Abstract
Introduction
Borderline personality disorder (BPD) is a severe disorder with high morbidity and mortality, but unknown etiology. Childhood abuse has been proposed as an etiological factor, but the mechanism by which an abuse history could influence risk for BPD has not been determined. The aim of this study was to determine whether the Tryptophan Hydroxylase 1 gene is related to BPD in a clinical sample, and whether TPH1 genotypes or haplotypes moderate the relationship between abuse history and BPD.
Methods
Three hundred ninety-eight patients diagnosed with mood disorders were genotyped for TPH1 G-6526A promoter polymorphism (rs4537731) and the A218C intron 7 polymorphism (rs1800532) and a set of ancestry informative markers, assessed for DSM IV diagnoses, and assessed for a history of physical and/or sexual abuse.
Results
Patients with a diagnosis of BPD were more likely to be risk allele carriers (A alleles at both loci) than the non-BPD group. Logistic regression analysis predicting BPD diagnosis with both single SNPs and haplotypes showed significant interaction effects between genotype and abuse history. Poisson regression predicting the number of BPD diagnostic criteria met with the same predictor set also included a significant interaction term. Risk allele carriers with a history of abuse had an increased likelihood of a BPD diagnosis.
Conclusion
Variation in TPH1may increase risk for developing BPD as a result of childhood abuse. Elements of BPD pathology may be due in part to a genetically influenced serotonergic dysfunction, which in turn may lead to a differential response to environmental stressors.
Keywords: TPH1, tryptophan hydroxylase, A218C, G-6526A, Borderline Personality Disorder, Childhood Abuse, Suicide, Impulsivity, Impulsiveness
INTRODUCTION
Borderline Personality Disorder (BPD) is a serious, disabling condition with prevalence rates of approximately 0.7 – 1.8% of the population overall (Samuels et al. 2002; Torgersen et al. 2001). It is the most commonly diagnosed personality disorder in clinical samples, affecting an estimated 15 % of all patients in outpatient treatment (Widiger and Weissman 1991). It is also a condition with significant morbidity and mortality, with up to 80% of patients engaging in some form of self-injurious behavior (Zisook et al. 1994), and a completed suicide rate of approximately 10% (McGlashan 1986). Although little is known about the etiology of BPD, a substantial literature exists on the putative etiological correlates of the disorder. These can be broadly grouped into three main categories: family history of psychopathology, personality traits, and environmental stressors (Trull et al. 2000). Most research attention has been focused on the role of childhood trauma.
Since Herman and van der Kolk (Herman and van der Kolk 1987) first hypothesized that trauma might be an important etiological factor in BPD, many researchers have reported an association between childhood trauma and BPD, and several meta-analyses (Fossati et al. 1999) and literature reviews (Goodman and Yehuda 2002) have concluded that traumatic experiences in childhood are an important etiological factor in at least a subset of BPD cases. However, estimates of the size of this subset vary considerably. For example, in their 1999 meta-analysis, Fossati (Fossati et al. 1999) and colleagues reported only a modest overall effect size (r=.28), and concluded that their findings did not seem to support the notion that childhood sexual abuse was a major psychological risk factor or antecedent of BPD. In a later review, Goodman and Yehuda (Goodman and Yehuda 2002) concluded that BPD patients report a history of childhood sexual abuse with much greater frequency than non-BPD patients, and estimate that approximately 40 – 70 % of BPD patients have a history of childhood trauma. However, they also point out that these types of experiences are not uniquely associated with BPD.
The fact that a large percentage of individuals who report a history of childhood abuse will not develop BPD in later life (Collishaw et al. 2007) suggests that there are important moderators of the relationship between abuse and BPD that remain unelucidated. Recently, Goodman, New and Siever (Goodman et al. 2004) proposed an etiological model of BPD in which inherited susceptibility interacts with environmental stressors such as abuse and trauma to increase the risk for the development of BPD. In this study, we sought to test this model using single nucleotide polymorphisms in the Tryptophan Hydroxylase I gene (TPH1). Specifically, we tested the hypotheses that 1) previously identified TPH1 risk alleles will be associated with BPD, and that 2) a history of childhood abuse will be associated with a BPD diagnosis, but only in the presence of the TPH1 risk alleles.
We chose to examine TPH1 polymorphisms for several reasons. Tryptophan hydroxylase is the rate-limiting enzyme in the serotonin metabolic pathway, and is responsible for catalyzing the conversion of tryptophan to 5-hydroxytryptophan in the periphery, and to a lesser extent in the CNS (TPH2 is the predominant isoform in the CNS). Because of the importance of serotonin in the stress response (Holmes 2008), investigation of genes related to the serotonin system may provide important information concerning susceptibility to the effects of stress-inducing life events such as childhood trauma. Also, we have recently shown that the TPH1 A218C polymorphism could distinguish BPD patients from healthy controls (Wilson et al. 2009), suggesting that mutations in this gene may play a role in the etiology of BPD. In addition to its association with BPD, TPH1 polymorphisms have been associated with several traits that are characteristic of patients with the disorder, including hostility (Hennig et al. 2005; New et al. 1998; Reuter and Hennig 2005), anger (Baud et al. 2009), harm avoidance (Anghelescu et al. 2005), and impulsiveness (Staner et al. 2002). Finally, associations between TPH1 polymorphisms and suicidal (Bellivier et al. 2004; Liu et al. 2006a; Paik et al. 2000) and non-suicidal self-injurious behavior (Evans et al. 1327; Pooley et al. 2003) have been reported by several investigators. The fact that self-injurious behavior occurs with high frequency in patients with BPD (Simeon et al. 1992) also suggests an association with the disorder. While several studies have published results that conflict with some of these findings (Bennett et al. 2000; Kunugi et al. 1999; Zalsman et al. 2001), very few studies of these conflicting studies have accounted for BPD rates in their samples. As our initial findings with TPH1 and BPD suggest that the inconsistency could be due to varying rates of BPD in these samples, we also aimed to test this hypothesis with the current study. While both the G-6526A and the A218C polymorphisms have been extensively studied, neither has been examined in a sample including suicide attempters and nonattempters, with and without BPD. With the inclusiveness of our sample, our aim was to clarify some of the conflicting findings on TPH1, in the hope of better identifying the phenotype most strongly associated with this gene.
