SUMMARY
Malignant melanoma is characterized by frequent metastasis, however specific changes that regulate this process have not been clearly delineated. Although it is well known that Wnt signaling is frequently dysregulated in melanoma, the functional implications of this observation are unclear. By modulating β-catenin levels in a mouse model of melanoma that is based on melanocyte-specific Pten loss and BrafV600E mutation, we demonstrate that β-catenin is a central mediator of melanoma metastasis to lymph node and lung. In addition to altering metastasis, β-catenin levels control tumor differentiation and regulate both MAPK/Erk and PI3K/Akt signaling. Highly metastatic tumors with β-catenin stabilization are very similar to a subset of human melanomas. Together these findings establish Wnt signaling as a metastasis regulator in melanoma.
INTRODUCTION
Malignant melanoma is the most deadly form of skin cancer and its incidence is increasing both in the U.S. and worldwide (Purdue et al., 2008). Nearly all melanoma deaths are a result of metastasis, which can occur early in disease progression, even from thin primary tumors (Bedrosian et al., 2000). Recent advances in melanoma therapeutics have led to improved survival in patients with metastatic melanoma, however; prognosis is still poor for most patients (Hodi et al., 2010; Chapman et al., 2011). Despite an improving understanding of genetic alterations present in human melanomas, how these changes relate to the metastatic process is not well understood. Metastasis is difficult to study in vitro, making the use of mouse models central to the functional evaluation of the roles of genetic changes observed in human melanomas in the metastatic process.
β-catenin associates with E-cadherin as part of the adherens junction and can also interact with T-cell factor/lymphoid enhancing factor (TCF/LEF) to activate the transcription of target genes as part of the canonical Wnt signaling pathway. β-catenin mutations were originally found to be present in 23% of melanoma cell lines (Rubinfeld et al., 1997) and have since been consistently described in about 5% of primary, uncultured melanomas (Omholt et al., 2001; Demunter et al., 2002; Reifenberger et al., 2002; Forbes et al., 2010). Nuclear β-catenin, which is thought to reflect canonical Wnt pathway activation, is present in approximately 1/3 of human melanoma specimens (Rimm et al., 1999). The mechanisms of β-catenin dysregulation are likely diverse, but are at least in part due to mutations both in β-catenin as well as other Wnt pathway components (reviewed by Larue and Delmas, 2006). Subsequent analyses have also reported abnormal cellular localization of β-catenin in the cytoplasm and nucleus of melanomas (Silye et al., 1998; Sanders et al., 1999; Omholt et al., 2001; Kageshita et al., 2001; Demunter et al., 2002; Kielhorn et al., 2003; Tucci et al., 2007; Chien et al., 2009). The consequences of this dysregulation, as well as the functional implications of β-catenin activating mutations in vivo, remain somewhat unclear. For example, while Wnt signaling activation has been shown to contribute to melanoma formation in mice (Delmas et al., 2007), other studies have reported that increased β-catenin staining is associated with improved survival in melanoma patients (Chien et al., 2009). These findings suggest that the results of Wnt/β-catenin signaling activation are complex and likely context-dependent.
β-catenin is important for normal melanocyte biology and is required for survival of melanocytes from neural crest precursors (Hari et al., 2002). Part of this requirement relates to the direct transcriptional activation of MITF by β-catenin when Wnt signaling is activated. MITF is a transcription factor that regulates melanocyte function and survival. Similarly to β-catenin, MITF is dysregulated in melanoma, where it is has been shown to be amplified in 10% of primary and 21% of metastatic melanomas (Garraway et al., 2005). The functional impact of MITF amplification and overexpression in human melanoma are also somewhat unclear (Garraway et al., 2005; Ugurel et al., 2007; Nazarian et al., 2010). The implications of activation of both Wnt signaling and melanocytic differentiation in human melanomas remain unresolved and highlight the need for systematic in vivo evaluation of the relationship between Wnt/MITF alterations and disease progression in the context of genetic alterations commonly observed in human melanomas.
