Abstract
Objective
Clinical trials of bone marrow-derived stem cell therapy for the heart have yielded variable results. The basic mechanism(s) that underlie their potential efficacy remains unknown. In the present study, we evaluate the survival kinetics, transcriptional response, and functional outcome of intramyocardial bone marrow mononuclear cell (BMMC) transplantation for cardiac repair in murine myocardial infarction model.
Methods and Results
We utilized molecular-genetic bioluminescence imaging and high throughput transcriptional profiling to evaluate the in vivo survival kinetics and gene expression changes of transplanted BMMCs after their engraftment into ischemic myocardium. Our results demonstrate short-lived survival of cells following transplant, with less than 1% of cells surviving by 6 weeks post-transplantation. Moreover, transcriptomic analysis of BMMCs revealed non-specific upregulation of various cell regulatory genes with a marked downregulation of cell differentiation and maturation pathways. BMMC therapy caused limited improvement of heart function as assessed by echocardiography, invasive hemodynamics, and positron emission tomography (PET). Histological evaluation of cell fate further confirmed findings of the in vivo cell tracking and transcriptomic analysis.
Conclusions
Collectively, these data suggest that BMMC therapy, in its present iteration, may be less efficacious than once thought. Additional refinement of existing cell delivery protocols should be considered to induce better therapeutic efficacy.
Keywords: stem cells, bone marrow mononuclear cells, transcriptional profiling, molecular imaging, myocardial infarction
INTRODUCTION
Within the last several years, bone marrow (BM)-derived cell therapy for heart disease has achieved clinical reality. Numerous trials (several ongoing) have attempted to evaluate the efficacy and possible benefits that BM-derived therapy may afford 1–13. Yet, despite the prevalence of clinical cell therapy, little is known regarding the biological mechanisms by which BM cells might act to modulate myocardial function. In fact, fundamental issues such as optimal cell dosing, timing of delivery, and survivability following transplant remain ill-defined 14, 15.
While both animal and human studies have attempted to address mechanisms underlying cardiac cell therapy 16–19, a detailed exploration of BM cell survival kinetics in the setting of acute ischemic injury remains lacking. The interplay between the host environment and transplanted cells has not been studied in detail, and the early cellular response following cell delivery and implantation at a transcriptional level remains unknown. In this paper, we aim to address some of the aforementioned issues, primarily to investigate the efficacy of bone marrow mononuclear cell (BMMC) therapy for acute myocardial disease.
We utilize a clinically relevant model of acute myocardial ischemia-reperfusion (I/R) injury, coupled with molecular imaging techniques to test our hypothesis that the limited efficacy of cell therapy demonstrated in clinical trials may be the result of poor post-transplant cell survival. We further characterize the effects of the ischemic cardiac milieu upon transplanted cell behavior by gene expression analysis, revealing a marked down-regulation of differentiation pathways in BMMCs transplanted into the injured heart. Finally, we employ multi-modality functional assays such as echocardiography, positron emission tomography (PET), and invasive hemodynamics to test whether BMMC therapy induces any improvement in cardiac function in the setting of acute ischemic injury.
MATERIALS AND METHODS
Animals
Adult female FVB mice (n=97, Jackson Laboratories, Bar Harbor, MN) and male L2G85 reporter transgenic mice (n=40, Stanford University, Stanford, CA) were used. Transgenic animals were created on the FVB background to constitutively express both firefly luciferase and enhanced green fluorescence protein (Fluc-eGFP) driven by a constitutive ubiquitin promoter in all tissues and organs, including BM cell populations. Animal care was provided in accordance with the Stanford University School of Medicine guidelines and policies for the use of laboratory animals.
Study design
A diagrammatic overview of the study is given in Supplemental Figure I. Specifically, female FVB mice were mechanically ventilated with 2–3% isoflurane and 100% O2 and randomized into two groups: (a) ischemia/reperfusion (I/R) injury (n=69) by occlusion of left anterior descending (LAD) coronary artery for 45 minutes or (b) sham procedure with open thoracotomy and placement of peri-LAD suture but no LAD occlusion (n=28). I/R animals (n=36) received 5×106 BMMCs (harvested from male L2G85 transgenic donors) via three direct intra-myocardial injections at the infarct border zones with a total volume of 50 μL using a 29-gauge Hamilton syringe. Sham (n=28) animals were injected with 5×106 BMMCs at 3 places at the anterolateral wall. A subset of the I/R animals (n=33) received 50 μl of PBS in a similar distribution to serve as controls for cardiac functional analyses. Operations were performed by a microsurgeon with several years of experience with this model. Cell therapy was monitored by optical bioluminescence imaging (BLI) on days 2, 4, 7, 10, 14, 21, and 48 using D-Luciferin (300 mg/g body weight, delivered intraperitoneally). BLI results were validated ex vivo by RT-PCR analysis for the male Sry gene. Transplanted cells were harvested from a subset of animals in both I/R (n=9) and sham (n=8) groups 4 days following delivery by explanation of the heart followed by collagenase digestion and FACS-based collection of GFP+ cells. RNA was harvested and used for microarray analysis. Cardiac function was assayed by echocardiography, PET imaging, and invasive pressure-volume (PV) loop analysis. Histological analysis was performed on randomly selected hearts (n=5) to determine BMMC fate.
Preparation of BMMCs
BMMCs were harvested from the long bones of male L2G85 transgenic mice and isolated by centrifugation in a density cell separation medium (Ficoll-Hypaque) prior to cardiac injection as previously described 20.
Bioluminescence imaging (BLI) of BMMC transplantation
BLI was performed using the Xenogen In Vivo Imaging System (Alameda, CA) as previously described 21. See Supplemental Methods for further details.
Validation of BLI by PCR analysis of BMMC-injected hearts ex vivo
For ex vivo validation of BLI, a standard curve was first generated by correlating cycle counts from real-time polymerase chain reaction probing for the male Sry gene in female hearts injected with known numbers of male BMMCs as previously described 22. See Supplemental Methods for further details.
