Abstract
Objective
Small hyaluronan (HA) oligosaccharides serve as competitive receptor antagonists to displace HA from the cell surface and induce cell signaling events. In articular chondrocytes this cell signaling is mediated by the HA receptor CD44 and induces stimulation of genes involved in matrix degradation such as matrix metalloproteinases as well as matrix repair genes including collagen type II, aggrecan and HA synthase-2. The objective of this study was to determine changes in the expression and function of aggrecanases after disruption of chondrocyte CD44-HA interactions.
Methods
Bovine articular chondrocytes or bovine cartilage tissue were pre-treated with a variety of inhibitors of major signaling pathways prior to the addition of HA oligosaccharides. Changes in aggrecanase were monitored by real time reverse transcriptase-polymerase chain reaction and western blot analysis of ADAMTS4, ADAMTS5 and aggrecan proteolytic fragments. To test the interactions between ADAMTS4 and MT4-MMP, protein lysates purified from stimulated chondrocytes were subjected to co-immunoprecipitation.
Results
Disruption of chondrocyte CD44-HA interactions with HA oligosaccharides induced the transcription of ADAMTS4 and ADAMTS5 in time- and dose-dependent manner. The association of GPI-anchored MT4-MMP with ADAMTS4 was also induced in articular chondrocytes by HA oligosaccharides. Inhibition of the NF-κB pathway blocked HA oligosaccharides-mediated stimulation of aggrecanases.
Conclusions
Disruptive changes in chondrocyte-matrix interactions by HA oligosaccharides induce matrix degradation and elevate aggrecanases via the activation of the NF-κB signaling pathway.
Keywords: Hyaluronan, Hyaluronan oligosaccharides, CD44, ADAMTS4, ADAMTS5, MT4-MMP
The supramolecular organization of proteoglycans in extracellular matrices of vertebrate tissues involves hyaluronan (HA) as the backbone for assembly (1). In articular cartilage, HA functions to retain aggrecan in large link protein-stabilized proteoglycan aggregates (2, 3). Many connective tissue cells, including chondrocytes, exhibit a large HA and proteoglycan-rich pericellular matrix that is tethered to the cell surface via interactions with the HA receptor, CD44 (4, 5). For these cells, the constitutive retention of HA and proteoglycans at the cell surface represents the quiescent state as the binding of the HA alone does not serve as an inductive signal. HA oligosaccharides, of the size of a HA hexasaccharides or larger, can compete with the high molecular mass HA to CD44 (6-8) and competitively displace HA (and the proteoglycan-rich pericellular matrix) from the cell surface (1, 3). The physical loss of this cell-associated pericellular matrix and the uncoupling of HA from CD44, gives rise to signal transduction events with responses that differ depending on the cell type. In articular chondrocytes this cell signaling induces stimulation of genes involved in matrix degradation such as matrix metalloproteinases (MMPs) as well as matrix repair genes including collagen type II, aggrecan and HA synthase-2 (HAS-2) (9-13). Under these conditions, HA oligosaccharides function as specific antagonists of high molecular weight HA. The current model is that CD44 receptors are clustered in the plasma membrane by way of multivalent interactions with high molecular mass HA; this clustering is reversed by addition of excess HA oligosaccharides, displacing endogenous HA and generating monovalent interactions between HA oligosaccharides and CD44 (1, 3). The transmembrane receptor CD44 has a short intracellular tail domain but no inherent kinase activity (14). Thus, signaling events induced by HA oligosaccharides unclustering of CD44 likely involve the activation of CD44-associated proteins including kinases and cytoskeletal elements (15).
Degradation of aggrecan is an important manifestation of osteoarthritis (OA) (16). Aggrecan depletion in OA cartilage has been ascribed to increased proteolytic cleavage of the core protein at specific Glu-X bonds (17) mediated by endoproteinases termed aggrecanases. The two principal aggrecanases are members of a family of secreted zinc metalloproteinases referred to as ADAMTS (a disintegrin and metalloproteinase with thrombospondin type-1 motifs) and are characterized by ancillary domain containing one or more thrombospondin type 1 repeats. (18). Whilst there are six known aggrecanases (ADAMTS1, 4, 5, 8, 9, 15), in human OA cartilage activity appears to be primarily due to ADAMTS4 and ADAMTS5 (19). ADAMTS4 is a major aggrecanase expressed in the human OA cartilage (20). It was shown in a transfected chondrosarcoma lineage cell that proteolytic C-terminal truncation of ADAMTS4 activates its capacity to cleave the interglobular domain of aggrecan; this process can be mediated by the glycosylphosphatidylinositol-anchored membrane type 4-matrix metalloproteinase (MT4-MMP; aka MMP-17) (21). Both ADAMTS4 and ADAMTS5 can generate the G1-NITEGE392 and 393ARG-G2-ELE1499/EEE1685 products from aggrecan—degradation products that are found in articular cartilage, meniscal cartilage and soft tissues within the joint space. These domains can also be observed in the medium of cartilage explants (22).
In the present study, we examined the effects of disruption of chondrocyte CD44-HA interactions by HA oligosaccharides and found enhanced transcription of both aggrecanases and the accumulation of ITEGE neoepitope in the medium of cartilage explants. The association of MT4-MMP with ADAMTS4 was found by co-immunoprecipitation, and the formation of this complex was enhanced following treatment with HA oligosaccharides. Moreover, chemical inhibitors of NF-κB but not of the p38 MAP kinase pathway blocked the HA oligosaccharides-mediated stimulation of ADAMTS4 and ADAMTS5 mRNA expression.
