Abstract
Purpose
To compare liver ADC obtained with breath-hold and free-breathing diffusion weighted imaging (DWI) in healthy volunteers and patients with liver disease.
Materials and Methods
Twenty-eight subjects, 12 healthy volunteers and 16 patients (9 NAFLD, 7 chronic active HCV), underwent breath-hold (BH) and free-breathing (FB) DWI MRI at 1.5T. Pearson’s correlation coefficient was used to determine correlation while paired t-tests assessed differences between BH and FB ADC. Estimated bias was calculated using the Bland-Altman method.
Results
Liver ADC (×10−3 mm2/sec) was lower on BH for all groups (mean difference 0.36±0.20; p<0.01). ADC was higher in healthy volunteers (BH 1.80±0.18; FB 2.24±0.20) compared to NAFLD patients (BH 1.43±0.27; FB 1.78±0.28) (p<0.001) and HCV patients (BH 1.63±0.191; FB 1.88±0.12). Overall correlation between BH and FB ADC was (r =0.75), greatest in NAFLD (r =0.90) compared to the correlation in HCV (r =0.24) and healthy subjects (r =0.34). Bland-Altman plots did not show agreement in mean absolute difference and estimated bias between subjects.
Conclusion
Correlation between BH and FB liver ADC is moderate indicating that BH and FB should not be used interchangeably. Additionally, the lower ADC values in BH versus FB should be accounted for when comparing different liver DWI studies.
Keywords: Diffusion-weighted Imaging (DWI), Breath-hold (BH), Free-breathing (FB), Non-Alcoholic Fatty Liver Disease (NAFLD), Hepatitis C (HCV)
INTRODUCTION
The most common etiologies of chronic liver disease are nonalcoholic fatty liver disease (NAFLD), which affects almost 30% of the population in the United States, alcoholic fatty liver disease, hepatitis B infection and hepatitis C infection (1–3). The detection and staging of diffuse liver disease is primarily achieved through histological assessment of tissue obtained by liver biopsy. However, there is a current drive towards developing non-invasive quantitative techniques for evaluation of disease severity. Diffusion weighted MRI (DWI) is a promising technique which is under investigation for such a purpose. Researchers have shown that DWI and liver apparent diffusion coefficient (ADC) may be helpful in the detection and characterization of liver fibrosis (4–9). Major limitations to the widespread use of liver ADC in clinical practice is the lack of a standardized DWI protocol, susceptibility of the DWI sequence to artifacts, and a lack of sufficient data comparing DWI techniques. Optimization of DWI requires a compromise between strategies for improving signal to noise versus strategies to reduce motion artifact which are associated with increased or decreased scan time, respectively. Breath-hold (BH) single-shot (SS) echo-planar imaging (EPI) is a rapid sequence with a short examination time which reduces motion artifact but has inherent low signal-to-noise ratio. The other frequently used technique, free- breathing (FB) DWI, provides a good signal-to-noise ratio (SNR), however may suffer from motion artifact (10). To the best of our knowledge, there is no prior study which has compared liver ADC determined with BH and FB DWI in both patients and healthy subjects. Therefore, the purpose of our study was to compare liver ADC values obtained with BH and FB DWI in healthy volunteers and patients with chronic liver disease.
MATERIALS AND METHODS
Patients
Relevant Committee of Human Research approval was obtained, and the study was in compliance with the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all subjects for the purpose of this MR imaging study. Twenty-eight consecutive subjects were imaged and recruited as part of two prospective trials, one studying NAFLD subjects and one studying HCV/HIV co-infected subjects. Additionally, twelve healthy volunteers with no prior history or finding related to liver disease were recruited. Nine patients with biopsy proven nonalcoholic fatty liver disease were recruited for the NAFLD cohort study, four of whom had Ishak fibrosis stage greater/equal than two. The remaining seven patients had chronic active HCV infection (HCV RNA positive) with concomitant HIV infection. They were recruited from the Northern California site of the Women’s Interagency HIV Study (WIHS), an ongoing prospective study established in 1994 to investigate the progression of HIV infection in women with and at risk for HIV. The recruitment, study design and baseline characteristics of WIHS women have been described elsewhere (11, 12). Exclusion criteria were general contraindications to MR imaging, such as an implanted pacemaker or claustrophobia.
