Abstract
Acute β-adrenergic blockade increases aortic wave reflection, however, the mechanisms remain unclear. Evidence suggests that β-adrenergic receptor sensitivity in the peripheral vasculature differs between sexes. Therefore, the goal of this study was to examine whether β-adrenergic blockade alters aortic wave reflection to a similar extent in young men and women. In thirty-one subjects (16M/15F; 26±1 years) non-invasive aortic pressure waveforms were synthesized from high-fidelity radial pressure waveforms via applanation tonometry before and during systemic β-blockade (0.25 mg/kg bolus, followed by 0.004 mg/kg/min continuous infusion of propranolol). β-blockade increased aortic augmentation index and wave reflection amplitude (aortic augmented pressure) in both sexes (P<0.01). Although the increase in augmentation index was not significantly different between sexes (7.5 ± 1.1 vs. 4.6 ± 1.5%, P=0.07), the increase in aortic augmented pressure was greater in women compared to men(2.8 ± 0.5 vs. 1.4 ± 0.5 mmHg, P<0.05). Aortic augmentation index adjusted for a heart rate of 75 beats per minute increased in women (4.1 ± 1.1%, P<0.05) following β-blockade, whereas it was unchanged in men (0.6 ± 1.3%, P=0.33). Moreover, the change in aortic augmentation index was inversely associated to the change in heart rate only in men (r = -0.54, P<0.05). Our data suggest that: 1) aortic wave reflection is increased to a greater extent in women following systemic β-blockade; and 2) enhanced aortic wave reflection appears to be mediated by a reduced heart rate in men, whereas the mechanism is unclear in women.
Keywords: aortic wave reflection, blood pressure, beta-adrenergic receptors, sex
Introduction
Beta (β)-adrenergic blockers are among the oldest and most widely used antihypertensive agents currently available for clinical use. β-blocker therapy is effective in reducing brachial artery blood pressure (BP) and is often recommended for many high-risk patients with cardiovascular diseases.1 However, evidence suggests that β-blockers may be less effective than other antihypertensive drugs in reducing stroke and cardiovascular mortality despite similar BP reductions.2-4 One explanation for the less than desirable cardiovascular protection provided by β-blockers is their lack of effectiveness in reducing central aortic BP and wave reflection. Along these lines, treatment with traditional nonvasodilating β-blockers (i.e. atenolol) is associated with greater central aortic pressures and wave reflection compared with other antihypertensive drugs.5-11 It has been postulated that the β-blocker induced reduction in heart rate (HR) prolongs systolic ejection time and delays the peak of the outgoing wave, thus causing the pressure wave reflections to augment the central aortic systolic pressure wave.11 Therefore, the benefits of HR reduction may be negated by the simultaneous increase in aortic pressure and wave reflection.
Acute and prolonged β-blockade also leads to an increase in systemic vascular resistance.12-15 Presumably, this is due to a reduction in β-mediated peripheral vasodilation and/or to an unmasking of alpha (α)-adrenergic mediated vasoconstriction.14 Theoretically, an increased peripheral vasoconstriction could cause a shift of arterial reflection sites proximally, and contribute to the enhanced aortic wave reflection during β-blockade, independent of a reduced heart rate. Previous work indicates that peripheral vascular β-adrenergic receptor sensitivity is enhanced in young women compared to men of a similar age.16 Consequently, forearm vasoconstrictor responses to norepinephrine are blunted in young women due to concurrent β-adrenergic mediated vasodilatation. Thus it is possible that β-adrenergic receptor mediated vasodilatation is enhanced in young women and offsets the vasoconstrictor effects of norepinephrine. Therefore, sex specific differences may exist in wave reflection characteristics during β-blockade. With this information as background, we tested the hypothesis that acute β-blockade would increase aortic wave reflection in both sexes, but that the increase would be greater in women. Since the sex specific differences in β-adrenergic mediated vasodilatation have been demonstrated in younger adults,17 we chose to test our hypothesis in a similar age group. Although hypertension related β-blocker usage is less common in young adults compared to older individuals, these drugs are used clinically for the treatment of cardiac arrhythmias, migraines, and anxiety in younger patients.
