Abstract
Background
Sudden cardiac death (SCD) remains a major public health problem; however, its true burden remains unknown with widely variable estimates of its incidence. We aimed to examine the contemporary epidemiology and autopsy characteristics of SCD in an ethnically diverse community.
Methods
3 physicians reviewed all deaths age ≥ 20 years reported to the San Francisco Medical Examiner (ME) in 2007 for presentations fitting WHO SCD criteria -- within 1 hour of symptom onset (witnessed) or within 24 hours of being observed alive and symptom-free (unwitnessed). After comprehensive review of ME investigation, WHO SCDs were classified as sudden arrhythmic death (SAD) or non-arrhythmic death. Coronary artery disease (CAD) and cardiac mass were evaluated in all SADs undergoing autopsy and compared to demographically similar accidental trauma control deaths.
Results
We identified 252 WHO SCDs; 145 were SADs. Men had a 2.2-fold higher SAD rate (p<0.0005). Blacks had a 3.15-fold higher SAD rate compared to Caucasians (p = 0.003). Significant CAD was present in 38.9% of SADs and associated with higher SAD risk compared to control deaths (OR 2.58, 95% CI 1.12–5.97, p=0.026). Mean cardiac mass was linearly associated with risk for SAD in cases without significant CAD (OR 2.06 per 100g, 95% CI 1.43–2.98, p<0.0005).
Conclusions
In a diverse, urban population, SAD incidence varied substantially by gender and race. Significant CAD accounted for far fewer SADs than previous studies, but remained associated with a 2.6-fold higher risk as compared to control deaths. These findings may reflect the evolving contemporary epidemiology of SCD.
Keywords: sudden death, arrhythmias, coronary artery disease, epidemiology, disparities, risk factors
Introduction
Sudden cardiac death (SCD) is a feared threat to public health and one of the leading causes of mortality in the United States. Current estimates of its incidence range from 184,000 to 450,000 per year, accounting for up to 15% of total mortality in the United States.1–3 The majority of SCDs occur out-of-hospital in patients for whom SCD is the initial and lethal manifestation of coronary artery disease (CAD).4–6 Despite numerous advances in prevention and treatment of heart disease in the last 30 years, rates of SCD remain high.7 Recent epidemiologic studies of SCD have examined homogeneous and largely Caucasian populations employing variable definitions of SCD ranging from 1 to 24 hours of symptom onset. No contemporary data exist on SCD in ethnically diverse communities.
The objective of our study was to examine the contemporary characteristics and underlying etiologies of SCD in a diverse, urban community (San Francisco County) after usual comprehensive Medical Examiner evaluation. We take advantage of the County Medical Examiner’s Office as a robust surveillance method for every incident out-of-hospital SCD as defined by World Health Organization (WHO) criteria. We then sought to evaluate CAD and cardiac mass as risk factors for SCD as compared to a demographically similar group of control deaths, accidental trauma deaths, already undergoing autopsy. We hypothesized that: (1) the standard WHO criteria for SCD are highly inaccurate for actual sudden arrhythmic death (SAD), (2) rates of SAD in San Francisco differ substantially by race and gender, and (3) CAD has decreased in prevalence as the primary cause for SAD in our diverse population in the modern era.
Methods
Setting
San Francisco, California has a population of 757,604 (2007 est., 676,305 ≥ 20 years old and encompasses 46.69 square miles. The population is 52.2% male, 44.7% Caucasian, 6.7% Black, 14.0% Hispanic, 31.5% Asian, and 3.1% other (including American Indian, Alaska Native, Hawaiian, or Pacific Islander). Eighty-one percent of people have at least a high school degree, and 45% hold a college degree or higher, but over 10% of the population live below the poverty level.10 San Francisco is served by a single county Medical Examiner (ME), and all out-of-hospital deaths occurring within San Francisco are required by California law to be reported to the ME. In addition, all deaths without a physician willing or able to determine cause of death (which encompasses nearly all emergency department deaths) are reported and accepted as cases by the ME. For all cases, the ME undertakes standard procedure for collecting pertinent information (including past medical history, paramedic reports, emergency department records, hospital records, and scene investigation) prior to the determination for performing a full autopsy or a more limited external examination.