MATERIALS AND METHODS
Participants
A total of 398 unrelated research participants were included in this study, selected from a larger sample admitted to a multisite project on mood disorders and suicidal behavior. All participants were diagnosed with an Axis I mood disorder, either major depressive disorder (n=312) or a bipolar disorder (n=86). Ninety-eight participants were diagnosed with borderline personality disorder, while the remaining 300 either had no Axis II diagnosis (76%, n=226) or one or more non-BPD diagnoses (24%, n=74). Of the 24% with another Axis II disorder, 3.3% (n=10) had a Cluster A diagnosis, 6% (n=18) had a cluster B diagnosis, and 15% (n=45) had a Cluster C diagnosis. The BPD sample has been previously studied and compared to a sample of healthy controls (Wilson et al. 2009). All participants were of Hispanic (20.9%) or non-Hispanic Caucasian (79.1%) descent. We limited the racial and ethnic composition of the sample in an effort to reduce the possibility of sample stratification. The decision to include Hispanic Caucasians was made based on recent research showing relatively minimal genetic distance between self-identified Hispanics and individuals of Caucasian descent (Tang et al. 2005; Wilson et al. 2009). As Hispanic individuals generally have a background that includes varying proportions of European, Native American, and/or African ancestry (Bertoni et al. 2003) and can be of any race (American Community Survey, US Census Bureau, http://www.census.gov/prod/2007pubs/acs-03.pdf), eliminating individuals with self-reported Black Hispanic heritage as we did in this study likely further minimized this distance. Exclusion criteria were the presence of persisting psychotic symptoms or psychotic disorder diagnosis, history of severe head trauma, organic mental syndromes, medical illnesses that could affect the brain or increase intracranial pressure, or the presence of mental retardation or cognitive impairment that might interfere with the completion of the assessments or the obtaining of informed consent. The study was approved by all applicable institutional review boards, and all participants provided written informed consent.
Measures
Diagnoses were determined using the Structured Clinical Interview for DSM-III-R/DSM-IV, Patient Edition (Spitzer et al. 1990) and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (First et al. 1995). The presence or absence of physical or sexual childhood abuse before the age of 15 was determined by responses to three direct questions from the Columbia Demographic and Treatment History Interview (Brodsky et al., 2001): “Any history of physical and/or sexual abuse over lifetime” (yes/no); “If yes, describe and code: physical/sexual/both/not applicable”; “If yes, did abuse take place before age 15 years?” (yes/no/not applicable). Physical abuse was defined as any physical disciplinary measure inflicted by a parental figure or caregiver that was severe enough to leave marks such as welts or bruises. Sexual abuse was defined as any sexually inappropriate contact occurring between the participant and an adult, or between the participant and another minor at least five years older.
Participants were grouped according to whether they reported no history of abuse, a history of either physical or sexual abuse, or a history of both physical and sexual abuse. This categorical grouping was recently used by Binder and colleagues (Binder et al. 2008) in a study investigating gene × environment interactions and the risk of post-traumatic stress disorder with good results. Because of cell size limitations of some of the analyses, a dichotomous Yes/No abuse variable was used in all analyses. Models with significant interaction terms were rerun with the categorical variable if appropriate, in order to determine whether severity of abuse determined the strength of the effect. All interviews were conducted by at least a master’s level clinicians with specific training in the use of the SCID instruments and clinical measures.
Genotyping
All participants were genotyped for the G-6526A promoter polymorphism (rs4537731) and the A218C intron 7 polymorphism (rs1800532) of the TPH1 gene. A218C genotypes were available for the full sample, while G-6526A genotypes were available for 300 out of 398 cases (75/98 BPD, 225/300 without BPD). Genomic DNA was extracted from lymphocytes as described by Huang et al. (2003) and from epithelial cells from cheek swabs according to the manufacturer’s procedure, and amplified via polymerase chain reaction (PCR). PCR amplification was performed in 20 µl of reaction mixture containing 1× PCR buffer, 40–100 ng DNA, 2 mM MgCl2, 4% of DMSO (dimethyl sulfoxide), 50 nM of each dNTP, 0.8 U RedTaq polymerase (Sigma, St Louis, MO), and 40 ng of each primer. Forward primer (TPH5F) 5’-TGGCATTGAAGTAAGAGCAC-3’ and reverse primer (TPH5R) 5’-GTTTCATGCAGGTATTAGTG-3’) were used for rs4537731. Forward primer (Mann-45), 5’-GCCAGGAATTCATCAATGG-3', and reverse primer (Mann-46), 5’-CCACCACATACACACCCAAA-3’ were used for rs1800532. The reaction conditions were as follows: 95°C for 4 min, 30 cycles at 95°C for 40 s, 40 s at 54°C, 40s at 72°C and a final extension step at 72°C for 3 min. PCR amplifications were performed in a DNA Robocycler (Stratagene, La Jolla, CA). PCR fragments for rs4537731 were digested with the Sau3AI (or MboI) restriction enzyme, while fragments for rs1800532 were digested with the NheI restriction enzyme. Electrophoresis was performed in 1.4% agarose and the PCR amplification products visualized with UV light to determine genotype.
Ancestry Informative Markers
In order to control for any hidden population stratification, we genotyped a set of 186 unlinked markers using a custom designed Illumina GoldenGate 96-well format Sentrix® arrays (Hodgkinson et al. 2008). A total of 500ng of sample DNA was used per assay. All pre-PCR processing was performed using a TECAN liquid handling robot running Illumina protocols. Arrays were imaged using an Illumina Beadstation GX500 and the data analyzed using GenCall v6.2.0.4 and GTS Reports software v5.1.2.0 (Illumina). Ethnicity factor scores for each individual were estimated by STRUCTURE using 1,051 CEPH subjects as reference. Proportion of membership of each subject in each of 7 clusters was estimated. These scores correspond to 7 geographic regions and sum to 1. Factors were labeled by geographic region of ancestry ("Africa", "Europe", "Middle East", "Central Asia", "Far East Asia", "Oceania", and "America"). To adjust for ethnic stratification in the SNP-wise analyses with a minimum degrees of freedom lost, it was necessary to determine which of these factors differed by TPH1 genotypes. Logistic regression was performed for each individual SNP, with the presence of the minor allele as a response variable and “Europe”, “Africa”, “America”, “Middle East”, and “Central Asia” as predictor variables. “Far East Asia” and “Oceania” factors had very low mean scores overall in the sample (means< 0.01) and were excluded from all analyses a priori.
Haplotype Imputation
As the TPH1 gene is covered by one large haplotype block, we created haplotypes based on the two polymorphisms. Haplotypes were imputed using the SIMHAP package (Carter et al. 2008) for the R statistical software program (http://cran.r-project.org), using genotyping information from the subset of 300 subjects with both genotypes. Haplotypes were imputed using genotyping information from the entire subsample. For individuals heterozygous at either of the two loci, chromosomal assignments were determined using the haplotype with the highest estimated posterior probability. These data were used to create codes indicating the number of haplotypes for each individual (0, 1, or 2), which were then used as predictors in our statistical models. Statistical tests of haplotype main effects and interactions were conducted using the −6526G and 218C alleles as the base haplotype. This two-SNP haplotype is the most common in the Hapmap CEPH sample, which is a population from Utah with primarily northern and western European ancestry (Thorisson et al. 2005), with a frequency of .442. Linkage disequilibrium coefficients were determined using the JLIN Java program(Carter et al. 2006).
Statistical Analyses
Means and standard deviations were computed for all variables in the analyses, and all variables were checked for normality and outliers. In order to test for group differences on the demographic variables, chi-squares were computed for gender and race (Hispanic versus non-Hispanic), and a t-test for independent samples was computed for age. Chi-squares were computed for genotype, as well as for a dominant risk allele models (AA/GA versus GG, AA/AC versus CC). Although the G allele is the minor allele for the G-6526A polymorphism, previous research has identified the A wild-type allele as the risk allele at this locus (Liu et al. 2006a; Rotondo et al. 1999).