Methods
Subjects
A total of 31 young healthy subjects (16 males and 15 females) were studied. Subjects completed written informed consent and underwent a standard screening. All were healthy, non-obese, non-smokers, and were not taking any medications (except for oral contraceptives in some women). All of the studies were performed in the Clinical Research Unit laboratory at the Mayo Clinic, where ambient temperature was controlled between 22°C and 24°C. Studies were performed after an overnight fast and subjects refrained from exercise, alcohol, and caffeine for at least 24 h. Female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives.18, 19 All study protocols were approved by the Mayo Institutional Review Board and were performed according to the Declaration of Helsinki.
Measurements
Brachial artery blood pressure and heart rate measurements
A 20 gauge, 5 cm catheter was placed in the brachial artery of the left arm under sterile conditions after local anesthesia (2% lidocaine). The catheter was connected to a pressure transducer, which was positioned at the level of the heart and interfaced with a personal computer to monitor arterial pressure. A 3-lead ECG was used for continuous recording of HR. Beat-to-beat stroke volume was measured from the brachial artery using Modelflow analysis, which computes an aortic waveform based on nonlinear pressure–volume, pressure–compliance and pressure–characteristic impedance equations, incorporating age, sex, height and body mass.20 Cardiac output was calculated as stroke volume × HR and total peripheral resistance (TPR) was calculated as mean arterial pressure/cardiac output.
Pulse wave analysis
Following 15 minutes of supine rest, the assessment of arterial wave reflection characteristics was performed non-invasively using the SphygmoCor system (AtCor Medical, Sydney, Australia) as described previously.21 Briefly, high-fidelity radial artery pressure waveforms were recorded by applanation tonometry of the radial pulse in the right wrist using a “pencil type” micromanometer (Millar Instruments, Houston, Texas). The radial blood pressure (BP) and waveforms were calibrated from the systolic and diastolic brachial artery BP (catheter). A validated, generalized transfer function was used to generate the corresponding aortic pressure waveform.22 The generalized transfer function has been validated using both intra-arterially 22, 23 and non-invasively 24 obtained radial pressure waves.
Pulse wave analysis of the aortic pressure waveform provided the following key variables of interest; aortic pressures, aortic augmentation index (AIx), AIx adjusted for a HR of 75 (AIx@75bpm), round trip travel time of the forward traveling wave from the ascending aorta to the major reflection site and back (Δtp), and wasted LV pressure energy (Ew), which is the component of extra myocardial oxygen requirement due to early systolic wave reflection. 21, 25 Ew can be estimated as [(π/4)*(augmented pressure × Δtr)*1.333], where 1.333 is the conversion factor for mmHg/s to dynes·s/cm2 and Δtr is the systolic duration of the reflected wave. Augmented pressure (AG) is the amplitude of the reflected wave and is defined as the difference between the first (forward wave) and second systolic shoulders of the aortic systolic blood pressure. Only high-quality recordings, defined as an in-device quality index of over 80% (derived from an algorithm including average pulse height variation, diastolic variation, and the maximum rate of rise of the peripheral waveform), were accepted for analysis. In general, 2-3 measurements were performed in order to get two measurements with an acceptable quality index.
Systemic β-adrenergic blockade
We previously showed that the β1- and β2-adrenergic receptors in the peripheral vasculature contribute to peripheral resistance in women, but not in men.17 In the present study we aimed to block all β-adrenergic receptors on the vasculature to assess whether there would be sex specific changes in indices of aortic wave reflection. Therefore, non-selective β-blockade was achieved using intravenous infusion of propranolol. A 0.25 mg/kg bolus of propranolol (administered over 5 minutes) was followed by a continuous infusion of 0.004 mg/kg/min propranolol to maintain β-blockade. This dose of propranolol has been previously proven to cause total β-blockade in adult humans.26 All hemodynamic and applanation tonometry measurements were repeated ~15-20 minutes after the start of the propranolol infusion.
