Abstract
Previous human studies have shown that large-artery stiffness contributes to an age-related decrease in cardiovagal baroreflex sensitivity. Whether this is also true with sympathetic baroreflex sensitivity is unknown. We tested the hypothesis that sympathetic baroreflex sensitivity is associated with the stiffness of baroreceptor segments (the carotid artery and the aorta) in elderly individuals, and that sex affects this relationship. Sympathetic baroreflex sensitivity was assessed from the spontaneous changes in beat-by-beat diastolic pressure and corresponding muscle sympathetic nerve activity (microneurography) during supine rest in 30 men [69±1 (mean±SEM) years] and 31 women (68±1 years). Carotid artery stiffness (B-mode ultrasonography) and aortic stiffness (magnetic resonance imaging) were also determined. We found that elderly women had lower sympathetic baroreflex sensitivity than elderly men (–2.33±0.25 vs. –3.32±0.25 bursts·100 beats–1·mmHg–1; P=0.007). β-stiffness indices of the carotid artery and the aorta were greater in elderly women than in men (6.68±0.48 vs. 5.10±0.50 and 4.03±0.47 vs. 2.68±0.42; both P<0.050). Sympathetic baroreflex sensitivity was inversely correlated with carotid artery stiffness in both men and women (r=0.49 and 0.50, both P<0.05), while this relation was shifted in parallel upward (towards a reduced sensitivity) in women with no changes in the slope (0.26 vs. 0.24 a.u.). Sympathetic baroreflex sensitivity and aortic stiffness showed similar trends. Thus, barosensory artery stiffness seems to be one independent determinant of sympathetic baroreflex sensitivity in elderly men and women. The lower sympathetic baroreflex sensitivity in elderly women may predispose them to an increased prevalence of hypertension.
Keywords: baroreceptors, β-stiffness, muscle sympathetic nerve activity, aging, sex differences
Introduction
The arterial baroreflex is a primary mechanism through which the autonomic nervous system regulates blood pressure (BP). Previous human studies have shown that cardiovagal baroreflex sensitivity (BRS) is blunted in normal aging1-3 as well as in patients with hypertension.4, 5 The blunted cardiovagal BRS has been found to be a risk factor for life-threatening arrhythmias and a predictor of sudden cardiac death.6, 7 Thus, understanding the mechanisms underlying age-related changes in BRS has significant clinical relevance.
Since the deformation of the baroreceptors rather than direct intra-vessel pressure during acute changes in arterial pressure is required to initiate neural firing,8 the stiffness of large elastic arteries where the baroreceptors exist (the carotid artery and the aortic arch) could be associated with a decrease in BRS. Indeed, a number of studies have demonstrated that an age-related increase in the stiffness of these arteries is responsible for the decline in cardiovagal BRS.2, 3, 9-11 Whether this is also the case with sympathetic BRS is unknown.
It was recently reported that sympathetic BRS was correlated with cardiovagal BRS in healthy young women.12 On the other hand, O'Leary et al13 showed that the difference in mechanical change of the carotid artery between nitroprusside and phenylephrine injection was related to hysteresis of the cardiovagal baroreflex but not the sympathetic baroreflex. A similar observation was made during a cold pressor test,14 head-up tilt,15 and volume expansion,16 suggesting that the relation between arterial stiffness and sympathetic BRS is complex. Surprisingly, there is no information available regarding the relationship between the stiffness of the aorta and sympathetic BRS in humans. Recent evidence of sex differences in age-related increases of arterial stiffness17, 18 and in autonomic control of BP19-22 suggests that sex may affect the relationship between arterial stiffness and sympathetic BRS.
In the present study, we hypothesized that sympathetic BRS would be associated with both carotid artery and aortic stiffness in elderly people and that sex would affect these relationships. To test this hypothesis, we assessed sympathetic BRS from the linear correlation between muscle sympathetic nerve activity (MSNA) and beat-by-beat diastolic BP (DBP) during spontaneous breathing, and the stiffness of the carotid artery and the aorta with ultrasound imaging and magnetic resonance imaging (MRI), respectively. We then determined the relationships between these variables in all subjects as well as in men and women separately.
