Abstract
Objective
Tenascin (TN)-C is an extracellular matrix protein associated with injury and remodeling. Since Transforming Growth Factor (TGF)-β induces both TN-C and Insulin-Like Growth Factor Binding Protein (IGFBP)-3, we sought to determine the role of IGFBP-3 in mediating TGF-β’s effects on TN-C production and to assess the levels of TN-C in vivo in SSc-associated pulmonary fibrosis (PF).
Methods
Primary human lung fibroblasts were stimulated with TGF-β or IGFBP-3 in the presence or absence of specific siRNAs and chemical signaling cascade inhibitors. TN-C levels were examined in lung tissues of patients with Systemic Sclerosis (SSc)-associated pulmonary fibrosis using immunohistochemistry (IHC) and compared to those of normal donors. TN-C levels were quantified in serum from normal donors and patients with SSc with or without PF using ELISA.
Results
IGFBP-3 mediated TGF-β induction of TN-C. Direct induction of TN-C by IGFBP-3 occurred in a p38K-dependent manner. TN-C levels were abundant in SSc lung tissues and localized to subepithelial layers of the distal airways. No TN-C was detectable around proximal airways. Patients with SSc-associated pulmonary fibrosis had significantly greater levels of circulating TN-C compared to patients without this complication. Longitudinal samples obtained from patients with SSc before and after the onset of PF showed increased levels post-PF.
Conclusion
IGFBP-3, which is overexpressed in fibrotic lungs, induces production of TN-C by subepithelial fibroblasts. The increased lung tissue levels of TN-C parallel levels detected in sera of patients with SSc and lung fibrosis, suggesting that TN-C may be a useful biomarker for SSc-PF.
Introduction
TN-C, also called Hexabrachion, is an extracellular matrix glycoprotein with key functions in cell adhesion, fibroblast migration, and other processes related to tissue remodeling and wound healing (1,2,3). Although minimal levels of TN-C are observed in normal adult life, higher levels are seen under pathologic conditions such as certain cancers. Initially identified as myotendinous antigen in chicks, TN-C is the initial representative of the five-membered tenascin family of extracellular matrix (ECM) glycoproteins. Expression of TN-C is reportedly highest during embryogenesis. During neural development, TN-C is produced by glial and Schwann cells, and outside the nervous system it is abundantly expressed in the developing skeleton, vasculature, and connective tissues (4). In adults, TN-C expression is significantly reduced. Under normal non-pathologic conditions, induction of TN-C is associated with tissue regeneration and remodeling processes, particularly wound healing (1). In dermal fibroblasts, TN-C regulates cell migration in response to injury (2).
In vivo studies using mouse models have demonstrated an increase in TN-C mRNA in response to injury of lung airway epithelium. This increase is followed by a decrease to steady state levels after epithelial restoration. However, in cases of abortive repair, there is accumulation of TN-C in the sub-epithelial regions of airways (3). This suggests a role of TN-C in ECM remodeling, a hallmark of fibrogenesis. In another study where bleomycin was used to induce pulmonary fibrosis in rats, TN-C was detected 3 days after bleomycin administration and was restricted to areas of tissue inflammation (5). This and other findings suggest that TN-C is an early response ECM molecule implicated in pulmonary fibrotic disorders.
SSc is a connective tissue disease of unknown etiology characterized by organ fibrosis. Lung involvement in SSc is currently the leading cause of death in patients with this disease (6). Research on the pathogenesis of lung fibrosis in SSc has been hampered by the limited availability of lung tissues. We had previously reported increased levels of IGFBP-3 in fibrotic lungs (7). Our goal was to characterize the levels and localization of TN-C in SSc lungs and its regulation by IGFBP-3 in primary fibroblasts derived from these lung tissues. We also sought to determine whether IGFBP-3 mediates the effects of TGF-β, a potent inducer of fibrosis.
Materials and Methods
Tissues and Cells
Lung tissues were obtained from patients with SSc undergoing lung transplantation at the University of Pittsburgh Medical Center. All patients had a physician-confirmed diagnosis of SSc and met the American College of Rheumatology criteria for the diagnosis of SSc (8). Normal lung tissues were obtained from organ donors whose lungs were not used for transplant surgery. Consent was obtained using a protocol approved by the University of Pittsburgh Institutional Review Board. Primary fibroblasts were cultured from lung tissues and maintained in Dulbecco’s Modified Eagles Medium (DMEM) (Mediatech Inc. Manassas, VA) supplemented with 10% Fetal Bovine Serum (FBS) and 1% antibiotic-antimycotic (Invitrogen Life Technologies) as previously reported (7).
Fibroblast Stimulation
Actively growing human primary lung fibroblasts in early passage (P3–P5) were plated at a density of 2×105 cells per well in 6-well culture plates. After 24hrs, the cells were serum-starved in DMEM for 12–16 hours prior to stimulation with human recombinant IGFBP-3 (R and D Systems Inc., Minneapolis, MN) at a final concentration of 750ng/ml for the indicated time points. Control wells were treated with PBS. Conditioned media and lysates were harvested and evaluated for TN-C production using western blot analysis.
IGFBP-3 Silencing
Primary lung fibroblasts were plated at a density of 2×105 cells as described above. After 24hrs, the cells were transfected with IGFBP-3 siRNA, IGFBP-5 siRNA or a control (non-specific scrambled) siRNA (Ambion Inc. Austin, TX) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and following the manufacturer’s recommendation. At 18hrs post-transfection, the medium on the cells was switched to DMEM/ 0.5% FBS/1% antibiotic/anti-mycotic for 3hrs and stimulated with TGF-β for 48hrs at a concentration of 10ng/ml. Conditioned media and lysates were harvested and evaluated for TN-C and collagen type I by western blot.