Statistical Analyses
Group data are expressed as means ± SEM. Analysis of variance was used to analyze baseline differences between sexes. Changes in the continuous dependent variables were analyzed using repeated measures analysis of variance. When a significant group-by-time interaction was observed, within group comparisons between time points and between-group comparisons at each time point were performed using Tukey’s post hoc analysis. To access the relationship between changes in HR and TPR and AIx during β-blockade, linear regression analysis was performed and Pearson’s correlation coefficients calculated. All statistical analyses were performed using SigmaStat software (version 2.03, SPSS Inc). An α-level of P < 0.05 was required for statistical significance.
Results
Subject demographics are presented in Table 1. All 31 subjects completed the study protocol. Beat-to-beat stroke volume could not be calculated from the brachial artery waveform in three subjects (1M/2F). Therefore, TPR values were only calculated in 15 and 13 of the male and female subjects, respectively. Baseline (pre β-blockade) HR, peripheral and aortic blood pressures, and ejection duration were not different between sexes (Table 2). However, women demonstrated a higher AIx, AIx@75bpm, AG, and TPR compared to men (P < 0.01; Table 2). Consequently, Ew was greater in women compared to their male counterparts (P < 0.01, Table 2).
Table 1.
Demographic Variables in Men (n=16) and Women (n=15)
| Demographics | Men | Women |
|---|---|---|
| Age, y | 25 ± 1 | 28 ± 2 |
| Height, cm | 177 ± 1 | 164 ± 1* |
| Weight, kg | 77 ± 2 | 62 ± 1* |
| BMI kg/m-2 | 24.5 ± 0.5 | 23.1 ± 0.4* |
Data are mean ± SEM. BMI indicates body mass index.
P < 0.05 vs. men
Table 2.
Hemodynamic and Aortic Wave Reflection Variables in Men (n=16) and Women (n=15).
| Hemodynamic/wave reflection variables | Men (n=16) | Women (n =15) | ||
|---|---|---|---|---|
| Pre BB | During BB | Pre BB | During BB | |
| HR, bpm -1 | 58 ± 2 | 50 ± 2* | 62 ± 2 | 55 ± 2* |
| PSBP, mm Hg | 123 ± 3 | 119 ± 3* | 125 ± 3 | 121 ± 3* |
| PDBP, mm Hg | 69 ± 2 | 70 ± 2 | 70 ± 2 | 70 ± 2 |
| PPP, mm Hg | 53 ± 2 | 48 ± 2* | 55 ± 2 | 51 ± 2* |
| ASBP, mm Hg | 102 ± 2 | 101 ± 2 | 107 ± 3 | 107 ± 3 |
| ADBP, mm Hg | 70 ± 2 | 71 ± 2 | 71 ± 2 | 71 ± 2 |
| APP, mm Hg | 32 ± 2 | 32 ± 1 | 37 ± 2 | 37 ± 2 |
| PPA | 1.69 ± 0.03 | 1.62 ± 0.03* | 1.54 ± 0.05† | 1.44 ± 0.05*† |
| AG, mm Hg | -0.5 ± 0.8 | 0.9 ± 0.8* | 4.3 ± 1.5† | 7.1 ± 1.6*† |
| AIx, % | -2.2 ± 2.6 | 2.4 ± 2.7* | 9.7 ± 3.5† | 17.1 ± 3.2*† |
| AIx @ 75 bpm-1, % | -10.1 ± 2.6 | -9.5 ± 2.8 | 3.8 ± 3.3† | 7.9 ± 2.9*† |
| Δtp, msec | 158 ± 4 | 163 ± 3 | 153 ± 4 | 153 ± 3 |
| Ew, dyne · cm2 · s | -74 ± 152 | 233 ± 154* | 864 ± 305† | 1408 ± 345*† |
| Ejection Duration, msec | 328 ± 8 | 330 ± 3 | 339 ± 4 | 336 ± 4 |
| Ejection Duration, % | 31.7 ± 1.0 | 27.5 ± 0.7* | 35.2 ± 1.2† | 30.9 ± 1.0*† |
| TPR, mm Hg L min-1 | 15.6 ± 0.7 | 19.2 ± 0.9*† | 19.9 ± 1.0 | 23.3 ± 1.0*† |
Data are mean ± SEM. BB indicates beta blockade; HR, heart rate; PSBP, peripheral systolic blood pressure; PDBP, peripheral diastolic blood pressure; PPP, peripheral pulse pressure; ASBP, aortic systolic blood pressure; ADBP, aortic diastolic blood pressure; APP, aortic pulse pressure; PPA, pulse pressure amplification; AG, augmented pressure; AIx, aortic augmentation index, AIx @ 75 bpm, aortic augmentation index adjusted for a HR of 75bpm;, Δtp, round trip travel time of the forward traveling wave from the ascending aorta to the major reflection site and back Ew, wasted left ventricular energy; TPR, total peripheral resistance.