MATERIALS AND METHODS
Cell Culture
Chondrocytes were isolated from full thickness (~ 600 μm) slices of the articular surface of the metacarpophalangeal joints of 18-24 month old adult bovine steers as described previously (23). For other studies, the upper layer (~200 μm) of articular cartilage (representing the upper ~30% of the cartilage full thickness) was dissected and collected first, followed by the isolation of slices representing the middle and deeper zones (~400 μm; ~70%). Thickness measurements were made on histological sections prepared from representative cartilage slices cut to separate the upper zone from the middle/deep zone. Cartilage slices were digested in 0.2% Pronase (Calbiochem, San Diego, CA) in 1:1 mixture of DMEM/Ham’s F-12 medium (Mediatech, Herndon, VA; D-glucose concentration, 3151.0 mg/L) plus 10% fetal bovine serum (FBS; Thermo Scientific, Rockford, IL) for 1.5 hours at 37 °C, followed by overnight digestion with 0.025% collagenase-P (Calbiochem) in DMEM/Ham’s F-12 medium containing 10% FBS. Primary chondrocytes were plated as high density monolayers (1 × 107 cells/35 mm2 dishes) in DMEM/Ham’s F-12 medium supplemented with 50 units/ml penicillin (Mediatech), 50 μg/ml streptomycin (Mediatech) and 10% FBS and incubated at 37 °C in atmosphere containing 5% CO2.
Preparation of HA oligosaccharides
HA from rooster comb (Sigma, St Louis, MO) was used to generate HA oligosaccharides as described previously (9). HA disaccharides (HA2) were generated from the HA oligosaccharides by treatment with chondroitinase ABC (Sigma) (10).
Treatment of cells with chemical inhibitors of major signaling pathway
Chondrocytes were cultured in DMEM/F-12 with 10% FBS for 48 hours, and then brought to serum-free conditions by a gradual reduction to 1% FBS for 24 hours prior to the start of the experiment in serum-free media. Chondrocytes were incubated with 250 μg/ml HA oligosaccharides (or PBS as vehicle) for time periods between 0 and 48 hours. As a control, the chondrocytes were stimulated with HA disaccharides (250 μg/ml), which were generated from HA oligosaccharides. For signaling inhibition assays, chondrocytes were pretreated with inhibitors of NF-κB (1 μM Helenalin, Calbiochem); p38 mitogen-activated protein (MAP) kinase (5 μM SB203580, Calbiochem); MAP kinase kinase (MEK) (10 μM PD98059, Calbiochem) or; phosphatidylinositol-3-kinase (PI3K)/Akt (25 μM LY294002, Calbiochem) for 60 minutes and then, treated with 0 or 250 μg/ml HA oligosaccharides for 24 hours in the continuing presence or absence of each inhibitor. These inhibitor concentrations have previously been reported as effective in blocking activation of NF-κB, p38 MAPK and MEK (12) as well as Akt phosphorylation (13) in bovine articular chondrocytes following treatment with HA oligosaccharides.
Cartilage explant cultures
Full-thickness slices (~1 × 10 × 10 mm) of bovine articular cartilage were cultured for 2 days in 1.0 ml DMEM/Ham’s F-12 medium containing 10% FBS. The tissue slices were rinsed, replaced with serum-free DMEM/Ham’s F-12 and then incubated for 1, 3 or 5 days without or with 250 μg/ml HA oligosaccharides.
Real time reverse transcriptase-polymerase chain reaction (RT-PCR)
Following incubation in the absence or presence of HA oligosaccharides, 10 ng/ml IL-1β (R&D; Minneapolis, MN) or pathway inhibitors, total RNA was isolated from the bovine chondrocytes using TRIzol® Reagent (Invitrogen, Carlsbad, CA). Samples were reverse transcribed with Q-Script cDNA supermix reagents (Quanta BioSciences, Gaithersburg, MD) and amplified at 42 °C for 30 minutes. For real time RT-PCR, the PCR products were detected by RT2 Real Time™ SYBR® Green reagents (SA Biosciences, Frederick, MD). Primer-specific amplification was at 60 °C for 30 seconds with fluorescence quantification performed at 72 °C. The primer sequences were designed as follows: GAPDH: forward, 5′ATTCTGGCAAAGTGGACATCGTCG3′, reverse, 5′ATGGCCTTTCCATTGATGACGAGC3′; ADAMTS4: forward, 5′TCACTGACTTCCTAGACAATGG3′, reverse, 5′ACTGGCGGTCAGCGTCGTAGT3′; ADAMTS5: forward, 5′CACCGTGGCTCAGGAAATTG3′, reverse, 5′GGAGCCGAAATTTTCTTCACAGA3′ and; MT4-MMP: forward, 5′TGACCAAGTGGAACAAGAGG3′, reverse, 5′TGATGTCGCTCCAGACTTTG3′. All primers were custom made by Integrated DNA Technologies (Coralville, IA). Thermal cycling was performed on a Smart Cycler system (Cepheid, Sunnyvale, CA). Real time RT-PCR efficiency (E) for each primer set was calculated according to Rasmussen (24). The fold increase in copy numbers of mRNA was calculated as a relative ratio of target gene (either ADAMTS4, ADAMTS5 or MT4-MMP) to GAPDH, following the model of Pfaffl (25).
Western blot analyses
Following incubations, the conditioned media of chondrocyte monolayers or articular cartilage explant cultures were collected and clarified by centrifugation at 13,000 rpm for 15 minutes at 4 °C. The medium was concentrated 10-fold (chondrocytes) or 5-fold (cartilage explants) using Amicon ultra-0.5 ml centrifugal filters (Millipore, Billerica, MA) and stored at −80 °C. Equivalent volumes of the concentrated conditioned media were loaded and separated on Novex 4-12% gradient SDS-PAGE gels (Invitrogen). Following electroblot transfer onto nitrocellulose membranes and blocking in 5% nonfat dry milk, membranes were incubated with primary antibodies followed by HRP-conjugated secondary antibodies. Detection was performed using chemiluminescence (Novex ECL, Invitrogen). Specific antibodies used were goat anti-ADAMTS4 IgG (0.2 μg/ml, Santa Cruz Biotechnology Santa Cruz, CA), rabbit anti-ADAMTS5 IgG (0.2 μg/ml, Thermo Fisher Scientific) (26) and rabbit anti-ITEGE373 IgG (0.5 μg/ml) (22, 27). The blots were stripped using 0.76% Tris/2% SDS with 0.7% β-mercaptoethanol pH 6.8 for re-probing with an anti-β-actin antibody (AC-15; Sigma). Gel images were subjected to densitometric analysis using Image J software (http://rsb.info.nih.gov). Fold increase in pixel density was obtained by normalization of band intensity for each condition to untreated, control sample values. The relative band intensity of untreated 24-hour-cultured control values was set to 1.0.