Methods
Subjects
One hundred and seventy-five elderly individuals were contacted between August 2008 and September 2010; 92 of them were interested in participating in research and were screened, and 61 (30 men, 31 women) were eventually qualified and enrolled in this study. They were non-smokers and had no overt history of cardiovascular, neuromuscular, or renal diseases. Women taking hormone replacement therapy were excluded. All subjects gave their written informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas. Subjects’ physical characteristics and fasting glucose and insulin are presented in Table 1.
Measurements
Muscle sympathetic nerve activity
MSNA signals were obtained with microneurography.23, 24 In brief, a recording electrode was placed in the peroneal nerve at the popliteal fossa, and a reference electrode was placed subcutaneously 2-3 cm apart from the recording electrode. The nerve signals were amplified (70 000 to 160 000-fold), band-pass filtered (700 to 2000 Hz), full-wave rectified, and integrated with a resistance-capacitance circuit (time constant 0.1 sec). Criteria for adequate MSNA recording include: pulse synchrony; facilitation during hypotension phase of the Valsalva maneuver, and suppression during the hypertensive overshoot phase after release; increase in response to breath holding; and insensitivity to emotional stimuli.24
Hemodynamics
Heart rate (HR) was determined from lead II of the electrocardiogram (Hewlett-Packard) and beat-by-beat arterial BP was derived by finger photoplethysmography (Nexfin). Arm cuff BP was measured by electrosphygmomanometry (SunTech) with a microphone placed over the brachial artery to detect Korotkoff sounds. Beat-by-beat carotid and radial pressures were obtained with a pencil-sized tonometer containing a high-fidelity strain gauge transducer (SphygmoCor). Since the absolute levels of these arterial pressures are subjected to hold-down force, the pressure signals obtained by tomometry were calibrated by equating their DBP and mean BP (MBP) to those obtained at the brachial artery as previously described.25 Cardiac output (CO) was measured with the modified acetylene rebreathing technique.26 Stroke volume (SV) was calculated from CO divided by HR, and total peripheral resistance (TPR) was calculated as the quotient of MBP (DBP+[systolic BP (SBP)–DBP]/3) from arm cuff BP and CO, multiplied by 80, where all valuables were measured during rebreathing. CO, SV, and TPR were normalized to body surface area as cardiac index, stroke index, and TPR index.
Arterial images
Cross-sectional images of the right common carotid artery were captured using a phase-locked echo tracking system coupled to a B-mode ultrasonic imager (iE33, Philips), which allows sequential measurements of the arterial wall movement non-invasively through a high-resolution transducer (7.5 MHz) positioned at 2 cm proximal to the carotid bifurcation with simultaneous electrocardiogram recordings.27 MRI of the aortic arch was obtained in the transverse plane at the level of the right pulmonary artery using 1.5-T clinical magnetic resonance scanner (Gyroscan Intera, Philips) to assess aortic pulsative dimension.
Protocol
The experiment was performed in the morning ≥2 h after breakfast, ≥72 h after the last caffeinated or alcoholic beverage, and ≥24 h after strenuous physical activity in a quiet, environmentally controlled laboratory with an ambient temperature of ~25° C. The subject was placed in the supine position. At least 10 min after an acceptable nerve recording site had been found, baseline data were recorded for 6 min during spontaneous breathing to assess sympathetic BRS. Subsequently, two Valsalva maneuvers (40 mmHg for 20 sec) were performed to assess cardiovagal BRS, separated by >2 min of recovery. Since the day-to-day variability of MSNA is small and the reproducibility of the measurement is high,28 arterial stiffness was assessed on the next day in the morning. Baseline hemodynamics were measured in all subjects. Both carotid and brachial arterial pressures were obtained using tonometry and arm cuff BP at the brachial artery, followed by ultrasonography on the common carotid artery for the assessment of carotid artery stiffness. Throughout the entire experimental procedures, beat-by-beat BP, HR, and respiratory waveforms were recorded continuously. The aortic MRI (for the assessment of aortic stiffness) was performed at the University of Texas Southwestern Medical Center at Dallas.