MAP Kinase Inhibition
In order to delineate the signaling cascades that are involved in TN-C induction, we blocked the activation of MAP kinases using chemical inhibitors. Cells were plated at a density of 2×105 cells per well in 6-well culture plates. Inhibitors specific for p38 mitogen activated protein kinase (p38Ki; SB 203580), c-jun N-terminal kinase (JNKi; JNK Inhibitor II), phosphoinositide-3 kinase (PI3Ki; LY 294002) (Calbiochem/EMD Biosciences, La Jolla, CA) or the MAP kinase kinase (MEKi; U0126) (Cell Signaling, Danvers, MA) were added to cells under serum-free conditions, followed by stimulation with IGFBP-3 for 3hrs or TGF-β for 48hrs. Conditioned media and lysates were harvested and evaluated for TN-C and collagen via western blot.
Western Blot Analysis
Immunoblotting was done as previously described (7).
Antibodies used were anti-human Tenascin antibody (BC-24, Sigma-Aldrich, St. Louis MO), anti-IGFBP-3 and anti-collagen type I (Santa Cruz Biotech. Inc., Santa Cruz, CA), or anti-alpha tubulin (Abcam Inc., Cambridge, MA) as primary antibodies and horseradish peroxidase-linked anti-mouse (GE Healthcare Ltd., Little Chalfont, Buckinghamshire UK) or anti-goat (Santa Cruz Biotech. Inc., Santa Cruz, CA) antibody as a secondary antibody. Signals were detected using chemiluminescence (Perkin Elmer, Shelton, CT).
Immunohistochemistry
Lung tissue sections (6μm) were deparaffinized in xylenes and rehydrated in graded solutions of ethanol. Antigens were retrieved using 1mM EDTA. Immersing the sections in a solution of 3% hydrogen peroxide in methanol quenched endogenous peroxidases. Non-specific interactions were blocked using 5% goat serum/ 4% BSA in PBS. Sections were treated with a 1:100 dilution of anti-human TN-C antibody (Sigma, BC-24), followed by a biotinylated anti-mouse secondary antibody (GE Healthcare Ltd., Little Chalfont, Buckinghamshire UK). Signals were detected by a peroxidase /chromogen reaction. The sections were incubated with biotinylated horseradish peroxidase (Vectastain ABC Kit, Vector Labs Inc., Burlingame CA) followed by a chromogen substrate (Aminoethylcarbazole (AEC) Substrate Kit), (Zymed, San Francisco CA). Hematoxylin (Vector Labs) was used as a counterstain. Sections were mounted using an aqueous (Crystal Mount) mounting medium (Biomeda, Foster City CA). Sections were imaged on a Nikon Eclipse 800 microscope (Nikon Instruments, Inc., Huntley, IL) under identical camera settings.
Immunofluorescence
Lung tissue sections were treated as described above, except that non-specific interactions were blocked with 10%BSA in PBS. The following antibodies were used: anti-human IFGBP-3 (Gropep Ltd., Thebarton SA 5031, Australia), anti-human wide spectrum cytokeratin antibody (Abcam Inc., Cambridge, MA), and anti-human TN-C (Sigma, clone BC-24), fluorescently tagged (Alexa) anti-rabbit or anti-mouse secondary antibody (Invitrogen, Molecular Probes, Eugene, OR). Control sections were incubated with rabbit immunoglobulin G at a concentration of 1ug/ml as a negative isotype control (GE Healthcare Ltd., Little Chalfont, Buckinghamshire UK). Nuclei were detected using Hoechst nuclear stain (Sigma-Aldrich, St. Louis MO). Slides were mounted using an aqueous (Gel Mount) mounting medium (Biomeda, Foster City, CA). Images were captured using an Olympus Provis light microscope (Olympus America Inc., Melville, NY)
Measurement of TN-C in Human Serum
Sera from patients diagnosed with SSc were stored at −80°C. Serum samples from 62 SSc patients were evaluated. All had serum autoantibodies recognized to be associated with pulmonary fibrosis (PF): anti-topoisomerase I, anti-Th/To, or anti-U11/U12 RNP (9). Thirty-one (50%) had PF at some time during their disease course as confirmed by chest radiograph or high resolution CT scan of the lungs, and 31 had no evidence of PF (No PF). Among the latter, follow up after the date of the serum sample averaged 14.6 ± 6.0 years. The mean disease duration in years in these two groups of SSc patients, from the first symptom attributable to SSc to the time their blood sample was obtained, was 8.2 ± 7.3 for PF patients and 6.5 ± 7.5 for the no-PF patients (N.S.) An additional four patients who had paired serum samples pre-dating a diagnosis of PF (average 6.45 years, range 3.0–11.8 years) and after diagnosis of PF (average 6.43 years, range 0.1–10.6 years) were analyzed to evaluate for individual trends in TN-C serum levels. These serum samples were not obtained from the patients with end-stage disease who provided lung tissue samples at the time of lung transplantation. Instead, they represent a cross-section of SSc patients seen in our outpatient Scleroderma Clinic. Sera from 10 healthy donors were used as controls. Levels of TN-C in sera were quantified using an ELISA kit that detects the high molecular weight variant of TN-C (Immuno-Biological Labs. Inc., Gunma Japan).
Statistical Analysis
The student’s t-test and Wilcoxon test were used where appropriate.