P < 0.01 vs. pre BB.
P < 0.05 vs. men at same time point.
During systemic β-blockade via propranolol infusion, HR and peripheral systolic and pulse pressures were reduced in both sexes (P < 0.01; Table 2). Aortic pressures were unchanged by β-blockade in both groups. The reduction in peripheral pulse pressure without commensurate changes in aortic pulse pressure resulted in a decreased pulse pressure amplification (P < 0.01; Table 2). AIx, AG, Ew and TPR were increased during β-blockade in both sexes (P < 0.01; Table 2). However, the increase in AG (2.8 ± 0.5 vs. 1.4 ± 0.5 mmHg, P < 0.05) and Ew (544 ± 116 vs. 308 ± 101 dyne · s/cm2, P ≤ 0.05) were greater in women during β-blockade compared to men. Although the increase in AIx was not significantly greater in women compared to men during β-blockade (7.5 ± 1.1 vs. 4.6 ± 1.5 %, P = 0.07; Figure 1A), the increase in AIx @75bpm was substantially greater in women (4.1 ± 1.1 vs. 0.6 ± 1.2%, P < 0.05; Table 2 and Figure 1B). The change (Δ) in AIx was inversely associated to ΔHR in men (r = -0.54, P < 0.05; Figure 2), but not in women (r = -0.19, P = 0.50). Conversely, ΔAIx was positively related to ΔTPR in women (r = 0.50, P < 0.05), but not in men (r = 0.17, P = 0.54)
Figure 1.
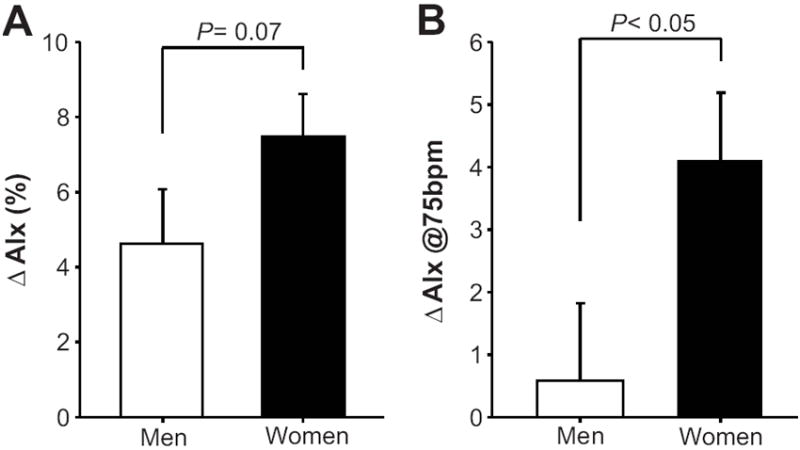
Changes (Δ) from baseline (pre β- blockade) in A) aortic augmentation index B) and aortic augmentation index adjusted for a HR of 75bpm during β-blockade in young men (open bars) and women (black bars). Data are mean ± SEM.
Figure 2.
