibers distal to the point of transsection or injury [36–37, 40–42]. This process involves initial axonal beading, axolemma breakdown, organelle accumulation, and finally the breakdown of myelin and oligodendrocyte apoptosis. The cellular debris is cleared by activated microglia and glial scar is formed by astrocytes [76]. This glia infiltration, which restricts AD and decreases MD [77] differentiates Wallerian degeneration from mere loss of fibers and myelin. Local ischemic lesions, such as WM lesions typical for the healthy older population [1–2], may be the primary lesions from which Wallerian degeneration originates in the aging brain [3], although decline in FA associated with healthy aging appears not to be dependent entirely on WM lesions [53].
Finally, the Bennett et al. [51] and Burzynska et al. [53] studies reported that some WM regions of older adults showed a decrease in FA accompanied by a decrease in AD and MD, without a difference in RD (e.g., cerebral peduncles, in Table 1). Lowered FA driven by decreased AD is considered a marker of acute and primary axonal damage and was observed, in human patients, in the early post-injury phase after corpus callosotomy [78] and in optic neuritis [79], as well as in various animal models of axonal injury [33, 80–85]. The extent of acute axonal injury in normal aging is not clear, but its origin may be ischemic and related to development of WM lesions. Bennett et al. proposed that a decrease in FA and AD with no significant change in RD and MD may be also a result of disrupted macrostructural reorganization of the fibers, such as less coherent fiber alignment.
2.4 Limitations in interpreting axial and radial diffusivity
As noted previously (Section 2.1), it is difficult to distinguish components of the microstructural pathology based on DTI indices alone. Although analyzing multiple DTI indices simultaneously provides the most information, the link to neurobiology remains incomplete at this time. In the case of chronic WM lesions, for example, two post-mortem studies of multiple sclerosis patients, combining DTI with histology, reported that myelin content and axonal count are strongly correlated [74–75]. Specifically, lesions in these patients were characterized by lower myelin content, axonal loss, and gliosis, expressed by lower FA and higher MD, RD and AD values. Furthermore, in multiple regression analyses conducted on each DTI index, both myelin content an axonal count were concurrent predictors of all indices except AD. This latter variable was related to myelin content but not to axonal count [74]. Thus, RD is sensitive but not specific to demyelination and may be more appropriately considered as a marker of overall tissue integrity [74].
Recent findings suggest that partial volume effects (i.e., incorrect assignment of a voxel as WM) may have a significant effect on the estimation of age-related effects. Vos et al. [86] found that estimated diffusivity parameters are differentially affected depending on whether partial volume represented CSF, WM with different principal eigenvector orientation, or GM. In addition, the partial volume effect varied as a function of fiber bundle volume, orientation, and curvature. In view of the WM structural changes associated with aging, Vos et al. recommend including these structural variables as covariates in analyses of age-related effects. Similarly, Miller et al. [87] compared ultra-high resolution DTI images (0.73 mm isotropic voxels), acquired post-mortem, to data blurred to resemble the more typical scan resolution of 2–3.5 mm3, and found that the lower resolution images led WM tracts to appear thinner than they actually were, due to partial volume effects. Other investigations, however, that have included post-processing methods to specifically address partial volume effects, have reported that age-related differences in DTI measures remain detectable following consideration of partial volume effects [71, 88]. Thus, although partial voluming related to age-related atrophy clearly affects thin tracts such as fornix (Table 1) [3, 53], age-related differences in diffusivity in other WM structures likely reflect true effects of age on microstructure.
3. Cognitive performance and WM integrity in aging
During healthy aging, behavioral measures of fluid cognitive abilities (those relying on elementary perceptual speed and sensory/motor functioning) tend to decline, whereas measures of crystallized cognitive abilities (those relating to knowledge and expertise) exhibit little or no decline until very late in life [89–92]. Imaging studies of WM volume and pathology, particularly studies of the lesions that appear as WM hyperintensities, have long indicated a relation between WM integrity and fluid cognition, particularly in samples of older adults [19–20, 93–98]. Building on initial reports by O’Sullivan et al. [69] and Sullivan et al. [49], evidence from DTI is rapidly accumulating to indicate that microstructural integrity of normal appearing WM has some role in age-related decline in cognitive functioning in healthy adults [16–18].
In a previous review of DTI studies of cognitive aging [16], we discussed several interpretive issues that currently limit the characterization of the specific relation between WM integrity and cognitive aging. Among these were the focus, in previous research, on the overall variance in outcome measures rather than the age-related variance. That is, few studies have either a) investigated the degree to which individual differences in WM integrity share age-related variance in cognitive performance; or b) directly compared different age groups in the correlation between WM integrity and cognition. Salthouse [99] has reviewed these and related issues, particularly the failure of previous studies to evaluate competing models of the relations among neuroanatomy, age, and cognition. Salthouse proposed that, at the present time, the evidence for concluding that aspects of brain structure are neuroanatomical substrates of age-related cognitive decline is weak. For example, if the relation between a neuroanatomical variable and cognition is reduced when the effect of age is controlled statistically, different interpretations are possible. This pattern may occur because age has a mediating role in the relation between the brain and cognition [100]. Alternatively, the neuroanatomical variable and cognition may actually be independent and only apparently related to each other by virtue of their relations to age.
Recent empirical investigations have taken disparate approaches to characterizing the relation between WM integrity and cognition in aging. In Table 2, we summarize the results of 29 published DTI studies addressing this relation; this summary adds 11 published reports to a corresponding table in our previous review [16]. We identified these studies from PubMed searches using the terms “white matter integrity AND aging” and “diffusion tensor imaging AND aging AND cognitive.” From this output, we selected articles that were empirical investigations of WM integrity-cognition effects in healthy older adults. Articles that used global rather than regional DTI measures [101–104] or used a measure of WM integrity other than FA [65, 105] were excluded. In this version of the table, we focus specifically on the three cognitive domains of executive function (e.g., maintaining a relevant task set and inhibiting distraction), processing speed, and memory, which is a division that is often used in studies of WM and aging [103–104, 106–109].
Table 2.