Results
Silencing IGFBP-3 Blocks TGF-β-induced TN-C
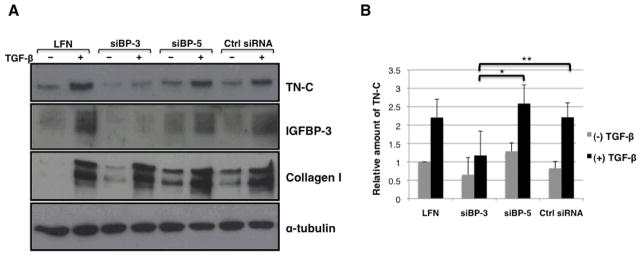
We had previously reported that that TGF-β directly induces IGFBP-3 production in primary lung fibroblasts (7). We therefore sought to determine whether IGFBP-3 mediates the effects of TGF-β on ECM production and TN-C in primary lung fibroblasts. To determine whether TN-C induction by TGF-β is mediated by and requires IGFBP-3, normal primary lung fibroblasts were transfected with IGFBP-3 siRNA and then stimulated with human recombinant TGF-β for 48hrs. SiRNA specific for IGFBP-5 was used as a related protein control siRNA. Conditioned media were evaluated for TN-C expression via western blot. Cell lysates were also collected and anti-alpha tubulin antibody was used as an internal control. Figure1A shows that cells treated with lipofectamine only showed baseline expression of TN-C. IGFBP-3 silencing also resulted in a clear reduction of TGF-β-induced IGFBP-3 production. Silencing IGFBP-3 inhibited TGF-β-induced production of TN-C, but had no effect on TGF-β-induced collagen levels suggesting that IGFBP-3 mediates TGF-β-induction of TN-C and that TGF-β induction of collagen occurs independently of IGFBP-3. Figure1B is a graphical representation of our data showing that silencing of IGFBP-3 results in a 55% reduction in TGF-β-stimulated TN-C secretion when compared to silencing of IGFBP-5 (siBP-5) and a 47% reduction when compared to a control non-specific siRNA (Control siRNA). The amount of secreted TN-C is reduced to baseline levels upon silencing IGFBP-3, even with TGF-β stimulation. The modulation of Tenascin-C protein levels is not paralleled by changes in steady-state mRNA levels (data not shown).
Figure 1.
A. IGFBP-3 siRNA (siBP-3) blocks TGF-β-induced TN-C expression in primary human fibroblasts. Primary lung fibroblasts in passage 3–5 were transfected with siRNA with or without subsequent stimulation with TGF-β. Conditioned media were collected after 48hrs and evaluated for TN-C expression by western blot (n = 3). IGFBP-3 siRNA efficiently and specifically silenced IGFBP-3 and showed no effect on TGF-β-induced collagen expression (LFN = lipofectamine). B. Graphical presentation of data shown in panel A (TN-C). Data are shown as ratios to baseline TN-C expression. * p = 0.004; ** p = 0.011.
Induction of TN-C in Primary Fibroblasts
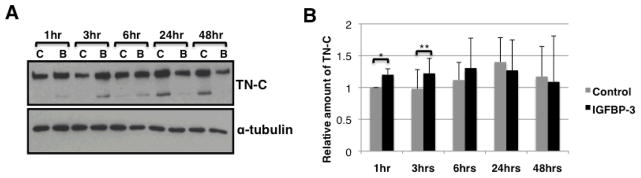
We then sought to determine whether IGFBP-3 could induce TN-C independently of TGF-β. Normal primary lung fibroblasts were stimulated with human recombinant IGFBP-3. Conditioned media were collected at the indicated time points and evaluated for TN-C protein levels using western blot. TN-C levels increased up to 6 hrs post-stimulation (Figure 2A). IGFBP-3-induced production of TN-C peaked at 3 hrs post-stimulation. Data were reproduced using primary lung fibroblasts from four different donors. Graphical presentation of these results (Figure 2B) shows that IGFBP-3 stimulation results in a statistically significant increase in TN-C secretion up to 3 hrs, suggesting that IGFBP-3 induces TN-C production independently of TGF-β. TN-C steady state mRNA levels remained unchanged under all treatment conditions (data not shown).
Figure 2.
A. Recombinant IGFBP-3 increases in TN-C secretion by normal fibroblasts. Normal primary lung fibroblasts in passages 3–5 were serum-starved overnight then stimulated with vehicle control (‘C’) or human recombinant IGFBP-3 (‘B’) at a concentration of 750ng/ml for the indicated time points. Conditioned media were collected and evaluated for TN-C levels using western blot. B. Graphical representation of data in panel A. Data are shown as ratios to baseline TN-C expression. * p = 0.03; ** p = 0.04.
Signaling Pathways Mediating TN-C Induction
Since IGFBP-3 induces TN-C production, we sought to indentify the signaling pathways mediating the inducing effects. We also determined whether TN-C induction by IGFBP-3 and TGF-β involve overlapping signaling pathways. Normal primary lung fibroblasts were stimulated with IGFBP-3 or TGF-β in the presence or absence of selected chemical pathway inhibitors. TN-C levels were examined by western blot. Levels of another ECM protein, collagen type I, were assessed for comparison. Figure 3 shows that addition of the JNK inhibitor significantly blocked TGF-β-induced TN-C protein levels. No significant effects on TN-C mRNA levels were observed with any of the signaling cascade chemical inhibitors (data not shown). Treatment with P38K inhibitor reduced both IGFBP-3 and TGF-β-mediated induction of TN-C, suggesting that the P38 MAP kinase pathway is a convergent pathway shared by both IGFBP-3 and TGF-β for the induction of TN-C. In contrast, collagen induction by both IGFBP-3 and TGF-β was blocked following inhibition of JNK and MEK (Figure 3). This identifies MEK as an alternate signaling cascade involved in regulation of another ECM component by both IGFBP-3 and TGF-β.