We used the Generalized Linear Model to run several types of analyses. Analyses were run with both individual genotypes and each of the two SNP haplotypes in the subset. We used a logistic binomial model to analyze the relationship between abuse history, genotype, and BPD diagnosis, controlling for age, gender, and attempter status, testing both additive and dominant genetic models. We also used a Poisson model to analyze the relationship between abuse history, genotype, and number of DSM-IV BPD criteria met (BPD criteria was available for a subset of the full sample, 373 of 398) with similar covariates and model assumptions. We used a dichotomous abuse variable (presence/absence) in all primary GLM models. Models with significant findings were reanalyzed using a categorical abuse history variable (none, physical or sexual, both physical and sexual) to assess whether severity of abuse history was a significant determinant of outcome. In order to adjust for multiple testing, we conducted a permutation-based analysis to empirically determine adjusted p-values for all covariates and interaction terms in the GLM models with significant findings based on the method of Wagner et al (2008). We conducted 1,000 permutations for each model, and present both original and adjusted p-values for the interaction terms. Differences in the levels of self-reported impulsiveness and hostility were analyzed with univariate ANOVA, comparing both the risk allele groupings and genotypes in the full sample as well as in the individual comparison groups (BPD versus no-BPD).
RESULTS
Participant Demographics and Descriptive Statistics
Demographic information comparing the two groups is presented in Table 1. The BPD group was younger than the control group and had more females. There was no difference in racial/ethnic composition of the groups (BPD 23.5% vs. No BPD 22.6% Hispanic Caucasian, χ2 = <1.0, df = 1, p = ns). Genotype frequencies for both SNPs were comparable in males and females in the full sample (G-6526A χ2 = 1.26, df = 2, ns, A218C χ2 = 1.25, df = 2, ns), the BPD group (G-6526A χ2 < 1.00, df = 2, ns, A218C χ2 < 1.00, df = 2, ns), and the non-BPD group (G-6526A χ2 = 1.05, df = 2, ns, A218C χ2 = 3.47, df = 2, p = .18). Consequently, males and females were combined for all analyses. There was no difference in Axis I mood disorder diagnoses between the groups, although there was more than twice the percentage of suicide attempters in the BPD group (72.4% vs. 31.0%, χ2 = 52.38, df = 1, p = .000). Because of this substantial disparity, in addition to including attempter status as a covariate in our analyses, we also confirmed our findings with statistical models including only suicide attempters.
TABLE 1.
Demographic and Clinical Characteristics by Group
| Characteristic | BPD (n= 98) |
N-BPD (n= 300) |
t / χ2 | df |
|---|---|---|---|---|
| Age | 34.4±10.5 | 42.8±11.8 | −6.31*** | 396 |
| Total Education | 15.2±2.8 | 15.2±3.1 | < 1.0 | 394 |
| Female (%) | 81 (82.7) | 190 (63.3) | 12.69*** | 1 |
| Race | ||||
| Non-Hispanic | 78 (79.6) | 242 (80.7) | < 1.0 | 1 |
| Hispanic | 20 (20.4) | 58 (19.4) | ||
| Abuse History | 55 (56.1) | 123 (41.0) | 6.83** | 1 |
| None | 43 (43.9) | 177 (59.2) | 17.72** | 3 |
| Physical | 16 (16.3) | 58 (19.4) | ||
| Sexual | 15 (15.3) | 37 (12.4) | ||
| Both | 24 (24.5) | 27 (9.0) | ||
Note. BPD = Borderline Personality Disorder.
p < .01,
p < .000.
Genotype Frequencies
Genotype and haplotype frequencies and statistics are presented in Tables 2, 3, and 4. Genotypes were in Hardy Weinberg equilibrium in the full sample (G-6526A χ2 =< 1.0, df=2, ns; A218C χ2 =< 1.0, df=2, ns) as well as in BPD (G-6526A χ2 < 1.0, df=2, ns; A218C χ2 =2.65, df=2, p=.27) and non-BPD (G-6526A χ2 < 1.0, df=2, ns; A218C χ2 < 1.0, df=2, ns) cases independently. As an additional data check, we also examined HWE in males and females, as well as in Hispanics and non-Hispanics in the full sample and found no deviations. The SNPs were in strong linkage disequilibrium in our sample (D’=.79). There were no significant group differences in either the number of −6526A allele carriers (AA/GA genotypes, χ2<1.0, df=1, ns) or the G-6526A genotype frequencies (χ2<1.0, df=2, ns). The BPD group had a higher frequency of 218A allele carriers (AA/AC genotypes) than the control group (χ2=5.61, df=1, p=.02), as well as a significant difference in A218C genotype frequencies (χ2=5.86, df=2, p=.05). Although neither SNP was related to suicidal behavior in the BPD group, the A218C polymorphism was associated with suicide in the N-BPD group (χ2=9.06, df=2, p=.01). Interestingly, for both SNPs, only genotype frequencies in the N-BPD nonattempter group were similar to data published from the HapMap project on the CEPH population. All other subgroups deviated to some degree toward increased percentages of risk allele carriers.
TABLE 2.
Genotype and Allele Frequencies by Group: G-6526A
| Group | Allele Frequencies |
Additive Risk Model |
Dominant Risk Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G (%) |
A (%) |
GG (%) |
GA (%) |
AA (%) |
χ2 | df | GG (%) |
GA/AA (%) |
χ2 | df | |
| BPD (n=75) | .373 | .627 | 10 (13.3) | 36 (48.0) | 29 (38.7) | < 1.0 | 2 | 10 (13.3) | 65 (86.7) | < 1.0 | 1 |
| No-BPD (n=225) | .416 | .584 | 38 (16.9) | 111 (49.3) | 76 (33.8) | 187 (16.9) | 38 (83.1) | ||||
| BPD Attempters (n=54) | .370 | .630 | 7 (13.0) | 26 (48.1) | 21 (38.9) | < 1.0 | 2 | 7 (13.0) | 47 (87.0) | < 1.0 | 1 |
| BPD Nonattempters (n=21) | .381 | .619 | 3 (14.3) | 10 (47.6) | 8 (38.1) | 3 (14.3) | 18 (85.7) | ||||
| N-BPD Attempters (n=71) | .345 | .655 | 7 (9.9) | 35 (49.8) | 29 (40.8) | 4.57a | 2 | 7 (9.9) | 64 (90.1) | 3.65a | 1 |
| N-BPD Nonattempters (n=154) | .448 | .552 | 31 (20.1) | 76 (49.4) | 47 (30.5) | 107 (20.1) | 47 (79.9) | ||||
| HapMap | .449 | .551 | .203 | .492 | .305 | ||||||
Note. BPD = Borderline Personality Disorder.
< .10,
p < .05,
p < .01.
TABLE 3.