Summary of aging studies examining relationships between fractional anisotropy (FA) and cognitive performance
| Cognitive Domain | Brain Region | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frontal | CCg | Inf Front | IC | Mid | Sup | CCs | Par | Occ | |
| Executive Function | |||||||||
| Younger and Older | |||||||||
| Composite measures [63–64, 121] | |||||||||
| Raw bivariate | — | 0.62 | 0.34 | 0.53 | 0.65 | 0.28 | 0.24 | — | — |
| Age partialed | — | 0.06 | — | — | 0.06 | — | — | — | — |
| Set shift/task switch [108, 116–117, 202] | |||||||||
| Raw bivariate | 0.40 | 0.65 | 0.61 | — | 0.22 | 0.45 | — | 0.40 | 0.26 |
| Age partialed | 0.25 | — | 0.52 | — | 0.23 | 0.46 | — | 0.29 | 0.23 |
| Working memory [108, 202] | |||||||||
| Raw bivariate | 0.42 | 0.30 | — | — | 0.18 | — | — | 0.47 | 0.04 |
| Age partialed | 0.14 | — | — | — | −0.04 | — | — | 0.18 | 0.03 |
| Memory span [108, 202] | |||||||||
| Raw bivariate | 0.13 | 0.28 | — | — | 0.02 | — | — | 0.06 | 0.02 |
| Age partialed | −0.02 | — | — | — | 0.00 | — | — | 0.02 | 0.06 |
| Older only | |||||||||
| Composite measures [203–206] | |||||||||
| Raw bivariate | 0.38 | 0.30 | — | — | 0.33 | 0.29 | — | 0.30 | — |
| Age partialed | 0.26 | 0.02 | 0.15 | — | 0.07 | 0.20 | — | 0.05 | — |
| Set shift/task switch [54, 69, 116] | |||||||||
| Raw bivariate | 0.41 | — | 0.63 | — | 0.09 | 0.47 | — | 0.32 | — |
| Age partialed | 0.09 | — | — | — | −0.34 | — | — | −0.04 | — |
| Working memory [54, 207] | |||||||||
| Raw bivariate | 0.09 | — | 0.57 | — | — | 0.41 | — | — | 0.28 |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Memory span [54] | |||||||||
| Raw bivariate | — | 0.57 | — | — | — | — | — | — | — |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Fluency [69, 207–209] | |||||||||
| Raw bivariate | 0.09 | — | — | — | 0.69 | 0.20 | — | 0.37 | 0.20 |
| Age partialed | 0.04 | — | — | — | 0.61 | — | — | 0.28 | — |
| Reasoning [207–209] | |||||||||
| Raw bivariate | 0.13 | — | — | — | — | 0.16 | — | 0.23 | 0.10 |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Stroop Interference [210] | |||||||||
| Raw bivariate | — | — | — | — | 0.64 | — | — | — | — |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Processing Speed | |||||||||
| Younger and Older | |||||||||
| Composite measures [63–64, 121] | |||||||||
| Raw bivariate | — | 0.64 | 0.60 | 0.54 | 0.66 | 0.42 | 0.35 | — | — |
| Age partialed | — | 0.13 | 0.34 | — | 0.19 | — | — | — | — |
| Digit Symbol [62] | |||||||||
| Raw bivariate | 0.30 | 0.19 | −0.01 | 0.14 | 0.15 | 0.17 | 0.24 | — | 0.25 |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Simple/choice reaction time [57, 202, 211] | |||||||||
| Raw bivariate | 0.35 | 0.51 | — | 0.31 | 0.16 | — | 0.51 | 0.35 | 0.05 |
| Age partialed | 0.17 | — | — | — | 0.05 | — | — | 0.19 | 0.05 |
| Finger tapping/movements [49, 62, 202] | |||||||||
| Raw bivariate | 0.22 | — | — | — | 0.42 | — | — | 0.41 | 0.18 |
| Age partialed | 0.19 | 0.10 | 0.03 | 0.35 | 0.11 | 0.12 | 0.09 | 0.17 | 0.20 |
| Visual search [112] | |||||||||
| Raw bivariate | — | 0.68 | 0.41 | — | — | 0.40 | 0.38 | — | — |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Older only | |||||||||
| Composite measures [203] | |||||||||
| Raw bivariate | 0.32 | — | — | — | 0.42 | — | — | 0.34 | — |
| Age partialed | 0.01 | — | — | — | 0.02 | — | — | −0.06 | — |
| Digit symbol [207] | |||||||||
| Raw bivariate | 0.14 | — | — | — | — | 0.36 | — | — | 0.10 |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Simple/choice reaction time [57, 207, 212] | |||||||||
| Raw bivariate | 0.20 | 0.63 | — | 0.55 | — | 0.43 | — | — | 0.01 |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Finger tapping/movements [213] | |||||||||
| Raw bivariate | — | — | — | — | — | — | 0.71 | — | — |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Memory | |||||||||
| Younger and Older | |||||||||
| Free recall [108] | |||||||||
| Raw bivariate | — | — | — | — | — | — | — | — | — |
| Age partialed | — | — | — | 0.36 | 0.30 | — | — | — | — |
| Paired-associate learning [108] | |||||||||
| Raw bivariate | — | — | — | — | — | — | — | — | — |
| Age partialed | — | — | — | 0.43 | — | — | — | — | — |
| Implicit learning [118] | |||||||||
| Raw bivariate | 0.46 | — | — | — | — | — | — | — | — |
| Age partialed | — | — | — | — | — | — | — | — | — |
| Semantic categorization [16] | |||||||||
| Raw bivariate | — | — | — | — | — | — | — | — | — |
| Age partialed | — | 0.46 | — | — | — | — | 0.52 | — | — |
| Older only | |||||||||
| Free recall [206, 208–209, 214] | |||||||||
| Raw bivariate | 0.14 | — | — | — | 0.35 | 0.12 | — | 0.46 | −0.08 |
| Age partialed | — | −0.01 | 0.26 | — | 0.09 | 0.18 | — | 0.58 | — |
| Recognition [52, 54] | |||||||||
| Raw bivariate | — | — | 0.27 | — | — | 0.02 | 0.29 | — | — |
| Age partialed | 0.49 | 0.49 | — | — | — | — | — | — | — |
| Paired-associate learning [54] | |||||||||
| Raw bivariate | — | — | 0.43 | — | — | 0.47 | — | — | — |
| Age partialed | — | — | — | — | — | — | — | — | — |
Note. Values are mean effect sizes (Pearson r) for effects reported in the referenced studies in the cognitive domains of Executive Function, Processing Speed, and Memory. Results are separated according to whether the FA-cognition relationships were examined across both younger and older adults combined or only among older adults (data are not presented from the few studies that also examined the FA-cognition relationships for younger adults separately). The mean effect sizes obtained with age-related variance included in the model (i.e., raw bivariate values) are presented separately from those that were partialed for the effect of age. Moderate effect sizes and larger (> 0.30) are listed in bold font.
Mean effect sizes were computed following the method of Rosenthal and Dimatteo [110]. Pearson r values were obtained from F and Spearman p statistics (in the latter case via conversion to standard normal deviate Z) when necessary, and r values were averaged after transforming them to weighted Fisher z scores. In some cases, r values were adjusted such that positive relationships indicate where better performance is associated with higher FA.
Front = frontal white matter (including forceps minor, anterior pericallosal white matter, and fronto-subcortical tracts); CCg = genu of the corpus callosum (including total corpus callosum); Inf Front = inferior frontal white matter (including inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and uncinate fasciculus); IC = internal capsule (including anterior, genu, and posterior subregions); Mid = middle white matter (including the body of the corpus callosum, fornix, external capsule, corticospinal tract, and temporal white matter); Sup = superior white matter (including cingulum bundle, superior longitudinal fasciculus, and centrum semiovale); CCs = splenium of the corpus callosum (including temporal subregions of the corpus callosum); Par = parietal white matter (including posterior pericallosal white matter); and Occ = occipital white matter (including forceps major).
Table 2 presents mean effect sizes [110] for relationships between FA and cognitive performance across nine brain regions. For each of the cognitive domains, results are presented separately for studies in which FA-cognition relationships were reported for both younger and older adults, or in older adults alone (i.e., when only older adults were examined or were reported separately from younger adults). Bold font indicates effect sizes that are moderate or larger (≥ 0.30). The reported data primarily suggest a stronger relation between FA and cognition for executive function and processing speed than for memory. This pattern has been noted previously [109, 111], but few direct comparisons among cognitive domains have been conducted. The cognitive domains in Table 2 are complex and likely to rely on multiple brain regions; at the present time, the WM-cognition relation does not exhibit a high degree of regional specificity [16, 108]. Within individual studies, however, reliable differentiation of specific information processing components has been observed. Madden et al. [55], for example, reported that FA within the genu and splenium was correlated with the efficiency of semantic memory retrieval but not with perceptual-motor speed. Bennett et al. [112] found that frontoparietal FA correlated with visual search speed, independently of perceptual-motor speed. The anterior-posterior gradient of age-related decline in FA also appears to correlate differentially with tests of speed and executive function (anterior) versus memory (posterior) [54, 108].