Data analysis
Data were sampled at 625 Hz and stored on personal computer with a commercial data acquisition system (Biopac). Off-line data analyses were performed using signal-processing software (LabView). Beat-by-beat HR was calculated from the R-R interval (RRI) measured by electrocardiogram. Beat-by-beat SBP and DBP were obtained from the arterial-pressure waveform. Sympathetic bursts were identified by a computer program,14 and then confirmed by an experienced microneurographer. The integrated neurogram was normalized by assigning a value of 100 to the largest amplitude of a sympathetic burst during the 6-min baseline.29 Burst area was measured as the area under the curve of each sympathetic burst of the normalized integrated neurogram on a beat-by-beat basis. The number of bursts per minute (burst frequency), the number of bursts per 100 heart beats (burst incidence), and total burst area per minute and per 100 beats (total MSNA) were used as quantitative indices.
Sympathetic BRS was assessed using the slope of the linear correlation between MSNA and DBP during spontaneous breathing, and cardiovagal BRS was assessed by RRI and SBP during the Valsalva maneuvers after adjusting all signal and physiological delays (see the Online Supplement at http://hyper.ahajoutnals.org). Detailed assessments of carotid artery and aortic stiffness were also reported in the Online Supplement.
Statistical analysis
Values are expressed as means±SEM. Linear regression analysis was used to evaluate the correlation between arterial stiffness and sympathetic BRS. Data between men and women were compared using unpaired t-tests. If normality tests and/or equal variance tests failed, we compared the differences between sexes using Mann-Whitney Rank Sum Tests. β-stiffness of the aorta calculated with transferred aortic pressure and direct carotid pressure for men and women was evaluated using two-way repeated ANOVA. A P value of <0.05 was considered statistically significant.
Results
Hemodynamics and Neural Variables
Table 2 depicts supine resting hemodynamics and MSNA. There was no difference between sexes in arm cuff SBP and MBP. Women had lower arm DBP, CO, and SV, but higher HR compared with men (all, P<0.05). Cardiac index showed no sex difference (P=0.392) while stroke index was significantly smaller in elderly women than men (P<0.001). TPR trended higher in elderly women than men (P=0.099), but TPR index did not differ between sexes (P=0.458). Although MSNA burst frequency tended to be higher in elderly women (P=0.078), there were no significant differences in burst incidence and total MSNA between sexes.
Baroreflex Sensitivity and Arterial Stiffness
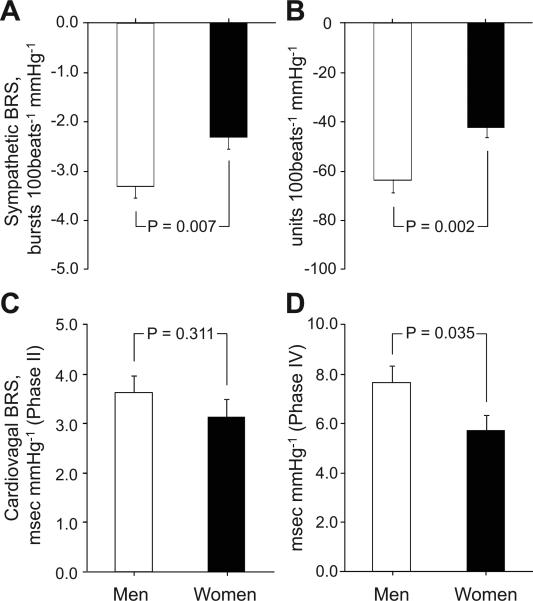
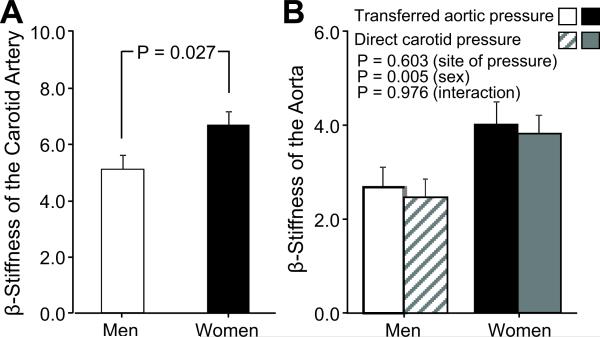
Sympathetic and cardiovagal BRS data are shown in Figure 1. Elderly men had higher sympathetic BRS than elderly women. There were no significant sex differences in cardiovagal BRS assessed during phase II of the Valsalva maneuver; however, cardiovagal BRS was greater in elderly men than women during phase IV. Elderly men had lower β-stiffness indices than women in both the carotid artery and aorta (Figure 2).