MR Imaging
All subjects were scanned with a 1.5T HDx scanner (GE Medical Systems, Milwaukee, WI) using an eight-channel body phased-array coil (GE Medical Systems, Milwaukee, WI). Anti-peristaltic and contrast agents were not administered. The MR imaging protocol included two axial diffusion-weighted, single-shot, echo-planar imaging sequences as follows: 1) BH DWI: sequential slice acquisition; acquisition time was 24 seconds, 3 direction BH DWI with parallel imaging, acceleration factor, 2; TR/TE, 2000/70.3 ms; matrix size, 128×128; field of view, 36–48 cm, corresponding to an in-plane acquisition voxel size of 0.28 to 0.375mm; slice/gap 10/2 mm; number of signal averages, 3; number of slices, 9; b-values, 0, 600 s/mm2; bandwidth, 1953 Hz/pixel; and spectral-spatial excitation of the water peak; one dummy scan was acquired at the beginning of the sequence. 2) FB DWI: sequential slice acquisition; acquisition time was 5 minutes, 6 direction FB DWI sequence; TR/TE, 7000/87.8 ms; matrix size, 128×128; field of view, 36–48 cm, corresponding to an in-plane acquisition voxel size of 0.28 to 0.375 mm; slice/gap 10/2 mm; number of signal averages, 6; number of slices, 9; b-values, 0, 600 s/mm2; bandwidth, 1953 Hz/pixel; and spectral-spatial excitation of the water peak; No dummy scans or pre-pulses was applied to the FB acquisition.
Image analysis
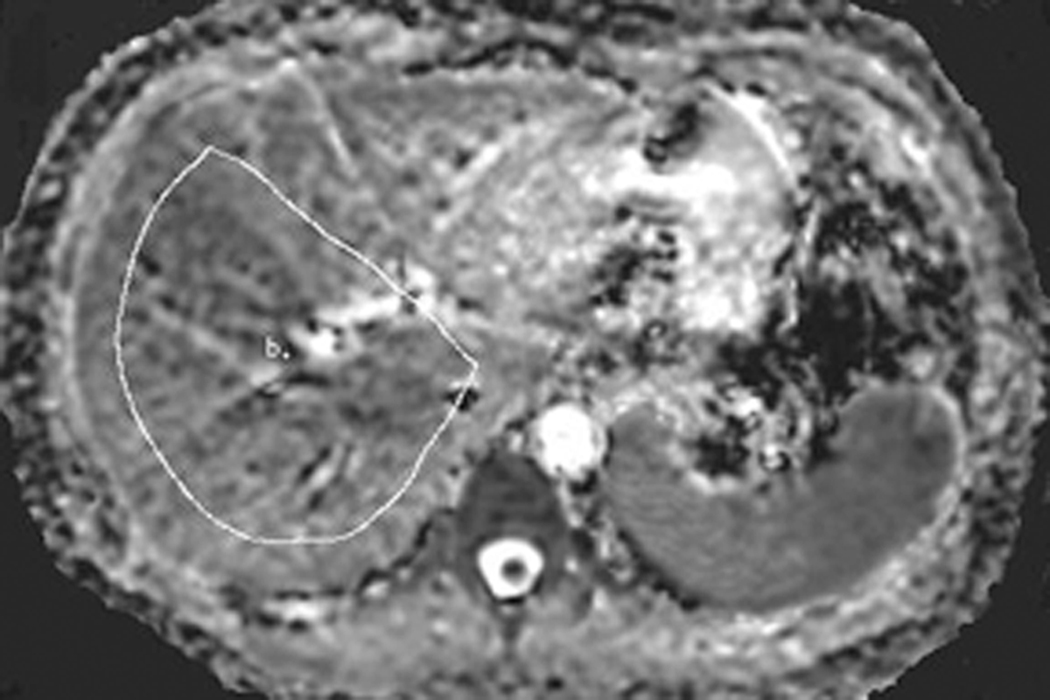
Liver ADC maps were generated on a voxel-by-voxel basis using a software program developed in-house to obtain ADC maps for each scan using a UNIX Solaris Workstation (Sun Microsystems, Sunnyvale, California). Only the right hepatic lobe was evaluated since it is well established that the left lobe is much more susceptible to image degradation due to cardiac and respiratory motion (10, 14). The ADC measurements were obtained with large free-form regions-of-interest drawn to include as much of the right lobe of the liver as possible while avoiding the edges of the liver. Large ROIs were used to permit greater liver signal averaging and potentially reduce variability due to local regional differences in ADC (15). A total of three large ROIs were drawn: at the level of the portal vein confluence and on the adjacent axial levels (12mm level above and below image centered on portal vein confluence) immediately above and below the portal vein confluence for the BH and FB sequences on anatomically matched locations in the same subjects [Figure 1]. All the ROIs were drawn with the same researcher with 2 years of experience in liver MR interpretation. The average ADC was calculated from these 3 large ROIs for BH and for FB sequences.
Figure 1.
57 year old woman with nonalcoholic fatty liver disease. The ADC map demonstrates placement of a large ROI to include as much of the right lobe as possible on an axial image including vessels but avoiding the liver periphery.