A new feature of Table 2 is that mean effect sizes are presented separately for: a) the raw bivariate correlations between FA and cognition, without age included in the model; and b) analyses that controlled statistically for the effect of age or age group (i.e., age partialed values). Thus, by comparing the raw bivariate and age-partialed values, we can estimate the degree to which WM integrity and cognition share age-related variance. That is, some form of age dependence is implied when the effect size for the WM-cognition relation decreases from the raw bivariate to the age-partialed version.
The current selection of published research yielded 28 raw bivariate correlations between FA and cognition that were both ≥ 0.30 (in bold font) and were accompanied by an age-partialed analysis. For 23 of those correlations, the raw correlation decreased to below 0.30 when age was partialed, which was less than half the size of the original raw value for 18 of the correlations. That is, in nearly two-thirds of cases with an FA-cognition effect of moderate or greater size, age shared more than 50% of the variance in cognition that was related to FA.
Independently of those 28 correlations with both raw and age-partialed values, Table 2 also contains 15 correlations from studies that only reported the age-partialed effects. Of these 15, eight were of at least moderate size (≥ 0.30) and seven were smaller in magnitude. Thus, studies that reported only age-partialed effects yielded approximately equal numbers of smaller and moderate effect sizes for the WM-cognition relations. From this finding, as well as from the previously discussed comparison of raw bivariate and age-partialed values, we conclude that age shares a substantial portion of the of the relation between WM integrity and cognition. As Salthouse [99] has noted, however, from this pattern alone we cannot determine whether age is directly influencing a relation between WM and cognition. The FA and cognitive variables may be independent but both related to age. In addition, by partialing age, the contribution of other variables that are associated with age (e.g., health status variables such as aerobic capacity) is also removed [113–115].
Whereas Table 2 illustrates the effect of age in the relation between WM integrity and cognition, several investigations have taken a different approach, using multiple regression and related techniques to identify the role of WM integrity in the relation between age and cognition. Following the addition of FA to models of the age-cognition relation, the proportion age-related variance is substantially reduced, yielding evidence for WM integrity (particularly within frontoparietal regions) as a mediating variable in age-related decline in the efficiency of semantic categorization [55], shifting attentional sets [116–117], and implicit learning [118]. Zahr et al. [64], however, compared these two approaches within the same data set and reported that the attenuation of the relation between FA and working memory, by age, was at least as great in magnitude as the attenuation of the age-related variance by FA. Thus, WM integrity and working memory may have independent relations to age, without age exhibiting a directly causal role in the WM integrity-working memory relation [99].
The identification of specific causal relations is possible in path-analytic and related techniques (e.g., structural equation modeling; SEM) that investigate the direct and indirect pathways of covariation among multiple variables [119]. Charlton et al. [120] have explored SEM models for the relations among age, cognition, and MD (as a measure of WM integrity) in a sample of adults 50–90 years of age. The results indicated that WM integrity (from a relatively large ROI: centrum semiovale and periventricular WM) mediated the age-related decline in working memory performance. Burgmans et al. [12] described an SEM model in which AD from a large ROI (WM in the parietal, temporal, and occipital lobes) mediated the age-related decline in pattern and letter comparison speed. Voineskos et al. [121] used DTI tractography rather than ROIs and developed an SEM model in which tract-specific FA mediated the relation between age and various forms of cognitive performance. These more sophisticated statistical methods will undoubtedly be valuable for understanding the role of WM integrity in cognitive aging. Their application, however, will need to be tempered by awareness that modeling is more appropriate for comparing different, competing models than for describing or exploring data [122–123], and that measures of WM from different tracts may not be independent but instead reflect shared variability in WM integrity across tracts [103, 124].
4. White matter integrity in geriatric neuropsychology
Although the main focus of this review is healthy aging, WM integrity plays an important role in many age-associated neurocognitive disorders, ranging from Alzheimer’s disease and other forms of dementia, to late-life depression, and the effects of stroke and traumatic brain injury [125–126]. Gold (this issue) provides a more detailed review of issues relating to Alzheimer’s disease and mild cognitive impairment. Measures from DTI, including tractography, are beginning to offer insights into neurological disease diagnosis and treatment planning [127– 129].
4.1 Alzheimer’s disease and other forms of dementia
A fundamental question is whether Alzheimer’s disease differs qualitatively from normal brain aging. Several DTI studies suggest that Alzheimer’s-related WM changes are not simply an exaggeration of the anterior-posterior gradient of normal aging but instead involve other brain regions (e.g., temporal) [130–133]. Both decreased FA and increased MD in the hippocampus and temporal regions appear to be associated with the episodic memory deficits that characterize the earliest stages of this disease [134–135]. Multivariate analyses of whole-brain WM have suggested that mean diffusivity maps perform slightly better than FA maps in classification of individuals with Alzheimer’s disease versus normal elderly [136]. Interestingly, even asymptomatic individuals with a family history of Alzheimer’s disease show reduced FA in regions typically affected by the disease, which suggests that WM integrity may be sensitive to Alzheimer’s-related pathology years before disease onset [137]. Data from DTI have also assisted in characterizing the transition from a preclinical to manifest disease [132, 138–139]. Research with DTI has also clarified some of the clinical and neuropathological differences between Alzheimer’s disease and other dementias, including frontotemporal dementia [140], vascular dementia [141], and dementia associated with Parkinson’s disease [142–144].
4.2. Late-life depression
Depression is the most prevalent psychiatric disorder and is particularly problematic in older adulthood because of its association with cognitive impairment and other adverse outcomes [145–146]. Region of interest based studies of late-life depression have found lower FA for depressed individuals relative to controls, particularly in frontal brain regions [147–148]; however, whole brain, voxel-based approaches have reported more widely distributed decrements in WM integrity [149–150]. Some studies have also found higher MD throughout the brain of older adults relative to controls, but most prominently in frontal regions [151]. The WM differences associated with late-life depression have also been associated with worse neuropsychological performance on tests of executive function (Stroop, Tower of Hanoi) and processing speed, although regional localization varies [150–152].
4.3 Plasticity of white matter
The prospect that WM integrity may increase in response to cognitive training has important implications for diagnosing and treating age-related cognitive disorders. Lovden et al. [106] compared DTI measures for younger and older adults before and after an intensive training regimen (101 1-hr sessions over 180 days), on tests of working memory, episodic memory, and perceptual speed. Relative to a no-training control group, individuals in the cognitive training group exhibited greater increase in FA and decrease in MD, in the genu of the corpus callosum. Further, the magnitude of the training-related improvements in WM integrity was as great for older adults as for younger adults. This type of plasticity of WM has also been demonstrated following brain surgery in humans. Yogarajah et al. [153] investigated epilepsy patients before and after temporal lobe resection and noted both positive and negative changes in WM integrity following surgery. In particular, larger increases in AD in the ventro-medial language network after the surgery were associated with smaller fall in post-operative verbal fluency, suggestive of structural reorganization. Sidaros et al.[154] and Bendlin et al. [155] examined the utility of DTI measures for assessing recovery following traumatic brain injury. Although the changes in WM integrity following brain injury are not entirely consistent, and a complete model of adult brain reorganization associated with injury is not available, DTI will be valuable for further advances on these issues. The concept of plasticity is particularly important in view of the results from fMRI, in healthy older adults, which suggest that some patterns of functional brain activity represent a direct response to structural changes [153, 156–158].