Genotype and Allele Frequencies by Group: A218C
| Group | Allele Frequencies |
Additive Risk Model |
Dominant Risk Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A (%) |
C (%) |
AA (%) |
AC (%) |
CC (%) |
χ2 | df | AA/AC (%) |
CC (%) |
χ2 | Df | |
| BPD (n=98) | .485 | .515 | 19 (19.4) | 57 (58.2) | 22 (22.4) | 5.86* | 2 | 76 (76.6) | 22 (22.4) | 5.62* | 1 |
| N-BPD (n=300) | .413 | .587 | 54 (18.0) | 140 (46.7) | 106 (35.3) | 194 (64.7) | 106 (35.3) | ||||
| BPD Attempters (n=71) | .493 | .507 | 13 (18.3) | 44 (62.0) | 14 (19.7) | 1.65 | 2 | 57 (80.3) | 14 (19.7) | 1.10 | 1 |
| BPD Nonattempters (n=27) | .463 | .537 | 6 (22.2) | 13 (48.1) | 8 (29.6) | 19 (70.4) | 8 (29.6) | ||||
| N-BPD Attempters (n=93) | .484 | .516 | 26 (28.0) | 38 (40.9) | 29 (31.2) | 9.06** | 2 | 64 (68.8) | 29 (31.2) | 1.02 | 1 |
| N-BPD Nonattempters (n=207) | .382 | .618 | 28 (13.5) | 102 (49.3) | 77 (37.2) | 130 (62.8) | 77 (37.2) | ||||
| HapMap | .398 | .602 | .186 | .424 | .390 | ||||||
Note. BPD = Borderline Personality Disorder.
p < .05,
p < .01.
TABLE 4.
Estimated Haplotype Frequencies by Group: −6526/218
| BPD | N-BPD | ||||
|---|---|---|---|---|---|
| Haplotype | Frequency | SE | Frequency | SE | |
| h.GC | .323 | .038 | .376 | .023 | |
| h.AA | .450 | .041 | .369 | .023 | |
| h.AC | .177 | .031 | .215 | .019 | |
| h.GA | .050 | .018 | .040 | .009 | |
Note. BPD = Borderline Personality Disorder.
Single SNP Analysis
Our first set of analyses modeled the relationship between BPD diagnosis (Y/N), abuse history (Y/N), and genotype for each of the SNPs independently, controlling for attempter status, gender, and age, under both additive and dominant risk allele models. In the first subset, predicting BPD diagnosis by G-6526A, the dominant risk allele model (AA/GA versus GG) provided the best fit to the data. The overall model was significant (χ2=65.50, df=6, p=.000), as was the interaction term (Wald=4.46, df=1, p=.035/adjp=.036). Neither the main effect for abuse history nor the main effect for genotype was significant once the interaction term was included in the model. Results were similar when the model included the categorical abuse variable (genotype × abuse interaction term Wald=4.45, df=1, p=.023/adjp=.025). In the second subset, predicting BPD diagnosis by A218C, the dominant risk allele model (AA/AC versus CC) also provided the best fit to the data. The overall model was significant (χ2=100.32, df=7, p=.000), as was the interaction term (Wald = 8.06, df=1, p = .005/adjp=.003). The main effect for abuse history was significant (Wald = 6.06, df=1, p = .014/adjp=.011), although the main effect for genotype was not. As with the −6256 analysis, results were similar when including the categorical abuse variable (genotype × abuse interaction term Wald=5.70, df=1, p=.017/adjp=.016). Risk of a BPD diagnosis for carriers of the A allele at both loci was increased in the presence of an abuse history. In addition, an increase in severity of abuse was associated with increased risk of a BPD diagnosis (see Figures 1 and 2). Neither the main effect for genotype nor the interaction term was significant in any of the additive risk models. Of note was the fact that in all of these analyses, the main effect for abuse history was not significant when the interaction term was included in the model.
Figure 1.
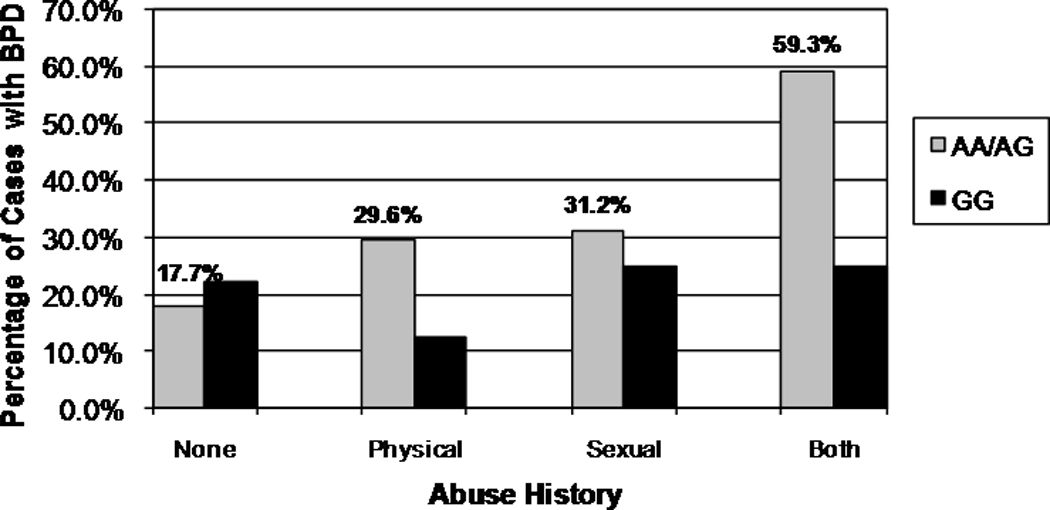
Percentage BPD Diagnosed Individuals by Group – G-6526A
Figure 2.
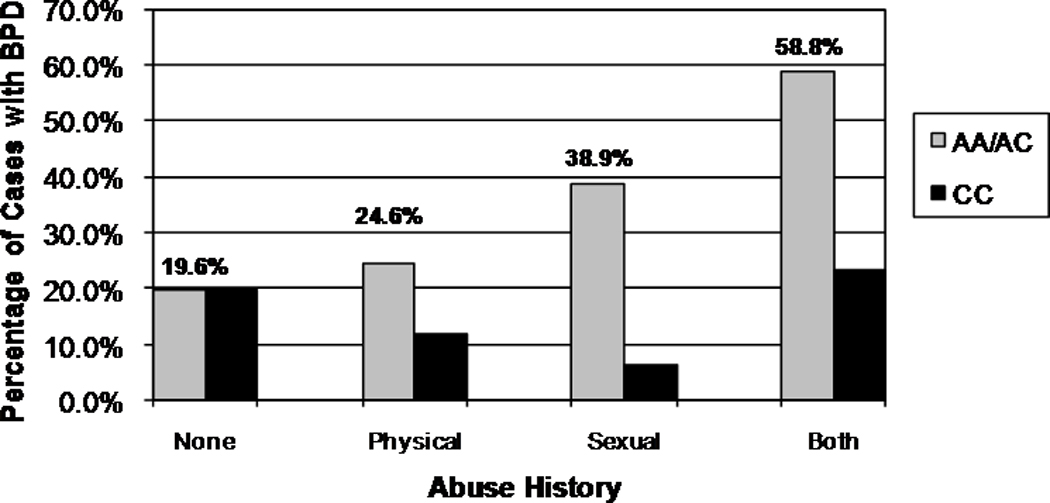
Percentage BPD Diagnosed Individuals by Group – A218C
The second set of analyses modeled the relationship between SCID-II BPD criteria, abuse history, and genotype for each of the SNPs independently as well as for the two-SNP haplotype, controlling for attempter status, gender, and age, again under both additive and dominant risk allele models. For the G-6526A polymorphism, none of the models resulted in a significant genetic effects or gene × abuse interaction terms. For the A218C polymorphism, the overall test of the dominant model was significant (χ2=68.95, df=7, p=.000), as was the interaction term (Wald=4.56, df=1, p=.033). The interaction between genotype and abuse history predicted the number of DSM-IV BPD criteria met across the entire sample, after controlling for age, sex, and attempter status. There were no significant effects in the model run with the categorical abuse variable.