Statistical analysis
All statistical analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, NC). The mean and standard deviation for demographic characteristics (age and BMI) and average liver ADC values for BH and FB sequences were calculated for the total population and by liver disease status. Differences in demographic characteristics were assessed by one-way analysis of variance using the Student Newman Keuls test for multiple comparisons. The paired t-test was used to detect differences in ADC values obtained through BH and FB techniques. Average liver ADC values were compared between healthy volunteers and patients by t-test using the Student Newman Keuls test to address multiple comparisons. ADC values were normally distributed for each subgroup.
Correlation and agreement in BH and FB measurement techniques were evaluated for the different subject groups. Pearson’s correlation coefficient was used to assess the correlation between average liver ADC values measured on BH and FB sequences. The estimated bias (mean difference between BH and FB liver ADC measurements) and 95% confidence intervals (limits of agreement) were then calculated and plotted using the Bland-Altman method (16, 17). The significance of variation in liver ADC difference between BH and FB across liver disease status was evaluated using the Brown-Forsythe test. A p-value of <0.05 indicated statistical significance.
RESULTS
The study population was primarily female accounting for 42% of healthy volunteers, 89% of NAFLD patients and 100% of HCV patients. NAFLD (mean age 52, range 33–71) and HCV (mean age 51, range 42–59) patients were significantly older than healthy volunteers (mean age 32, range 22–58) (p<0.05). BMI was significantly higher among NAFLD patients (mean 31.7, range 23.6–46.6) compared to both healthy volunteers (mean 22.4, range 20.0–25.5) and HCV patients (mean 24.2, range 19.1–32.6) (p<0.05). Grade of steatosis in NAFLD subjects was 0 for 3, 1 for 3, 2 for 0 and 3 for 3 subjects. Ishak fibrosis stage in this group was 0 for 5, 1 for 0, 2 for 1, 3 for 2 and stage 4 for 1 subject.
The BH and FB average ADC values and their differences are shown in Table 1, Figure 2 and Figure 3. Liver ADC was significantly lower on BH compared to FB DWI for all groups with a mean difference of 0.36± 0.20 × 10−3 (p<0.01).
Table 1.
Average right lobe liver ADC (×10−3 mm2/sec) and ADC decrease (reduction) between FB and BH DWI.
| BMI mean±SD |
Avg ADC in BH mean±SD |
Avg ADC in FB mean±SD |
ADC Reduction mean±SD |
|
|---|---|---|---|---|
| Healthy [n=12] | 22.4± 1.7‡ | 1.80± 0.18† | 2.24± 0.20† | 0.43± 0.22* |
| All Patients [n=16] | 28.4± 7.96 | 1.52± 0.25 | 1.82± 0.22 | 0.30± 0.16* |
| NAFLDs [n=9] | 31.7± 8.1‡ | 1.43± 0.27† | 1.78± 0.28† | 0.35± 0.12* |
| HCVs [n=7] | 24.2± 5.8‡ | 1.63± 0.19 | 1.88± 0.12† | 0.24± 0.20* |
| Entire Population [n=28] | 25.9±6.7 | 1.64± 0.26 | 2.00± 0.30 | 0.36± 0.20* |
Body mass index (BMI), breath-hold (BH), free-breathing (FB), non-alcoholic fatty liver disease (NAFLD), chronic active hepatitis C (HCV),
denotes significant difference between NAFLD and other 2 groups (p<0.05),
denotes significant difference between Healthy volunteers and other groups (p< 0.01),
denotes significant difference between BH and FB (p<0.01).
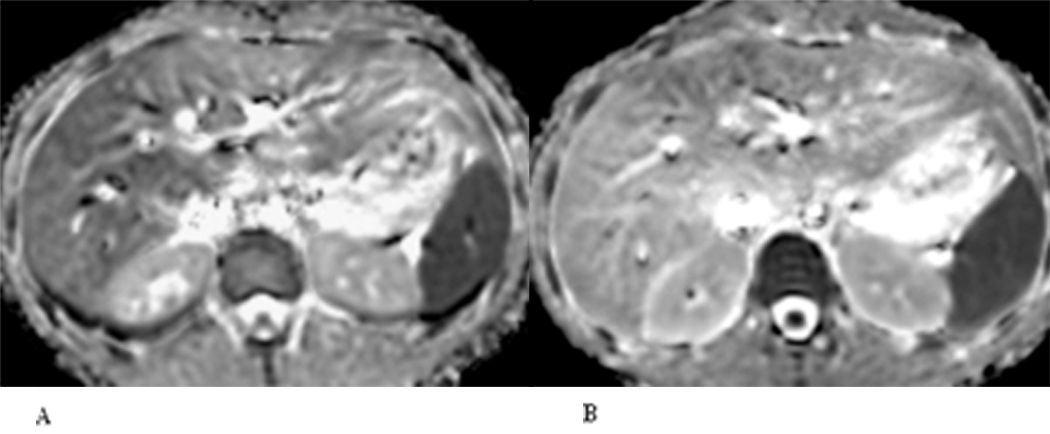
Figure 2.