5. Technical developments in DTI
Advances in the technology of DTI can contribute significantly to both defining the neurobiological mechanisms of WM integrity in aging and identifying clinical translation. As discussed previously (Section 2), a difficulty in understanding age-related decline in FA is the presence of crossing fibers within a voxel, which may not always be captured at the level of spatial resolution available. Thus, a decline in the structural integrity of WM may be difficult to distinguish from a change in the local coherence of fibers [37–38, 51]. Similarly, atrophy and related structural changes associated with aging may contribute to estimates of age-related differences in DTI measures [86–87]. Several types of improvements in DTI resolution, and the integration of DTI and fMRI measures, promise to provide a more accurate characterization of age-related WM effects.
5.1. Correction for geometric distortions in single-shot-EPI-based DTI data
Most DTI data in investigations of aging and cognition are acquired with single-shot echo-planar imaging (EPI) pulse sequence, which has superior imaging speed and is relatively insensitive to intra-scan motion. However, it is well known that the spatial resolution and spatial accuracy are lower in EPI, as compared with other clinical MRI pulse sequences. Specifically, DTI data obtained with EPI are geometrically distorted, resulting from both subject-dependent susceptibility field gradients and subject-independent eddy currents. One approach to reducing distortion is to use a twice-refocused DTI sequence [159], but a more effective and complete correction for both susceptibility and eddy current related distortions relies on extra reference scans, in the form of field inhomogeneity maps that correspond to the various diffusion weighting directions [160]. Using this latter approach, Truong et al. [161] demonstrated that, by adding sensitivity encoding (SENSE) DTI acquisition and a reconstruction algorithms with higher-order polynomial correction, significantly improved spatial accuracy and signal-to-noise ratio can be obtained.
Recently, Truong et al. [162] further demonstrated that eddy currents-induced distortions have a complex time-domain pattern. Truong et al. proposed an alternative dynamic B0 mapping and off-resonance correction method that measures the exact spatial, temporal, and diffusion-weighting direction dependence of the susceptibility- and eddy current-induced magnetic fields. As a result, both susceptibility effects and time-varying eddy current artifacts can be effectively corrected, yielding DTI data with the highest possible spatial fidelity and accuracy. Figure 4 shows color-coded FA maps in three representative slices with no correction (A), static correction (B), and dynamic correction (C). The uncorrected FA maps in three representative slices show severe susceptibility-induced distortions, particularly near the genu of the corpus callosum (Figure 4A, dashed lines), as well as eddy current-induced FA errors, most prominently at the anterior and posterior edges of the brain (arrows). The conventional static off-resonance correction can only correct for the susceptibility-induced distortions, and for the eddy current-induced FA errors at the posterior edge of the brain, but not at the anterior edge (Figure 4B, arrows). In contrast, the dynamic off-resonance correction can more effectively correct for all artifacts (Figure 4C).
Figure 4.
Color-coded fractional anisotropy (FA) maps, with red = right–left, green = anterior– posterior, blue = superior–inferior. In three axial slices, the dashed lines and arrows highlight regions with severe susceptibility-induced distortions and eddy current-induced FA errors, respectively [162]. A = no correction. B = static correction; C = dynamic correction. Adapted with permission from Neuroimage, Volume 57/Issue 4, T.-K. Truong, N.-k. Chen, & A. W. Song, Dynamic correction of artifacts due to susceptibility effects and time-varying eddy currents in diffusion tensor imaging, pp. 1343–1347. Copyright 2011, with permission from Elsevier.
5.2. Beyond single-shot EPI acquisition: Alternative imaging pulse sequences for DTI of high spatial-resolution
Several studies have shown that alternative imaging pulse sequences can be used to acquire DTI data with significantly improved spatial-resolution, relative to single-shot EPI based DTI. First, high-resolution DTI with significantly reduced geometric distortion and motion-related artifact can be achieved through acquiring segmented k-space data along the readout direction (i.e., readout-segmented EPI or RS-EPI), in contrast to conventional multi-shot EPI with k-space segmentation along the phase-encoding direction [163–164]. Second, the self-navigated interleaved spiral (SNAILS) sequence for high-resolution DTI, developed by Liu et al. [165] improves spatial resolution by using a multi-shot, fat-saturated diffusion-weighted spin echo sequence that oversamples the center of k-space, providing an inherent motion compensation capability. Third, Pipe et al. [166] showed that high-resolution and high-quality DTI data can be obtained by integrating two sequences: fast spin echo imaging (which reduces distortion), and the periodically rotated overlapping parallel lines with enhanced reconstruction (i.e., PROPELLER), which increases tolerance to intra-scan motion. It is also feasible to integrate a multi-shot EPI sequence and PROPELLER motion-artifact reduction, producing high-resolution DTI with reduced artifact [167].
5.3. Beyond single- tensor model: Diffusion measurement in q-space and at higher angular-resolution
Within the DTI framework, significant advances in spatial resolution have been achieved by using extended scanning periods with post-mortem brains [87], and by using the long-range information contained in the fibers tracts, at higher MRI field strengths [168–169]. The diffusion tensor model, however, relies on the assumption of Gaussian diffusion [170], with a single diffusion tensor within a voxel, which may not always be valid. To address this, researchers have been using different mathematical frameworks for measuring diffusion to incorporate non-Gaussian diffusion and as a result can better resolve multiple diffusion tensors within the same voxel (angular resolution).
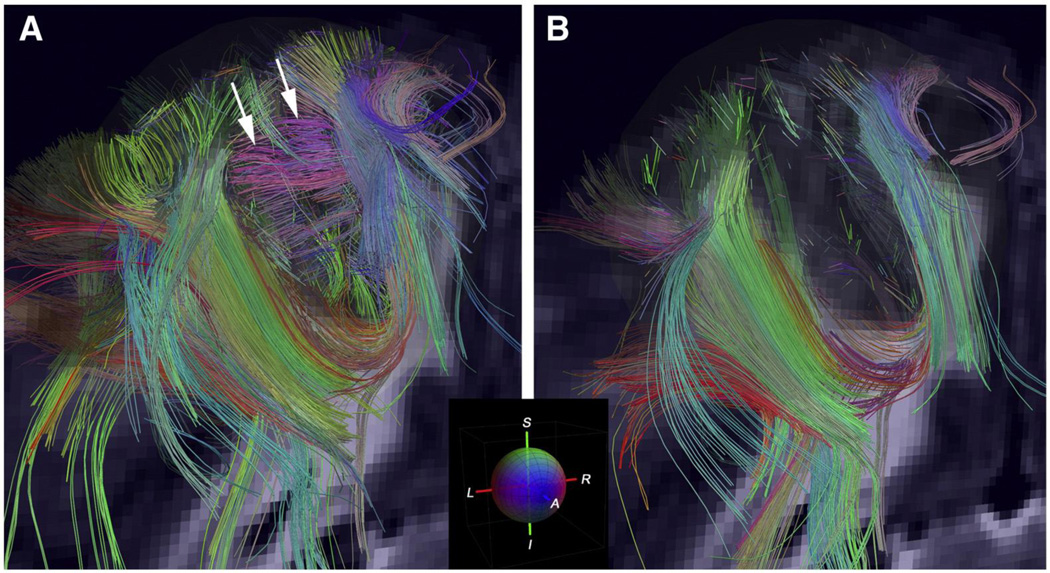
The initial methods for non-Gaussian diffusion measurement include q-space imaging, in which the probability density function for molecular diffusion is estimated without the assumption of a Gaussian distribution [171–172]. More recent approaches include diffusion spectrum imaging (DSI) [173–175], high-angular-resolution diffusion imaging [176–177], q-ball imaging [178], and generalized diffusion tensor imaging (GDTI) methods [179], among others. These methods can more accurately resolve crossing fibers, as compared with the conventional DTI and in some cases can resolve multiple axon directions within a single voxel. For example, Figure 5 shows that more fibers can be correctly identified using DSI, in comparison to the conventional DTI model [175]. The patterns of crossing fibers observed from these advanced imaging methods correspond to those obtained, in other data sets, from autoradiography [174], although validation within the same brain is needed [180]. In addition, these new methods tend to require extensive scanning time, and thus more efficient data acquisition techniques will be needed for applications in vivo.