In this study, we utilized a conditional mouse model of melanoma based on melanocyte-specific Pten loss and the BrafV600E activating mutation in order to help clarify the role of Wnt/β-catenin signaling and downstream factors such as MITF in melanoma formation and progression. By either inactivating or stabilizing β-catenin in these melanomas, the functional role of β-catenin was specifically evaluated. The mouse models of melanoma generated in this study were closely characterized and compared to human melanomas with analogous genetic changes.
RESULTS
β-catenin loss inhibits melanoma formation in Pten/Braf-driven melanomas
In order to evaluate the role of canonical Wnt signaling and β-catenin in melanoma formation and progression, we used a previously characterized conditional mouse model of melanoma based on melanocyte-specific Pten-inactivation and the BrafV600E activating mutation (Dankort et al., 2009). In this model (henceforth referred to as the “Pten/Braf” model), genetic recombination of floxed alleles and melanoma formation occurs after topical application of 4-hydroxtamoxifen (4-HT). Further, mice develop melanoma from endogenous melanocytes, closely recapitulating the complex nature of tumor initiation and formation in a physiologically accurate microenvironment. In the Pten/Braf model, just these two genetic changes are sufficient to cause melanoma in 100% of mice within 1 month of generalized tumor induction by topical application of 4-HT (Figure 1A; Dankort et al., 2009).
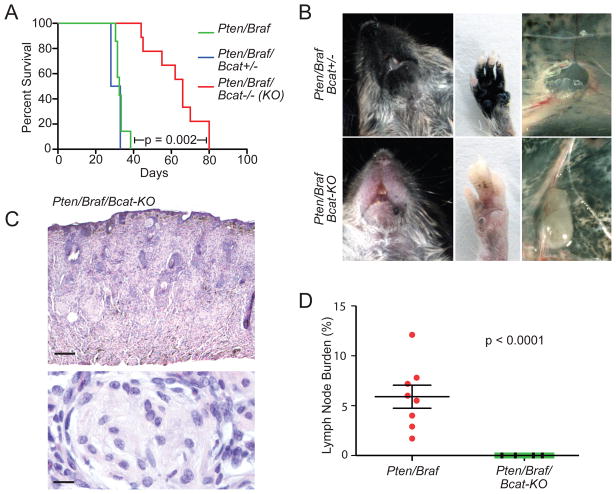
Figure 1. β-catenin loss inhibits melanoma formation in Pten/Braf-driven melanomas.
(A) Survival analysis of Pten/Braf cohorts after perinatal/generalized tumor induction.
(B) Comparison of Pten/Braf/Bcat+/− (top) and Pten/Braf/Bcat-KO (bottom) tumor burden at 28 days of age. Metastasis to inguinal lymph node is also shown (right panels).
(C) Hematoxylin and eosin (H&E) stained Pten/Braf/Bcat-KO flank tumor, scale 300 μM (top) and 10 μM (bottom).
(D) Quantification of metastasis to the inguinal lymph nodes in Pten/Braf/Bcat-KO mice (n=13) compared to Pten/Braf mice (n=8). Error bars represent standard error of the mean (SEM).
See also Figure S1.
To study the role of endogenous β-catenin in melanoma formation and progression, β-catenin was inactivated in the Pten/Braf model by using a previously characterized conditional knockout Ctnnb1 allele (Brault et al., 2001; referred to as Bcat-KO). In these mice, wildtype β-catenin is expressed in melanocytes until Cre-mediated recombination results in β-catenin inactivation at the time of tumor induction. Pten/Braf/Bcat+/− or Pten/Braf/Bcat−/− (KO) mice were treated perinatally with topical 4-HT in order to induce generalized recombination in melanocytes. In mice in which both copies of β-catenin were inactivated, a significant increase in median survival was observed relative to the Pten/Braf mice (Figure 1A). Although pigmented lesions did eventually develop in Pten/Braf/Bcat-KO mice, melanoma formation was significantly delayed, especially on footpads and peri-oral skin and mucosa (Figure 1B). Histologically, Pten/Braf/Bcat-KO melanomas invade deep into the dermis and subcutis. In contrast to Pten/Braf melanomas, which are pigmented, these tumors show only rare pigmented tumor cells and exhibit focal nerve sheath-like histopathological features (Figure 1C).