Co-immunoprecipitation
Bovine articular chondrocytes (1 × 107 cells/35 mm2 dish) were stimulated with 250 μg/ml HA oligosaccharides for times indicated, or cultured without HA oligosaccharides for 24 hours as control. Cells were rinsed in cold PBS and lysed on ice for 10 minutes in 100 μl of lysis buffer [10 mM Tris-HCl/0.15 M NaCl, pH 7.5 with 1% NP-40, 1 mM EDTA and protease inhibitor cocktail (Thermo Fisher Scientific)]. The mixture was centrifuged at 13,000 rpm for 10 minutes at 4 °C and the supernatant collected as the cell lysates. For immunoprecipitations, magnetic protein G beads were conjugated by mixing goat anti-ADAMTS4 IgG (2 μg) with Dynabeads® (Invitrogen) (1.5 mg) in 200 μl of PBS for 10-minutes at room temperature with rotation. The cell lysates were incubated with washed Dynabeads®-antibody complexes for 10 minutes at room temperature with rotation to allow antigen to bind to the complexes. The supernatants from this reaction were collected, concentrated to 50% volume by centrifugal spin filter prior to analysis as the unbound fraction. The Dynabeads®-antibody-antigen complexes were washed 3 times and the antigens eluted with 20 μl of elution buffer (50 mM glycine, pH 2.8 along with 10 μl of NuPAGE LDS Sample Buffer (Invitrogen) and reducing agent), followed by heating for 10 minutes at 70 °C. Eluted supernatants (bound fraction) were collected in a magnetic field apparatus and aliquots loaded by equivalent volume onto Novex 4-12% gradient SDS-PAGE gels for western blot analysis. Samples were processed by electrophoreses and electroblot as described above and analyzed by western blotting using rabbit anti-MT4-MMP IgG (0.2 μg/ml, Santa Cruz Biotechnology). The experiment was performed three times, with similar results obtained in each experiment.
Statistical analysis
All data were obtained from at least three independent experiments performed in triplicate. Statistical significance was determined using the Student’s t test. A p-value of less than 0.05 was considered significant.
RESULTS
HA oligosaccharides stimulate the expression of ADAMTS4 and ADAMTS5 mRNA
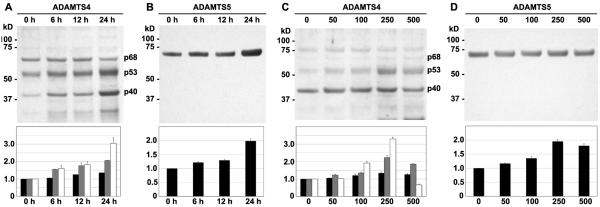
Primer sets for real time RT-PCR amplification and quantification of bovine ADAMTS4, ADAMTS5 and GAPDH were designed. Amplifications efficiencies for each primer set were determined allowing the comparison of mRNA copy numbers between treated versus control cultures, normalized to GAPDH. Chondrocytes were incubated in serum-free conditions for 24 hours in the absence of HA oligosaccharides or for 6, 12, 24 or 48 hours in the presence of 250 μg/m HA oligosaccharides, and processed for total RNA. As shown in Fig 1A, as early as 3 hours of incubation with HA oligosaccharides there was a 2.9-fold increase in ADAMTS4 mRNA copy number as compared to untreated control chondrocytes. This effect of HA oligosaccharides was time-dependent with the maximum enhancement observed at the 24-hour time point (11.3-fold increase). As shown in Fig 1B, HA oligosaccharides also affected a statistically significant increase in ADAMTS5 mRNA expression by 12 hours, reaching a maximal 2.9 fold increase at the 24-hour time point. HA-oligosaccharide mediated stimulation of aggrecanase mRNA was transient and copy numbers returned to basal levels at the 48-hour time point (Fig. 1A, B). HA oligosaccharides affected a concentration-dependent increase in aggrecanase, reaching statistical significance at 250 μg/ml following 6 hours of treatment (white bars) and 100 μg/ml following 24 hours of treatment (black bars, Fig. 1C, D). As a control, the same HA oligosaccharides were predigested with chondroitinase ABC to generate HA disaccharides. Incubation of chondrocytes with HA disaccharides at 250 μg/ml resulted in no stimulation of ADAMTS4 or ADAMTS5 mRNA (Fig. 1C, D). These results suggest that the aggrecanase stimulatory activity observed is due to a reagent sensitive to chondroitinase digestion and not a contaminant. The maximum levels of stimulation of ADAMTS4 and ADAMTS5 mRNA induced by HA oligosaccharides (11.2-fold and 2.8-fold, respectively, Fig. 1) were substantial but lower than values obtained in parallel cultures treated with 10 ng/ml IL-1β for 24 hours. In these cells, IL-1β treatment resulted in a 22-fold increase in ADAMTS4 and 7.1-fold increase in ADAMTS5 mRNA as compared to control chondrocytes (data not shown).
Figure 1. Time course and concentration dependency of aggrecanase mRNA stimulation by HA oligosaccharides.
The fold change in mRNA copy number for ADAMTS4 (panel A) or ADAMTS5 (panel B) are shown for chondrocytes cultured for 24 hours in the absence (Ctr) or, 1 to 48 hours in the presence of 250 μg/m HA oligosaccharides. Values represent the average ± S.D. of data derived from triplicate cultures. Chondrocytes treated for 6 hours (white bars) or 24 hours (black bars) with 0 to 500 μg/ml HA oligosaccharides or 250 μg/ml HA disaccharides (HA2) were analyzed for fold changes in mRNA copy number for ADAMTS4 (panel C) or ADAMTS5 (panel D). Values represent the average ± S.D. of data derived from triplicate cultures. *P<0.05, **P<0.01 respectively as measured using an unpaired Student’s t test.