Figure 5.
A = Diffusion spectrum imaging (DSI) tractography of monkey cerebral cortex showing fibers within the white matter of the cortical gyrus, and radiate fibers within adjacent gyri of the cerebral cortex (arrows); B = DTI tractography of a corresponding region shows fewer fibers within the white matter of the gyrus and no radiate fibers within the cortex [175]. Reprinted from Neuroimage, Volume 41/Issue 4, V.J. Wedeen, R.P. Wang, J.D. Schmahmann, T. Benner, W.Y.I. Tseng, G. Dai, D.N. Pandya, P. Hagmann, H. D'Arceuil, & A.J. de Crespigny, Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers, pp. 1267–1277, Copyright 2008, with permission from Elsevier.
5.4. Toward tissue specificity: Axon- and myelin-specific DTI measures
Age-related decline in WM integrity tends to be more pronounced in RD than in AD [54–55, 71], consistent with a prominent role for decreased myelin integrity in aging [65–66]. Multiple DTI indices concomitantly vary with adult age (Table 1), however, and assigning specific neurobiological interpretation to DTI parameters is problematic (Sections 2.1, 2.4) [37–38].
The MRI pulse sequences may be modified in a way that the derived tensor measures specifically reflect physiological information of myelin. A recent study by Avram et al. [181] described a magnetization transfer (MT) prepared stimulated-echo DTI technique that is sensitive to the microanatomy of myelin tissue. The short echo time enabled by the stimulated-echo acquisition preserves significant signal weighting from myelin water of short T2 time constant, and the MT preparation further provides differentiating sensitization to this signal. This myelin water weighted DTI has the potential to lead to improved assessment of myelin pathology and detection of degradation in myelin microanatomy.
One recently developed method, the AxCaliber technique [182–183], will be valuable in characterizing changes of white-matter microstructure related to aging, particularly with respect to axonal populations with differing diameters. The AxCaliber technique provides a noninvasive estimate of diameter distribution within each voxel, based on the differences in restricted diffusion, across populations of axons, at different diffusion times. Therefore, by changing the diffusion time from short to long one can probe the relative contributions of different subpopulations, reflecting axonal diameter distribution, within each voxel. Larger diameter axons generally occur in pathways supporting rapid neural transmission, such as the pyramidal (corticospinal) tract. Single-cell recording of pyramidal tract neurons in the cat has demonstrated significant reduction in conduction velocity with age [184]. Human post-mortem studies, however, are divided as to whether age-related volumetric decline is more pronounced for larger myelinated axons [45] or more thinly myelinated ones [44, 47, 185]. The integrity of smaller, later myelinating fibers within association (e.g., prefrontal) cortex is correlated with maximum motor speed [105]. In view of the central role of processing speed in theories of cognitive aging [186–187], and the relation between FA and speed (Table 2), AxCaliber is a particularly promising method for investigations of aging and cognition.
5.5. Toward brain activations: Functional DTI
In contrast to functional MRI with BOLD contrast, which measures hemodynamic responses associated with neuronal activity, DTI is a structural imaging modality that assesses the integrity of WM. Although neuroimaging research typically assumes that sensory and perceptual experience derives from neuronal activity, recent evidence suggests that detectable changes in WM occur in response to sensory stimulation. Mandl et al.[188] reported that changes occur in FA occur selectively in relevant WM tracts following sensory stimulation, when analyzed as a 200 sec time course. During and following the administration of a 1 min tactile or visual stimulus, changes occurred in the thalamocortical tracts for the tactile stimulus and in the splenium and optic radiations for the visual stimulus. The FA changes were relatively small in magnitude (~1%) and comprised both positive and negative effects, but Mandl et al. proposed that the positive changes (stimulation-related increases in FA) represent local fiber activity, possibly glial cell swelling, although the specific biophysical mechanisms remain unknown.
5.6. Toward constructing a Brain Connectome: Graph theory representation of DTI based connectivity maps
The quantitative maps (e.g., FA maps) derived from DTI data include valuable physiological information for different brain regions. These data are derived either from an a priori selection of relevant regions (e.g. tractography) or from an unbiased, voxelwise analysis across the whole brain (e.g., TBSS). However, the DTI data alone do not define the higher-order structure among WM tracts.
Recently, researchers have been using new mathematical approaches, particularly graph-theory analysis [189–190], to assess the overall topographical network properties (e.g., the global efficiency) of FA maps and to estimate the relative strength of the connections among tracts. Gong et al. [191], for example, analyzed DTI data from 80 healthy young adults and found that the DTI derived topographical measures of connectivity networks resemble that of a small-world architecture, which is characterized by greater local interconnectivity relative to a random network. Iturria-Medina et al. [192] used DTI based graph-theory analysis to measure the overall network properties of the right and left hemispheres. Their results indicate that the right hemisphere is more efficient and interconnected, whereas the left hemisphere comprises regions that are more central or indispensable (e.g., language) for the whole-brain structural network. In a graph-theory study of individual differences in DTI tractography data, Li et al. [193] found that individuals with higher intelligence scores exhibited WM networks with a shorter characteristic path length and a higher global efficiency, indicating a more efficient parallel information transfer in the brain. The changes in WM integrity associated with normal aging, however, have not as yet been explored from a graph-theory perspective.
Researchers in the area of BOLD resting-state activity have demonstrated reliable differences in resting state functional networks associated with aging in healthy adults [194–197]. These differences, predominantly a reduction in connectivity in the default mode network of regions, centered in medial prefrontal and posterior cingulate cortical regions, reflect age-related differences in the resting state activity of the brain. Structural WM connectivity, as assessed from DTI, appears to be an anatomical constraint on this functional connectivity [198–200]. In addition, DTI data suggest that age-related changes in cognition represent the combined influences of WM integrity and resting state functional connectivity, not only within the default mode network but also within networks involving prefrontal and subcortical regions [107, 109, 201]. Further studies that integrate DTI and fMRI measures will be valuable in characterizing the structure-function relations within the human brain connectome.
6. Conclusion
Information from DTI provides a unique perspective on cognition in aging by contributing several indices of the microstructural integrity of cerebral WM. To date, a wide range of research investigations suggests that, even in the absence of significant disease, individual differences in WM integrity share a substantial portion of age-related variance in cognitive abilities, particularly those related to elementary perceptual speed and executive function. Further, age-related differences in WM integrity vary across brain regions, with older adults exhibiting relatively greater decline (lower FA, higher diffusivity) in more anterior and superior brain regions, which may reflect the later development of myelination in these regions. Although preliminary findings suggest that the age-related effects derive more directly from changes in myelin structure than in axonal integrity, additional technical developments in DTI resolution and tissue specificity are needed to define the neurobiological mechanisms of WM changes associated with aging. At the interpretive level, it will be necessary to move beyond describing individual correlations among variables, to testing competing models of the relations among WM integrity, aging, and cognition. Initial evidence supports one model in which decline in cerebral WM integrity directly contributes to the less efficient performance of fluid cognitive abilities that occurs during healthy aging.
Highlights.
-
>
We review diffusion tensor imaging (DTI) of white matter (WM) integrity in aging.
-
>
Even without disease, age-related decline occurs in both cognition and WM integrity.
-
>
Age-related decline in WM integrity interrupts communication among brain networks.
-
>
Disconnection of brain network may contribute to normal cognitive decline with age.