Microarray analysis of injected BMMCs
Heart-BMMC complexes were harvested from I/R (n=5) and sham (n=4) groups 4 days following transplantation and subjected to collagenase digestion on a Langendorff apparatus as previously described 23. The resultant cell slurry was FACS sorted, and 40,000 to 100,000 eGFP+ cells collected for RNA isolation (RNeasy purification columns, Qiagen, CA). RNA purity was confirmed by analysis on the Agilent 2100 Bioanalyzer using RNA Pico Chips (Agilent, Sunnyvale, CA). RNA was amplified using two rounds of linear amplification (Agilent amplification kit) per manufacturer’s instructions. RNA hybridizations were performed using the Agilent Mouse (Development) Oligo Microarray G4120A platform, consisting of 20,371 60-mer oligonucleotides representing over 20,000 known mouse genes and derived largely from sequences from the National Institute on Aging cDNA 7.4K and 15K mouse clone sets 24. A common reference consisting of RNA derived from whole 17.5-day mouse embryos was utilized as previously described 25. Significance analysis of microarrays (SAM) was performed in a two-class, unpaired fashion in order to identify genes that were significantly up- or down-regulated between I/R and sham groups, using a false discovery rate (FDR) cutoff of 5%. Gene lists were analyzed with web-based DAVID 26 and GoMiner 27 cluster-function analysis tools to identify enriched categories of gene ontology (p < 0.05 by Fisher Exact Test, with murine background). Proprietary software (Agilent, Menlo Park, CA) was utilized to generate ball and stick gene tree diagrams relating parent-daughter relationships between gene ontology (GO)-terms, degree of significance of GO-term up/down regulation, and number of genes significantly up/down regulated within a GO term.
Echocardiographic determination of left ventricular contractility
Please refer to the Supplemental Methods section.
Positron emission tomography analysis
A subset of animals from both I/R (BMMC and PBS groups; n=11/group) underwent myocardial PET imaging using [18F]-fluorodeoxyglucose ([18F]-FDG) radiotracer. Imaging was acquired with the P4 Concorde MicroPETsystem. Animals were injected with [18F]-FDG (144±33 μCi), and images from 60 to 75 minutes after injection were reconstructed by filtered back-projection algorithm. Myocardial perfusion was calculated as ROI-derived [18F]-FDG percentage injected dose per gram of tissue (%ID/g) as previously described 28. See Supplemental Methods for further details.
Invasive cardiovascular hemodynamics
Animals receiving either BMMC (n=15) or PBS (n=13) following I/R injury were assayed 6-weeks following surgery. Please refer to the Supplemental Methods section for further details.
Flow cytometry analysis
Please refer to the Supplemental Methods section.
Tissue fixation and immunohistochemical analysis
Please refer to the Supplemental Methods section.
Statistical analysis
Experimental results are expressed as mean ± SEM. Linear regression analysis was performed to determine correlation between 2 variables. Repeated measures ANOVA with post-hoc testing and non-paired Student’s T-test were used where appropriate. The level of significance was defined as p < 0.05. Microarray statistical analysis was carried out as described above.
RESULTS
In vitro analysis of BMMCs shows robust reporter gene expression
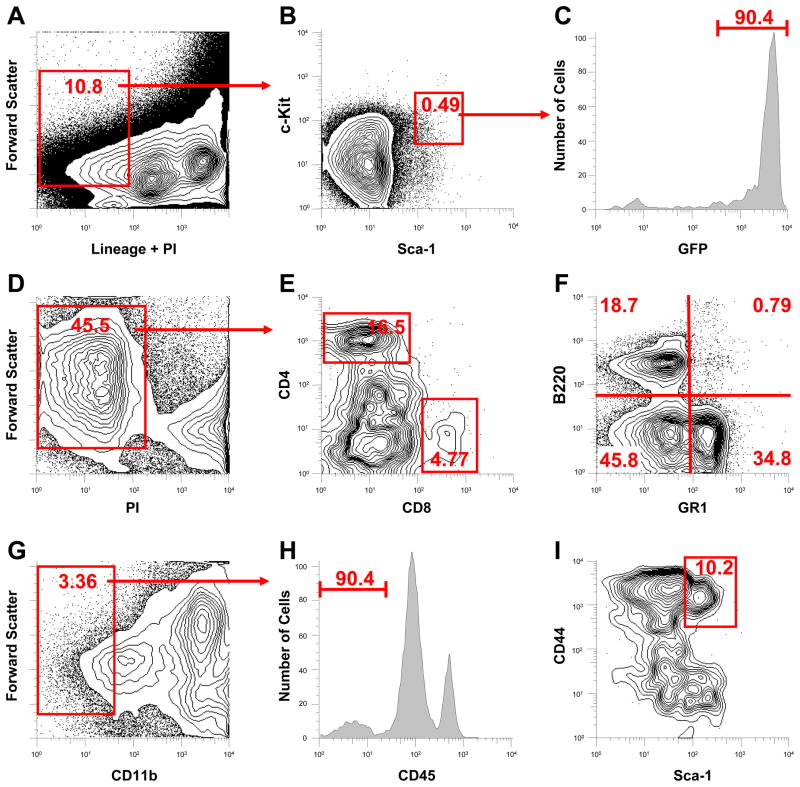
We have previously described the L2G transgenic mice, which constitutively express both the firefly luciferase (Fluc) and enhanced green fluorescent protein (eGFP) reporter genes 20, 22. Cells derived from these mice can be easily tracked when transplanted into wild-type (WT) mice by whole-body bioluminescence imaging (Fluc) or histological sections (eGFP). To confirm prior reports and establish that L2G-derived BMMCs were similar to genetically unmodified wild-type BMMCs, we performed marrow phenotyping by FACS analysis. Approximately 0.058±0.009% of the mononuclear cell fraction possessed the classical hematopoietic stem cell phenotype (Lin−, c-kit+, Sca-1+; Figure 1A, 1B). Over 90% of this population expressed GFP (Figure 1C). L2G-derived marrow also possessed the classical fractions of CD8+ and CD4+ cells (Figure 1D, 1E). Granulocyte and B-cell lineage cells were also present within the L2G marrow (Figure 1F), as were a typical proportion of mesenchymal cells, defined as CD11b−, CD45−, Sca-1+, CD44+ (Figure 1G-1I). Age and sex matched comparison of marrow from L2G versus wild-type mice (n = 3 per group) revealed similar KLS fractions, which are defined as c-kit+, Sca-1+, Linlow/− (WT: 0.061 vs L2G: 0.056%, p=NS; Figure 2A, 2B). Further analysis demonstrated L2G-derived mononuclear cells to be Thy 1.1 intermediate and Flk-2 negative (Figure 2C, 2D). To confirm that the reporter gene expression of the L2G-derived cells was consistent and reliable, we performed in vitro reporter gene expression assays. Over 90% of L2G cells exhibited robust eGFP, compared to no detectable fluorescence in WT-derived marrow (Figure 2E). eGFP expression was confirmed by direct confocal microscopy of cells immediately following harvest, demonstrating strong and uniform eGFP expression within the cytosol (Figure 2F). Luciferase reporter gene activity was assessed by in vitro imaging of BMMCs as well as by firefly luciferase assay. Imaging of known concentrations of cells revealed a robust correlation between cell number and BLI signal (Figure 2G, 2H; r2=0.99). In vitro firefly luciferase assay yielded similar results (Figure 2I) with a significant correlation between cell number and Fluc expression (r2=0.98).