Pten/Braf melanomas metastasize to draining lymph nodes in 100% of mice treated perinatally with 4-HT (Dankort et al., 2009). However, when β-catenin is inactivated, metastatic tumor cells in draining lymph nodes become undetectable, even at up to 80 days of age (Figure 1B and 1D). These findings suggest that endogenous β-catenin is important both in melanoma formation, as well as subsequent spread of tumor cells to draining lymph nodes. Melanomas did not form when β-catenin was inactivated in the context of either BrafV600E mutation, or Pten loss alone, demonstrating that β-catenin inactivation does not promote tumorigenesis in the context of these individual oncogenic changes (Figure S1A).
β-catenin stabilization accelerates Pten/Braf-driven melanomagenesis
Activation of the canonical Wnt signaling pathway results in stabilization, accumulation, and nuclear translocation of β-catenin. In the nucleus, β-catenin interacts with the Lef1/Tcf family of transcription factors leading to transcriptional activation of target genes such as: MYC, CCND1 (Cyclin D1), and AXIN2, as well as melanocyte-specific transcripts such as MITF (Larue and Delmas, 2006; MacDonald et al., 2009). Mutations to exon 3 phosphorylation sites, which regulate β-catenin stability, provide an alternate means for increasing β-catenin activity even in the absence of Wnt ligands (Omholt et al., 2001; Demunter et al., 2002; Reifenberger et al., 2002). In light of the inhibitory effect of β-catenin loss in Pten/Braf melanomas and the prevalence of β-catenin stabilizing mutations in human melanomas, we tested the effects of β-catenin stabilization in the Pten/Braf tumor model. In order to do this, Pten/Braf mice were crossed with mice bearing the previously characterized Ctnnb1loxex3 allele (Harada et al., 1999). Prior to recombination, wildtype β-catenin is expressed in melanocytes, but after Cre-mediated excision of exon 3, a stabilized (degradation-resistant) version of β-catenin is expressed (Bcat-STA allele). Mutations in human melanomas almost always affect exon 3 phosphorylation sites (Forbes et al., 2010) resulting in analogous stabilization of β-catenin.
After generalized induction of recombination, Pten/Braf/Bcat-STA mice exhibit a reduced survival relative to Pten/Braf mice (Figure 2A–B). By the time of weaning, Pten/Braf/Bcat-STA mice are moribund with heavily pigmented tumors (Figure 2C–D). Necropsy revealed enlarged, pigmented lymph nodes in all mice, which were confirmed to contain metastatic melanoma (Figure 2C and 2E). Upon histological examination, tumors were markedly pigmented throughout their entire thickness and tumor cells could be found surrounding and directly abutting the microvasculature.
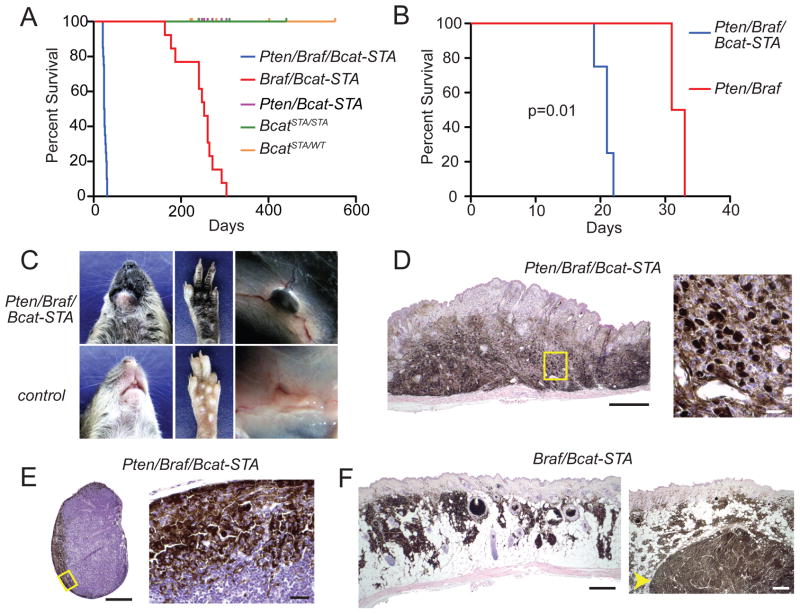
Figure 2. β-catenin stabilization accelerates Pten/Braf-driven melanomagenesis.