Figure 3.
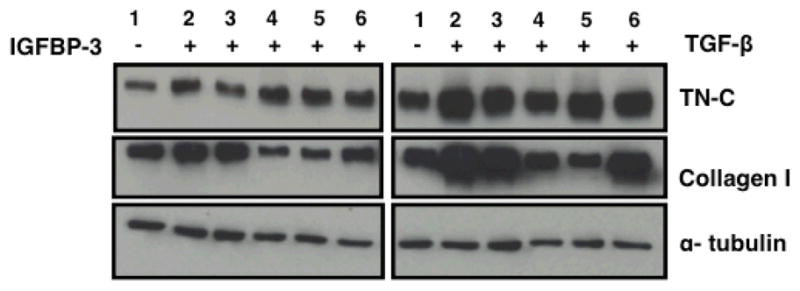
Inhibition of the p38 MAP kinase pathway blocks IGFBP-3 induction of TN-C. Normal lung fibroblasts in passage 5 were serum starved overnight, then treated with the indicated pathway inhibitors followed by IGFBP-3 (left panel) or TGF-β(right panel). Cellular lysates were harvested and used in western blot. Lanes 1 & 2: vehicle; Lane 3: p38Ki; Lane 4: JNKi; Lane 5: MEKi; Lane 6: PI3Ki. The p38 kinase inhibitor (p38ki) caused a decrease in IGFBP-3 induction of TN-C. TGF-β induction of TN-C was reduced by inhibitors of the JNK and p38K inhibitors. JNKi, MEKi, and PI3Ki, but not p38Ki, caused a reduction in IGFBP-3-induced collagen expression.
TN-C is Increased in vivo in SSc-Associated Lung Fibrosis
We had previously reported increased levels of IGFBP-3 in fibrotic lung tissues (7). Having shown that IGFBP-3 induces TN-C production in vitro, we then examined in vivo TN-C levels in normal and fibrotic lung tissues using IHC. A distinct pattern for TN-C expression was observed in fibrotic lung sections (Figure 4A). TN-C was abundant around airways of SSc lungs with a subepithelial distribution, whereas TN-C levels in normal lung tissues were weakly detectable and relatively homogeneous in distribution. The level and localization pattern of TN-C in fibrotic lungs were compared to those of fibronectin, an ECM molecule whose expression is increased in fibrotic tissues, using serial sections. Fibronectin expression appeared uniform throughout the lung tissues, but TN-C expression was confined to subepithelial regions of airways and largely undetected in other areas (Figure 4B). To determine if the increased subepithelial expression of TN-C is restricted to smaller distal airways, we examined TN-C levels in proximal airways from the same individuals. Figure 4C shows that TN-C is not detectable in proximal airways of normal donors or patients with SSc, suggesting that its abnormal expression is restricted to distal airways.
Figure 4.
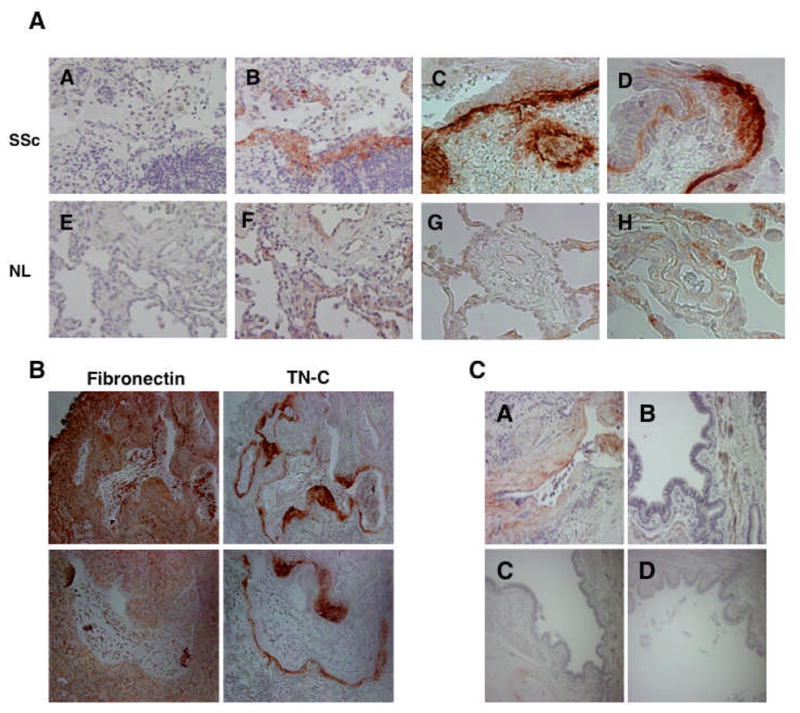
A. Immunohistochemical detection of TN-C in lung tissues. TN-C is abundant in regions underlying epithelial linings in fibrotic systemic sclerosis (SSc) lungs (panels A–D) compared to normal lung (NL) (panels E–H). Mouse isotype antibody control (panels A & E); TN-C (panels B–D and F–H). Images were taken at 200X (panels A–C and E–G) and 400X (panels D & H) magnification. TN-C levels and distribution were examined in 4 independent SSc and 4 independent normal donor samples. B. Comparison of TN-C and Fibronectin distribution in serial SSc lung sections. Fibronectin is ubiquitously detected in SSc lungs, while TN-C is localized to subepithelial layers and fibroblastic foci. Upper panels, 100X magnification. Lower panels; 200X magnification. C. TN-C is abundant around small distal airways of SSc lung (A) as compared to large proximal airways (B and C). TN-C expression in proximal airways of SSc lung is comparable to that seen in normal lung proximal airways (D). A & B, 200x magnification; C & D; 100x magnification.