Figure 1. Characterization of L2G bone marrow.
Analysis of lineage negative (Lin−) fraction of bone marrow (A) reveals 0.058% of L2G marrow cells are of the “classical” hematopoietic stem cell (HSC) phenotype (B, Lin−, c-kit+, Sca-1+). L2G HSCs express GFP at high levels (C). Analysis of marrow (D) demonstrates significant fractions of CD8+ and CD4+ cells (E), as well as granulocyte lineage (Gr-1+) and B-cell lineage (B220) expressing cells (F). The fraction of mesenchymal stem cells (CD11b−, CD45−, Sca-1+, CD44+) is 0.025%, comparable to wild type (G–I).
Figure 2. Comparison of L2G marrow versus wild type and in vitro analysis of reporter gene expression.
Lineage negative populations from L2G (A) and age/sex-matched wild type mice (B) demonstrate similar frequencies of hematopoietic stem cell (Lin−, c-kit+, Sca-1+) fractions. Thy 1.1 (C) and Flk-2 (D) analysis of HSCs from L2G mice demonstrate similar profiles to those reported for wild type. (E) GFP signal from HSC from wild type (black line) and L2G (green line) demonstrates strong GFP expression by transgenic mice. (F) Confocal microscopy reveals robust GFP expression by bone marrow mononuclear cells (BMMC) from L2G mice (scale bar = 5 μm). In vitro analysis of Fluc reporter gene expression by L2G85 BMMCs demonstrates robust correlation between bioluminescent imaging and cell number (G) as quantified further by linear regression analysis of imaging signal versus cell number (H, R2=0.99) and Fluc enzyme activity versus cell number (I, R2=0.98).
In vivo imaging of BMMCs transplanted into normal and ischemic myocardium reveals differential patterns of survival
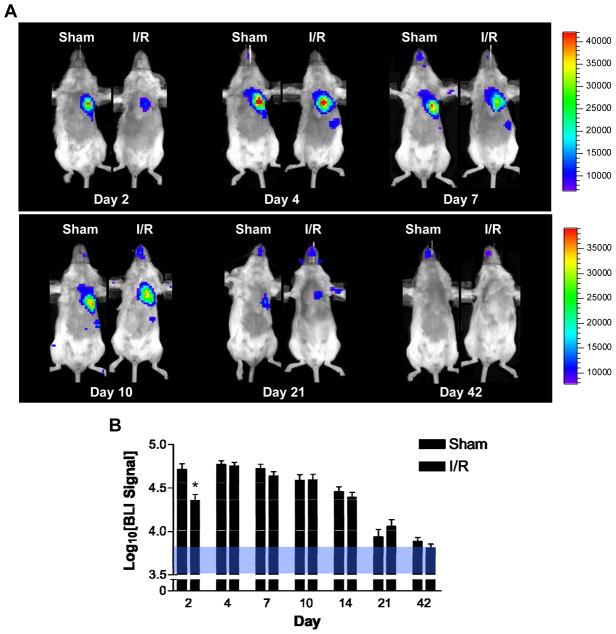
To elucidate the survival kinetics of BMMCs transplanted into the acutely injured heart, BLI was used to image cell fate for up to 6 weeks following transplantation (Figure 3A). In both non-injured and ischemia-reperfusion (IR) injured hearts, cell signal followed a quadratic trend over time, peaking at day 4, and falling significantly by day 10 through 21 (Multivariate ANOVA, p=0.001; Figure 3B). Ischemic injury worsened early BMMC survival, as demonstrated by significantly lower signal in the IR group compared to the sham group on day 2 (4.35±0.06 vs. 4.72±0.07 log[p/s/cm2/sr], p=0.037). By day 4, signal strength in the ischemic hearts increased significantly to reach similar values to those in non-ischemic hearts (4.77±0.04 vs. 4.76±0.04 log[p/s/cm2/sr], p=NS), suggesting a transient, ischemia-induced proliferation of BMMCs. Signals in both groups demonstrated similar decay kinetics, decreasing significantly by day 21 (3.95±0.08 vs 4.00±0.07 log[p/s/cm2/sr], p=NS) and approaching near undetectable levels in the chest by day 42 (3.87±0.04 vs. 3.81±0.05 log[p/s/cm2/sr], p=NS). To test whether the observed decrease in cell signal following transplantation into the heart was host tissue-dependent, we transplanted BMMCs into the hindlimb of syngeneic FVB mice and quantified the cell signal over time. We detected similar results of cell loss compared to the cardiac I/R injections (Supplemental Figure II). Furthermore, to evaluate the hypothesis that a high degree of early cellular engraftment predicts efficacy of therapeutic outcome, we used BLI data measured on day 2 after transplant as a surrogate measure of cellular engraftment and stratified the BMMC transplant recipients into “high” versus “low” engraftment groups. Indeed, BLI signal intensity at day 2 correlated well with long-term survival and left ventricular function as assessed by echocardiography at week 6 (Supplemental Figure III).