To determine whether particular sub-populations of chondrocytes respond differently to HA oligosaccharides, articular chondrocytes were isolated from the upper layers (~30%) and the lower, middle-deep zone layers of bovine cartilage. Cells isolated from these two zones as well as full-thickness cartilage slices were incubated for 24 hours without or with 250 μg/ml HA oligosaccharides and analyzed for changes in ADAMTS4 and ADAMTS5 mRNA expression. As shown Figure 2, chondrocytes derived from the upper middle / superficial layers were more responsive to HA oligosaccharides as compared to chondrocytes isolated from the middle and deep zones. Chondrocytes derived from full thickness slices reflected an intermediate level of responsiveness.
Figure 2. HA oligosaccharide-mediated stimulation of aggrecanase mRNA expression in chondrocytes derived from different layers of articular cartilage.
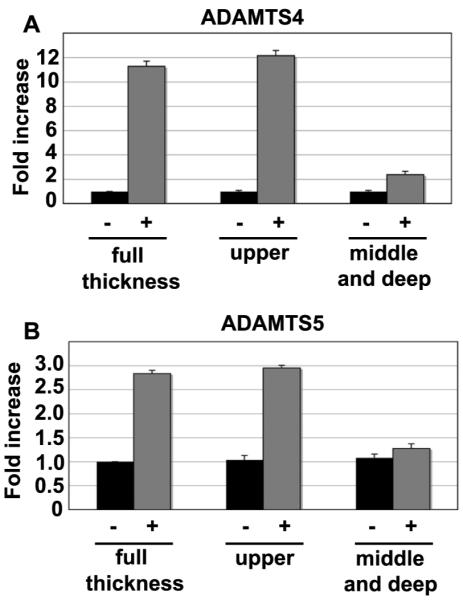
Chondrocytes were isolated from full thickness, the upper layers (upper 30%) or, the middle to deep zone layers (lower 70%) of articular cartilage, and cultured for 24 hours with (+) or without (−) 250 μg/ml HA oligosaccharides. The fold change in ADAMTS4 mRNA copy number (panel A) and ADAMTS5 mRNA copy number (panel B) values, between control and treated cultures were determined. Values shown represent the average ± S.D. of data derived from triplicate cultures. *P<0.05, **P<0.01 respectively as measured using an unpaired Student’s t test.
HA oligosaccharides enhance the levels of ADAMTS4 and ADAMTS5 protein released into the medium
Conditioned media from bovine articular chondrocyte cultures were analyzed by western blotting for aggrecanases. ADAMTS4 is synthesized in a pro-form (full-length; p100) which is processed in a multi-step manner, including furin-cleavage to a p68 form and subsequent conversion to species (p53 and p40 forms) with significant aggrecanase activity (Glu373-Ala374 cleaving activity) (21). As shown in Fig 3A, chondrocytes produced and secreted immunoreactive ADAMTS4 that was present as a p68, p53 and p40 species in the 24-hour media of control cultures. After incubation with HA oligosaccharides, there was an increase in ADAMTS4 released as compared to untreated chondrocytes, with increases in the p53 and p40 species the most prominent, especially at the 24-hour time point. The p53 and p40 species also increased in proportion to HA oligosaccharide concentration (Fig 3C) reaching a maximal level at 250 μg/ml. ADAMTS5 was visualized as a single 70 kD protein (Fig 3B). HA oligosaccharides also induced an increase in the level of ADAMTS5 present in the conditioned culture medium, a level that appears maximal following treatment of chondrocytes with 250 μg/ml HA oligosaccharides (Fig. 3B, 3D). Interestingly, no significant stimulation of aggrecanase protein was observed in cell lysates (data not shown).
Figure 3. Time course and concentration dependency of aggrecanase protein stimulation by HA oligosaccharides.
Shown are western blots of concentrated conditioned medium samples probed using antibodies specific for ADAMTS4, detecting ADAMTS4 species at 68, 53 and 40 kD (panels A and C) or for ADAMTS5 protein (panels B and D). Chondrocytes cultured for 24 hours in the absence (Ctr) or, for 6, 12 or 24 hours in the presence of 250 μg/m HA oligosaccharides are shown in panels A and B. Chondrocytes cultured for 24 hours with 0 to 500 μg/ml HA oligosaccharides are shown in panels C and D. Panels A – D depict representative western blots from one of three separate experiments. Detected bands were quantified by densitometric analysis and the normalized average ± SD fold change in pixel intensity for each condition, derived from three separate experiments (Panel A and D: black bar, p68; striped bar, p53; white bar, p40) .
HA oligosaccharides enhance the accumulation of aggrecanase degradation products, ITEGE-G1 domains
As a measure of aggrecanase activity, ADAMTS4 or ADAMTS5 generated fragments of aggrecan can be detected by use of antibodies that recognize the neoepitope G1 domain ITEGE (22). Accumulation of ITEGE-G1 domains released into the conditioned medium of bovine cartilage explant cultures was analyzed by western blotting. As shown in Fig 4, the ITEGE-G1 domain, present in the medium of control explants after 5 days, is represented as a heterogeneous band between 68-72 kD. This 68-72 kD band is similar in size as the ITEGE-G1 domain isolated from the conditioned medium of IL-1β treated bovine explant cultures [data not shown; also see Ariyoshi et al. (27)]. When cartilage explants were exposed to HA oligosaccharides for 1, 3 or 5 days, a progressive increase in the level of ITEGE-G1 domains released into the medium was observed (Fig 4). These results suggest that the ADAMTS-mediated aggrecan degradation has occurred in these tissues and increases with the duration of exposure of the tissues to HA oligosaccharides.