Acknowledgements
Preparation of this article was supported by grants R01 AG011622, R21 NS065344 and R01 EB009483, F32 AG038299, and K23MH087741 from the National Institutes of Health. We are grateful to Tim Salthouse for his comments on a previous version of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 3.Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition, 2nd ed. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- 5.Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- 6.Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed. New York: Psychology Press; 2008. pp. 1–54. [Google Scholar]
- 7.Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- 8.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen-Berg H, Behrens TE. Diffusion MRI: From quantitative measurement to In vivo neuroanatomy. San Diego, CA: Elsevier; 2009. [Google Scholar]
- 10.Jones DK. Diffusion MRI: Theory, methods, and applications. New York: Oxford University Press; 2011. [Google Scholar]
- 11.Mori S. Introduction to diffusion tensor imaging, Elsevier. Amsterdam. 2007 [Google Scholar]
- 12.Burgmans S, Gronenschild EH, Fandakova Y, Shing YL, van Boxtel MP, Vuurman EF, Uylings HB, Jolles J, Raz N. Age differences in speed of processing are partially mediated by differences in axonal integrity. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- 15.Johansen-Berg H, Behrens TE. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol. 2006;19:379–385. doi: 10.1097/01.wco.0000236618.82086.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in aging and age-related neurodegenerative disorders. In: Jones DK, editor. Diffusion MRI: Theory, methods, and applications. New York: Oxford University Press; 2011. pp. 624–643. [Google Scholar]
- 19.Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 20.Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 22.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51:565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. J Neurol Neurosurg Psychiatry. 2010;81:13–19. doi: 10.1136/jnnp.2008.167288. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan EV, Rohlfing T, Pfefferbaum A. Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking. Dev Neuropsychol. 2010;35:233–256. doi: 10.1080/87565641003689556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 27.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 28.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 29.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 30.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 31.Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys J. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32:1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- 36.Sun SW, Liang HF, Cross AH, Song SK. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage. 2008;40:1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler-Kingshott CA, Cercignani M. About "axial" and "radial" diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 39.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 40.Cohen-Adad J, El Mendili MM, Lehericy S, Pradat PF, Blancho S, Rossignol S, Benali H. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55:1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. [DOI] [PubMed] [Google Scholar]
- 41.Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 42.Thomalla G, Glauche V, Weiller C, Rother J. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2005;76:266–268. doi: 10.1136/jnnp.2004.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: sex differences. Neuroreport. 1996;7:1761–1764. doi: 10.1097/00001756-199607290-00013. [DOI] [PubMed] [Google Scholar]
- 44.Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- 45.Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Ann N Y Acad Sci. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- 46.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- 47.Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging. 1997;18:609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- 48.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- 51.Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6:705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- 60.Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 315–372. [Google Scholar]
- 61.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48:4155–4163. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage. 2009;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical "disconnection" in aging and Alzheimer's disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 68.Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 69.O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical "disconnection" as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- 70.Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. J Magn Reson Imaging. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Du AT, Hayasaka S, Jahng GH, Hlavin J, Zhan W, Weiner MW, Schuff N. Patterns of age-related water diffusion changes in human brain by concordance and discordance analysis. Neurobiol Aging. 2010;31:1991–2001. doi: 10.1016/j.neurobiolaging.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norris DG. The effects of microscopic tissue parameters on the diffusion weighted magnetic resonance imaging experiment. NMR in Biomedicine. 2001;14:77–93. doi: 10.1002/nbm.682. [DOI] [PubMed] [Google Scholar]
- 74.Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmierer K, Wheeler-Kingshott CA, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, Scaravilli F, Barker GJ, Tofts PS, Miller DH. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med. 2008;59:268–277. doi: 10.1002/mrm.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- 77.Wang S, Wu EX, Qiu D, Leung LH, Lau HF, Khong PL. Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res. 2009;69:1190–1198. doi: 10.1158/0008-5472.CAN-08-2661. [DOI] [PubMed] [Google Scholar]
- 78.Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- 79.Naismith RT, Xu J, Tutlam NT, Snyder A, Benzinger T, Shimony J, Shepherd J, Trinkaus K, Cross AH, Song SK. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology. 2009;72:589–594. doi: 10.1212/01.wnl.0000335766.22758.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng S, Hong Y, Zhou Z, Jinsong Z, Xiaofeng D, Zaizhong W, Yali G, Ying L, Yingjuan C, Yi H. Monitoring of acute axonal injury in the swine spinal cord with EAE by diffusion tensor imaging. J Magn Reson Imaging. 2009;30:277–285. doi: 10.1002/jmri.21825. [DOI] [PubMed] [Google Scholar]
- 81.Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- 82.Thuen M, Olsen O, Berry M, Pedersen TB, Kristoffersen A, Haraldseth O, Sandvig A, Brekken C. Combination of Mn(2+)-enhanced and diffusion tensor MR imaging gives complementary information about injury and regeneration in the adult rat optic nerve. J Magn Reson Imaging. 2009;29:39–51. doi: 10.1002/jmri.21606. [DOI] [PubMed] [Google Scholar]
- 83.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li J, Li XY, Feng DF, Gu L. Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur J Neurosci. 2011;33:933–945. doi: 10.1111/j.1460-9568.2010.07573.x. [DOI] [PubMed] [Google Scholar]
- 86.Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 55:1566–1576. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 87.Miller KL, Stagg CJ, Douaud G, Jbabdi S, Smith SM, Behrens TE, Jenkinson M, Chance SA, Esiri MM, Voets NL, Jenkinson N, Aziz TZ, Turner M, Johansen-Berg H, McNab JA. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- 89.Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- 90.Craik FIM, Salthouse TA. The handbook of aging and cognition. 3rd ed. New York: Psychology Press; 2008. [Google Scholar]
- 91.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 92.Salthouse TA. Mechanisms of age-cognition relations in adulthood. Hillsdale: NJ: Erlbaum; 1992. [Google Scholar]
- 93.DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 94.Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Crawford JR, Whalley LJ. Neuropsychologic correlates of brain white matter lesions depicted on MR images: 1921 Aberdeen Birth Cohort. Radiology. 2001;221:51–55. doi: 10.1148/radiol.2211010086. [DOI] [PubMed] [Google Scholar]
- 95.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 97.Rabbitt P, Scott M, Lunn M, Thacker N, Lowe C, Pendleton N, Horan M, Jackson A. White matter lesions account for all age-related declines in speed but not in intelligence. Neuropsychology. 2007;21:363–370. doi: 10.1037/0894-4105.21.3.363. [DOI] [PubMed] [Google Scholar]