This work was supported by grants from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research (KL2 RR024130) and the National Heart, Lung, and Blood Institute (R01 HL102090–01A1), both to ZHT. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Identification of WHO SCDs
All deaths reported to the ME and accepted as cases between January 1, 2007 and December 31, 2007 were reviewed by a panel of 3 physicians to identify all out-of-hospital SCDs for individuals ≥ 20 years. We also reviewed the death certificates of all deaths not reported to the ME to ascertain any out-of-hospital SCDs missed by the ME. The University of California, San Francisco Committee on Human Research approved this study protocol. Asian, black, Caucasian, or Hispanic ethnicity was determined by standard ME protocol -- using family report and/or pre-mortem self-report (when available). For all deaths reported to the ME, a narrative of circumstances and details at the time of death presentation were available. SCD was defined using WHO criteria: sudden, unexpected natural death in a person without any previous condition that would seem fatal and who died within 1 hour of symptom onset (event witnessed) or within 24 hours of having been observed alive and symptom-free (event unwitnessed).11 SCD cases were identified based solely on their characteristics at presentation to the ME as an out-of-hospital SCD. Deaths were excluded if they had a known history of non-cardiac chronic terminal illness (e.g., terminal cancer, end-stage renal disease) on the basis that such deaths were not unexpected. We also excluded deaths with an identifiable non-cardiac etiology (e.g., obvious recent drug use) on scene. We did not exclude patients based on other medical history including presence or absence of diabetes mellitus, hypertension, or other chronic non-terminal illnesses. For these WHO defined SCDs, we then performed a comprehensive review taking into account circumstances of death, available prior medical records, ME investigation (e.g. family and witness interviews), toxicology, and available autopsy data. Using these comprehensive data, WHO SCDs were adjudicated by the panel as either SAD or non-arrhythmic sudden deaths (e.g., congestive heart failure deaths, drug overdose, pulmonary embolism, occult cancer, etc.). Thus, SAD was defined as any SCD which met WHO criteria and no obvious non-arrhythmic cardiac cause of death was found (e.g., tamponade, myocardial rupture, acute valve failure, etc.).
Autopsy Evaluation and Comparison to Control Accidental Trauma Deaths
Autopsies were performed at the discretion of the ME upon receiving the case and typically performed when external examination alone does not reveal cause of death. Routine toxicology screening of urine, blood, liver tissue, and heart tissue for drugs was performed per standard ME practice. To estimate the prevalence of CAD and cardiac mass in the general population at risk for SCD, we identified a control group of demographically similar non-SCDs, accidental trauma deaths, the large majority of which underwent autopsy. All such accidental traumatic deaths of individuals age ≥ 20 years occurring in San Francisco in 2007 and receiving autopsy were included. We excluded homicides and suicides due to important differences in demographic characteristics and prevalent disease(s). Accidental trauma deaths are likely to occur randomly and by definition such deaths are not due to any underlying medical condition(s).
Hearts were excised and weighed. The major epicardial arteries and their main branches were cut transversely at 2-mm intervals and decalcified before sectioning if necessary. All segments were grossly examined for presence of luminal thrombus. Segments that had >50% cross-sectional luminal stenosis by visual inspection were submitted for light microscopy, histological examination, and morphometric measurements. Significant CAD was defined as ≥75% cross-sectional area reduction in at least one coronary artery; mild CAD was defined as < 75% area reduction in all coronary arteries. Active coronary lesion was defined as presence of disrupted coronary plaque, including erosion or rupture, luminal thrombus, or both.12
Statistical Methods
We used Poisson models to compare SAD incidence rates by gender and race/ethnicity; robust rather than model-based standard errors were used to account for over-dispersion of the outcome. In the case-control sample, we used a logistic model to assess the independent association of CAD with SAD, and linear models to evaluate the joint influence of case/control status and CAD on cardiac mass, both overall and allowing for interaction with case/control status. In the linear model, we accounted for non-linear age effects using a restricted cubic spline.