Figure 4. Time course of ITEGE-G1 accumulation in the medium of bovine explant cultures treated with HA oligosaccharides.
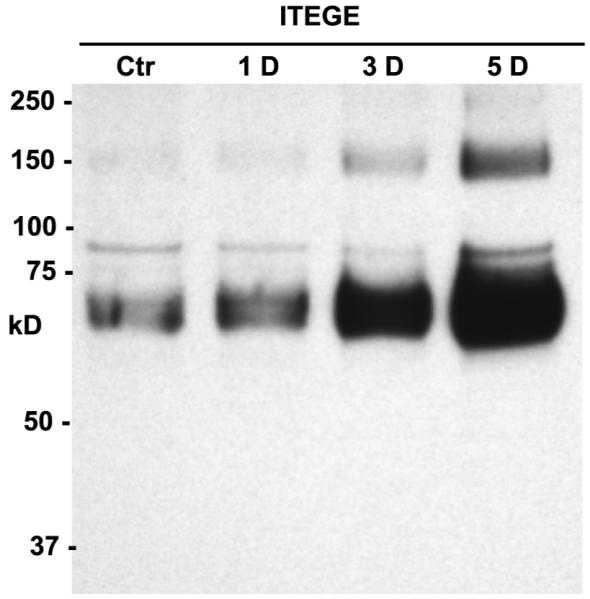
Bovine cartilage tissue was cultured as explants and treated for 5 days in the absence (Ctr) or, for 1, 3 or 5 days (1D, 3D, 5D respectively) in the presence of 250 μg/ml HA oligosaccharides. Shown is a representative western blot of concentrated conditioned medium probed using an anti-ITEGE neoepitope antibody. The image shown is representative of three separate experiments.
Interactions between MT4-MMP and ADAMTS4 are enhanced by treatment of chondrocytes with HA oligosaccharides
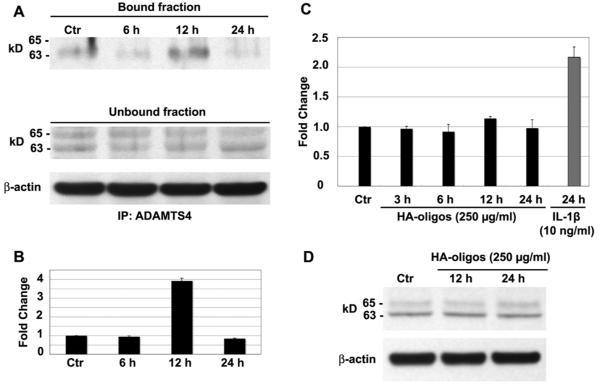
To examine whether association of GPI-anchored MT4-MMP and ADAMTS4 is also affected by HA oligosaccharide-induced activation of ADAMTS4 aggrecanase activity, we examined the interaction between ADAMTS4 and MT4-MMP by co-immunoprecipitation. Protein lysates were prepared and incubated with anti-ADAMTS4-conjugated magnetic beads, and the resulting bound and unbound fractions were analyzed by western blotting with anti-MT4-MMP antibody. As shown in Fig.5A, a single 63 kD band for MT4-MMP is detected in the ADAMTS4 immunoprecipitate of control and HA oligosaccharide treated chondrocytes. The level of MT4-MMP recovered from ADAMTS4 precipitates increased following HA oligosaccharides treatment of chondrocytes for 12 hours (Fig. 5A, 5B). MT4-MMP was also recovered in the unbound fraction but presented as a doublet band. These doublet bands are indicative of the 65 kDa (latent form) and 63 kDa (activated form) of MT4-MMP (28). No changes in the level of the doublet bands present in the unbound fraction were observed in immunoprecipitates of lysates from HA oligosaccharides treated chondrocytes. β-actin in unbound fraction also was not affected. Interestingly, the addition of HA oligosaccharides had no effect on the expression of MT4–MMP mRNA (Fig. 5C) or MT4-MMP protein (Fig. 5D). As a positive control, 24 hours of IL-1β treatment significantly increased MT4-MMP mRNA (Fig. 5C). These data suggest that HA oligosaccharides not only increase the synthesis of ADAMTS4 but, also promote the interaction of ADAMTS4 and the activated form (63 kD) of MT4-MMP.
Figure 5. Effect of HA oligosaccharides on MT4-MMP expression and interaction with ADAMTS4.
Chondrocytes were treated without (Ctr) or with 250 μg/ml HA oligosaccharides (HA-oligos) for indicated times. In panel A, cell lysates were immunoprecipitated using anti-ADAMTS4 and bound or unbound protein fractions probed using an anti-MT4-MMP antibody. Equivalent protein aliquots of cell lysates were also analyzed for β–actin. Panel B; bands detected in the bound fraction were quantified by densitometric analysis and the normalized average ± SD fold change in pixel intensity for each condition, derived from three separate experiments. Panel C; fold change in MT4-MMP mRNA copy number of chondrocytes treated 24 hours in the absence (Ctr) or, for times shown with HA oligosaccharides or IL-1β. Values shown represent the average ± SD of data derived from triplicate cultures. Panel D is a representative western blot (one of three separate experiments) probed for changes in MT4-MMP protein from lysates of chondrocytes incubated without (Ctr) or with HA oligosaccharides were. Equivalent protein aliquots of cell lysates were also analyzed for β–actin.