Haplotype Analyses
Haplotypes were imputed for a subset of cases with genotypes for both SNPS (n=300). The AA risk haplotype was used in all analyses. As with the single SNP analyses, the dominant genetic risk model provided the best fit to the data. In the analysis predicting BPD diagnosis by the −6256/218 haplotypes the overall model was significant (χ2=71.87, df=7, p=.000), as was the interaction term (Wald=5.74, df=1, p=.017/adjp=.024). Individuals with one or two AA haplotypes were at increased risk for a BPD diagnosis when a history of abuse was present. As with the single SNP analyses, none of the genetic or interaction terms were significant under the additive model, and neither of the Poisson models included significant genetic or genotype × abuse interaction terms. As with the individual SNP models, neither the main effect for abuse history nor haplotype were significant once the interaction term was included in the model.
As several investigators (Liu et al. 2006a; Roy et al. 2001; Tsai et al. 1999), including members of our group (Galfalvy et al. 2009), have reported an association between TPH1 polymorphisms and suicidal behavior, in addition to controlling for suicide attempter status in our analyses, we also reran the logistic haplotype analysis in the subset of cases and controls with a history of suicidal behavior (n=164) under both additive and dominant risk allele models. Results confirmed the associations found in the original analyses, with the dominant model providing the best fit to the data, a significant main effect for the AA haplotype (Wald=4.96, df=1, p=.026) and a significant interaction term (Wald=9.17, df=1, p=.002).
DISCUSSION
Our results suggest that TPH1 moderates the association between a history of childhood abuse and the risk for developing BPD later in life, and that increasing severity of abuse may be associated with increasing BPD risk in those carrying the TPH1 risk alleles. In addition, the interaction between abuse history and individual genotypes, as well as the two SNP haplotype, not only increased risk for a BPD diagnosis, but also seemed to influence severity of psychopathology, as indicated by the number of DSM-IV diagnostic criteria met for BPD. Notably, these associations were found both in the full sample, as well as in a restricted sample comprised of only suicide attempters. This finding suggests that the observed interaction between TPH1 genotype and abuse history were not due to the association between TPH1 and suicidal behavior observed in the non-BPD group. Also, as there was no significant difference in Axis I mood disorder diagnoses between the BPD and Non-BPD groups, it is unlikely that our results reflect an association between TPH1 and Axis I pathology.
Although there is limited research investigating the interaction between TPH1 and a history of abuse, an interesting study by Keltikangas-Jarvinen and colleagues (Keltikangas-Jarvinen et al. 2007) reported results that lend support to our findings in a study exploring whether TPH1 polymorphisms interact with hostile early childhood environment to increase the personality trait of harm avoidance in healthy controls. Using a measure that assessed for the presence of emotional rejection, inconsistent discipline, and maternal neglect, the authors found that the A allele of the A218C interacted with a hostile childhood rearing environment to increase harm avoidance in otherwise psychiatrically healthy women. As high levels of harm avoidance have been reported in personality studies of BPD patients (Korner et al. 2007), as well as in the offspring of mothers with BPD (Barnow et al. 2006), the results of this research lend support to our findings.
Similar to our previous study comparing this sample to healthy controls, we also found that BPD patients had higher frequencies of both TPH1 polymorphisms and the two SNP haplotype in comparison to depressed patients without BPD. While this is not a true replication of the initial finding, since we are comparing the same BPD sample to a second control sample of patients with mood disorders but without BPD, the fact that comparison to a clinical comparison sample also yielded significant findings further supports the view that TPH1 is associated with BPD. In addition, we found that while neither of the polymorphisms distinguished suicide attempters from non-attempters in the BPD group, the intron 7 polymorphism was significantly associated with suicidal behavior in the non-BPD group. Although these associations appear to be somewhat conflicting, they provide support for our view that TPH1 may be associated with an endophenotype that is associated with BPD, but that can also be more frequently found in suicidal individuals without BPD. Indeed, visual inspection of the genotype frequencies for the polymorphisms, and for the G-6526A polymorphism in particular, strongly suggests that this is the case. Genotype frequencies for the BPD groups and the non-BPD attempters are similar, whereas the non-BPD nonattempters have frequencies that are similar to healthy controls.
Our findings may help to integrate some of the inconsistent findings in the literature regarding the association between TPH1 and suicidal behavior. As with single SNP association studies, several investigators have reported associations between TPH1 haplotypes and suicidal behavior in several populations. For example, Lui and colleges (Liu et al. 2006b) reported that a haplotype containing A alleles of both the G-6526A and the A218C SNPs was most strongly related to suicide in a Chinese psychiatric sample of predominantly patients with schizophrenia. Also, in a recent study by our group (Galfalvy et al. 2009) examining the relationship between TPH1 and suicide in 343 patients with mood disorders, we found the A/A haplotype was associated with history of suicidal behavior, and significantly predicted suicide risk prospectively. Although the research including the G-6526A promoter polymorphism is limited, many studies including the A218C polymorphism have also found no association between TPH1 and suicidal behavior (Kunugi et al. 1999; Souery et al. 2001) or suicidal ideation in depressed individuals(Du et al. 2001). In addition, a recent meta-analysis of studies investigating the association between TPH1 and suicide (Saetre et al. 2009) reported that results from association studies were highly heterogeneous, and concluded that TPH1 polymorphisms seem to be associated with increased risk for disorders that have high rates of suicide, rather than specifically and directly increasing risk for suicidal behavior. This heterogeneity could be explained in part by varying rates of BPD in the samples included in these studies.
Although little is known about the effects of mutations in the TPH1 gene, the G-6526A promoter polymorphism has been shown to be functional. The polymorphism resides within a c-ETS transcription factor binding domain (Rotondo et al. 1999), and the A allele has been associated with an approximately 3 fold increase in gene transcription (Sun et al. 2005). As sequence analysis shows that the presence of the mutant allele activates the binding site, the finding that the A allele is associated with increased TPH1 expression suggests that the mutation leads to increased transcription of TPH1. In contrast to the G-6526A polymorphism, no functional studies have been undertaken with the A218C polymorphism. Because it is located in an intronic region, it does not lead to any functional change in protein coding. However, the polymorphism is also in a potential transcription factor (GATA-1) binding site. The GATA-1 transcription factor has been shown to increase transcription rates of some genes up to 100 fold (Wong et al. 2004), but also can act as a repressor (Raich et al. 1995). In either case, a binding site created via the transmission of the A allele at this locus could potentially have a significant effect on TPH gene expression.