24 year old healthy woman (healthy volunteer). A lower liver ADC value is reflected by the lower liver signal intensity on BH DWI (A) compared with FB DWI (B).
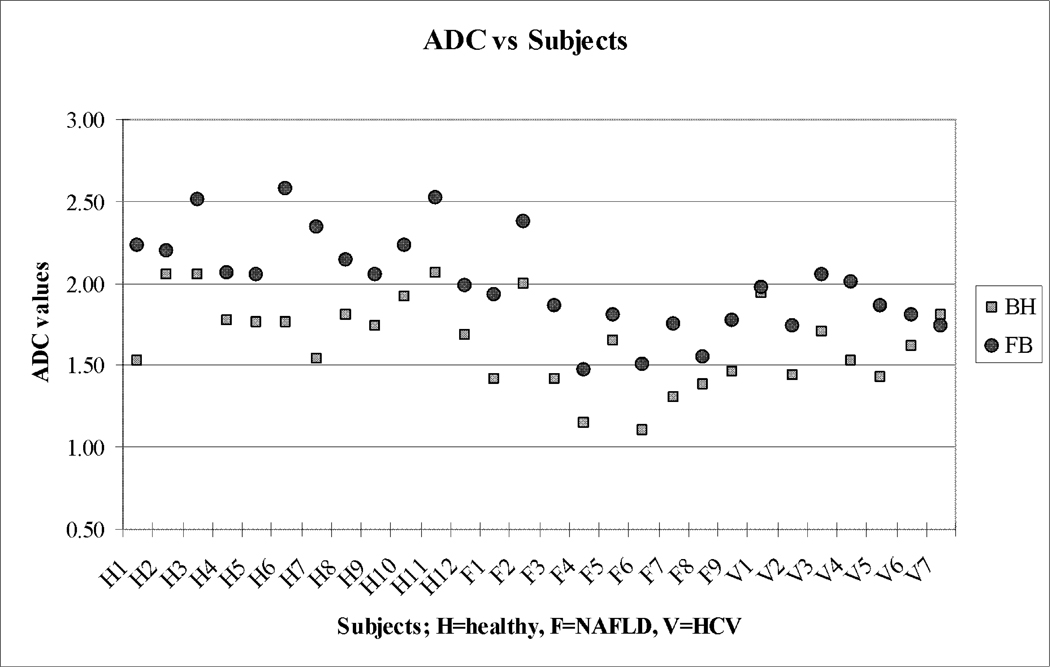
Figure 3.
Average right lobe liver ADC Values (×10−3 mm2/sec) of breath-hold compared to free breathing. ADC values were shown on Y axis and subjects on X axis. Note the FB ADCs are higher than the BH values for all but one case.
The liver ADC (×10−3 mm2/sec) was higher in healthy volunteers (BH 1.80±0.18; FB 2.24±0.20) compared to patients with NAFLD (BH 1.43±0.27; FB 1.78±0.28) (p<0.001), and HCV patients (BH 1.63±0.191; FB 1.88±0.12), although statistical significance was not attained with the HCV patient group.
A significant correlation between BH and FB liver ADC was detected for all study participants (r =0.75). However correlation in BH and FB measurements was strong for NAFLD patients (r =0.90) and weak and non-significant for both HCV patients (r =0.24) and healthy volunteers (r =0.34) (Table 2).
Table 2.
Pearson Correlation Coefficient of ADC values obtained with breath-hold and free-breathing DWI for different subject groups in the study.