Inhibitors of select signaling pathways block the stimulation of ADAMTS4 and ADAMTS5 by HA oligosaccharides
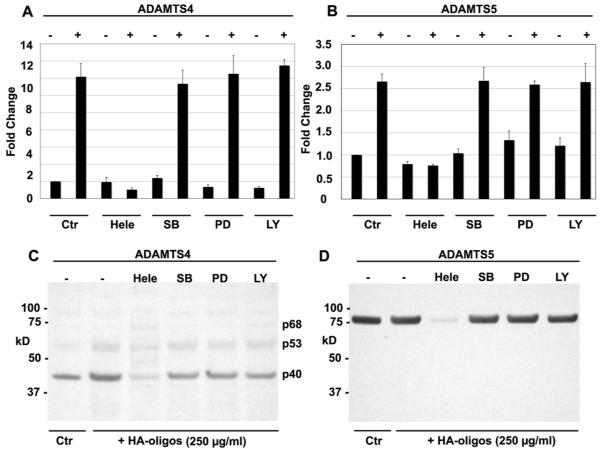
We have shown previously that chemical inhibitors of the NF-κB and p38 MAP kinase pathways block HA oligosaccharide-induced increases in MMP-13 mRNA, protein and caseinolytic activity in bovine articular chondrocytes (12). Conversely, inhibition of Akt activation (but not NF-κB, p38 MAP or p44/42 kinases) blocked HA oligosaccharides activation of HAS2 and HA production (13). To identify the general signaling pathway responsible for HA oligosaccharide induced stimulation of aggrecanase, bovine articular chondrocytes were pretreated with optimized concentrations of chemical pathway inhibitors. In control chondrocytes cultures treated with HA oligosaccharides but, without added inhibitor, there was an 11-fold and 2.7-fold increase in ADAMTS4 and ADAMTS5 mRNA, respectively following 24 hours of exposure to HA oligosaccharides (Fig. 6A, 6B). Helenalin (Hele) pre-treatment effectively blocked the stimulation of both ADAMTS4 and ADAMTS5 mRNA. In contrast, following pretreatment with SB203580, an increase in HA oligosaccharide-induced ADAMTS4 and ADAMTS5 mRNA expression was still observed. Additionally, neither the p44/42 MAP kinase inhibitor (PD98059) nor the PI3/Akt pathway inhibitor (LY294002) blocked the stimulation of ADAMTS4 or ADAMTS5 mRNA. As shown in Fig. 6C and 6D, pretreatment with helenalin also blocked the HA oligosaccharides-stimulated as well as basal levels of ADAMTS4 and ADAMTS5 protein recovered in conditioned media (lane 3), whereas SB203580, PD98059 or LY294002 had no effect on the accumulation of these aggrecanases. Lower levels of aggrecanase proteins present in the cell lysates also did not change (data not shown).
Figure 6. Effect of chemical inhibitors of signaling pathways on HA oligosaccharide-mediated stimulation of ADAMTS4 and ADAMTS5 in bovine articular chondrocytes.
The fold change in ADAMTS4 (panel A) or ADAMTS5 (panel D) mRNA copy number of chondrocytes preincubated without (Ctr) or with chemical inhibitors: Helenalin (Hele), SB203580 (SB), PD98059 (PD) or LY294002 (LY), 60 minutes prior to, and during 24 addition hours of incubation in the absence (−) or presence (+) of 250 μg/ml HA oligosaccharides. Values shown represent average ± S.D. of data derived from triplicate cultures. Conditioned medium from chondrocytes preincubated without (−) or with chemical inhibitors, 60 minutes prior to, and during 24 addition hours of incubation in the absence (Ctr) or presence of 250 μg/ml HA oligosaccharides (+ HA-oligos) were analyzed by western blotting and probed using anti-bodies specific for ADAMTS4 (panel C) or ADAMTS5 (panel D).
DISCUSSION
One of the early events associated with osteoarthritis is the loss of aggrecan from the cartilage. The data provided herein suggest that transcription and protein secretion of ADAMTS4 and ADAMTS5 are stimulated in bovine articular chondrocytes in response to HA oligosaccharide treatment. In addition, HA oligosaccharide treatment resulted in an increased association of ADAMTS4 with MT4-MMP, an increased release of the C-terminal truncated form of ADAMTS4 into the conditioned media of cultures and, an increase in aggrecanase-derived ITEGE neoepitope, all three events indicative of an overall enhancement in aggrecanase enzymatic activity. In this system, the stimulation of ADAMTS4 was more pronounced than that of ADAMTS5, similar to previous reports on IL-1α treated meniscal cartilage explants (29).
The question remains as to how chondrocytes, embedded deep within cartilage, sense or detect changes in the composition of the extracellular matrix—changes that require activation of matrix repair cycle. Such repair cycles could be initiated by a release of a matrix bound cytokines, feedback by damaged matrix macromolecules or, direct sensing by matrix receptors. Our work has focused on the latter, examining mechanisms whereby changes in multivalent occupancy of the hyaluronan receptor CD44 activate a cycle(s) of matrix repair. For these studies, small HA oligosaccharides are used as specific antagonists of CD44 interactions with HA-aggrecan-link protein complexes. As such, the HA oligosaccharides were used in this study to mimic events that might be expected to occur during wear-and-tear of the HA/aggrecan-rich extracellular matrix and the loss of cell-matrix interactions. Disaccharides of HA are too small to compete for binding of HA to CD44 and serve as an important negative control (9). As shown in Fig 1C and 1D, no stimulation of ADAMTS4 or ADAMTS5 was observed following treatment with HA disaccharides. Since these disaccharides are derived from the same HA oligosaccharide preparation, this demonstrates that the stimulation events observed are not due to potential contaminants within the oligosaccharide preparation. All of the stimulatory activity in the HA oligosaccharide preparation was susceptible to chondroitinase ABC.
The use of HA oligosaccharides as competitive antagonists of HA-CD44 interactions is not restricted to cartilage research. In the field of cancer biology, HA oligosaccharides have been shown to suppress tumor growth, induce tumor regression and in some cases inhibit metastasis (30-34). In these cancer models HA oligosaccharides are used as antagonists to selectively disrupt HA-CD44 interactions resulting in the activation of numerous signaling pathways. For example, in HA oligosaccharide treatment of anchorage independent murine carcinoma cells results in an inhibition of PI3kinase (coupled with an activation of PTEN), inhibition of Akt phosphorylation, followed by an inhibition of BAD, stimulation of caspase-3 and induction of apoptosis (31). Unlike the high levels of activated Akt found in many transformed cells, the level of phospho-Akt in chondrocytes is low at baseline and HA oligosaccharide treatment results in the activation of Akt (13). In human bladder carcinoma cells treated with HA oligosaccharides, the investigators observed an activation of Ras, followed by PKCξ, IκB kinase 1 and 2, leading to the inhibition of IκBα phosphorylation/degradation and the activation of NF-κB (35). Given that CD44 exhibits no intrinsic kinase or phosphatase activity (14) signal initiation is likely indirect, dependent on changes in CD44-associated intracellular elements.