Figure 3. Longitudinal bioluminescent imaging analysis of BMMC transplantation into normal and ischemic hearts reveals differential early survival patterns.
(A) Images following the same two animals (sham on left and ischemia-reperfusion [I/R] on the right) demonstrate significantly lower number of cells on day 2 in ischemic hearts. Ischemia also induced a proliferation response resulting in a rapid rise in BLI signal between days 2 and 4 in the I/R group (scales represent BLI signal in p/s/cm2/sr). Longitudinal cell survival was similar in both groups, reaching background levels by day 42. Average BLI signal from both sham (n=8) and I/R (n=8) groups are summarized in (B). Background signal range is given by the area shaded blue. Error bars represent SEM, *P < 0.05.
In vitro and in vivo TaqMan® analysis correlates with BLI
To validate and confirm the quantitative analysis of transplanted cell survival, we correlated BLI results to an established ex vivo methodology of quantifying cell survival following transplant, namely TaqMan® reverse-transcription quantitative PCR (RT-qPCR). To produce a standard curve, we performed qRT-PCR, probing for the Sry sequence in the genomic DNA isolated from whole female hearts injected with known numbers of male BMMCs ex vivo. Analysis revealed a robust correlation between cycle count and the number of transplanted male cells (r2=0.99, Figure 4A). We then examined hearts taken from animals following BMMC injection and BLI at various days post-transplantation, and thereby demonstrated a strong linear correlation between threshold cycle count and BLI signal (r2=0.99, Figure 4B, left vertical axis). Construction of this correlation curve then allowed for extrapolation of surviving cell number based on BLI signal (Figure 4B, right vertical axis). By this technique, the lower limit of cell detection was on the order of 1×102 cells, similar to prior reports for BLI imaging within the thorax 20, 22.
Figure 4. Validation of in vivo imaging by real time-PCR analysis of SRY containing L2G BMMCs transplanted into female recipient hearts.
(A) Ex vivo correlation between PCR cycle number (probing for SRY) for known number of male cells transplanted into female hearts demonstrates an R2 value of 0.99. (B) Regression analysis of BLI signal generated from hearts imaged in vivo (n=8) at different times following BMMC transplantation followed by real time PCR analysis for SRY demonstrates a robust correlation between BLI signal and SRY copy number (r2=0.95). Right axis gives expected cell number for given BLI signal based on ex vivo analysis depicted in (A).
Microarray analysis of transplanted cells
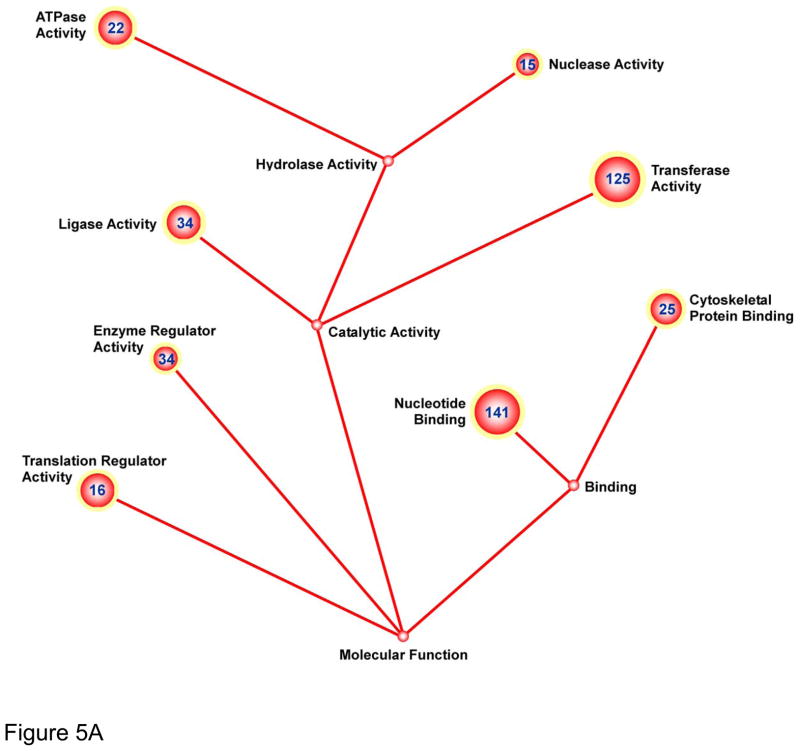
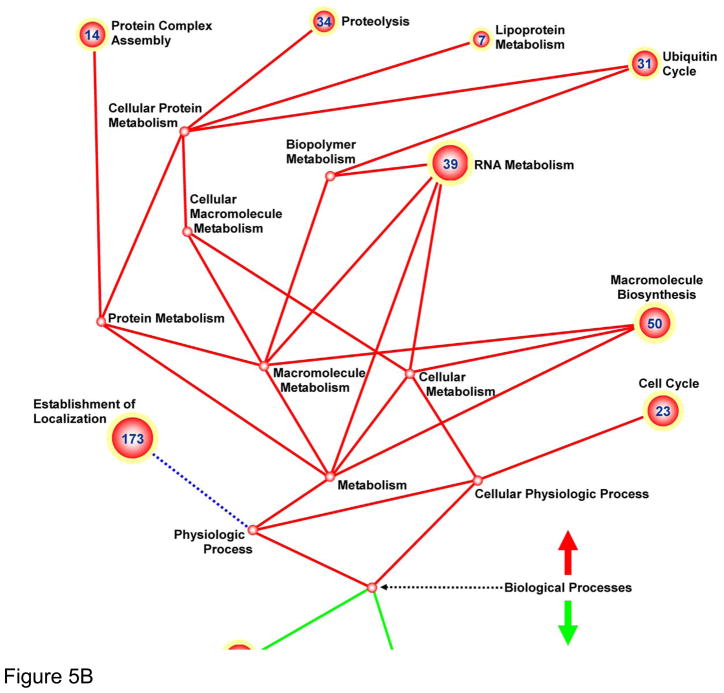
In order to gain insight into the transcriptional changes occurring within BMMCs after delivery into ischemic myocardium, we isolated them from explanted hearts and performed comprehensive transcriptional profiling analysis. Specifically, gene regulation was evaluated as a function of I/R injury, comparing the transcription response of cells injected into injured hearts against those injected into normal (sham-operated) animals. FACS sorting of Langendorff–digested I/R and sham heart cells resulted in collection of 40,000 to 100,000 GFP+ cells per heart (Supplemental Figure IV). Approximately 1,200 genes were found to be significantly upregulated when comparing I/R vs. sham-operated hearts, and 200 genes were significantly downregulated (Supplemental Tables I and II; q-value < 0.05). Functional cluster analysis by DAVID and GoMiner online-analysis yielded numerous up- and down- regulated enriched functional groups (full list of functional groups with degree of significance given in Supplemental