| Entire Population [n=28] |
Volunteers [n=12] |
All Patients [n= 16] |
NAFLD [n=9] |
HCV [n=7] |
|
|---|---|---|---|---|---|
| Correlation Coefficients | 0.75 | 0.34 | 0.77 | 0.90 | 0.24 |
| P-value | <0.0001 | 0.272 | 0.0005 | 0.001 | 0.598 |
Non-alcoholic fatty liver disease (NAFLD), chronic active hepatitis C (HCV), region of interest (ROI)
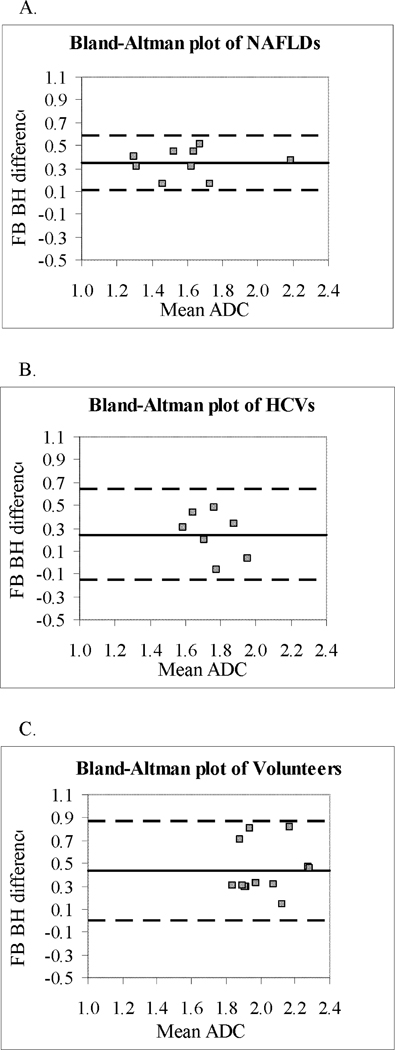
The Bland-Altman plots with 95% limits of agreement are shown for breath-hold and free-breathing average liver ADC differences in Figure 4. Bland-Altman plots demonstrate a lack of agreement in mean absolute difference between all groups. The differences in measurements obtained by BH and FB were significantly different from zero for NAFLD patients and healthy volunteers as demonstrated by 95% limits of agreement not including zero. The limits of agreement ranged between 0.11 to 0.59 in NAFLD patients, −0.15 to 0.64 in HCV patients, and 0.003 to 0.87 in healthy volunteers but significant differences in variance were not detected (p=0.58, Brown-Forsythe test).
Figure 4.
Bland-Altman plots of average liver ADC in (A) patients with NAFLD, (B) patients with HCV, and (C) healthy volunteers. The difference between the ADC with 2 breathing techniques (BH and FB) is plotted against the average of the ADC measurements made by the 2 breathing techniques. The central line represents the mean absolute difference between the two techniques, and the dashed lines represent the 95% limits of agreement. There is reduced scatter in NAFLD patients compared to healthy volunteers and compared to patients with HCV (not significant). ADC values on X and Y axis are (×10−3 mm2/sec).
DISCUSSION
Our study of BH and FB in healthy volunteers and patients with NAFLD and patients with HCV demonstrated several significant findings. First, BH ADC values were consistently and significantly lower than FB for all groups, p<0.01. Second, the correlation between BH and FB was highest and strong for NAFLD patients (0.90), but very weak and non-significant for HCV patients (0.24) and for healthy volunteers (0.34). Third, we observed a significantly lower liver ADC in patients with NAFLD compared to healthy volunteers (P<0.001), which may reflect underlying liver pathology as previously suggested in the literature (4–9). Limited research has been done to compare breathing techniques in DWI (18–21). These studies have focused primarily on the comparison of BH and respiratory triggered DWI in small numbers of healthy volunteers or patients with focal liver diseases. Furthermore, there is a lack of consensus with regard to which breathing technique is superior for DWI of the liver, which is compounded by technique and vendor related variation in ADC measurement. Although determining the superiority of FB versus BH DWI was beyond the scope of this study we wanted to establish if measurements of ADC from the two techniques were comparable and as such may be used interchangeably for patient follow-up. In our study, the mean liver ADC was 1.80± 0.18 × 10−3mm2/sec for healthy volunteers on BH DWI and was slightly higher compared to the BH ADC values reported by Kwee et al. (1.57–1.62 ×10−3mm2/sec) (18). There are a number of possible factors contributing to the difference in the ADC values in our study including our slightly higher b-value of 600 s/mm2 rather than 500 s/mm2, and a volume sampling difference from variation in slice thickness. In our study, we used a slice/gap of 10/2 mm compared to the thinner 7/1 mm used by Kwee et al (18), which may allow greater partial voluming with vessels. Other factors which may result in ADC differences include technical parameters related to different MRI scan manufacturers. While the impact of the combined different sequence and technical differences is not clearly understood, it does indicate the importance of such differences on ADC measurement.
Kwee et al. studied control subjects and (18) reported that respiratory triggered DWI was associated with significantly higher ADC in normal liver parenchyma but was less reproducible than FB or BH DWI. Furthermore, although ADC derived from FB and BH DWI was not found to be significantly different, the overall ADC values with FB DWI tended to be higher. Conversely, Kandpal et al. (19) reported greater signal to noise and accuracy of ADC with respiratory triggered DWI compared to BH imaging for detection of focal liver lesions. Nasu et al. (21) reported no significant difference in liver ADC obtained with FB and respiratory triggered DWI. Although there is a lack of consensus on the optimal DWI technique, there are advantages and disadvantages to both BH and FB DWI. The principal advantage of BH DWI is a short scan time while the principal advantage of FB DWI is a good signal to nose ratio, with the disadvantage of higher motion artifact (10). Respiratory triggered DWI is similar to FB DWI from the perspective of a long scan time but with reduced variation in the region imaged and in motion artifact. A direct comparison of BH to FB DWI has not previously been formally assessed.