In this study, the induction of aggrecanases by HA oligosaccharides was preferentially blocked by the NF-κB inhibitor, helenalin, an inhibitor that blocks NF-κB-DNA binding activity by selectively alkylating the p65 subunit of NF-κB (36). Helenalin has been reported to have alternative side reactions in some cell types including the generation of reactive oxygen species and activation of apoptosis (37). Although this was not directly investigated in this study, we have observed previously that helenalin treatment of control bovine articular chondrocytes had no effect on MMP-13-related activity as measured by caseinolytic zymography and fluorogenic substrate assay (12) or, in another study, by measuring changes in HAS-2 mRNA (13). Moreover, we have demonstrated previously that HA oligosaccharides activate NF-κB as measured by NF-κB EMSA, activation of a NF-κB responsive promoter element (in C28/I2 cells) and, the phosphorylation of IKKα and IKKβ (12). Thus, while care must be taken to interpret results based on all chemical inhibitors, several lines of evidence suggest that NF-κB is indeed activated in chondrocytes exposed to HA oligosaccharides. Other investigators have also reported the role of NF-κB in the stimulation of mRNA levels of aggrecanases (38) and iNOS (39).
In a recent study by Yatabe et al., chondrocytes derived from OA cartilage responded to IL-1α treatment by an elevation of the mRNA and protein expression of ADAMTS4 (40). Interestingly, including small HA oligosaccharides during the IL-1α treatment augmented the stimulation of ADAMTS4 expression. On the other hand, addition of polymer HA (2700 kDa HA) in combination with IL-1α reduced the stimulation, but did not return expression levels to baseline. This study supports our results that multivalent HA interactions with chondrocyte CD44 lowers the stimulated levels closer to baseline expression of aggrecanases whilst displacement of those interactions with HA oligosaccharides results in stimulation of aggrecanases.
Previous studies reported that C-terminal truncation enhances the aggrecanase and versicanase activities of ADAMTS4 (41). With further investigation, it was reported that ADAMTS4 activation involves the coordinated activity of GPI-anchored MT4-MMP (29). An original suggestion was made that in human chondrosarcoma cell line JJ012-TS4 stably transfected with ADAMTS4 and transiently co-transfected with MT4-MMP, interaction between MT4-MMP and ADAMTS-4 occurred at the cell surface, since following cleavage of GPI linkages with phosphatidylinositol-specific phospholipase C, the investigators were able to co-immunoprecipitate ADAMTS-4 and MT4-MMP from that supernatant (21). In this study using co-immunoprecipitation method, we demonstrated that endogenous ADAMTS4 can form a complex with MT4-MMP and that HA oligosaccharides-induced ADAMTS4 was present in bovine articular chondrocytes in association with MT4-MMP. This result suggested that HA oligosaccharides enhanced ADAMTS4 activity through GPI-anchored MT4-MMP mediated processing. Therefore, following disruption of HA-CD44 interactions, a combination of increased NF-κB signaling and increased ADAMTS4 may be coupled with interactions between active MT4-MMP, generating the activation of aggrecanase to result in elevated aggrecanase activity.
In conclusion, we demonstrated herein that HA oligosaccharides enhance the transcription of ADAMTS4 and ADAMTS5 in chondrocytes by activation of NF-κB signaling pathways. CD44-mediated signaling supports this activation and is likely critical to certain cell types such as chondrocytes. These results suggest that articular chondrocytes have the capacity to sense changes in cell surface HA-CD44 interactions, resulting in the initiation of a chondrocytic chondrolysis response. Additional studies are needed to examine the correlation of HA oligosaccharides and aggrecanases regarding cartilage metabolism. Whether fragmentation of HA occurs in vivo also remains to be determined. Nonetheless, several mechanisms can lead to a disruption of HA-cell interactions, all with similar results that include the activation of potent matrix metalloproteinases.
ACKNOWLEDGEMENTS
The authors thank Dr Amanda J Fosang of the Department of Paediatrics and Murdoch Childrens Research Institute at Melbourne University, Parkville, Australia for the anti-ITEGE antibody. Supported in part by NIH grants: RO1-AR43384 (WK), RO1-AR39507 (CBK).
Supported by the NIH (grant R01-AR-039507 to CBK and grant R01-AR-043384 to WK) from the National Institute of Arthritis, Musculoskeletal and Skin Diseases.