Table III). Functional groups were further sub-sorted into Molecular Function (Figure 5A) and Biological Processes (Figure 5B). The greatest number of genes up-regulated within the Molecular Function category were subsets of the “nucleotide binding”, “transferase activity”, “ligase activity”, and “enzyme regulator activity” GO-terms. Analogous genes were found to be similarly up-regulated within Biological Process, notably within the following GO-terms: “establishment of localization”, “RNA metabolism”, “cell cycle”, and “macromolecule biosynthesis”. Down-regulated genes were clustered within Biological Processes categories, with no significant down-regulation observed in Molecular Function. Analysis of significantly down-regulated genes was revealing. Specifically, they included numerous genes involved in “cell differentiation”, “cell fate commitment”, “development”, “pattern specification”, and “Wnt signaling”, suggesting that the ischemic environment of the host myocardium may hamper initiation of developmental, differentiation, and maturation pathways.
Figure 5. Genomic profiling of BMMCs transplanted into ischemic myocardium reveals down-regulation of differentiation and cell fate commitment pathways.
Ball-and-stick figures represent ontological cluster function analysis of gene expression changes. Enumerated spheres represent significantly up- or down-regulated groups of genes (GO-terms). Size of spheres correlates to the significance of enrichment of the GO term (sphere radius is proportional to −log10[p-value for GO-term]) and numbers within sphere designate number of genes within the GO-term significantly up or down-regulated. Solid lines represent parent-daughter relationship between terms; terms related otherwise are connected by dashed lines. Functional groups were further sub-sorted into Molecular Function (A) and Biological Processes (B).
Histological evaluation supports findings of molecular imaging and microarray analysis
After in vivo imaging, we performed histological analysis of explanted hearts at various time points following transplantation. As reported in prior studies 22, 29, transient occlusion of the LAD artery resulted in a typical pattern of I/R injury with transmural involvement of the myocardium in regions supplied by the main branches of the left coronary artery (Figure 6A). L2G-derived BMMCs were clearly present in the infarcted areas with a cytosolic pattern of GFP expression (Figure 6B, 6C). Evaluation of hearts harvested at 6 weeks post-implantation did not reveal persistent BMMCs (data not shown). As predicted by transcriptional profiling analysis, staining for cardiac-specific markers such as troponin (Figure 6D) and connexin (Figure 6E) revealed no histological evidence of transdifferentiation by the BMMCs at day 7 post-transplant. These findings are in agreement with those described in previous reports 30, 31.
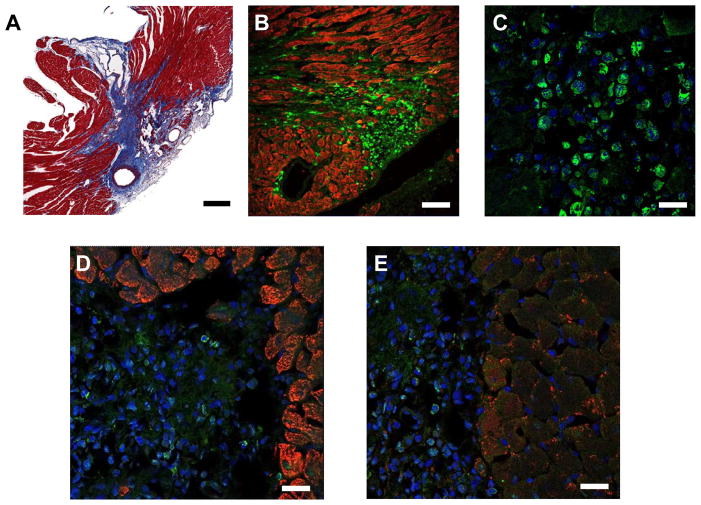
Figure 6. Histological evaluation of transplanted BMMCs confirms microarray analysis with no evidence of transdifferentiation.
(A) Masson-Trichome staining of heart 7 days following I/R injury demonstrates infarct zone emanating from area supplied by left anterior coronary artery (scale bar = 100 μm). (B) Low power view of heart 7 days following I/R injury followed by BMMC injection. Native myocardium is stained with troponin (red) and transplanted cells can be seen expressing the GFP reporter gene (green, scale bar = 100 um). (C) High power view of cells within infarct zone co-stained with DAPI (blue) demonstrate intra-cytosolic GFP expression (scale bar = 20 um). Staining for cardiac markers troponin (D) and connexin43 (E) reveal no expression by transplanted cells (scale bars = 30 μm).