- 98.van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, Pajak A, Sans S, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Launer LJ, Hofman A. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 99.Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011 doi: 10.1037/a0023262. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 101.Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, Null M, Fox PT. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 2010;49:1190–1199. doi: 10.1016/j.neuroimage.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Norden AG, de Laat KF, van Dijk EJ, van Uden IW, van Oudheusden LJ, Gons RA, Norris DG, Zwiers MP, de Leeuw FE. Diffusion tensor imaging and cognition in cerebral small vessel disease The RUN DMC study. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Penke L, Munoz Maniega S, Murray C, Gow AJ, Hernandez MC, Clayden JD, Starr JM, Wardlaw JM, Bastin ME, Deary IJ. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS. Imaging age-related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging. 2009;29:23–30. doi: 10.1002/jmri.21572. [DOI] [PubMed] [Google Scholar]
- 105.Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lovden M, Bodammer NC, Kuhn S, Kaufmann J, Schutze H, Tempelmann C, Heinze HJ, Duzel E, Schmiedek F, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 107.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A. Cortico-striatal connectivity and cognition in normal aging: A combined DTI and resting state fMRI study. Neuroimage. 2011;55:24–31. doi: 10.1016/j.neuroimage.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 110.Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- 111.Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 112.Bennett IJ, Motes MA, Rao NK, Rypma B. White matter tract integrity predicts visual search performance in young and older adults. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: recent findings and future directions. J Mol Neurosci. 2004;24:9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- 114.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 115.Marks BL, Madden DJ, Bucur B, Provenzale JM, White LE, Cabeza R, Huettel SA. Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci. 2007;1097:171–174. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- 116.Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perry ME, McDonald CR, Hagler DJ, Jr, Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47:2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Jr, Howard DV. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salthouse TA, Kausler DH, Saults JS. Utilization of path-analytic procedures to investigate the role of processing resources in cognitive aging. Psychol Aging. 1988;3:158–166. doi: 10.1037//0882-7974.3.2.158. [DOI] [PubMed] [Google Scholar]
- 120.Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 121.Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG, et al. Up the Garden Path: A critique of Penke and Deary (in press) and further exploration concerning the Charlton et al. (2008) path analysis relating loss of white matter integrity to cognition in normal aging. Neurobiol Aging. 2010 [Google Scholar]
- 123.Penke L, Deary IJ, et al. Some guidelines for structural equation modelling in cognitive neuroscience: the case of Charlton et al.'s study on white matter integrity and cognitive ageing. Neurobiol Aging. 2010;31:1656–1660. doi: 10.1016/j.neurobiolaging.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 124.Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P. Microstructural correlations of white matter tracts in the human brain. Neuroimage. 2010;51:531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baltan S. Ischemic injury to white matter: an age-dependent process. Neuroscientist. 2009;15:126–133. doi: 10.1177/1073858408324788. [DOI] [PubMed] [Google Scholar]
- 126.Schonberger M, Ponsford J, Reutens D, Beare R, O'Sullivan R. The relationship between age, injury severity, and MRI findings after traumatic brain injury. J Neurotrauma. 2009;26:2157–2167. doi: 10.1089/neu.2009.0939. [DOI] [PubMed] [Google Scholar]
- 127.Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- 128.Catani M. Diffusion tensor magnetic resonance imaging tractography in cognitive disorders. Curr Opin Neurol. 2006;19:599–606. doi: 10.1097/01.wco.0000247610.44106.3f. [DOI] [PubMed] [Google Scholar]
- 129.Filley CM. White matter and behavioral neurology. Ann N Y Acad Sci. 2005;1064:162–183. doi: 10.1196/annals.1340.028. [DOI] [PubMed] [Google Scholar]
- 130.Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 131.Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SA. White matter tract integrity in aging and Alzheimer's disease. Hum Brain Mapp. 2009;30:1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scola E, Bozzali M, Agosta F, Magnani G, Franceschi M, Sormani MP, Cercignani M, Pagani E, Falautano M, Filippi M, Falini A. A diffusion tensor MRI study of patients with MCI and AD with a 2-year clinical follow-up. J Neurol Neurosurg Psychiatry. 2010;81:798–805. doi: 10.1136/jnnp.2009.189639. [DOI] [PubMed] [Google Scholar]
- 133.Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Pena-Gomez C, Bargallo N, Molinuevo JL, Bartres-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 135.Sexton CE, Mackay CE, Lonie JA, Bastin ME, Terriere E, O'Carroll RE, Ebmeier KP. MRI correlates of episodic memory in Alzheimer's disease, mild cognitive impairment, and healthy aging. Psychiatry Res. 2010;184:57–62. doi: 10.1016/j.pscychresns.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 136.Friese U, Meindl T, Herpertz SC, Reiser MF, Hampel H, Teipel SJ. Diagnostic utility of novel MRI-based biomarkers for Alzheimer's disease: diffusion tensor imaging and deformation-based morphometry. J Alzheimers Dis. 2010;20:477–490. doi: 10.3233/JAD-2010-1386. [DOI] [PubMed] [Google Scholar]
- 137.Bendlin BB, Ries ML, Canu C, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC. White matter is altered with parental family history of Alzheimer's disease. Alzheimers Dement. 2010;6:394–403. doi: 10.1016/j.jalz.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor imaging. AJNR Am J Neuroradiol. 2009;30:893–899. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wee CY, Yap PT, Li W, Denny K, Browndyke JN, Potter GG, Welsh-Bohmer KA, Wang L, Shen D. Enriched white matter connectivity networks for accurate identification of MCI patients. Neuroimage. 2011;54:1812–1822. doi: 10.1016/j.neuroimage.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain. 2009;132:2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zarei M, Damoiseaux JS, Morgese C, Beckmann CF, Smith SM, Matthews PM, Scheltens P, Rombouts SA, Barkhof F. Regional white matter integrity differentiates between vascular dementia and Alzheimer disease. Stroke. 2009;40:773–779. doi: 10.1161/STROKEAHA.108.530832. [DOI] [PubMed] [Google Scholar]
- 142.Matsui H, Nishinaka K, Oda M, Niikawa H, Kubori T, Udaka F. Dementia in Parkinson's disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:177–181. doi: 10.1111/j.1600-0404.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- 143.Gattellaro G, Minati L, Grisoli M, Mariani C, Carella F, Osio M, Ciceri E, Albanese A, Bruzzone MG. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, Udaka F. Wisconsin Card Sorting Test in Parkinson's disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:108–112. doi: 10.1111/j.1600-0404.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 145.Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13:105–117. doi: 10.1097/01.nrl.0000252947.15389.a9. [DOI] [PubMed] [Google Scholar]
- 146.Potter GG, McQuoid DR, Steffens DC, Welsh-Bohmer KA, Krishnan KR. Neuropsychological correlates of magnetic resonance imaging-defined subcortical ischemic depression. International Journal of Geriatric Psychiatry. 2008;24:219–225. doi: 10.1002/gps.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 148.Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 149.Yuan Y, Zhang Z, Bai F, Yu H, Shi Y, Qian Y, Zang Y, Zhu C, Liu W, You J. White matter integrity of the whole brain is disrupted in first-episode remitted geriatric depression. NeuroReport. 2007;18:1845–1849. doi: 10.1097/WNR.0b013e3282f1939f. [DOI] [PubMed] [Google Scholar]
- 150.Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, Hrabe J, Kanellopoulos D, Shanmugham BR, Alexopoulos GS. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shimony JS, Sheline YI, D'Angelo G, Epstein AA, Benzinger TL, Mintun MA, McKinstry RC, Snyder AZ. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schermuly I, Fellgiebel A, Wagner S, Yakushev I, Stoeter P, Schmitt R, Knickenberg RJ, Bleichner F, Beutel ME. Association between cingulum bundle structure and cognitive performance: an observational study in major depression. Eur Psychiatry. 2010;25:355–360. doi: 10.1016/j.eurpsy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 153.Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, Alexander DC, Symms MR, Koepp MJ, Duncan JS. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133:2348–2364. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sidaros A, Engberg A, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 155.Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Sherman JE, Johnson SC. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42:503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: a review and future directions. Neuropsychol Rev. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21:657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- 159.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 160.Chen B, Guo H, Song AW. Correction for direction-dependent distortions in diffusion tensor imaging using matched magnetic field maps. Neuroimage. 2006;30:121–129. doi: 10.1016/j.neuroimage.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 161.Truong TK, Chen B, Song AW. Integrated SENSE DTI with correction of susceptibility- and eddy current-induced geometric distortions. Neuroimage. 2008;40:53–58. doi: 10.1016/j.neuroimage.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Truong TK, Chen NK, Song AW. Dynamic correction of artifacts due to susceptibility effects and time-varying eddy currents in diffusion tensor imaging. Neuroimage. 2011;57:1343–1347. doi: 10.1016/j.neuroimage.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62:468–475. doi: 10.1002/mrm.22024. [DOI] [PubMed] [Google Scholar]