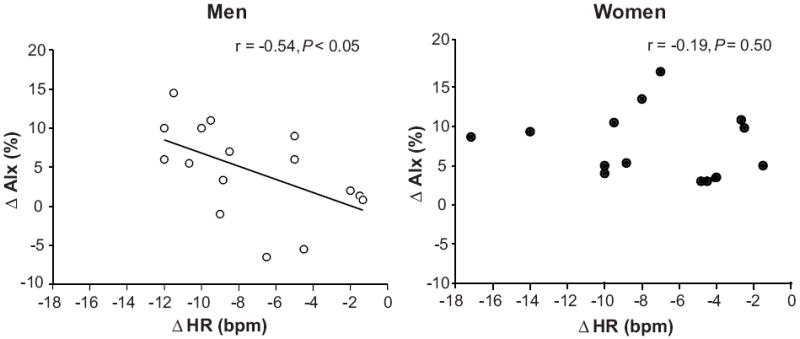
Linear regression analysis of the relationship between changes HR and AIx during β-adrenergic blockade in men and women. The correlations demonstrate that changes in AIx during β-adrenergic blockade were associated with reductions in HR in young men, whereas no association was observed in young women.
Discussion
The major new findings of the current study were 1) acute β-adrenergic blockade increased aortic wave reflection in young men and women, 2) aortic wave reflection was increased to a greater extent in women during systemic β-blockade; and 3) the increase in aortic wave reflection during acute systemic β-blockade appeared to be caused by different mechanisms between sexes. That is, increases in aortic wave reflection characteristics in men during systemic β-blockade were predominantly driven by a reduction in HR, whereas other mechanisms in addition to a reduced HR likely contributed to the enhanced wave reflection in women. Taken together these data suggest that β-blocking drugs may affect central artery hemodynamics differently in men and women.
Our finding that acute β-blockade with intravenous propranolol enhanced aortic wave reflection in young healthy adults is in agreement with a previous report in hypertensive patients.14 However, those authors did not report whether there were any sex differences in aortic wave reflection during β-blockade. Moreover, it should also be noted that all hemodynamic measurements (in the previous report) were made after premedication with chlorapheniramine. Therefore, it is unclear whether the changes in aortic hemodynamics during β-blockade were affected by prior antihistamine administration. To our knowledge this is the only other study to examine the acute effects of β-blockade on aortic wave reflection.
Long-term treatment with traditional nonvasodilating β-blockers (e.g. atenolol) is associated with increased central aortic pressures and wave reflection compared with alternative antihypertensive therapy.5-11 From a clinical standpoint this is particularly relevant, since increased AIx is a strong independent risk marker for premature CAD and all cause and cardiovascular mortality.27, 28 Along these lines, a meta-analysis of nine studies which included atenolol trials demonstrated that mortality was significantly higher with this agent, despite similar reductions in brachial blood pressure, compared with other antihypertensive drugs.29
Increases in aortic wave reflection during both short- and long-term β-blockade have been attributed to their heart rate lowering effect. It has been postulated that the reduced heart rate prolongs systolic ejection duration and allows the reflected wave a greater opportunity to appear in late systole rather than in diastole, thus increasing indices of wave reflection (i.e. AIx).14, 30 However, this concept would require pulse wave velocity (PWV) to remain unchanged during β-blockade. In this context, some studies have demonstrated an effect of β-blockers to lower aortic PWV31-33, whereas others have not.7 In the present study AIx was increased during propranolol infusion in both men and women (Table 2). However, after adjusting the AIx for heart rate (i.e. AIx@75bpm) only the women showed a significant increase (Table 2 and Figure 1B). Moreover, the change in AIx was inversely related to the change in HR during β-blockade in men, but not in women (Figure 2). Therefore, men with a greater increase in AIx had a greater reduction in HR during β-blockade. These findings suggest that increases in aortic wave reflection in young men during β-blockade are due to a reduced heart rate, whereas additional factors likely contribute to an enhanced AIx in young women.