Figure 1.
Sympathetic baroreflex sensitivity (BRS) calculated from burst incidence (A) and total MSNA (B) during spontaneous breathing in the supine position, and cardiovagal BRS assessed during phase II (C) and phase IV (D) of the Valsalva maneuver in elderly men and women. Values are means±SEM.
Figure 2.
β-stiffness indices of the carotid artery assessed using ultrasonography (A) and the aorta assessed using magnetic resonance images with the aortic pressure computed by transfer function from radial artery and with the carotid artery pressure directly measured by tonometory (calibrated by brachial BP) (B) in elderly men and women. Values are means±SEM.
Relationship between Sympathetic Baroreflex Sensitivity and Arterial Stiffness
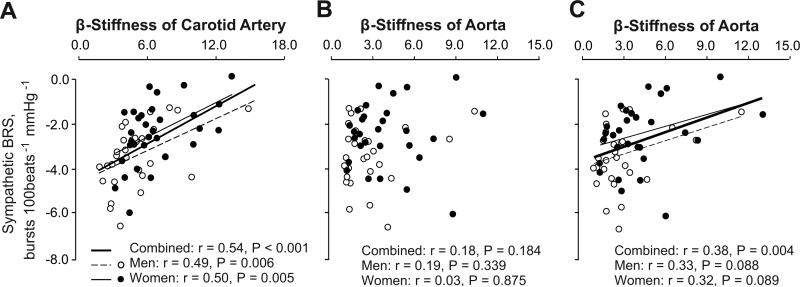
Sympathetic BRS was significantly and inversely correlated with β-stiffness index of the carotid artery in all, as well as in men and women separately (Figure 3A). The slope of this relationship was 0.26 in women and 0.24 a.u. in men, while the y-intercept was –4.05 in women and –4.55 bursts·100 beats–1·mmHg–1 in men. Sympathetic BRS was not correlated with β-stiffness index of the aorta calculated with aortic pressure derived from transfer function analysis in any groups (Figure 3B). Conversely, sympathetic BRS was correlated with β-stiffness of aorta calculated with carotid artery pressure in all subjects together, though with less statistical certainty in men and women examined separately (Figure 3C). This relation was shifted in parallel upward (towards a reduced sensitivity) in women with no changes in the slope.
Figure 3.
Linear regression analysis of the inter-individual relationship between sympathetic BRS and β-stiffness of the carotid artery (A; 30 men, 31 women) and the aorta (28 and 29) calculated with aortic pressure derived from transfer function analysis (B) and carotid artery pressure (C) in all subjects and in elderly men (○) and women (●), separately.
Discussion
Our major findings are: (1) sympathetic BRS was lower and β-stiffness indices of both the carotid artery and aorta were higher in elderly women than men; (2) sympathetic BRS was correlated with carotid artery stiffness and aortic stiffness in all subjects, as well as in elderly men and women separately; and (3) the slope of the correlation between sympathetic BRS and carotid artery and aortic stiffness was similar between sexes, however, the line relating these two variables was shifted in parallel upward in elderly women. Thus, barosensory artery stiffness seems to be one independent determinant of sympathetic BRS in elderly men and women. The lower sympathetic BRS in elderly women may predispose them to an increased prevalence of hypertension.
Sex Differences in Baroreflex Sensitivity
Recent studies suggested that there was no sex difference in sympathetic BRS in young individuals.12, 20, 30, 31 However, our data in elderly subjects showed that women had lower sympathetic BRS than men, suggesting that age-related changes in sympathetic baroreflex function may be different between sexes. Similar sympathetic BRS across all ages was reported in men during steady state changes in BP with a neck chamber method32 and continuous phenylephrine infusion.33 Conversely, lower sensitivity in elderly men than young men was observed during dynamic changes in BP by the Valsalva maneuver.34 Although a limited number of studies have included women, Studinger et al35 found that age did not affect integrated sympathetic BRS in men and women during bolus injection of nitroprusside and phenylephrine. The discrepancy between our results and those of Studinger et al may be attributable to different methods used for the assessment of sympathetic BRS. However, it has been demonstrated that pharmacological and spontaneous BRS values are closely correlated in most instances.36 Another possible explanation for the discrepancy may be age differences. Our subjects were older than those in the study of Studinger et al (i.e., mean age 68 vs. 62 yrs). It has been shown that sympathetic activity increases with age while the increment is greater in women, especially after menopause, compared with men.37 Resting MSNA was much lower in the study by Studinger et al (22 bursts·min-1 both in men and women) than ours (39 in men and 42 in women). Additionally, we found that resting MSNA trended greater in elderly women than men, while Studinger et al did not. It is likely that BP is regulated near the threshold of the baroreflex curve, particularly in elderly women in our study, where the sensitivity is lower than the maximal point38, and therefore, we found sex differences in sympathetic BRS.