- 164.Holdsworth SJ, Skare S, Newbould RD, Bammer R. Robust GRAPPA-accelerated diffusion-weighted readout-segmented (RS)-EPI. Magn Reson Med. 2009;62:1629–1640. doi: 10.1002/mrm.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu C, Bammer R, Kim DH, Moseley ME. Self-navigated interleaved spiral (SNAILS): Application to high-resolution diffusion tensor imaging. Magn Reson Med. 2004;52:1388–1396. doi: 10.1002/mrm.20288. [DOI] [PubMed] [Google Scholar]
- 166.Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med. 2002;47:42–52. doi: 10.1002/mrm.10014. [DOI] [PubMed] [Google Scholar]
- 167.Wang FN, Huang TY, Lin FH, Chuang TC, Chen NK, Chung HW, Chen CY, Kwong KK. PROPELLER EPI: an MRI technique suitable for diffusion tensor imaging at high field strength with reduced geometric distortions. Magn Reson Med. 2005;54:1232–1240. doi: 10.1002/mrm.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Calamante F, Tournier JD, Jackson GD, Connelly A. Track-density imaging (TDI): super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage. 2010;53:1233–1243. doi: 10.1016/j.neuroimage.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 169.Calamante F, Tournier JD, Heidemann RM, Anwander A, Jackson GD, Connelly A. Track density imaging (TDI): Validation of super resolution property. Neuroimage. 2011;56:1259–1266. doi: 10.1016/j.neuroimage.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 170.Basser PJ. Relationships between diffusion tensor and q-space MRI. Magn Reson Med. 2002;47:392–397. doi: 10.1002/mrm.10052. [DOI] [PubMed] [Google Scholar]
- 171.Assaf Y, Cohen Y. Structural information in neuronal tissue as revealed by q-space diffusion NMR spectroscopy of metabolites in bovine optic nerve. NMR Biomed. 1999;12:335–344. doi: 10.1002/(sici)1099-1492(199910)12:6<335::aid-nbm581>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 172.King MD, Houseman J, Roussel SA, van Bruggen N, Williams SR, Gadian DG. q-Space imaging of the brain. Magn Reson Med. 1994;32:707–713. doi: 10.1002/mrm.1910320605. [DOI] [PubMed] [Google Scholar]
- 173.Gilbert RJ, Wedeen VJ, Magnusson LH, Benner T, Wang R, Dai G, Napadow VJ, Roche KK. Three-dimensional myoarchitecture of the bovine tongue demonstrated by diffusion spectrum magnetic resonance imaging with tractography. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1173–1182. doi: 10.1002/ar.a.20387. [DOI] [PubMed] [Google Scholar]
- 174.Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 175.Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 176.Frank LR. Anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med. 2001;45:935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- 177.Frank LR. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med. 2002;47:1083–1099. doi: 10.1002/mrm.10156. [DOI] [PubMed] [Google Scholar]
- 178.Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. Diffusion MRI of complex neural architecture. Neuron. 2003;40:885–895. doi: 10.1016/s0896-6273(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 179.Liu C, Mang SC, Moseley ME. In vivo generalized diffusion tensor imaging (GDTI) using higher-order tensors (HOT) Magn Reson Med. 2010;63:243–252. doi: 10.1002/mrm.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Catani M. From hodology to function. Brain. 2007;130:602–605. doi: 10.1093/brain/awm008. [DOI] [PubMed] [Google Scholar]
- 181.Avram AV, Guidon A, Song AW. Myelin water weighted diffusion tensor imaging. Neuroimage. 2010;53:132–138. doi: 10.1016/j.neuroimage.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 182.Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59:1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barazany D, Basser PJ, Assaf Y. In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. Brain. 2009;132:1210–1220. doi: 10.1093/brain/awp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xi MC, Liu RH, Engelhardt JK, Morales FR, Chase MH. Changes in the axonal conduction velocity of pyramidal tract neurons in the aged cat. Neuroscience. 1999;92:219–225. doi: 10.1016/s0306-4522(98)00754-4. [DOI] [PubMed] [Google Scholar]
- 185.Terao S, Sobue G, Hashizume Y, Shimada N, Mitsuma T. Age-related changes of the myelinated fibers in the human corticospinal tract: a quantitative analysis. Acta Neuropathologica. 1994;88:137–142. doi: 10.1007/BF00294506. [DOI] [PubMed] [Google Scholar]
- 186.Madden DJ. Speed and timing of behavioral processes. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5th ed. San Diego, CA: Academic Press; 2001. pp. 288–312. [Google Scholar]
- 187.Salthouse TA, Madden DJ. Information processing speed and aging. In: Deluca J, Kalmar J, editors. Information processing speed in clinical populations. New York: Psychology Press; 2007. pp. 221–241. [Google Scholar]
- 188.Mandl RC, Schnack HG, Zwiers MP, van der Schaaf A, Kahn RS, Hulshoff Pol HE. Functional diffusion tensor imaging: measuring task-related fractional anisotropy changes in the human brain along white matter tracts. PLoS ONE. 2008;3:e3631. doi: 10.1371/journal.pone.0003631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 190.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 191.Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Iturria-Medina Y, Perez Fernandez A, Morris DM, Canales-Rodriguez EJ, Haroon HA, Garcia Penton L, Augath M, Galan Garcia L, Logothetis N, Parker GJ, Melie-Garcia L. Brain hemispheric structural efficiency and interconnectivity rightward asymmetry in human and nonhuman primates. Cereb Cortex. 2011;21:56–67. doi: 10.1093/cercor/bhq058. [DOI] [PubMed] [Google Scholar]
- 193.Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5 doi: 10.1371/journal.pcbi.1000395. e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the "default network" in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 196.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:1–13. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004 a;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Teipel SJ, Bokde AL, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, Herpertz SC, Moller HJ, Hampel H. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49:2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 200.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen NK, Chou YH, Song AW, Madden DJ. Measurement of spontaneous signal fluctuations in fMRI: adult age differences in intrinsic functional connectivity. Brain Struct Funct. 2009;213:571–585. doi: 10.1007/s00429-009-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- 203.Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- 204.Charlton RA, Barrick TR, Lawes IN, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 2010;46:474–489. doi: 10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 205.Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, Przybelski SA, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Petersen RC, Jack CR., Jr Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011 doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- 208.Shenkin SD, Bastin ME, MacGillivray TJ, Deary IJ, Starr JM, Wardlaw JM. Childhood and current cognitive function in healthy 80-year-olds: a DT-MRI study. Neuroreport. 2003;14:345–349. doi: 10.1097/00001756-200303030-00010. [DOI] [PubMed] [Google Scholar]
- 209.Shenkin SD, Bastin ME, Macgillivray TJ, Deary IJ, Starr JM, Rivers CS, Wardlaw JM. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovasc Dis. 2005;20:310–318. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- 210.Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- 211.Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- 212.Davis SW, Kragel J, Madden DJ, Cabeza R. The architecture of cross-hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr123. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fling BW, Walsh CM, Bangert AS, Reuter-Lorenz PA, Welsh RC, Seidler RD. Differential callosal contributions to bimanual control in young and older adults. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Charlton RA, Barrick TR, Markus HS, Morris RG. The relationship between episodic long-term memory and white matter integrity in normal aging. Neuropsychologia. 2010;48:114–122. doi: 10.1016/j.neuropsychologia.2009.08.018. [DOI] [PubMed] [Google Scholar]