The exact mechanisms underlying the enhanced aortic wave reflection during β-blockade in women are unclear, but there are several possible explanations. First, the greater aortic wave reflection in women during β-blockade could have resulted from an increase in arterial PWV as a consequence of changes in arterial stiffness. Along these lines, increases in aortic PWV causes early return of the reflected wave from peripheral reflecting sites and thus results in a greater AIx. A limitation of the present study was that we did not assess aortic PWV. Therefore, we are unable to evaluate whether the greater increases in aortic wave reflection characteristics in women during β-blockade were related to differences in aortic stiffness. Second, decreases in heart rate can prolong systolic ejection time and increase the likelihood that pressure wave reflections will augment the outgoing pressure wave during systole. In the present study the decrease in heart rate during propranolol infusion was similar between men and women. Additionally, systemic β-blockade did not alter the absolute systolic ejection duration in men and women (Table 2). When expressed relative (%) to the duration of the cardiac cycle, the change in systolic ejection duration during β-blockade was similar between sexes (Table 2). Taken together these results suggest that differences in the timing of systolic ejection likely do not explain the enhanced aortic wave reflection in women during β-blockade. Another potential cause for the enhanced aortic wave reflection during β-blockade in women might be related to differences in peak aortic blood flow velocity. Klinke et al.34 demonstrated that propranolol decreases the peak aortic blood flow velocity in men. However, it is currently unclear if sex related differences in the aortic blood velocity during propranolol exist.
Lastly, acute and prolonged β-blockade increase systemic vascular resistance, 12-15 likely by reducing the β-mediated peripheral vasodilation and/or by unmasking a degree of α-adrenergic mediated vasoconstriction.14 It appears that the β-adrenergic receptors are either more sensitive or up-regulated in young women vs. men.16 Moreover, we have recently demonstrated that the β-adrenergic receptors offset α-adrenergic vasoconstriction in young women but not young men.17 Along these lines, young men demonstrate a positive relationship between muscle sympathetic nerve activity (MSNA) and TPR, whereas this relationship is absent in young women. However, during β-blockade the relationship between MSNA and TPR became positive in young women.17 Therefore, women may have greater peripheral vasoconstriction (in addition to heart rate changes) in response to non-selective β-blockade. The increased peripheral vasoconstriction could cause a shift of arterial reflection sites proximally, which in turn would result in earlier wave reflection and thereby enhance aortic wave reflection in women. In this context, the ΔTPR during β-blockade in the present study was related to the ΔAIx in women, but not in men.
Experimental considerations
In the present study propranolol (a non-selective β-adrenergic antagonist) was used to examine the impact of β-blockade on indices of aortic wave reflection. Therefore, we can not extrapolate the current findings to other β-blocking drugs, especially newer vasodilating β-blockers (i.e. nebivolol and carvedilol). These β-blockers have been shown to have favorable effects on peripheral vascular resistance,35, 36 arterial distensibility,37 aortic pressures, wave reflection characteristics and stiffness. 10, 31, 32, 38 Therefore the use of vasodilatory β-blockers may offset any deleterious hemodynamic effects of heart rate reduction by decreasing wave reflection from the periphery. It is also important to note that with the development of newer and more selective β-blockers the use of propranolol as an antihypertensive agent has been reduced over the last several years. However, propranolol and other non-selective β-blockers are still commonly used in the treatment of cardiac arrhythmias, benign tremors, migraines, and heart failure.
The current findings are limited to young normotensive men and women. However, previous findings have demonstrated that acute β-blockade (via propranolol) increases aortic wave reflection in hypertensive patients.14 Unfortunately, other than age the authors did not report information related to patient demographics or if any possible sex related differences existed in the hemodynamic responses to β-blockade in their subjects. Therefore, it is unclear whether the sex differences observed in the present study can be extrapolated to other populations with or at risk for cardiovascular disease (i.e. hypertension) that would likely receive β-blockers. Lastly, the findings of the current study are limited to the effects of acute β-blockade on central artery hemodynamics. Whether similar sex differences exist in response to long-term β-blockade therapy needs to be examined further.