Results
Rates of WHO SCD and SAD
During the study period, 6246 deaths occurred in San Francisco; 4258 deaths were reported to the San Francisco medical examiner, 1988 were not reported. Death certificates of all 1988 deaths not reported were examined for place and any cardiac cause(s) of death in an attempt to identify out-of-hospital SCDs missed by ME surveillance. 90 of these 1988 deaths had primary cardiovascular cause of death; of these, 89 occurred in the inpatient, hospice, or nursing home setting. Thus only 1 possible out-of-hospital SCD identified by standard death certificate criteria was potentially missed by ME surveillance.
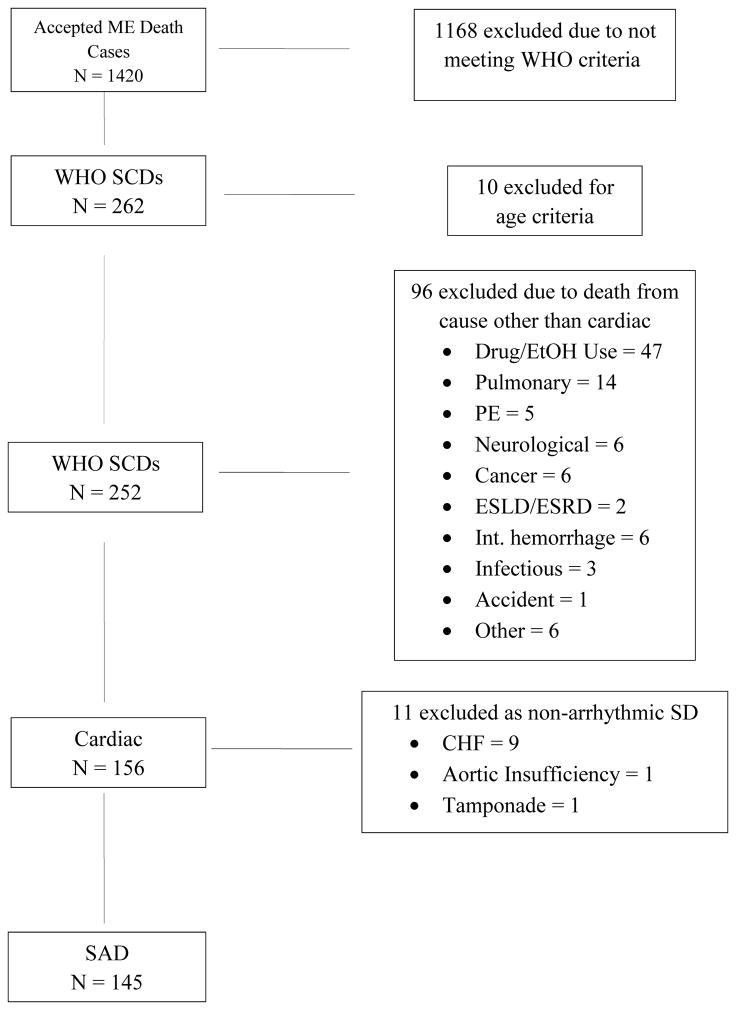
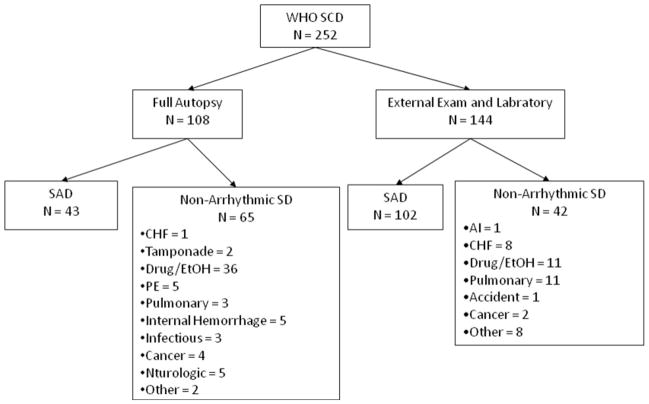
The ME accepted 1420 of 4258 deaths as cases; deaths not accepted as cases were deaths in hospital inpatients (exclusive of emergency department deaths), hospice, and subjects who were in recent (within 21 days) physician attendance. From these 1420 deaths, we identified 252 cases of WHO-defined SCD (incidence of 37.3 per 100,000; mean age 62.2 years, 70.2% male), accounting for 4.4% of overall mortality (see Figure 1 and Table 1). Of these 252 WHO SCDs, 156 deaths were determined to be due to cardiac etiology. Autopsies were performed in 108 of 252 (43%) WHO SCDs; 40% of autopsied and 71% of non-autopsied WHO SCDs were determined to be due to arrhythmic etiology (see Figure 2). Eleven deaths were due to non-arrhythmic cardiac causes -- 9 due to congestive heart failure (massive pulmonary edema), and 1 each due to tamponade and acute aortic insufficiency. Thus SAD accounted for 145 of the 252 WHO-defined SCDs, representing an incidence of 21.4 per 100,000 persons and a positive predictive value for WHO criteria for arrhythmic etiology of 57.5%. No cases of hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, or anomalous coronary arteries were found. The most common non-cardiac causes in WHO SCDs were drug or alcohol overdose found by positive toxicology (47), followed by pulmonary causes (14) and occult metastatic cancer (6).