Multi-modality assessment of cardiac function following I/R injury
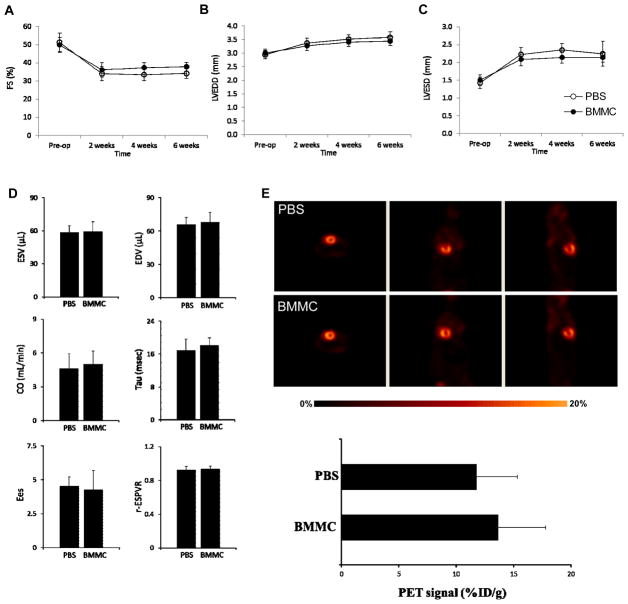
Given the mixed results of prior studies (both animal and clinical) evaluating cardiac function following BMMC transplant 32, 33, we chose to employ multi-modality analysis to determine if BMMC transplantation improved myocardial function in our model. Echocardiography was utilized to assess myocardial function, while PET perfusion imaging was used to evaluate possible changes in myocardial perfusion. Echocardiography (Figure 7A) revealed a significant decrease in fractional shortening (FS) 2 weeks following I/R injury in both PBS (51.3±5.0% to 34.1±3.8%, p < 0.05) and BMMC treated groups (49.8±4.0% to 36.1±3.9%, p < 0.05). There was a trend towards preserved FS in the BMMC group through week 6, although this did not reach statistical significance (37.9±2.5% vs 34.2±2.7%, p=NS). Evaluation of diastolic and systolic function by echocardiographic assessment of left ventricular end-diastolic dimension (LVEDD) and end-systolic dimension (LVESD) revealed a similar trend toward improvement of diastolic and contractile dynamics of BMMC-treated hearts (Figure 7B, 7C). Invasive hemodynamics confirmed our echocardiographic findings, revealing no statistically significant difference in end-diastolic and end-systolic volumes at week 6 (Figure 7D, top row), although a trend towards improvement was observed in the BMMC-treated group. Other measurements of cardiac output and contractility such as left ventricular time relaxation constant (tau), end-systolic elastance (EES), and end-systolic pressure-volume relationship (ESPVR) were also similar between PBS- and BMMC-treated groups (Figure 7D, bottom panels). Finally, PET [18F]-FDG imaging analysis also revealed no significant increase in myocardial viability following BMMC transplant. Although PET signal was mildly increased in hearts treated with BMMCs compared to PBS alone (Figure 7E), the difference in viability signal did not achieve statistical significance (13.6±4.1 vs 11.7±3.5 %ID/g, p=NS).
Figure 7. Functional analysis reveals no long-term improvement following BMMC therapy.
Echocardiography demonstrates trend towards improved ejection fraction (A) in hearts receiving BMMC versus PBS. Assessment of (B) end-systolic and(C) end-diastolic diameters suggests mild improvement of systolic and diastolic function, respectively. However, these trends did not achieve statistical significance. (D) Invasive hemodynamic analysis confirms echocardiography revealing no significant difference between various functional measures (ESV; end-systolic volume, EDV; end-diastolic volume, CO; cardiac output, Tau; ventricular relaxation time constant, Ees; end-systolic elastance, r-ESPVR; correlation coefficient of the end-systolic pressure volume relationship) as assessed for hearts receiving BMMC versus PBS. (E) PET [18F]-FDG imaging of hearts receiving PBS (top) versus BMMC (bottom row) demonstrates no significant improvement of myocardial viability following BMMC therapy. PET imaging signals are quantified as a histogram below the images.
DISCUSSION
Since its introduction as a therapeutic concept over 10 years ago, BMMC therapy has been initiated in a variety of formats worldwide. Most clinical trials have yielded mixed results 32, 33. Two meta-analysis of 18 and 10 trials, respectively, evaluating the effect of intracoronary infusion of BMMCs for the treatment of acute MI have shown marginal benefits at best (≈3–4% increase in LVEF) 34, 35. Given that autologous cell preparations represent a therapeutic product of far greater complexity than traditional drugs, in-depth study of their in vivo behavior is required. However, despite the ongoing clinical work, detailed molecular analyses of BMMCs after transplantation into myocardium remain unknown. In particular, little is known regarding the early biological activity of BMMCs following their delivery into a novel myocardial environment, including the early transcriptional events that might dictate cell survival and therapeutic efficacy.
In the present study, we have attempted to address these issues and provide insight as to how BMMC therapy can be improved for enhanced success in the clinic realm (Supplemental Figure 1). We utilized molecular techniques and a clinically relevant model of acute ischemic myocardial injury to demonstrate that 1) the ischemic myocardial environment induces a proliferative response in the transplanted cell population, but long-term engraftment is limited, 2) transcriptional profiling of cells transplanted into the heart reveals up-regulation of numerous “house-keeping” and cell proliferation genes, but a marked down-regulation of pathways regulating differentiation and maturation, and 3) transplanted cells induce a mild, transient improvement in cardiac function that does not appear to be long-lasting.