In our study, BH ADC values were consistently lower than FB ADC values for all except one subject. A possible explanation for higher liver ADC values with FB compared to BH DWI may be signal contribution by adjacent vessels or other tissues as a consequence of respiratory motion between the different acquisitions. The effect of cardiac motion on liver DWI is recognized and is far more pronounced in the left than the right lobe of the liver (10, 14). Kwee et al (14), reported signal decrease ratios between systole and diastole of 25.5% in the left lobe and 17.3% in the right lobe in 3 volunteers. Nasu et al (21, 22) described pseudo-anisotropy artifact when using respiratory triggered DWI, which the authors hypothesize to originate in localized hepatic movement, such as extension, contraction and rotation. Although pseudo-anisotropy was observed in patients with and without cirrhosis, it was more often found to be present in patients without cirrhosis and was associated with higher ADC. As such, the authors suggest that respiratory and cardiac motion is more likely to result in motion artifact in a less rigid or non-cirrhotic liver and consequent higher liver ADC. While we used breath-hold and free-breathing rather than respiratory triggered DWI, the principle of pseudo-anisotropy artifact may have contributed to the higher ADC observed with free-breathing DWI and may also be relevant to the higher ADC observed in healthy volunteers. We observed a moderate overall correlation between BH and FB ADC values (0.77), which is concordant with prior studies comparing other breathing techniques for DWI (10, 15, 18–20) and supporting the notion that liver ADC obtained with different breathing techniques varies depending on the technique and should not be interchanged. We also observed the highest correlation of BH with FB ADC in NAFLD subjects (0.90). A possible explanation for such a difference in BH and FB ADC correlation between the subject groups may be related to respiratory effort or pseudo-anisotropy, particularly since BH and FB ADC differences were most disparate between healthy volunteers and patients with NAFLD. A further explanation, may be related to a reduced pulmonary function observed in over-weight and obese individuals (23, 24), and the patients with NAFLD in this study had significantly higher BMI than the healthy controls and HCV patients (p<0.05, Student Newman Keuls). Thus these NAFLD subjects may demonstrate less diaphragmatic and consequent liver motion with respiration compared to healthy or non-obese subjects, which would result in a greater approximation of liver sampling on FB sequences and less partial voluming of vessels. It is also possible that differences in hepatic venous flow pattern between healthy subjects and patients may affect the correlation of BH ADC to FB ADC. With progression of fibrosis, hepatic venous flow decreases (which has been reported in studies using Doppler sonography to assess hepatic blood flow) (25–30). Hagiwara et al (30), reported significant differences for all perfusion MR imaging-estimated parameters in patients with advanced liver fibrosis, except for absolute portal venous blood flow and absolute total liver perfusion; a significant increase in absolute arterial blood flow, arterial fraction, distribution volume, mean transit time and significant decrease in portal venous fraction was observed . Since DWI is influenced by perfusion, the averaged signal associated with FB DWI may be reduced due to such a decrease in blood flow. Also, the FB ADC may be influenced by the affects of altered blood flow patterns in the liver with respect to respiration (inspiration increases flow velocity in portal and hepatic vein in healthy subjects) (29), such that a dampened alteration in hepatic venous blood flow with respect to respiration in patients with chronic liver disease may result in a reduced difference between BH and FB DWI. When considering causal effects of ADC differences between sequences, it is important to consider the impact of the sequence parameters. In a study of 35 patients and 20 volunteers, Shiehmorteza et al, (13), reported that shot number in a SE EPI sequence may influence ADC, such that the application of a “dummy” b=0 scan or scans may need to be acquired to attain a steady-state prior to further DWI acquisitions. Our data was acquired at 1.5T whereas the data from Shiehmorteza et al, (13) was acquired at 3T with its concomitant higher T1 and greater difference between fully relaxed signals and steady-state signals. Also, our data was acquired with three signal averages and one dummy scan (BH) or six signal averages (FB) compared to their single acquisition. Additionally, we scanned one subject with multiple b=0 acquisitions following the same protocol as the FB and BH diffusion sequences. Comparison of the signal intensity across the timetime points demonstrated that the signals were less than 3% different. Thus, our data likely suffers little effect from the potential lack of being in steady-state. Even with this limitation, we found a significantly higher ADC with FB than BH acquisitions. The incremental benefit of normalization techniques including comparison of other organs (spleen or muscle) relative to liver also requires investigation.