Figure 1.
Determination of Sudden Arrhythmic Deaths
SF ME = San Francisco Medical Examiner; WHO = World Health Organization; SCD = Sudden cardiac death; SAD = Sudden arrhythmic death; PE = Pulmonary embolism; ESLD = End-stage liver disease; ESRD = End-stage renal disease, CHF = Congestive heart failure.
Table 1.
Demographic Characteristics of WHO SCDs and SADs and Reference Populations
| WHO SCDs | SAD Total | Trauma Total | San Francisco Population | Autopsied SADs evaluated for CAD | Autopsied Trauma Deaths evaluated for CAD | |
|---|---|---|---|---|---|---|
| Total Age ≥ 20 | 252 | 145 | 228 | 676,305 | 36 | 191 |
| Age | 62.2 ± 16.3 | 67.4 ± 16.6 | 49.9 ± 16.8 | 53.9 ± 17.3 | 49.2 ± 17 | |
| Age Range | 24 – 99 | 24 – 99 | 20 – 95 | 24 – 92 | 20 – 93 | |
| Male | 177 (70.2%) | 102 (70.3%) | 168 (73.7%) | 347,368 (51.4%) | 29 (80.5%) | 139 (72.7%) |
| Ethnicity | ||||||
| Caucasian | 107 (42.5%) | 66 (45.5%) | 126 (55.3%) | 328,799 (48.6%) | 21 (58.3%) | 103 (53.3%) |
| Black | 65 (25.8%) | 27 (18.6%) | 48 (21.1%) | 42,667 (6.3%) | 7 (19.4%) | 39 (20.4%) |
| Hispanic | 21 (8.3%) | 11 (7.6%) | 23 (10.1%) | 81,577 (12.1%) | 3 (8.3%) | 20 (10.5%) |
| Asian | 57 (22.6%) | 39 (26.9%) | 29 (12.7%) | 205,969 (30.5%) | 5 (13.9%) | 27 (14.1%) |
Figure 2.
Stratification of WHO SCDs by Autopsy Status
SAD = Sudden arrhythmic death; SD = Sudden death; CHF = Congestive heart failure; PE = Pulmonary embolism; AI = Aortic insufficiency
Rates of SAD by Ethnicity
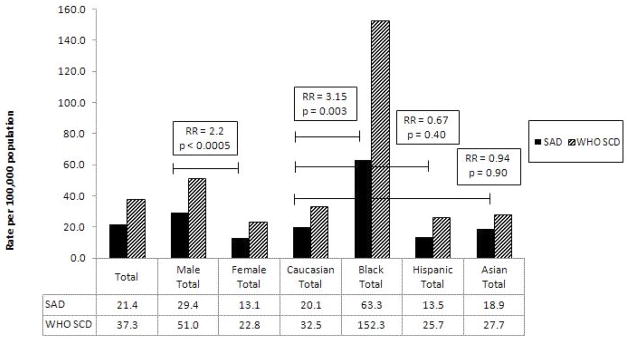
Among WHO SCDs determined to be of arrhythmic etiology (SADs), incidence was 2.2-fold higher among men (29.4 per 100,000 vs. women 13.1 per 100,000 p<0.0005) (see Figure 3). Rates of SAD were highest among black men (70.9 per 100,000) and lowest among Hispanic females (7.9 per 100,000). Blacks (7.4% of SF population) suffered a 3.15-fold (95% CI: 1.49–6.67, p = 0.003) higher rate of SAD compared to Caucasians (43.8% of S.F. population); the comparable rates for Asians and Hispanics were 0.94 (95% CI: 0.37–2.42, p = 0.90) and 0.67 (95% CI: 0.27–1.69, p = 0.40) that of the reference Caucasian rate, respectively.