Localization of TN-C and IGFBP-3 in Fibrotic Lungs
Since both IGFBP-3 and TN-C are overexpressed in fibrotic lungs, we used double immunofluorescence to determine if the two molecules are co-localized. A wide spectrum anti-human cytokeratin antibody was used to identify epithelial cells. TN-C was detected in regions immediately adjacent to cytokeratin suggesting expression by fibroblasts adjacent to epithelial cells (Figure 5A). TN-C and IGFBP-3 showed no co-localization in lung tissues. Airway epithelial cells expressed IGFBP-3 whereas TN-C was detected in subepithelial layers (Figures 5B & 5C). Furthermore, TN-C was expressed in fibroblastic foci-like areas in SSc lung tissues (Figure 5A, white arrow).
Figure 5.
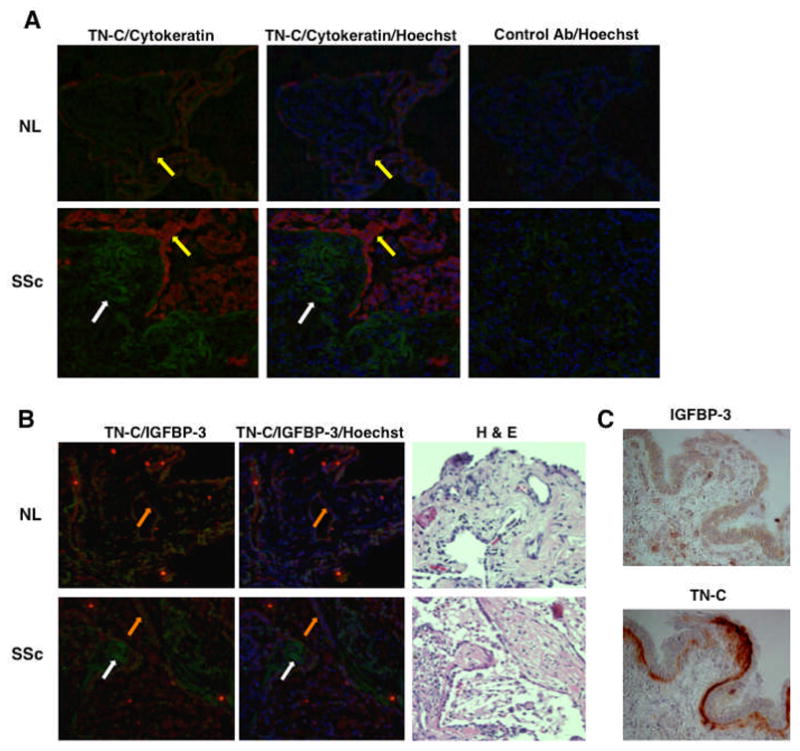
A. Immunofluorescent localization of TN-C. TN-C (green color; white arrows) is abundant in areas adjacent to and underlying epithelial cells identified by wide-spectrum cytokeratin (red color; yellow arrows). Mouse and rabbit isotype antibodies were used as controls (left panels). Hoechst was used to detect nuclei. B. Immunofluorescent localization of IGFBP-3. TN-C (green color; white arrows) is abundant in regions underlining IGFBP-3-expressing epithelial cells (red color; orange arrows). There is no detectable co-localization of TN-C and IGFBP-3. Hoechst was used to detect nuclei. H & E stained serial sections are shown in the left panels. C. Immunohistochemical detection of IGFBP-3 and Tenascin-C in serial sections of lung tissues from a patient with SSc.
Increased Serum Levels of TN-C in SSc Patients with Pulmonary Fibrosis
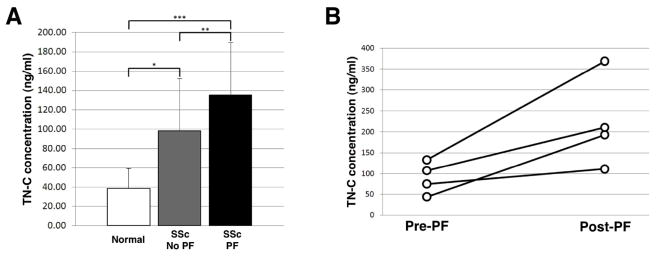
To determine if increased lung tissue levels of TN-C reflect circulating levels of the protein, we measured TN-C levels in sera from patients with SSc with and without pulmonary fibrosis and compared them to levels in sera of controls. TN-C levels in control sera were dramatically lower than those of SSc patients (Figure 6A). The mean serum TN-C level in SSc patients with pulmonary fibrosis was significantly higher (133.0 ± 55.4 ng/ml) than in SSc patients without this complication (99.4 ± 54.7 ng/ml). The follow-up period of patients with PF was 6±4yrs whereas patients without PF had a follow-up of 12±6yrs. There was no significant difference in the ages of controls compared to the SSc patients without or with PF (45.11 ± 9.19 yrs vs. 42.74 ± 8.83 yrs and 47.55 ± 7.72 yrs, respectively). These data suggest that circulating TN-C levels are higher in SSc patients than normal donors and that the levels increase in the presence of PF. TN-C levels were measure in paired serum samples 6.45 ± 3.80 years prior to and 6.43 ± 4.50 years after the development of clinically evident PF from an additional four SSc patients. TN-C levels in sera obtained following the development of PF showed an average 2.65-fold increase (range 1.49–4.39) compared to samples from the same patients prior to the development of PF (p = 0.03) (Figure 6B). Thus, increasing circulating TN-C reflects the development of SSc-associated pulmonary fibrosis.