The mechanism by which differential expression levels of the TPH1 gene could exert an effect on CNS serotonergic function is not well understood. Tryptophan hydroxylase is a critical precursor enzyme in the serotonin metabolic pathway, and TPH1 mutations, particularly if they led to changes in gene expression or enzyme functionality, could influence serotonin levels. However, as research has shown that TPH2 is the predominant isoform in the adult CNS, while TPH1 is primarily active in the periphery (Sakowski et al. 2006), it is difficult to establish a direct link between TPH1 activity and CNS serotonin. Anecdotal evidence from animal studies suggests several possible indirect connections. First, animal studies have shown that chronic restraint stress can lead to significant increases in CNS TPH1 expression in adulthood (Abumaria et al. 2008; Abumaria et al. 2007), which suggests that differences in TPH1 functionality may be more evident in the context of stress induced gene transcription. Alternatively, animal studies have shown that TPH1 is expressed much more prominently in the brain during the late prenatal developmental period, and that TPH1 may play a role in the development of the serotonin system via an influence on neuron number and growth (Nakamura et al. 2006; Rind et al. 2000). Differences in TPH1 expression or functionality could have an effect on prenatal serotonin levels, which could in turn lead to subtle deficiencies in serotonergic functioning in later life. Research on the effects of antidepressant medication use during the prenatal period has demonstrated the deleterious effects of elevated serotonin exposure in utero, and has been associated with several adverse outcomes, including alterations in HPA reactivity (Oberlander et al. 2008).
The significant interaction terms in our analyses raise the question of how the putative genetic liability created by mutations in the TPH1 gene would impact the experience of, and the neurobiological consequences of childhood trauma. Many investigators have shown that childhood trauma is associated with significant neurobiological sequelae (for review, see Teicher et al. (Teicher et al. 2002). While the mechanism underlying these sequelae are not entirely clear, recent research has shown an association between post-trauma cortisol levels and hippocampal volume reductions over a 12–18 month period in children with PTSD (Carrion et al. 2007). This suggests that the prolonged activation of the HPA axis and increased exposure to stress hormones that are consequences of ongoing abuse can have long term neurobiological consequences on brain development and function. Because of serotonin’s role in the stress response system (Chaouloff 2000; Chaouloff et al. 1999), individual differences in serotonin production could alter the capacity to mitigate this effect. Animal studies have shown that among serotonin’s many functions, its release during stress serves to attenuate the damaging effects of corticosteroids by increasing mineralocorticoid receptor density in the hippocampus (Gesing et al. 2000; Mitchell et al. 1990) via binding at the 5-HT7 receptor (Laplante et al. 2002), and thus serves to increase the resiliency through increasing the modulatory capacity of the stress response system. It is possible that any reduction in the capacity for serotonin synthesis under basal and/or stressful conditions could lead to a reduced capacity to offset the damaging effects of stress, and thus to more severe neurobiological consequences. Again, more research is needed if we are to better understand what is likely a complex interplay between genetic liabilities and environmental factors.
This study has several limitations. First, the modest size of the sample included in this research means our findings must be replicated and validated. While association studies are often difficult to replicate, the fact that our findings are consistent with the large literature on TPH1 polymorphism associations increases the likelihood that our findings are valid. Also, the fact that we studied only two polymorphisms means that we did not capture all the known variation in the TPH1 gene. Several additional tagging SNPs are required in order to achieve this. Finally, TPH1 allele frequencies are known to vary considerably between ethnic groups, and even between northern and southern Europe (Nielsen et al. 1997). While we made an effort to control for hidden stratification using structured association methods, it is possible that there is additional unknown stratification in our sample that may have contributed to the results.
Despite these limitations, our results suggest that the TPH1 is associated with an underlying risk for both suicidal behavior and borderline personality disorder, and that TPH1 moderates the relationship between childhood trauma and risk for developing BPD later in life. Additional research on the neurobiological effects of genetic variation in the TPH1 gene will be important in understanding the manner in which TPH1 influences risk for both suicidal behavior and BPD, as well as in determining more definitively whether TPH1 mutations create a genetic vulnerability that leads to greater neurobiological sequelae as a result of childhood abuse or trauma, and a consequent increase in risk for BPD. Research focused on determining the effects of variation at the A218C locus is also of particular importance.
ACKNOWLEDGEMENTS
This work was funded in part by grants from the National Institute of Mental Health (MH 48514, MH62185, MH59710, MH61017, and MH62665) and the National Institute on Alcohol Abuse and Alcoholism (AA15630).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors of this manuscript have no financial or other relationships that are relevant to the subject matter of this research or the results described herein.
References
- Abumaria N, Ribic A, Anacker C, Fuchs E, Flugge G. Stress upregulates TPH1 but not TPH2 mRNA in the rat dorsal raphe nucleus: identification of two TPH2 mRNA splice variants. Cellular & Molecular Neurobiology. 2008;28:331–342. doi: 10.1007/s10571-007-9259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumaria N, Rygula R, Hiemke C, Fuchs E, Havemann-Reinecke U, Ruther E, Flugge G. Effect of chronic citalopram on serotonin-related and stress-regulated genes in the dorsal raphe nucleus of the rat. European Neuropsychopharmacology. 2007;17:417–429. doi: 10.1016/j.euroneuro.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Anghelescu I, Klawe C, Fehr C, Singer P, Schleicher A, Himmerich H, Hiemke C, Dahmen N, Szegedi A. The TPH intron 7 A218C polymorphism and TCI dimension scores in alcohol-dependent patients: hints to nonspecific psychopathology. Addictive Behaviors. 2005;30:1135–1143. doi: 10.1016/j.addbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Barnow S, Spitzer C, Grabe HJ, Kessler C, Freyberger HJ. Individual Characteristics, Familial Experience, and Psychopathology in Children of Mothers With Borderline Personality Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;Vol 45:965–972. doi: 10.1097/01.chi.0000222790.41853.b9. [DOI] [PubMed] [Google Scholar]