Figure 3.
Rates of SAD and WHO SCD by Gender and Ethnicity
RR is for SAD. RR = relative risk; SAD = Sudden arrhythmic death; SCD = Sudden cardiac death
Coronary Artery Disease in SADs and Trauma Control Deaths
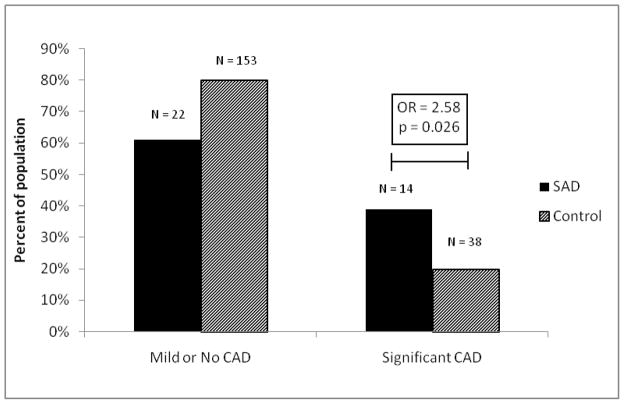
We identified 145 SADs and 228 accidental trauma controls (Table 1). Of these, 36 SADs (mean 53.9 years, 80.5% male) and 191 controls (mean 49.2 years, 72.7% male) underwent autopsy evaluation for CAD and cardiac mass. Due to decomposition or other technical factors such as significant cardiac trauma (37 controls), CAD was not evaluated in all autopsied cases. Significant CAD was found in 14 SADs (38.9%) and 38 controls (19.9%), p = 0.018 (see Figure 4). Only 1 of 36 (2.7%) SADs and none of 191 controls had an active coronary lesion, p=0.16. In a logistic model adjusting for age, sex, race, and BMI, the OR for the association of significant CAD with SAD was 2.58 (95% CI 1.12–5.97, p=0.026).
Figure 4.
Coronary Artery Disease in Autopsied SADs and Controls
OR = Odds ratio; SAD = Sudden arrhythmic death; CAD = Coronary artery disease; Control = Accidental trauma death
Cardiac Mass in SADs and Trauma Control Deaths
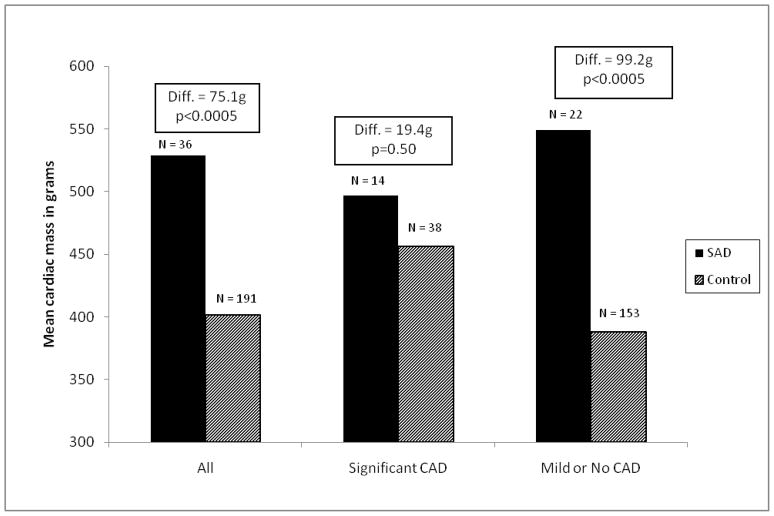
Mean cardiac mass was higher in SADs compared to controls by 75.1g (95% CI 37.4–112.9, p<0.0005), after adjustment for age, sex, race/ethnicity, and BMI; however, this difference varied by presence of significant CAD on autopsy (interaction p=0.026). Specifically, cardiac mass was significantly higher in SADs than controls among persons without significant CAD (difference 99.2g, 95% CI 54.1–144.3, p<0.0005), but was similar among those with significant CAD (difference 19.4g, 95% CI 37.7–76.6, p=0.50) (see Figure 5). After adjustment for age and BMI, cardiac mass was linearly associated with risk for SAD: OR 2.06 per 100 g among persons without significant CAD, 95% CI 1.43–2.98, p<0.0005; OR 1.25 per 100 g among those with significant CAD, 95% CI 0.81–1.92, p=0.32; p=0.08 for interaction with significant CAD. Results were similar in analyses using cardiac mass index (cardiac mass divided by BMI), rather than regression adjustment for BMI.