We recently found in healthy young individuals that cardiovagal BRS during BP falls (phase II of the Valsalva maneuver) was similar between sexes; however, women had lower cardiovagal BRS during BP rises (phase IV) than men.20 Similar results were found in elderly subjects in the current study. Advancing age decreases cardiovagal BRS even in the healthy population,1-4 men and women seem to have a similar decline in cardiovagal BRS, whereas the rate of increase in arterial stiffness is greater in women.17, 18, 39 One animal study40 showed that cardiovagal BRS was lower in female than male rats only during BP rises with phenylephrine, and the difference disappeared by infusion of atropine but not propranolol, suggesting that the sex difference in cardiovagal BRS was caused by a lower vagal response to baroreceptor activation. Thus, sex differences in cardiovagal BRS, which impaired the correction of hypertension in women41 may originate in the neural component of the baroreflex, altering the relationship between sympathetic and cardiovascular BRS in the elderly compared to the young12 (see Figure S1 in the Online Supplement).
The Relation between Sympathetic Baroreflex Sensitivity and Arterial Stiffness
We found that sympathetic BRS was inversely correlated with the barosensory artery stiffness. To our knowledge, there have been only two studies which simultaneously assessed sympathetic BRS and arterial stiffness;13, 35 however, neither study investigated the correlation between these two variables. O'Leary et al13 reported that the hysteresis during drug-induced changes in BP occurred in the mechanical change of the carotid artery but not sympathetic BRS in middle-aged subjects; therefore, they concluded that there was no association between sympathetic BRS and carotid artery stiffness. Conversely, Studinger et al35 described the reduction in integrated sympathetic BRS and a greater sensitivity of the neural component in elderly compared to young subjects during decreasing BP, suggesting that there was an association between integrated sympathetic BRS and carotid artery stiffness. Since all subjects in our study were seniors, we cannot determine the effects of aging per se on the relationship of sympathetic BRS and arterial stiffness. Within the elderly, sympathetic BRS was lower as carotid and aortic arterial stiffness was higher, which supports the idea proposed by Studinger et al.
This significant correlation was also shown separately in elderly men and women; however, some important sex differences existed. The line relating sympathetic BRS and artery stiffness in elderly women was shifted in parallel upward (less sensitive) compared to elderly men, but the slope of the linear regression line was similar between sexes. These data suggested that the effect of artery stiffness, serving as the mechanical component of sympathetic BRS, is similar between elderly men and women (if the effect were different between sexes, this slope would have been different). However, the higher artery stiffness did not account entirely for the lower sympathetic BRS in elderly women (if the higher arterial stiffness were the only cause of the lower sensitivity, the relation in elderly women would have been shifted right-upward on the same regression line of elderly men). We used DBP and MSNA as the input and output variables, respectively, which contained both the mechanical and neural components of the baroreflex (Figure S2). Therefore, the offset of this relation may indicate the difference of the neural component. Since Studinger et al demonstrated that the neural component of sympathetic BRS increased in the elderly,35 the lower sympathetic BRS in elderly women found in this study seems to be attributable to the lower increase in the neural component, as well as the higher stiffness of the barosensory artery during BP falls.