Figure 6.
A. Serum TN-C levels are significantly higher in SSc patients with pulmonary fibrosis compared to patients without pulmonary fibrosis. Sera were collected from patients diagnosed with SSc with and without pulmonary fibrosis. Serum TN-C levels were measured by ELISA and compared to TN-C levels in healthy individuals. * p = 0.0001; ** p = 0.009; *** p = 1.1 × 10−8. B. Serum TN-C levels are increased in serum samples from 4 patients with PF compared to serum from the same patients prior to the development of PF. The difference in pre- and post-PF TN-C levels was significant (p = 0.03).
Discussion
TN-C is a hexameric molecule with alternatively spliced monomers ranging in molecular weight from 190 to 220kDa. Deposited in the ECM, TN-C has been found to regulate cell growth and migration (2, 10). Published studies have identified TN-C as an early response molecule that is induced upon injury and degraded during tissue repair (3). TN-C is considered a key factor in tissue remodeling and has become a molecule of interest in fibrotic diseases. In this study, we report the novel observation that IGFBP-3 mediates TGF-β induction of TN-C and can directly induce TN-C production in human primary lung fibroblasts. We also report increased levels of TN-C in lung tissues with SSc-associated lung fibrosis with a distinctive subepithelial distribution.
Pulmonary fibrosis in SSc is characterized by excessive ECM deposition and fibroblast proliferation/accumulation. TGF-β is the most potent and extensively studied pro-fibrotic factor implicated in the pathogenesis of fibrotic diseases (11). TGF-β contributes to the fibrotic process by increasing matrix production (12) and is one of the known inducers of TN-C (13,14). As early as 1988, Pearson et al. demonstrated that addition of TGF-β to chick embryo fibroblasts resulted in a 4-fold increase in TN-C secretion (15). Verrechia et al. subsequently reported that TGF-β induces production of TN-C in human dermal fibroblasts (14). We had previously reported that TGF-β is also a potent inducer of IGFBP-3 (7). We now show that IGFBP-3 mediates TGF-β-induced TN-C expression but can also directly induce TN-C. Our results identify IGFBP-3 as a new mediator of TGF-β’s pro-fibrotic effects. Based on our findings, it is clear that IGFBP-3 mediates TGF-β inducing effects on TN-C but not collagen, suggesting a unique pathway for TN-C regulation.
Abnormally high levels of TN-C have been reported in lung tissues of patients with pulmonary fibrosis, particularly usual interstitial pneumonia or UIP (16), suggesting a role for TN-C in the development and/or progression of fibrotic pulmonary disorders or as a biomarker of lung fibrosis. A possible consequence of increased TN-C expression during injury is the recruitment of fibroblasts to the site of tissue damage. TN-C is expressed in cardiac muscle after myocardial infarction and mediates tissue remodeling by recruiting fibroblasts and inducing matrix metalloproteinases (17). Trebaul et al reported that TN-C facilitates migration of fibroblasts to the site of tissue damage (2). In support of these observations, TN-C null mice have reduced myofibroblast recruitment following induction of hepatic fibrosis (18).
Down regulation of TN-C following tissue repair is thought to be largely due to proteolytic degradation (2) by matrix metalloproteinase 7 (19) and other enzymes. Proteolytic degradation of full-length TN-C therefore could serve as a means of mitigating TN-C-associated inflammatory and migratory responses to injury. Thus, in addition to increased production, insufficient TN-C degradation may also contribute to abnormal repair, allowing TN-C to accumulate at epithelial margins. Since TN-C is a substrate of several matrix metalloproteases (MMP), post-translational modification and reduced degradation is another mechanism by which IGFBP-3 may increase TN-C levels in fibrosis. In that context, not only is IGFBP-3 known to modulate the activity of several MMPs, but it may also regulate levels and/or activity of tissue inhibitors of MMP (TIMP), thus altering the balance of MMP/TIMP and thus reducing the degradation of matrix proteins such as TN-C that can then contribute to aberrant tissue repair and fibrosis. In fact, evaluation of epithelial lining fluids from patients diagnosed with UIP, sarcoidosis, and allergic bronchiole-alveolitis (hypersensitivity pneumonitis) demonstrated elevated levels of TN-C compared to those of healthy controls (20). Also, immunoreactivity studies of bronchial biopsies have shown higher levels of TN-C in the sub-epithelial basement membranes of patients with chronic asthma as opposed to healthy controls (21). We reported increased IGFBP-3 in airway epithelial cells of asthmatics, which correlated with disease severity, and in patients with idiopathic pulmonary fibrosis (7, 22). Thus IGFBP-3 produced by epithelial cells basolaterally can induce TN-C production of underlying subepiethlial fibroblasts, thus maintaining a vicious cycle of a fibrosis-promoting milieu.