Figure 5.
Mean Cardiac Mass in Autopsied SADs and Controls
Diff = Mean difference in cardiac mass; SAD = Sudden arrhythmic death; CAD = Coronary artery disease; Control = Accidental trauma death
Discussion
This investigation sought to determine the characteristics of SCD in an ethnically diverse, urban community which may presage forthcoming rapid national demographic shifts in the United States in which no majority ethnicity is projected by 2042.13 Using a robust surveillance method for all consecutive SCDs fitting standard, widely accepted WHO criteria, we found a rate of WHO SCD that is comparable to recent estimates. Only 1 (0.4%) possible out-of-hospital SCD was not reported to the ME. After excluding non-cardiac and nonarrhythmic causes by ME investigation, true SAD accounted for only 57.5% of WHO SCDs. SAD varied substantially by gender and race with higher incidence in men and blacks. Among SADs undergoing autopsy, significant CAD accounted for 38.9% of cases, less than half of historical estimates, but was still associated with a 2.6-fold higher risk of SAD as compared to accidental trauma control deaths. Cardiac mass was also significantly higher in SADs as compared to controls, but linearly associated with risk for SAD among only those without CAD.
The somewhat lower rate of SAD may be due to several factors and reflect recent trends: (1) the effects of contemporary treatment of heart disease, including recent widely adopted primary prevention implantable cardioverter defibrillators (ICDs) in patients with heart failure (2) earlier recognition of sudden cardiac arrest (SCA) resulting in bystander and early resuscitation, (3) the diversity of the San Francisco population which has a significant proportion of Asian and Hispanic residents, both with lower rates of SCD as compared to Caucasians, (4) the exclusion of SCA survivors, who have been included in some studies, and (5) a concerted effort to exclude non-cardiac sudden deaths by reviewing all records to exclude cases with a known terminal illness or with obvious signs of a non-cardiac cause of death. These factors together may explain the observed lower incidence of true SAD as compared to older estimates of SCD/SCA in more homogeneous populations.
Our findings corroborate previous studies that have also demonstrated the poor specificity of the WHO definition of SCD for SAD, the most relevant phenotype from a public health standpoint and the only one prevented by ICDs. This study highlights the need for refinement of criteria for identification of SCD as true arrhythmic etiology (SAD) accounted for just over half of SCDs as defined by standard, widely accepted criteria. Despite attempts to exclude suspected and obvious non-cardiac deaths prior to autopsy, a non-cardiac cause of sudden death was found in nearly two-thirds of all autopsied WHO SCDs.
By midcentury, Caucasians are estimated to no longer represent the majority ethnic group in the United States, with rapid increases in Hispanic and Asian populations.13 As the population diversifies, investigation in diverse communities is essential to further define racial differences in disease. We demonstrate that Asians and Hispanics (combined 46% of SF population) have significantly lower rates of SCD and SAD while blacks have a greater than 3-fold higher rate as compared to Caucasians. Indeed, this may partially explain an overall WHO SCD rate (37.3/100,000) more comparable to rates recently observed in homogeneous Asian populations (Okinawa, Japan: 37/100,00017) than predominantly Caucasian populations (Oregon, USA: 53/100,000,9 Ireland: 51.2/100,00016). A higher rate of SCD in blacks has been previously noted but has been reported inconsistently. While our study confirms these prior observations, further investigation is needed to determine the reason(s) for this disparity.