Some limitations of the present study include: first, the number of subjects in the NAFLD and HCV patient groups were relatively small, which may account for the lack of significance when comparing healthy subjects with HCV patients. However, we still observed a significantly lower liver ADC in the patients with NAFLD compared to healthy volunteers, and the purpose of our study was to assess ADC measurements with respect to BH and FB techniques across different subject types rather than assess severity or presence of liver disease. Second, most of the patients were women; the direction of any potential bias this may incur is unclear. Third, healthy subjects were younger than other groups; and the lower correlation between BH and FB ADC may reflect a difference in respiratory effort, although the correlation between techniques was also low for the HCV group of older age. Fourth, we acquired diffusion images with only two b-values (0s/mm2 and 600s/mm2). While two b values are generally accepted as a minimum requirement for ADC calculation recent advances in technology have permitted generation of ADC maps using three or more b values which may help elucidate the importance of micro-circulation changes in liver ADC. Fifth, the FB sequence was obtained using six diffusion-encoding directions while the BH used only three. Early data of ours used six directions for calculating an ADC and this was continued in the current study for comparison purposes. The BH sequence did not include 6 directions due to time and signal to noise constraints. Previous DTI studies of the liver have shown the ADC to be isotropic (15, 28). Therefore the three additional diffusion-encoding directions were deemed to have similar impact as having an additional acquisition, increasing the signal to noise of the data. Sixth, no pre-pulse or dummy scans were acquired in the FB acquisition to ensure steady-state. As the TR was long (6 sec) and there were 6 acquisitions averaged, and as data from one individual did not show increased signal in the first b=0 acquisition versus a second b=0 acquisition, this effect is likely minimal in this study. Seventh, in this study respiratory triggered technique was not investigated and compared to BH and FB.
In conclusion, the correlation between BH and FB liver ADC is moderate at best such that BH and FB DWI should not being used interchangeably. Additionally, the lower ADC values in BH versus FB DWI should be accounted for when comparing different liver DWI studies.
ACKNOWLEDGEMENT
The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Grant Support: Funding from grants: NIH: K23 AI-66943, UO1 AI-034989, MO1-RR-00083, and R01 DK061738-SI (NMB) and R01 DK074718-01 and a UCSF Hellman Family Award.
REFERENCES
- 1.Clark JM, Diehl AM. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124:248–250. doi: 10.1053/gast.2003.50032. [DOI] [PubMed] [Google Scholar]
- 2.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 4.Amano Y, Kumazaki T, Ishihara M. Single-shot diffusion-weighted echo-planar imaging of normal and cirrhotic liver using a phased-array multicoil. Acta Radiol. 1998;39:440–442. doi: 10.1080/02841859809172460. [DOI] [PubMed] [Google Scholar]
- 5.Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008;28:89–95. doi: 10.1002/jmri.21227. [DOI] [PubMed] [Google Scholar]
- 6.Girometti R, Furlan A, Bazzocchi M, et al. Diffusion-weighted MRI in evaluating liver fibrosis; a feasibility study in cirrhotic patients. Radiol Med (Torino) 2007;112:394–408. doi: 10.1007/s11547-007-0149-1. [DOI] [PubMed] [Google Scholar]
- 7.Taouli B, Tolia AJ, Losada M, et al. Diffusion-weighted MRI for quantification for liver fibrosis: preliminary experience. AJR. Am J Roentgenol. 2007;189:799–806. doi: 10.2214/AJR.07.2086. [DOI] [PubMed] [Google Scholar]
- 8.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with single-shot echo-planar imaging in the upper abdomen: preliminary clinical experience in 61 patients. Abdom Imaging. 1999;24:456–461. doi: 10.1007/s002619900539. [DOI] [PubMed] [Google Scholar]
- 9.Zhou ML, Yan FH, Xu PJ, et al. Comparative study on clinical and pathological changes of liver fibrosis with diffusion-weighted imaging. Zhonghua Yi Xue Za Zhi. 2009;89(7):1757–1761. [PubMed] [Google Scholar]