TN-C-incited fibroblast proliferation may lead to hyperplasia. Tenascin plaques are observed in association with hyperplastic type II alveolar epithelial cells in biopsies of active cryptogenic fibrosing alveolitis (aka idiopathic pulmonary fibrosis, IPF) (23). In another study using idiopathic pulmonary fibrosis (IPF) lung tissues, TN-C was not only abundantly expressed in fibroblastic foci of areas of active fibrosis, but also in the basement membranes adjacent to metaplastic epithelia of honeycomb cysts (24). Increased expression of TN-C is reported in both deep and superficial dermis of involved SSc skin (25). Focal localization of TN-C has also been observed in nodular scleroderma lesions (26), suggesting that localized production of TN-C is a feature of remodeling. Our findings support these reports and extend them. We demonstrate an accumulation of TN-C in the subepithelial layers of small distal airways of fibrotic SSc lung tissues. TN-C immunoreactivity has a distinct pattern of localization around smaller airways, which appear distorted, but is not expressed in more proximal airways. Thus, TN-C expression in fibrotic lung tissues appears in areas of recurring injury.
TGF-β has been exhaustively studied as a fibrotic mediator. It is a known inducer of the ECM components fibronectin and collagen. Microarray data indicate that TGF-β also induces TN-C mRNA expression (14). Other studies have demonstrated that application of TGF-β-neutralizing antibody results in lowered TN-C protein expression in rats with irradiation-induced fibrosis (27). Based on MAP kinase inhibitor studies in chick embryonic skin fibroblasts, the p38K signaling cascade has been identified as one of the pathways required for induction of TN-C by TGF-β(28). From such studies it can be deduced that TGF-β may act via different pathways to induce TN-C expression. Our findings confirm these reports and extended them in primary human lung fibroblasts by demonstrating that this induction is mediated to a large extent by the JNK signaling pathway and to a lesser extent via the p38K pathway. Both of these signaling cascades are stress-activated and mediate wound-healing processes. It is therefore not surprising that these cascades are required for TN-C induction.
We previously demonstrated that TGF-β induces production and secretion of IGFBP-3 by primary fibroblasts (7). We now show that IGFBP-3 mediates TGF-β stimulation of TN-C expression. In fact, IGFBP-3 silencing restored TN-C levels to baseline even in the presence of TGF-β. IGFBP-3 has been reported to mediate the growth promoting effects of TGF-β. For example, IGFBP-3 mediates TGF-β-induced inhibition of cancer cell proliferation (29). In a different study, the use of IGFBP-3 neutralizing antibody blocked TGF-β-induced growth of human bronchial airway smooth muscle cells (30). Also, IGFBP-3 was found to mediate TGF-β-induced proliferation of colon cancer cells (31). This leads us to conclude that IGFBP-3 plays a pivotal role in mediating TGF-β effects.
Elevated levels of TN-C have been reported in sera of patients with cryptogenic organizing pneumonia (32) as well as in bronchoalveolar lavage (BAL) fluids of patients with UIP, sarcoidosis and other fibrotic lung diseases (20). These results imply a role of TN-C in the pathogenesis of chronic lung disease, particularly pulmonary fibrosis. We show increased serum levels of TN-C in SSc patients with pulmonary fibrosis compared to SSc patients without pulmonary fibrosis. The cellular source of the circulating TN-C in SSc-associated PF and other fibrotic lung diseases has not been identified. We also show a significant increase in circulating TN-C levels in SSc patients following the onset of PF compared to the same patients prior to the development of PF. Our findings, in concert with those reported by Histomi et al and Kaarteenaho-Wiik et al, suggest that elevated serum TN-C levels can serve as markers of lung fibrosis in SSc and related diseases (20, 32).
In conclusion, we report that IGFBP-3 mediates TGF-β-induced TN-C production and can directly stimulate TN-C expression. Furthermore, increased subepithelial focal expression of TN-C in vivo in human lung tissues is adjacent to IGFBP-3 producing epithelial cells, suggesting that IGFBP-3 secreted by epithelial cells induces TN-C production and deposition by underlying subepithelial fibroblasts. We further demonstrate that TN-C can serve as a circulating marker of pulmonary fibrosis in patients with SSc.
Acknowledgments
This work was supported in part by NIH/NIAMS grant R01 AR050840 and P30 DK072506. The authors would like to thank Mary Lucas and Noreen Fertig for technical assistance.