Footnotes
Conflict of interest: All authors declare no conflict of interest
REFERENCES
- 1.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 2.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–70. [PubMed] [Google Scholar]
- 3.Knudson CB, Knudson W. Hyaluronan and CD44: modulators of chondrocyte metabolism. Clin Orthop. 2004;427:152–62. [PubMed] [Google Scholar]
- 4.Knudson W, Aguiar DJ, Hua Q, Knudson CB. CD44-anchored hyaluronan-rich pericellular matrices: An ultrastructural and biochemical analysis. Exp Cell Res. 1996;228:216–28. doi: 10.1006/excr.1996.0320. [DOI] [PubMed] [Google Scholar]
- 5.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–13. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 6.Underhill CB, Toole BP. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979;82:475–84. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudson W, Bartnik E, Knudson CB. Assembly of pericellular matrices by COS-7 cells transfected with CD44 homing receptor genes. Proc Natl Acad Sci USA. 1993;90:4003–07. doi: 10.1073/pnas.90.9.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–75. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 9.Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrogenic chondrolysis. Arthritis Rheum. 2000;43:1165–74. doi: 10.1002/1529-0131(200005)43:5<1165::AID-ANR27>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharide-induced activation of transcription factors in bovine articular chondrocytes. Arthr Rheum. 2005;52:800–09. doi: 10.1002/art.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacob S, Knudson CB. Hyaluronan fragments activate nitric oxide synthase and the production of nitric oxide by articular chondrocytes. Int J Biochem Cell Biol. 2006;38:123–33. doi: 10.1016/j.biocel.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFkappaB and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2006;281:17952–60. doi: 10.1074/jbc.M602750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz I, Ariyoshi W, Takahashi N, Knudson CB, Knudson W. Hyaluronan oligosaccharide treatment of chondrocytes stimulates expression of both HAS-2 and MMP-3, but by different signaling pathways. Osteoarthritis Cartilage. 2010;18:447–54. doi: 10.1016/j.joca.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponta H, Sherman L, Herrlich P. CD44: From adhesion molecules to signaling regulators. Nature Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 15.Girish KS, Kemparaju K, Nagaraju S, Vishwanath BS. Hyaluronidase inhibitors: a biological and therapeutic perspective. Curr Med Chem. 2009;16(18):2261–88. doi: 10.2174/092986709788453078. [DOI] [PubMed] [Google Scholar]
- 16.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 17.Arner EC, Pratta MA, Trzaskos JM, Decicco CP, Tortorella MD. Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-degradaing activity. J Biol Chem. 1999;274(10):6594–601. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- 18.Apte SS. A Disintegrin-like and Metalloprotease (Reprolysin-type) with Thrombospondin Type 1 Motif (ADAMTS) Superfamily: Functions and Mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56(2):575–85. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 20.Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathology International. 2007;57:703–11. doi: 10.1111/j.1440-1827.2007.02167.x. [DOI] [PubMed] [Google Scholar]
- 21.Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–51. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- 22.Ilic MZ, Handley CJ, Robinson HC, Mok MT. Mechanism of catabolism of aggrecan by articular cartilage. Arc Biochem Biophys. 1992;294:115–22. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- 23.Nishida Y, Knudson CB, Kuettner KE, Knudson W. Osteogenic protein-1 promotes the synthesis and retention of extracellular matrix within bovine articular cartilage and chondrocyte cultures. Osteoarthritis Cartilage. 2000;8:127–36. doi: 10.1053/joca.1999.0281. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen TB, Uttenthal A, de Stricker K, Belak S, Storgaard T. Development of a novel quantitative real-time RT-PCR assay for the simultaneous detection of all serotypes of foot-and-mouth disease virus. Arch Virol. 2003;148:2005–21. doi: 10.1007/s00705-003-0145-2. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–91. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- 27.Ariyoshi W, Knudson CB, Luo N, Fosang AJ, Knudson W. Internalization of aggrecan G1 domain neoepitope ITEGE in chondrocytes requires CD44. J Biol Chem. 2010;285:36216–24. doi: 10.1074/jbc.M110.129270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J Biol Chem. 1999;274:34260–6. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- 29.Lemke AK, Sandy JD, Voigt H, Dreier R, Lee JH, Grodzinsky AJ, et al. Interleukin-1alpha treatment of meniscal explants stimulates the production and release of aggrecanase-generated, GAG-substituted aggrecan products and also the release of pre-formed, aggrecanase-generated G1 and m-calpain-generated G1-G2. Cell Tissue Res. 2010;340:179–88. doi: 10.1007/s00441-010-0941-4. [DOI] [PubMed] [Google Scholar]
- 30.Ward JA, Huang L, Guo H, Ghatak S, Toole BP. Perturbation of hyaluronan interactions inhibits malignant properties of glioma cells. Am J Pathol. 2003;162:1403–9. doi: 10.1016/S0002-9440(10)64273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–20. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 32.Toole BP. Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin Cancer Res. 2009;15:7462–68. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosono K, Nishida Y, Knudson W, Knudson CB, Naruse T, Suzuki Y, et al. Hyaluronan Oligosaccharides Inhibit Tumorigenicity of Osteosarcoma Cell Lines MG-63 and LM-8 in Vitro and in Vivo via Perturbation of Hyaluronan-Rich Pericellular Matrix of the Cells. Am J Pathol. 2007 doi: 10.2353/ajpath.2007.060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slomiany MG, Dai L, Tolliver LB, Grass GD, Zeng Y, Toole BP. Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides. Clin Cancer Res. 2009;15:7593–601. doi: 10.1158/1078-0432.CCR-09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald KA, Bowie AG, Skeffington BS, O’Neill LA. Ras, protein kinase C zeta, and I kappa B kinases 1 and 2 are downstream effectors of CD44 during the activation of NF-kappa B by hyaluronic acid fragments in T-24 carcinoma cells. J Immunol. 2000;164:2053–63. doi: 10.4049/jimmunol.164.4.2053. [DOI] [PubMed] [Google Scholar]
- 36.Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem. 1998;273:33508–16. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- 37.Berges C, Fuchs D, Opelz G, Daniel V, Naujokat C. Helenalin suppresses essential immune functions of activated CD4+ T cells by multiple mechanisms. Mol Immunol. 2009;46:2892–901. doi: 10.1016/j.molimm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Chockalingam PS, Varadarajan U, Sheldon R, Fortier E, LaVallie ER, Morris EA, et al. Involvement of protein kinase Czeta in interleukin-1beta induction of ADAMTS-4 and type 2 nitric oxide synthase via NF-kappaB signaling in primary human osteoarthritic chondrocytes. Arthritis Rheum. 2007;56:4074–83. doi: 10.1002/art.23043. [DOI] [PubMed] [Google Scholar]
- 39.Chu SC, Marks-Konczalik J, Wu HP, Banks TC, Moss J. Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters. Biochem Biophys Res Commun. 1998;248:871–8. doi: 10.1006/bbrc.1998.9062. [DOI] [PubMed] [Google Scholar]
- 40.Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68:1051–8. doi: 10.1136/ard.2007.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002;277:11034–41. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]