Our molecular imaging results confirm earlier observations from cell therapy studies employing chronic ischemia models that cell survival is, at best, limited to a few weeks 20, 22. Several other investigators have postulated that limited survival may partially explain why BMMC therapy has not achieved the success suggested by early animal studies 32, 36, 37. This observation highlights one of the major hurdles facing BMMC therapy in that, even in ideal conditions such as our control animal group (i.e., syngeneic, non-injured myocardium), transplanted cell grafts persisted for a limited time in host tissue. These survival data also raise concern regarding the hypothesis that improvement in cardiac function following cell therapy may be due to secretion of paracrine factors by the transplanted cells 38, 39. If paracrine signaling is a primary mechanism underlying improved cardiac function, intuitively long-term secretion from a sufficient number of surviving cells would be more beneficial for sustained benefit.
Although long-term survival of BMMCs following acute ischemic injury is limited, early post-transplantation cell behavior appears a dynamic and active process. Utilizing molecular imaging and gene profiling techniques, we were able to discern a differential pattern of early post-transplant survival and proliferation of BMMCs in the I/R injured hearts as compared to non-ischemic myocardium, confirming previous finding 21. The implication that the inflammatory nature of the post-infarcted myocardium may affect transplanted cell behavior is rather intuitive and there is evidence to show that the intense inflammatory reaction occurring after myocardial infarction may produce chemokines responsible for lymphocyte tracking and stem cell homing to the damaged myocardium 22, 40, 41. However, the post-infarct inflammatory milieu also appears to have a dual effect. On the one hand, it serves to activate homing and promote a proliferative response amongst transplanted cells as observed in the present study. On the other hand, it may create a hostile environment, resulting in indiscriminate cellular activation and turning cells away from stable maturation fates 21, 31. Given the results of the present study, investigations into the optimal BM cell fraction, cell dose, timing, and delivery modality are expected to add much-needed insight into an improved strategy for making cell transplantation an effective therapeutic tool 42. In addition, understanding the response of transplanted cells to their new host environment will be essential to exploit the possible therapeutic potential of BMMCs. It is with this premise that we chose to examine the early transcriptional events within BMMCs as described in this study.
The gene-profiling findings presented here represent a novel dataset in the growing body of literature examining BMMC therapy for heart disease. To our knowledge, this is the first study evaluating (on a transcriptional level) the transcriptional response of BMMCs in the ischemic heart. Our gene-ontology analysis shows that the overwhelming cellular response to transplantation is to simply survive in the new environment, rather than activate pathways implicated in engraftment and long-term survival. Specifically, we saw no significant activation of long-term proliferation, differentiation, or maturation pathways in the transplanted BMMCs. Rather, BMMCs activated macromolecule synthesis and cellular machinery genes, suggesting a fraught attempt to mitigate cell death. Of particular interest are the GO terms demonstrating the most robust upregulation, such as “cell cycle”, “cytoskeletal protein binding”, and “establishment of localization”. These groups encompass genes responsible for rapid cell growth and division, confirming the cell behavior observed through BLI and lending credence to the notion of a provocative inflammatory milieu. Of equal importance are the findings of significantly down-regulated groups of genes clustering in the areas of “development”, “cell differentiation”, “pattern specification”, and “cell fate commitment”. These provide transcriptional-level evidence that BMMCs were not able to adopt mature cardiac phenotypes shortly after transplantation in our acute ischemia model 30. However, the interpretation of the array analysis has some limitations. BMMCs are a heterogeneous cell population representing portions of hematopoietic cells as well as macrophages, granulocytes, and natural killer cells 20. Therefore, we cannot completely exclude the possibility that the changes observed in the array data may be impacted by changes in the surviving cell population over the time as opposed to transcriptional changes in specific cell populations. Further studies injecting purified cell populations would be needed to tease out the transcriptomic response of specific cell lineages.
In summary, we present a multimodal evaluation of BMMC therapy in a clinically relevant animal model. We have detailed the earliest transcriptional events following transplant, described long-term transplanted cell survival kinetics, and characterized the eventual effects upon cardiac function following transplant. Our findings mirror present clinical experience, which shows limited functional improvement following BMMC therapy 33, 34. The survival data provided by BLI suggest that therapeutic efficacy may be limited by insufficient cell survival, while our genomic data suggest very active transplanted cellular machinery responding to an acutely inflamed host tissue environment. Successful therapy generating long-term improvement in cardiac function may very well depend on modulation of both the host environment and the transplanted cell transcriptional response to ensure optimal BMMC engraftment and survival.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
We would like to acknowledge funding support from the National Institutes of Health grants F32-HL-084982 (AYS), German Research Foundation DFG-Hu-1857/1-1 (BCH), NIH EB009689 (JCW), NIH HL093172 (JCW), and Burroughs Wellcome Foundation (JCW).
Footnotes
DISCLOSURES
None.