It has been reported that acute coronary-related events account for the majority of sudden cardiac deaths in the adult population and prior research suggests that approximately 80% of adults with SCD have severe CAD.21–26 However, in this community-based study, only one of 36 autopsied SADs had an active coronary lesion as the cause of SAD while significant CAD was found in fewer than 40% of SADs. Nevertheless, significant CAD still imparted a 2.6-fold higher risk of SCD as compared to accidental trauma deaths. These results are in notable contrast to the 80% prevalence of significant CAD found in a recent autopsy series of 71 sudden unexpected deaths in adults aged 25–64 years in Minnesota between 2001–2004.8 However, the case definition used in that study, a symptom duration up to 24 hours, may bias towards ischemic (i.e., pump failure or acute MI) rather than arrhythmic causes of death, whereas the WHO definition (symptom onset < 1 hour) may be more specific for SAD, whether ischemic or non-ischemic. In addition, as with prior reports on the prevalence of CAD in SCD,21–26 cases were uniformly Caucasian (96%) and male (86%); thus considerable differences in case demographics and definition may together account for the disparity in CAD prevalence found in sudden deaths in Minnesota vs. San Francisco. Another possibility is that we have underestimated the contribution of CAD due to the exclusion in our analysis of WHO SCDs deemed cardiac/arrhythmic without autopsy. Indeed, 40% of autopsied WHO SCD cases were determined to be arrhythmic in etiology compared to 71% of non-autopsied WHO SCD cases. This suggests that cases in which CAD was thought to be a more likely cause of SCD were less likely to undergo full autopsy. On the other hand, the fact that 60% of autopsied SCDs found non-cardiac cause suggests that many of the SCDs deemed arrhythmic without autopsy may actually have been non-cardiac had autopsy been performed.
Previous autopsy series of selected SCDs have reported inconsistent findings with regard to cardiac mass. In our population, we found a higher mean cardiac mass in SADs as compared to controls but this difference varied by the presence of significant CAD. We demonstrate a specific linear relationship between cardiac mass and increased SAD risk among those without significant CAD, but not in those with significant CAD. Cardiac mass may therefore be a useful adjunct in risk stratification for SAD in those without CAD.
Strengths and Limitations
The major strength of this study is the use of a robust surveillance method for nearly all SCDs in a diverse, urban community, incorporating comprehensive analysis including autopsy to provide data not available in prior studies based on death certificate measures of SCD. The study is limited by its retrospective design and an autopsy rate of 43% for WHO SCDs, although this is notably more than triple the respective 11.6% and 14% autopsy rates of SCDs in the recent studies of community SCDs in Oregon9 and sudden deaths in VALIANT,28 and similar to the 62% autopsy rate in the Minnesota study.8 As a retrospective study, our data are also limited by the standard practice of the medical examiner in the reviewed year. Of the 4258 deaths reported to the ME in 2007, 1420 were accepted as ME cases. In San Francisco, all out-of-hospital deaths, including deaths occurring in the emergency department, require report to the ME. Cases not accepted were typically inpatient hospital deaths, hospice deaths, and deaths for whom a primary physician was willing to sign a death certificate, usually because death was expected (e.g. metastatic cancer) or the patient was seen within 21 days of death. Thus, we may have missed a number of SCDs for which a physician signed a death certificate with assumed cardiac cause. However, we have likely captured the large majority of SCDs in 2007 as evidenced by a SCD incidence which is comparable to other recent reports.
Conclusions
In summary, our results demonstrate the low specificity of WHO criteria for true arrhythmic sudden death, substantial ethnic and gender differences in burden of disease, a far lower contribution of CAD to SAD than previous reports in homogeneous populations, and the potential role for cardiac mass in risk stratification of SAD for those without CAD. These findings illustrate the evolving, contemporary epidemiology of SAD in a diverse population.
Acknowledgments
We are gratefully indebted to the investigators and staff of the San Francisco Medical Examiner’s Office for assistance with data collection. We thank Karl Sporer, M.D. for providing access to San Francisco Fire Department paramedic reports.