- Baud P, Perroud N, Courtet P, Jaussent I, Relecom C, Jollant F, Malafosse A. Modulation of anger control in suicide attempters by TPH-1. Genes, Brain and Behavior. 2009;8:97–100. doi: 10.1111/j.1601-183X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Chaste P, Malafosse A. Association between the TPH gene A218C polymorphism and suicidal behavior: a meta-analysis. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2004;124:87–91. doi: 10.1002/ajmg.b.20015. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, McMahon WM, Watabe J, Achilles J, Bacon M, Coon H, Grey T, Keller T, Tate D, Tcaciuc I, Workman J, Gray D. Tryptophan hydroxylase polymorphisms in suicide victims. Psychiatric Genetics. 2000;10:13–17. doi: 10.1097/00041444-200010010-00003. [DOI] [PubMed] [Google Scholar]
- Bertoni B, Budowle B, Sans M, Barton SA, Chakraborty R. Admixture in Hispanics: distribution of ancestral population contributions in the Continental United States. Human Biology. 2003;75:1–11. doi: 10.1353/hub.2003.0016. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL. Stress Predicts Brain Changes in Children: A Pilot Longitudinal Study on Youth Stress, Posttraumatic Stress Disorder, and the Hippocampus. Pediatrics. 2007;199:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Carter KW, McCaskie PA, Palmer LJ. JLIN: A Java based Linkage Disequilibrium plotter. BMC Bioinformatics. 2006;7:60. doi: 10.1186/1471-2105-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter KW, McCaskie PA, Palmer LJ. SimHap GUI: An intuitive graphical user interface for genetic association analysis. BMC Bioinformatics. 2008;9:557–559. doi: 10.1186/1471-2105-9-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Serotonin, stress, and corticoids. Journal of Psychopharmacology. 2000;14:139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B. Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse & Neglect. 2007;31:211–229. doi: 10.1016/j.chiabu.2007.02.004. [see comment] [DOI] [PubMed] [Google Scholar]
- Du L, Bakish D, Hrdina PD. Tryptophan hydroxylase gene 218A/C polymorphism is associated with somatic anxiety in major depressive disorder. Journal of Affective Disorders. 2001;65:37–44. doi: 10.1016/s0165-0327(00)00274-3. [DOI] [PubMed] [Google Scholar]
- Evans J, Reeves B, Platt H, Leibenau A, Goldman D, Jefferson K, Nutt D. Impulsiveness, serotonin genes and repetition of deliberate self-harm (DSH) Psychological Medicine. 1327;30:1327–1334. doi: 10.1017/s0033291799002822. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbons M, Williams JBW, Benjamin L. User's guide for the Structured Clinical Interview for for DSM-IV Axis II Personality Disorders (SCID-II) 1995 [Google Scholar]
- Fossati A, Madeddu F, Maffei C. Borderline Personality Disorder and childhood sexual abuse: A meta-analytic study. Journal of Personality Disorders. 1999;Vol 13:268–280. doi: 10.1521/pedi.1999.13.3.268. [DOI] [PubMed] [Google Scholar]
- Galfalvy H, Huang YY, Oquendo MA, Currier D, Mann JJ. Increased risk of suicide attempt in mood disorders and TPH1 genotype. Journal of Affective Disorders. 2009;115:331–338. doi: 10.1016/j.jad.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesing A, Bilang-Bleuel A, Droste SK, Linthorst ACE, Holsboer F, Reul JMHM. Psychological Stress Increases Hippocampal Mineralocorticoid Receptor Levels: Involvement of Corticotropin-Releasing Hormone. Journal of Neuroscience. 2000;21:4822–4829. doi: 10.1523/JNEUROSCI.21-13-04822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, New A, Siever L. Trauma, Genes, and the Neurobiology of Personality Disorders. Annals of the New York Academy of Sciences. 2004;1032:104–116. doi: 10.1196/annals.1314.008. [DOI] [PubMed] [Google Scholar]
- Goodman M, Yehuda R. The relationship between psychological trauma and borderline personality disorder. Psychiatric Annals. 2002;32:337–345. [Google Scholar]
- Hennig J, Reuter M, Netter P, Burk C, Landt O. Two types of aggression are differentially related to serotonergic activity and the A779C TPH polymorphism. Behavioral Neuroscience. 2005;119:16–25. doi: 10.1037/0735-7044.119.1.16. [DOI] [PubMed] [Google Scholar]
- Herman JL, van der Kolk BA. Traumatic antecedents of borderline personality disorder. Washington, D.C: American Psychiatric Press; 1987. [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neuroscience & Biobehavioral Reviews. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltikangas-Jarvinen L, Puttonen S, Kivimaki M, Elovainio M, Rontu R, Lehtimaki T. Tryptophan hydroxylase 1 gene haplotypes modify the effect of a hostile childhood environment on adulthood harm avoidance. Genes, Brain, & Behavior. 2007;6:305–313. doi: 10.1111/j.1601-183X.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- Korner A, Gerull F, Stevenson J, Meares R. Harm avoidance, self-harm, psychic pain, and the borderline personality: life in a "haunted house". Comprehensive Psychiatry. 2007;48:303–308. doi: 10.1016/j.comppsych.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Ishida S, Kato T, Sakai T, Tatsumi M, Hirose T, Nanko S. No evidence for an association of polymorphisms of the tryptophan hydroxylase gene with affective disorders or attempted suicide among Japanese patients. American Journal of Psychiatry. 1999;156:774–776. doi: 10.1176/ajp.156.5.774. [DOI] [PubMed] [Google Scholar]
- Laplante P, Diorio J, Meaney MJ. S erotonin regulates hippocampal glucocorticoid receptor expression via a 5-HT7 receptor. Developmental Brain Research. 2002;139:199–203. doi: 10.1016/s0165-3806(02)00550-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Li H, Qin W, He G, Li D, Shen Y, Shen J, Gu N, Feng G, He L. Association of TPH1 with suicidal behaviour and psychiatric disorders in the Chinese population. Journal of Medical Genetics. 2006a;43 doi: 10.1136/jmg.2004.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li H, Qin W, He G, Li D, Shen Y, Shen J, Gu N, Feng G, He L. Association of TPH1 with suicidal behaviour and psychiatric disorders in the Chinese population. Journal of Medical Genetics. 2006b;43:1–6. doi: 10.1136/jmg.2004.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH. The Chestnut Lodge follow-up study. III. Long-term outcome of borderline personalities. Archives of General Psychiatry. 1986;43:20–30. doi: 10.1001/archpsyc.1986.01800010022003. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Rowe W, Boksa P, Meaney MJ. Serotonin Regulates Type II Corticosteroid Receptor Binding in Hippocampal Cell Cultures. Journal of Neuroscience. 1990;10:1745–1752. doi: 10.1523/JNEUROSCI.10-06-01745.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, Xiu Y, Ohtsuji M, Lin QS, Nishimura H, Hasegawa H, Hirose S. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. Journal of Neuroscience. 2006;26:530–534. doi: 10.1523/JNEUROSCI.1835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Gelernter J, Yovell Y, Trestman RL, Nielsen DA, Silverman J, Mitropoulou V, Siever LJ. Tryptophan hydroxylase genotype is associated with impulsive-aggression measures: a preliminary study. American Journal of Medical Genetics. 1998;81:13–17. doi: 10.1002/(sici)1096-8628(19980207)81:1<13::aid-ajmg3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Jenkins GL, Stefanisko KM, Jefferson KK, Goldman D. Sequence, splice site and population frequency distribution analyses of the polymorphic human tryptophan hydroxylase intron 7. Brain Research. Molecular Brain Research. 1997;45:145–148. doi: 10.1016/s0169-328x(96)00304-x. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau R, Mayes L, Riggs W, Rurak D, Papsdorf M, Misri S, Weinberg J. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Human Development. 2008;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, Toh K, Kim J, Lee C. TPH gene may be associated with suicidal behavior, but not with schizophrenia in the Korean population. Human Heredity. 2000;50:365–369. doi: 10.1159/000022942. [DOI] [PubMed] [Google Scholar]