We found no correlation between sympathetic BRS and aortic stiffness calculated with aortic pressure derived from transfer function analysis. However, when directly measured carotid artery pressure (calibrated by brachial BP) was used for calculation of aortic stiffness, we found that although the value was similar to that calculated using transferred aortic pressure, the relationship between sympathetic BRS and aortic stiffness became more clearly discernable. This discrepancy may be caused by an artifact of the general transfer function, especially in the elderly population. It seems to be more reasonable to use carotid artery pressure rather than transferred central pressure for calculation of aortic stiffness in our study (Table S1). Certainly, we recognize that carotid artery pressure is different from real aortic pressure. Sander et al42 found that the reduction in sympathetic activity during phenylephrine injection with (isolated aortic baroreceptor loading) and without (both carotid and aortic baroreceptor loading) neck pressure was similar, indicating that aortic baroreceptors play a more important role in BP control. One previous animal study showed that the aortic baroreceptors were mainly involved in the control of high BP, whereas at lower pressures the major control occurred through the carotid baroreflexes.43 To determine the relative contribution of carotid and aortic baroreceptors in BP regulation, future studies are needed, perhaps with direct invasive measurements of vascular pressures.
Limitations
First, sympathetic BRS was evaluated during spontaneous breathing with peripheral (finger) DBP, and therefore, the entire baroreflex stimulus-response curve to central BP cannot be determined. We used the binning method to reduce the impact of non-baroreflex influence, which allowed us to reveal the physiological modulation of sympathetic control around the prevailing and operating point.20 Conversely, we could not completely solve the discrepancy between peripheral and central BP, although we used finger DBP which showed the least error compared to finger SBP (Table S2).44 Second, aortic stiffness was evaluated with MRI, which could be limited by low spatial resolution. The aortic size may affect the calculation of aortic stiffness (Table S3). However, the reproducibility for the analysis of aortic MRI in our laboratory was high while there seemed to be small sex differences (the typical error of the intra-operator variability of the manual calculation: 2.1% for men and 1.8% for women). Third, the range of the neural component is so wide, containing afferent sites including barosensory segments, central nervous system, and efferent sites, that we cannot determine which site(s) have been affected by sex (Figure S2).
Perspectives
Large epidemiological studies have demonstrated the higher prevalence of hypertension and the lower BP control rate in elderly women than men.45, 46 Since baroreflex function is blunted in hypertensive patients,4, 5 the lower sympathetic BRS in elderly women than men, combined with the lower cardiovagal BRS during BP rises shown in this study, may be one major mechanism underlying sex differences in hypertension and responses to antihypertensive medications. The lower BP control function in elderly women caused by the higher stiffness of the carotid artery and less sympathetic baroreflex buffering has clinical implications, because the relationship between BP and cardiovascular events is more pronounced in people aged ≥65 years.47 However, which component of the sympathetic baroreflex--arterial stiffness, MSNA response, or sympathetic vascular transduction--is responsible for the risks of hypertension in elderly women remains to be determined in future studies.
Supplementary Material
Acknowledgments
We are grateful to the study volunteers for their participation. We also thank Jeffrey L. Hastings, M. Dean Palmer, Peggy Fowler, Murugappan Ramanathan, Cyrus Oufi, and Wade Wang for their valuable laboratory assistance.
Funding Sources
This study was supported by the National Institutes of Health grant R01 HL091078.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Lansimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol. 1998;84:576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- 2.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281:H284–289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 3.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104:1627–1632. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- 4.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 5.Lage SG, Polak JF, O'Leary DH, Creager MA. Relationship of arterial compliance to baroreflex function in hypertensive patients. Am J Physiol. 1993;265:H232–237. doi: 10.1152/ajpheart.1993.265.1.H232. [DOI] [PubMed] [Google Scholar]
- 6.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: A predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 7.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr., Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: Implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 8.Brown AM. Receptors under pressure. An update on baroreceptors. Circ Res. 1980;46:1–10. doi: 10.1161/01.res.46.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Chesterton LJ, Sigrist MK, Bennett T, Taal MW, McIntyre CW. Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant. 2005;20:1140–1147. doi: 10.1093/ndt/gfh808. [DOI] [PubMed] [Google Scholar]