- 10.Naganawa S, Kawai H, Fukatsu H, et al. Diffusion-weighted imaging of the liver: technical challenges and prospects for the future. Magn Reson Med Sci. 2005;4:175–186. doi: 10.2463/mrms.4.175. [DOI] [PubMed] [Google Scholar]
- 11.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 13.Shiehmorteza M, Sirlin CB, Wolfson T, et al. Effect of shot number on the calculated apparent diffusion coefficient in phantoms and in human liver in diffusion-weighted echo-planar imaging. J Magn Reson Imaging. 2009;30:547–553. doi: 10.1002/jmri.21861. [DOI] [PubMed] [Google Scholar]
- 14.Kwee TC, Takahara T, Niwa T, Ivancevic MK, Herigault G, Van Cauteren M, Luijten PR. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. MAGMA. 2009 Oct;22(5):319–325. doi: 10.1007/s10334-009-0183-1. [DOI] [PubMed] [Google Scholar]
- 15.Colagrande S, Pasquinelli F, Mazzoni LN, Belli G, Virgili G. MR-diffusion weighted imaging of healthy liver parenchyma: repeatability and reproducibility of apparent diffusion coefficient measurement. J Magn Reson Imaging. 2010;31(4):912–920. doi: 10.1002/jmri.22117. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8(1):307–310. [PubMed] [Google Scholar]
- 17.Ludbrook J. Statistical technique for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol. 2002;29:527–536. doi: 10.1046/j.1440-1681.2002.03686.x. [DOI] [PubMed] [Google Scholar]
- 18.Kwee T, Takahara T, Koh DM, Nievelstein R, Luijten P. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging. 2008;28:1141–1148. doi: 10.1002/jmri.21569. [DOI] [PubMed] [Google Scholar]
- 19.Kandpal H, Sharma R, Madhusudhan KS, Singh Kapoor K. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: Comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol. 2009;192:915–922. doi: 10.2214/AJR.08.1260. [DOI] [PubMed] [Google Scholar]
- 20.Taouli B, Sandberg A, Stemmer A, et al. Diffusion-weighted imaging of the liver: Comparison of navigator triggered and breathhold acquisitions. Magn Reson Imaging. 2009;30:561. doi: 10.1002/jmri.21876. [DOI] [PubMed] [Google Scholar]
- 21.Nasu K, Kuroki Y, Sekiguchi R, Nawano S. The effect of simultaneous use of respiratory triggering in diffusion weighted imaging of the liver. Magn Reson Med Sci. 2006;5:129–136. doi: 10.2463/mrms.5.129. [DOI] [PubMed] [Google Scholar]
- 22.Nasu K, Kuroki Y, Fujii H, Minami M. Hepatic pseudo-anisotropy: a specific artifact in hepatic diffusion-weighted images obtained with respiratory triggering. MAGMA. 2007 Oct;20(4):205–211. doi: 10.1007/s10334-007-0084-0. [DOI] [PubMed] [Google Scholar]
- 23.Saxena Y, Sidhwani G, Upmanyu R. Abdominal obesity and pulmonary functions in young Indian adults: a prospective study. Indian J Physiol Pharmacol. 2009;53:318–326. [PubMed] [Google Scholar]
- 24.Piper AJ, Grunstein RR. Big breathing: the complex interaction of obesity, hypoventilation, weight loss, and respiratory function. J Appl Physiol. 2010;108:199–205. doi: 10.1152/japplphysiol.00713.2009. [DOI] [PubMed] [Google Scholar]
- 25.Zekanovic D, Ljubicic N, Boban M, et al. Doppler ultrasound of hepatic and system hemodynamics in patients with alcoholic liver cirrhosis. Dig Dic Sci. 2010;55:458–466. doi: 10.1007/s10620-009-0760-1. [DOI] [PubMed] [Google Scholar]
- 26.Bolognesi M, Sacerdoti D, Mescoli C, et al. Different hemodynamic patterns of alcoholic and viral endstage cirrhosis: analysis of explanted liver weight, degree of fibrosis and splanchnic Doppler parameters. Scand J Gastroentrol. 2007;42:256–262. doi: 10.1080/00365520600880914. [DOI] [PubMed] [Google Scholar]
- 27.Iwao T, Toyonaga A, Oho K, et al. Value of Doppler ultrasound parameters of portal vein and hepatic artery in the diagnosis of cirrhosis and portal hypertension. Am J Gastroenterol. 1997;92:1012–1017. [PubMed] [Google Scholar]
- 28.Kok T, Van Der Jagt EJ, Haagsma EB, et al. value of Doppler ultrasound in cirrhosis and portal hypertension. Scand J Gastroentrol Suppl. 1999;230:82–88. doi: 10.1080/003655299750025598. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovici N, Navot N. The relationship between respiration, pressure and flow distribution in the vena cava and portal and hepatic veins. Surg Gynecol Obstet. 1980;151:753–763. [PubMed] [Google Scholar]
- 30.Hagiwara M, Rusinek H, Lee VS, Losada M, Bannan MA, Krinsky GA, Taouli B. Advanced liver fibrosis: diagnosis with 3D whole-liver perfusion MR imaging--initial experience Radiology. 2008;246(3):926–934. doi: 10.1148/radiol.2463070077. [DOI] [PubMed] [Google Scholar]