References
- 1.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the topcare-ami trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 3.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 4.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The boost randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 5.Meluzin J, Janousek S, Mayer J, Groch L, Hornacek I, Hlinomaz O, Kala P, Panovsky R, Prasek J, Kaminek M, Stanicek J, Klabusay M, Koristek Z, Navratil M, Dusek L, Vinklarkova J. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128:185–192. doi: 10.1016/j.ijcard.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 6.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 7.van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, de Roos A, Fibbe WE, Zwaginga JJ, Boersma E, Schalij MJ, Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: A randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 8.Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, Feng X, Zhang J, Duan Y, Wang J, Liu D, Wang H. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with st-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J. 2009;30:1986–1994. doi: 10.1093/eurheartj/ehp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled boost (bone marrow transfer to enhance st-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 10.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with st-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 11.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kogler G, Wernet P, Muller H-W, Kostering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The iact study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 12.Beeres SLMA, Bax JJ, Dibbets P, Stokkel MPM, Zeppenfeld K, Fibbe WE, van der Wall EE, Schalij MJ, Atsma DE. Effect of intramyocardial injection of autologous bone marrow-derived mononuclear cells on perfusion, function, and viability in patients with drug-refractory chronic ischemia. The Journal of Nuclear Medicine. 2006;47:574–580. [PubMed] [Google Scholar]
- 13.Perin EC, Dohmann HFR, Borojevic R, Silva SA, Sousa ALS, Silva GV, Mesquita CT, Belem L, Vaughn WK, Rangel FOD, Assad JAR, Carvalho AC, Branco RVC, Rossi MID, Dohmann HJF, Willerson JT. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:II-213–21. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen PK, Lan F, Wang Y, Wu JC. Imaging: Guiding the clinical translation of cardiac stem cell therapy. Circ Res. 2011;109:962–979. doi: 10.1161/CIRCRESAHA.111.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen IY, Wu JC. Cardiovascular molecular imaging: Focus on clinical translation. Circulation. 2011;123:425–443. doi: 10.1161/CIRCULATIONAHA.109.916338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 17.Meluzin J, Vlasin M, Groch L, Mayer J, Kren L, Rauser P, Tichy B, Hornacek I, Sitar J, Palsa S, Klabusay M, Koristek Z, Doubek M, Pospisilova S, Lexmaulova L, Dusek L. Intracoronary delivery of bone marrow cells to the acutely infarcted myocardium. Optimization of the delivery technique. Cardiology. 2009;112:98–106. doi: 10.1159/000141462. [DOI] [PubMed] [Google Scholar]
- 18.Dohmann HFR, Perin EC, Takiya CM, Silva GV, Silva SA, Sousa ALS, Mesquita CT, Rossi M-ID, Pascarelli BMO, Assis IM, Dutra HS, Assad JAR, Castello-Branco RV, Drummond C, Dohmann HJF, Willerson JT, Borojevic R. Transendocardial autologous bone marrow mononuclear cell injection in ischemic heart failure: Postmortem anatomicopathologic and immunohistochemical findings. Circulation. 2005;112:521–526. doi: 10.1161/CIRCULATIONAHA.104.499178. [DOI] [PubMed] [Google Scholar]
- 19.Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, Metens T, da Costa AM, Mahmoudabady M, Egrise D, Blocklet D, Mazouz N, Naeije R, Heyndrickx G, McEntee K. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. The Journal of Thoracic and Cardiovascular Surgery. 2009;138:646–653. doi: 10.1016/j.jtcvs.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 20.van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, Contag CH, Robbins RC, Wu JC. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swijnenburg R-J, Govaert JA, van der Bogt KEA, Pearl JI, Huang M, Stein W, Hoyt G, Vogel H, Contag CH, Robbins RC, Wu JC. Timing of bone marrow cell delivery has minimal effects on cell viability and cardiac recovery after myocardial infarction/clinical perspective. Circulation: Cardiovascular Imaging. 2010;3:77–85. doi: 10.1161/CIRCIMAGING.109.872085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 24.Carter MG, Hamatani T, Sharov AA, Carmack CE, Qian Y, Aiba K, Ko NT, Dudekula DB, Brzoska PM, Hwang SS, Ko MSH. In situ-synthesized novel microarray optimized for mouse stem cell and early developmental expression profiling. Genome Research. 2003;13:1011–1021. doi: 10.1101/gr.878903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabibiazar R, Wagner RA, Liao A, Quertermous T. Transcriptional profiling of the heart reveals chamber-specific gene expression patterns. Circulation Research. 2003;93:1193–1201. doi: 10.1161/01.RES.0000103171.42654.DD. [DOI] [PubMed] [Google Scholar]
- 26.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat Protocols. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.Zeeberg B, Feng W, Wang G, Wang M, Fojo A, Sunshine M, Narasimhan S, Kane D, Reinhold W, Lababidi S, Bussey K, Riss J, Barrett J, Weinstein J. Gominer: A resource for biological interpretation of genomic and proteomic data. Genome Biology. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyama H, Ichise M, Liow JS, Vines DC, Seneca NM, Modell KJ, Seidel J, Green MV, Innis RB. Evaluation of anesthesia effects on [18f]fdg uptake in mouse brain and heart using small animal pet. Nucl Med Biol. 2004;31:251–256. doi: 10.1016/S0969-8051(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011;121:3689–3700. doi: 10.1172/JCI45709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KBS, Ismail Virag J, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 31.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 32.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 33.Hansson EM, Lindsay ME, Chien KR. Regeneration next: Toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Lipinski MJ, Biondi-Zoccai GGL, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim H-S, Kang H-J, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. Journal of the American College of Cardiology. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Archives of Internal Medicine. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 36.Wu JC. Molecular imaging: Antidote to cardiac stem cell controversy. J Am Coll Cardiol. 2008;52:1661–1664. doi: 10.1016/j.jacc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Beitnes JO, Gjesdal O, Lunde K, Solheim S, Edvardsen T, Arnesen H, Forfang Kr, Aakhus S. Left ventricular systolic and diastolic function improve after acute myocardial infarction treated with acute percutaneous coronary intervention, but are not influenced by intracoronary injection of autologous mononuclear bone marrow cells: A 3 year serial echocardiographic sub-study of the randomized-controlled astami study. European Journal of Echocardiography. 2011;12:98–106. doi: 10.1093/ejechocard/jeq116. [DOI] [PubMed] [Google Scholar]
- 38.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (sfrp2) is the key akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proceedings of the National Academy of Sciences. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. The FASEB Journal. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 40.Frangogiannis NG, Entman ML. Chemokines in myocardial ischemia. Trends in Cardiovascular Medicine. 2005;15:163–169. doi: 10.1016/j.tcm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular Research. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 42.Hung T-C, Suzuki Y, Urashima T, Caffarelli A, Hoyt G, Sheikh AY, Yeung AC, Weissman I, Robbins RC, Bulte JM, Yang PC. Multimodality evaluation of the viability of stem cells delivered into different zones of myocardial infarction. Circulation: Cardiovascular Imaging. 2008;1:6–13. doi: 10.1161/CIRCIMAGING.108.767343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.