References
- 1.Mackie EJ, Halfter W, Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988;107:2757–67. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans. 2007;35:695–7. doi: 10.1042/BST0350695. [DOI] [PubMed] [Google Scholar]
- 3.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2009;40:633–42. doi: 10.1165/rcmb.2008-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiquet M, Fambrough DM. Chick myotendinous antigen II: a novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol. 1984;98:1937–46. doi: 10.1083/jcb.98.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Young SL, Clarke McIntosh J. Induction of tenascin in rat lungs undergoing bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 1998;274:1049–57. doi: 10.1152/ajplung.1998.274.6.L1049. [DOI] [PubMed] [Google Scholar]
- 6.Steen VD, Medsger TA. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Pilewski JM, Feghali-Bostwick CA, Liu L, Henry AC, Knauer AV. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166:399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subcommitee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 9.Fertig N, Domsic RT, Rodriguez-Reyna T, Kuwana M, Lucas M, Medsger TA, Jr, et al. Anti-U11/U12 RNP antibodies in systemic sclerosis: a new serological marker associated with pulmonary fibrosis. Arthritis Rheum. 2009;61:958–65. doi: 10.1002/art.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.End P, Panayotou G, Entwistle A, Waterfield MD, Chiquet M. Tenascin: a modulator of cell growth. Eur J Biochem. 1992;209:1041–51. doi: 10.1111/j.1432-1033.1992.tb17380.x. [DOI] [PubMed] [Google Scholar]
- 11.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–82. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Norman JT, Shrivastav S, Lucio-Cazana J, Kopp JB. In vitro models of TGF-β-induced fibrosis suitable for high-throughput screening of antifibrotic agents. Am J Physiol Renal Physiol. 2007;293:631–40. doi: 10.1152/ajprenal.00379.2006. [DOI] [PubMed] [Google Scholar]
- 13.Chiquet-Ehrismann R, Kalla P, Pearson CA. Participation of tenascin and transforming growth factor-β in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 1989;49:4322–25. [PubMed] [Google Scholar]
- 14.Verrecchia F, Chu ML, Mauviel A. Identification of Novel TGF-b/Smad Gene Targets in Dermal Fibroblasts using a Combined cDNA Microarray/Promoter Transactivation Approach. J Biol Chem. 2001;276:17058–62. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 15.Pearson CA, Pearson D, Shibahara S, Hofsteenge J, Chiquet-Ehrismann R. Tenascin: cDNA and induction by TGF-β. Embo J. 1988;7:2977–2981. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaarteenaho-Wiik R, Tani T, Sormunen R, Soini Y, Virtanen I, Paakko P. Tenascin immunoreactivity as a prognostic marker in usual interstitial pneumonia. Am J Respir Crit Care Med. 1996;54:511–18. doi: 10.1164/ajrccm.154.2.8756830. [DOI] [PubMed] [Google Scholar]
- 17.Imanaka-Yoshida K, Hiroe M, Yoshida T. Interaction between cell and extracellular matrix in heart disease: multiple roles of tenascin-C in tissue remodeling [Review] Histol Histopathol. 2004;19:517–25. doi: 10.14670/HH-19.517. [DOI] [PubMed] [Google Scholar]
- 18.El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol. 2007;211:86–94. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
- 19.Siri A, Knäuper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem. 1995;270:8650–54. doi: 10.1074/jbc.270.15.8650. [DOI] [PubMed] [Google Scholar]
- 20.Kaarteenaho-Wiik R, Mertaniemi P, Sajanti E, Soini Y, Pääkkö P. Tenascin is increased in epithelial lining fluid in fibrotic lung disorders. Lung. 1998;176:371–80. doi: 10.1007/pl00007619. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen A, Altraja A, Kämpe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med. 1997;156:951–58. doi: 10.1164/ajrccm.156.3.9610084. [DOI] [PubMed] [Google Scholar]
- 22.Veraldi KL, Gibson BT, Yasuoka H, Myerburg MM, Kelly EA, Balzar S, et al. Role of insulin-like growth factor binding protein-3 in allergic airway remodeling. Am J Respir Crit Care Med. 2009;180:611–17. doi: 10.1164/rccm.200810-1555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace WA, Howie SE, Lamb D, Salter DM. Tenascin immunoreactivity in cryptogenic fibrosing alveolitis. J Pathol. 1995;175:415–20. doi: 10.1002/path.1711750409. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn C, Mason RJ. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol. 1995;147:1759–69. [PMC free article] [PubMed] [Google Scholar]
- 25.Lacour JP, Vitetta A, Chiquet-Ehrismann R, Pisani A, Ortonne JP. Increased expression of tenascin in scleroderma. Br J Dermatol. 1992;127:328–34. doi: 10.1111/j.1365-2133.1992.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani H, Taniguchi H, Sakakura T, Shimizu M. Nodular scleroderma: focally increased tenascin expression differing from that in the surrounding scleroderma skin. J Dermatol. 1995;4:267–71. doi: 10.1111/j.1346-8138.1995.tb03384.x. [DOI] [PubMed] [Google Scholar]
- 27.Wehrhan F, Rödel F, Grabenbauer GG, Amann K, Brückl W, Schultze-Mosgau S. Transforming growth factor beta 1 dependent regulation of tenascin-C in radiation impaired wound healing. Radiother Oncol. 2004;72:297–303. doi: 10.1016/j.radonc.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Chiquet M, Sarasa-Renedo A, Tunç-Civelek V. Induction of tenascin-C by cyclic tensile strain versus growth factors: distinct contributions by Rho/ROCK and MAPK signaling pathways. Biochim Biophys Acta. 2004;1693:193–204. doi: 10.1016/j.bbamcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Oh Y, Müller HL, Ng L, Rosenfeld RG. Transforming growth factor-beta-induced cell growth inhibition in human breast cancer cells is mediated through insulin-like growth factor-binding protein-3 action. J Biol Chem. 1995;270:13589–92. doi: 10.1074/jbc.270.23.13589. [DOI] [PubMed] [Google Scholar]
- 30.Cohen P, Rajah R, Rosenbloom J, Herrick DJ. IGFBP-3 mediates TGF-1-induced cell growth in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:545–51. doi: 10.1152/ajplung.2000.278.3.L545. [DOI] [PubMed] [Google Scholar]
- 31.Kansra S, Ewton DZ, Wang J, Friedman E. IGFBP-3 mediates TGF1 proliferative response in colon cancer cells. Int J Cancer. 2000;87:373–78. doi: 10.1002/1097-0215(20000801)87:3<373::aid-ijc10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Hisatomi K, Sakamoto N, Mukae H, Hayashi T, Amenomori M, Ishimoto H, et al. Elevated levels of tenascin-C in patients with cryptogenic organizing pneumonia. Intern Med. 2009;48:1501–7. doi: 10.2169/internalmedicine.48.2233. [DOI] [PubMed] [Google Scholar]