- Pooley EC, Houston K, Hawton K, Harrison PJ. Deliberate self-harm is associated with allelic variation in the tryptophan hydroxylase gene (TPH A779C), but not with polymorphisms in five other serotonergic genes. Psychological Medicine. 2003;33:775–783. doi: 10.1017/s0033291703007463. [DOI] [PubMed] [Google Scholar]
- Raich N, Clegg CH, Grofti J, Romeo PH, Stamatoyannopoulos G. GATA1 and YY1 are developmental repressors of the human E-globin gene. The EMBO Journal. 1995;14:801–809. doi: 10.1002/j.1460-2075.1995.tb07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Hennig J. Pleiotropic effect of the TPH A779C polymorphism on nicotine dependence and personality. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2005;134:20–24. doi: 10.1002/ajmg.b.30153. [DOI] [PubMed] [Google Scholar]
- Rind HB, Russo AF, Whittemore SR. Developmental regulation of tryptophan hydroxylase messenger RNA expression and enzyme activity in the raphe and its target fields. Neuroscience. 2000;101:665–677. doi: 10.1016/s0306-4522(00)00402-4. [DOI] [PubMed] [Google Scholar]
- Rotondo A, Schuebel K, Bergen A, Aragon R, Virkkunen M, Linnoila M, Goldman D, Nielsen D. Identification of four variants in the tryptophan hydroxylase promoter and association to behavior. Molecular Psychiatry. 1999;4:360–368. doi: 10.1038/sj.mp.4000578. [DOI] [PubMed] [Google Scholar]
- Roy A, Rylander G, Forslund K, Asberg M, Mazzanti CM, Goldman D, Nielsen DA. Excess tryptophan hydroxylase 17 779C allele in surviving cotwins of monozygotic twin suicide victims. Neuropsychobiology. 2001;43:233–236. doi: 10.1159/000054895. [DOI] [PubMed] [Google Scholar]
- Saetre P, Lundmark P, Hansen T, Rasmussen HB, Djurovic S, Melle I, Andreassen OA, Werge T, Agartz I, Hall H, Terenius L, Jönsson EG. The tryptophan hydroxylase 1 (TPH1) gene, schizophrenia susceptibility, and suicidal behavior: A multi-centre case-control study and meta-analysis. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics advanced online publication. 2009 doi: 10.1002/ajmg.b.30991. [DOI] [PubMed] [Google Scholar]
- Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Research. 2006;1:11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Samuels JE, Eaton WW, Bienvenu OJ, Brown C, Costa PT, Nestadt G. Prevalence and correlates of personality disorders in a community sample. British Journal of Psychiatry. 2002;180:536–542. doi: 10.1192/bjp.180.6.536. [DOI] [PubMed] [Google Scholar]
- Simeon D, Stanley B, Frances A, Mann JJ, Winchel R, Stanley M. Self-mutilation in personality disorders: psychological and biological correlates. Am J Psychiatry. 1992;149:221–226. doi: 10.1176/ajp.149.2.221. [DOI] [PubMed] [Google Scholar]
- Souery D, Van Gestel S, Massat I, Blairy S, Adolfsson R, Blackwood D, Del-Favero J, Dikeos D, Jakovljevic M, Kaneva R, Lattuada E, Lerer B, Lilli R, Milanova V, Muir W, Nothen M, Oruc L, Papadimitriou G, Propping P, Schulze T, Serretti A, Shapira B, Smeraldi E, Stefanis C, Thomson M, Van Broeckhoven C, Mendlewicz J. Tryptophan hydroxylase polymorphism and suicidality in unipolar and bipolar affective disorders: a multicenter association study. Biological Psychiatry. 2001;49:405–409. doi: 10.1016/s0006-3223(00)01043-x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for the DSM-IIII-R/DSM-IV – Patient Edition. New York: New York State Psychiatric Institute., New York; 1990. [Google Scholar]
- Staner L, Uyanik G, Correa H, Tremeau F, Monreal J, Crocq MA, Stefos G, Morris-Rosendahl DJ, Macher JP. A dimensional impulsive-aggressive phenotype is associated with the A218C polymorphism of the tryptophan hydroxylase gene: a pilot study in well-characterized impulsive inpatients. American Journal of Medical Genetics. 2002;114:553–557. doi: 10.1002/ajmg.10405. [DOI] [PubMed] [Google Scholar]
- Sun HS, Fann CS, Lane HY, Chang YT, Chang CJ, Liu YL, Cheng AT. A functional polymorphism in the promoter region of the tryptophan hydroxylase gene is associated with alcohol dependence in one aboriginal group in Taiwan. Alcoholism: Clinical & Experimental Research. 2005;29:1–7. doi: 10.1097/01.alc.0000150635.51934.6d. [DOI] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A, Pankow JS, Province MA, Hunt SC, Boerwinkle E, Schork NJ, Risch NJ. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics. 2005;76:268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatric Clinics of North America. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Thorisson GA, Smith AV, Krishnan L, Stein LD. The International HapMap Project Web site. Genome Research. 2005;15:1591–1593. doi: 10.1101/gr.4413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Archives of General Psychiatry. 2001;Vol 58:590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clinical Psychology Review. 2000;Vol 20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Wang YC. Tryptophan hydroxylase gene polymorphism (A218C) and suicidal behaviors. Neuroreport. 1999;10:3773–3775. doi: 10.1097/00001756-199912160-00010. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Weissman MM. Epidemiology of borderline personality disorder. Hosp Community Psychiatry. 1991;42:1015–1021. doi: 10.1176/ps.42.10.1015. [DOI] [PubMed] [Google Scholar]
- Wilson ST, Stanley B, Brent DA, Oquendo MA, Huang YY, Mann JJ. The tryptophan hydroxylase I A218C polymorphism is associated with diagnosis, but not suicidal behavior, in borderline personality disorder. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2009;150B:202–207. doi: 10.1002/ajmg.b.30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EY, Lin JG, Forget BG, Bodine DM, Gallagher PG. Sequences downstream of the erythroid promoter are required for high level expression of the human alpha-spectrin gene. Journal of Biological Chemistry. 2004;279:55024–55033. doi: 10.1074/jbc.M408886200. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Frisch A, King RA, Pauls DL, Grice DE, Gelernter J, Alsobrook J, Michaelovsky E, Apter A, Tyano S, Weizman A, Leckman JF. Case control and family-based studies of tryptophan hydroxylase gene A218C polymorphism and suicidality in adolescents. American Journal of Medical Genetics. 2001;105:451–457. doi: 10.1002/ajmg.1406. [DOI] [PubMed] [Google Scholar]
- Zisook S, Goff A, Sledge P, Shuchter SR. Reported suicidal behavior and current suicidal ideation in a psychiatric outpatient clinic. Annals of Clinical Psychiatry. 1994;6:27–31. doi: 10.3109/10401239409148836. [DOI] [PubMed] [Google Scholar]