Abbreviations List
- SCD
Sudden cardiac death
- SAD
Sudden arrhythmic death
- SCA
Sudden cardiac arrest
- CAD
Coronary artery disease
- PE
Pulmonary embolism
- ESLD
End-stage liver disease
- ESRD
End-stage renal disease
- CHF
Congestive heart failure.
- ME
Medical examiner
- WHO
World Health Organization
Footnotes
Disclosures:
This research was funded by grants from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research (KL2 RR024130) and the National Heart, Lung, and Blood Institute (R01 HL102090-01A1), both to ZHT. There are no relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.State-specific mortality from sudden cardiac death--United States, 1999. MMWR Morb Mortal Wkly Rep. 2002;51:123–126. [PubMed] [Google Scholar]
- 3.Myerburg RJ. Scientific gaps in the prediction and prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2002;13:709–723. doi: 10.1046/j.1540-8167.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: The Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Gagnon DR, Cupples LA. Epidemiology of sudden coronary death: Population at risk. Can J Cardiol. 1990;6:439–444. [PubMed] [Google Scholar]
- 6.Rea TD, Eisenberg MS, Becker LJ, Murray JA, Hearne T. Temporal trends in sudden cardiac arrest: A 25-year emergency medical services perspective. Circulation. 2003;107:2780–2785. doi: 10.1161/01.CIR.0000070950.17208.2A. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 8.Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: Results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed July 14, 2010];US Census Bureau San Francisco County quickfacts. http://quickfacts.census.gov/qfd/states/06/06075.html.
- 11.Report of a working group parts I and II Copenhagen. Denmark: Regional office for Europe World Health Organization; 1969. Working group on ischemic heart disease registers. [Google Scholar]
- 12.Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92:1701–1709. doi: 10.1161/01.cir.92.7.1701. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Census Bureau. [Accessed 7/14/10]; http://www.census.gov/newsroom/releases/archives/population/cb08-123.html.
- 14.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 15.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in new york city. The pre-hospital arrest survival evaluation (PHASE) study. JAMA. 1994;271:678–683. [PubMed] [Google Scholar]
- 16.Byrne R, Constant O, Smyth Y, Callagy G, Nash P, Daly K, Crowley J. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the west of Ireland. Eur Heart J. 2008;29:1418–1423. doi: 10.1093/eurheartj/ehn155. [DOI] [PubMed] [Google Scholar]
- 17.Tokashiki T, Muratani A, Kimura Y, Muratani H, Fukiyama K. Sudden death in the general population in Okinawa: Incidence and causes of death. Jpn Circ J. 1999;63:37–42. doi: 10.1253/jcj.63.37. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Schatzkin A. Sudden death: Lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 19.Goraya TY, Jacobsen SJ, Belau PG, Weston SA, Kottke TE, Roger VL. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000;75:681–687. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 20.Gillum RF. Sudden cardiac death in Hispanic Americans and African Americans. Am J Public Health. 1997;87:1461–1466. doi: 10.2105/ajph.87.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss AJ, Goldenberg I. Prevention of sudden cardiac death: Need for a plaque stabilizer. Am Heart J. 2010;159:15–16. doi: 10.1016/j.ahj.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 23.Myerburg RJ, Interian A, Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–19F. doi: 10.1016/s0002-9149(97)00477-3. [DOI] [PubMed] [Google Scholar]
- 24.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death. Structure, function, and time-dependence of risk. Circulation. 1992;85:I2–10. [PubMed] [Google Scholar]
- 25.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: Epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–1197. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 26.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 27.Burke AP, Farb A, Liang YH, Smialek J, Virmani R. Effect of hypertension and cardiac hypertrophy on coronary artery morphology in sudden cardiac death. Circulation. 1996;94:3138–3145. doi: 10.1161/01.cir.94.12.3138. [DOI] [PubMed] [Google Scholar]
- 28.Pouleur AC, Barkoudah E, Uno H, Skali H, Finn PV, Zelenkofske SL, Belenkov YN, Mareev V, Velazquez EJ, Rouleau JL, Maggioni AP, Kober L, Califf RM, McMurray JJ, Pfeffer MA, Solomon SD. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]