- 10.Lenard Z, Studinger P, Kovats Z, Reneman R, Kollai M. Comparison of aortic arch and carotid sinus distensibility in humans--relation to baroreflex sensitivity. Auton Neurosci. 2001;92:92–99. doi: 10.1016/S1566-0702(01)00309-5. [DOI] [PubMed] [Google Scholar]
- 11.Mattace-Raso FU, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias-Smale S, Reneman RS, Hoeks AP, Hofman A, Witteman JC. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: The rotterdam study. J Hypertens. 2007;25:1421–1426. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]
- 12.Dutoit AP, Hart EC, Charkoudian N, Wallin BG, Curry TB, Joyner MJ. Cardiac baroreflex sensitivity is not correlated to sympathetic baroreflex sensitivity within healthy, young humans. Hypertension. 2010;56:1118–1123. doi: 10.1161/HYPERTENSIONAHA.110.158329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Leary DD, Steinback CD, Cechetto AD, Foell BT, Topolovec JC, Gelb AW, Cechetto DF, Shoemaker JK. Relating drug-induced changes in carotid artery mechanics to cardiovagal and sympathetic baroreflex control. Can J Physiol Pharmacol. 2005;83:439–446. doi: 10.1139/y05-030. [DOI] [PubMed] [Google Scholar]
- 14.Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol. 2002;282:H1717–1723. doi: 10.1152/ajpheart.00899.2001. [DOI] [PubMed] [Google Scholar]
- 15.O'Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol. 2003;88:769–774. doi: 10.1113/eph8802632. [DOI] [PubMed] [Google Scholar]
- 16.Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1658–1662. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- 17.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 18.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens. 2001;19:2205–2212. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 20.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol. 2009;587:2019–2031. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: Implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 23.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 26.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol. 2007;103:867–874. doi: 10.1152/japplphysiol.01106.2006. [DOI] [PubMed] [Google Scholar]
- 27.Izumi S, Muano T, Mori A, Kika G, Okuwaki S. Common carotid artery stiffness, cardiovascular function and lipid metabolism after menopause. Life Sci. 2006;78:1696–1701. doi: 10.1016/j.lfs.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- 30.Tank J, Diedrich A, Szczech E, Luft FC, Jordan J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension. 2005;45:1159–1164. doi: 10.1161/01.HYP.0000165695.98915.9a. [DOI] [PubMed] [Google Scholar]
- 31.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- 32.Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol. 1992;263:H798–803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- 33.Davy KP, Tanaka H, Andros EA, Gerber JG, Seals DR. Influence of age on arterial baroreflex inhibition of sympathetic nerve activity in healthy adult humans. Am J Physiol. 1998;275:H1768–1772. doi: 10.1152/ajpheart.1998.275.5.H1768. [DOI] [PubMed] [Google Scholar]
- 34.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Baroreflex control of muscle sympathetic nerve activity is attenuated in the elderly. J Auton Nerv Syst. 1998;73:182–185. doi: 10.1016/s0165-1838(98)00128-3. [DOI] [PubMed] [Google Scholar]
- 35.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol. 2009;587:2049–2057. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: A nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol. 2010;298:H816–822. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol. 1998;275:R1600–1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- 38.Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T, Charkoudian N. Hysteresis in the sympathetic baroreflex: Role of baseline nerve activity. J Physiol. 2011;589:3395–3404. doi: 10.1113/jphysiol.2011.208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The framingham heart study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Rahman AA. Gender difference in baroreflex-mediated bradycardia in young rats: Role of cardiac sympathetic and parasympathetic components. Can J Physiol Pharmacol. 1999;77:358–366. [PubMed] [Google Scholar]
- 41.Tyden G. Aspects of cardiovascular reflex control in man. An experimental study. Acta Physiol Scand Suppl. 1977;448:1–62. [PubMed] [Google Scholar]
- 42.Sanders JS, Ferguson DW, Mark AL. Arterial baroreflex control of sympathetic nerve activity during elevation of blood pressure in normal man: Dominance of aortic baroreflexes. Circulation. 1988;77:279–288. doi: 10.1161/01.cir.77.2.279. [DOI] [PubMed] [Google Scholar]
- 43.Pelletier CL, Clement DL, Shepherd JT. Comparison of afferent activity of canine aortic and sinus nerves. Circ Res. 1972;31:557–568. doi: 10.1161/01.res.31.4.557. [DOI] [PubMed] [Google Scholar]
- 44.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: A validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 45.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the us adult population. Results from the third national health and nutrition examination survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 46.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 47.National high blood pressure education program working group National high blood pressure education program working group report on hypertension in the elderly. Hypertension. 1994;23:275–285. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.