Perspectives
Several studies have reported that antihypertensive medications can have substantially different effects on central aortic pressure and wave reflection, despite a similar impact on brachial blood pressure. 7, 8, 10, 11, 39, 40 Along these lines, conventional β-blockers appear to be less effective in protecting against cardiovascular events compared to other antihypertensive agents. 2, 11, 41 The reduced effectiveness of β-blockers is thought to be a consequence of the failure to reduce aortic blood pressures and wave reflection. Our current findings suggest that the negative effects (i.e. increased aortic wave reflection) of conventional β-blockers may be greater in women. Interestingly, data from a recent, large (~18,000 patients) international study in patients with currently treated or newly diagnosed hypertension suggest women receive β-blocker therapy more frequently than men.42 Taken together with the present data, this might suggest that sex should be considered prior to β-blocker therapy, especially when non-selective β-blockers are considered. To this point, future studies should address whether long term β-blockade has a greater impact on aortic wave reflection in women compared with men.
Acknowledgments
The authors are grateful to the study volunteers for their participation. We also thank Shelly, Roberts, Jean Knutson, Karen Krucker, Christopher Johnson, Nancy Meyer, and Pam Engrav for their technical assistance.
Sources of Funding This study was supported by National Institutes of Health grants HL083947 (MJJ, NC), HL46493 (MJJ), AR55819 (DPC), DK082424 (TBC), and CTSA RR-024150 (Mayo Clinic), and by Mayo Clinic Research Subcommittee Early Career Development Supplement (TBC) and American Heart Association grant 2170087 (ECH).
Footnotes
Disclosures None.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (life): A randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 3.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the anglo-scandinavian cardiac outcomes trial-blood pressure lowering arm (ascot-bpla): A multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 4.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 5.Dhakam Z, McEniery CM, Yasmin, Cockcroft JR, Brown MJ, Wilkinson IB. Atenolol and eprosartan: Differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–219. doi: 10.1016/j.amjhyper.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Kamran H, Salciccioli L, Bastien C, Castro P, Sharma A, Lazar JM. Effect of beta blockers on central aortic pressure in african-americans. J Am Soc Hypertens. 2011;5:94–101. doi: 10.1016/j.jash.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–413. doi: 10.1161/HYPERTENSIONAHA.109.133801. [DOI] [PubMed] [Google Scholar]
- 8.Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–123. doi: 10.1016/j.amjhyper.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Olafiranye O, Qureshi G, Salciccioli L, Weber M, Lazar JM. Association of beta-blocker use with increased aortic wave reflection. J Am Soc Hypertens. 2008;2:64–69. doi: 10.1016/j.jash.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Polonia J, Barbosa L, Silva JA, Bertoquini S. Different patterns of peripheral versus central blood pressure in hypertensive patients treated with beta-blockers either with or without vasodilator properties or with angiotensin receptor blockers. Blood Press Monit. 2010;15:235–239. doi: 10.1097/MBP.0b013e32833c8a64. [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the conduit artery function evaluation (cafe) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 12.Hansson L, Zweifler AJ, Julius S, Hunyor SN. Hemodynamic effects of acute and prolonged beta-adrenergic blockade in essential hypertension. Acta Med Scand. 1974;196:27–34. doi: 10.1111/j.0954-6820.1974.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 13.Lund-Johansen P. Hemodynamic consequences of long-term beta-blocker therapy: A 5-year follow-up study of atenolol. J Cardiovasc Pharmacol. 1979;1:487–495. doi: 10.1097/00005344-197909000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Ting CT, Chen CH, Chang MS, Yin FC. Short- and long-term effects of antihypertensive drugs on arterial reflections, compliance, and impedance. Hypertension. 1995;26:524–530. doi: 10.1161/01.hyp.26.3.524. [DOI] [PubMed] [Google Scholar]
- 15.Ulrych M, Frohlich ED, Dustan HP, Page IH. Immediate hemodynamic effects of beta-adrenergic blockade with propranolol in normotensive and hypertensive man. Circulation. 1968;37:411–416. doi: 10.1161/01.cir.37.3.411. [DOI] [PubMed] [Google Scholar]
- 16.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 17.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: The role of the beta-adrenergic receptors. J Physiol. 2011 August 22; doi: 10.1113/jphysiol.2011.212753. published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 19.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: Fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010;235:111–118. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 21.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Current opinion in cardiology. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher D, Adji A, O’Rourke MF. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J Hypertens. 2004;17:1059–1067. doi: 10.1016/j.amjhyper.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto J, Nichols WW, O’Rourke MF, Imai Y. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: Influence of arterial wave reflection. Am J Hypertens. 2008;21:329–333. doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- 26.Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab. 2001;86:4440–4444. doi: 10.1210/jcem.86.9.7855. [DOI] [PubMed] [Google Scholar]
- 27.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 28.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 29.Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: Is it a wise choice? Lancet. 2004;364:1684–1689. doi: 10.1016/S0140-6736(04)17355-8. [DOI] [PubMed] [Google Scholar]
- 30.Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol. 2008;52:1482–1489. doi: 10.1016/j.jacc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 31.Dhakam Z, Yasmin, McEniery CM, Burton T, Brown MJ, Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–356. doi: 10.1097/HJH.0b013e3282f283c9. [DOI] [PubMed] [Google Scholar]
- 32.Mahmud A, Feely J. Beta-blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–667. doi: 10.1038/ajh.2008.156. [DOI] [PubMed] [Google Scholar]
- 33.Asmar RG, London GM, O’Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: A comparison with atenolol. Hypertension. 2001;38:922–926. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 34.Klinke WP, Christie LG, Nichols WW, Ray ME, Curry RC, Pepine CJ, Conti CR. Use of catheter-tip velocity--pressure transducer to evaluate left ventricular function in man: Effects of intravenous propranolol. Circulation. 1980;61:946–954. doi: 10.1161/01.cir.61.5.946. [DOI] [PubMed] [Google Scholar]
- 35.Dunn CJ, Lea AP, Wagstaff AJ. Carvedilol. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disorders. Drugs. 1997;54:161–185. doi: 10.2165/00003495-199754010-00015. [DOI] [PubMed] [Google Scholar]
- 36.Kamp O, Sieswerda GT, Visser CA. Comparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertension. Am J Cardiol. 2003;92:344–348. doi: 10.1016/s0002-9149(03)00645-3. [DOI] [PubMed] [Google Scholar]
- 37.McEniery CM, Schmitt M, Qasem A, Webb DJ, Avolio AP, Wilkinson IB, Cockcroft JR. Nebivolol increases arterial distensibility in vivo. Hypertension. 2004;44:305–310. doi: 10.1161/01.HYP.0000137983.45556.6e. [DOI] [PubMed] [Google Scholar]
- 38.Kampus P, Serg M, Kals J, Zagura M, Muda P, Karu K, Zilmer M, Eha J. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension. 2011;57:1122–1128. doi: 10.1161/HYPERTENSIONAHA.110.155507. [DOI] [PubMed] [Google Scholar]
- 39.Chen CH, Ting CT, Lin SJ, Hsu TL, Yin FC, Siu CO, Chou P, Wang SP, Chang MS. Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension. 1995;25:1034–1041. doi: 10.1161/01.hyp.25.5.1034. [DOI] [PubMed] [Google Scholar]
- 40.Pannier BM, Guerin AP, Marchais SJ, London GM. Different aortic reflection wave responses following long-term angiotensin-converting enzyme inhibition and beta-blocker in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:1074–1077. doi: 10.1046/j.1440-1681.2001.03570.x. [DOI] [PubMed] [Google Scholar]
- 41.Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta-blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: A systematic review and meta-analysis. Am J Ther. 2009;16:133–142. doi: 10.1097/MJT.0b013e31817fd87e. [DOI] [PubMed] [Google Scholar]
- 42.Thoenes M, Neuberger HR, Volpe M, Khan BV, Kirch W, Bohm M. Antihypertensive drug therapy and blood pressure control in men and women: An international perspective. J Hum Hypertens. 2010;24:336–344. doi: 10.1038/jhh.2009.76. [DOI] [PubMed] [Google Scholar]