(A) Survival analysis of cohorts with β-catenin stabilization after perinatal tumor induction.
(B) Survival analysis of Pten/Braf littermates, either with or without β-catenin stabilization, after perinatal tumor induction.
(C) Tumor phenotype in Pten/Braf/Bcat-STA mice (top panels) compared to non-tumor prone mice (bottom panels) at 21 days of age.
(D) H&E stained Pten/Braf/Bcat-STA flank tumor, scale 500 μM. Right panel: high power of outlined field, scale 20 μM.
(E) H&E stained tumor-draining lymph node from a Pten/Braf/Bcat-STA mouse, scale 400 μM. High power of outlined field, scale 20 μM, right panel.
(F) H&E stained nevi (left panel) and tumor (right panel, arrow) from a Braf/Bcat-STA mouse, scale 500 μM (left) and 500 μM (right).
See also Table S1.
Melanomas also formed in mice with Braf activation and β-catenin stabilization in the presence of wildtype Pten after generalized induction of recombination. These tumors, in contrast to the Pten/Braf/Bcat-STA tumors, had a very long latency, with a median survival of 250 days (Figure 2A). Braf/Bcat-STA tumors generally grew very slowly and were heavily pigmented (Figure 2F). Approximately 10% of tumors exhibited more rapid growth, which was always accompanied by relative loss of melanocytic differentiation antigen expression (Table S1). In contrast to the Pten/Braf/Bcat-STA model, neither manifestation of the Braf/Bcat-STA model exhibited distant metastasis.
β-catenin signaling controls the ability of melanomas to metastasize to lung, bowel, and spleen
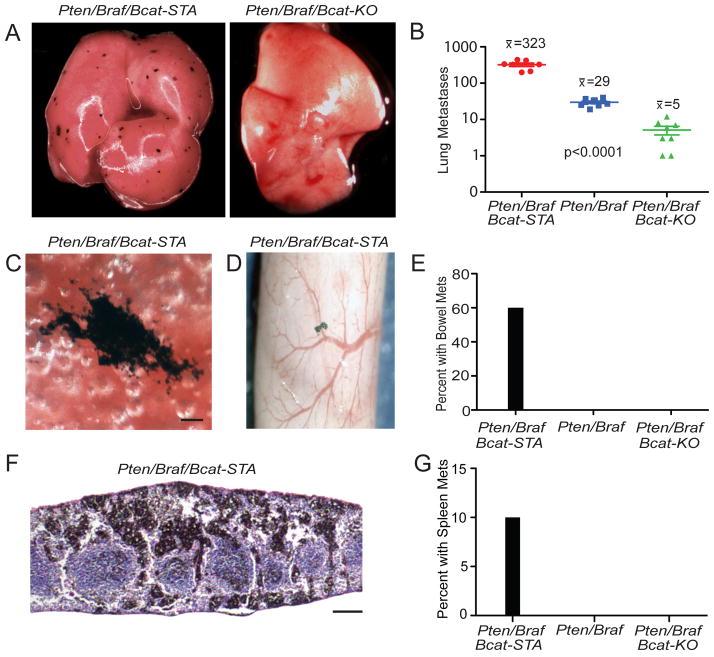
As most melanoma deaths are due to metastasis to distant organs, we characterized the pattern of visceral metastasis in Pten/Braf melanomas with altered β-catenin status. In Pten/Braf/Bcat-STA mice, lung metastases were present at nearly a 100-fold greater frequency than Pten/Braf/Bcat-KO mice (Figures 3A–B and S2A–C). The metastases were irregular in shape and ranged in size from <0.1mm to >0.5 mm (Figures 3C and S2A–C). These pigmented metastases stained strongly for the melanocytic marker Tyrp1 (Figure S2D–E). β-catenin stabilization in Pten/Braf melanomas also resulted in metastasis to other visceral sites such as bowel and spleen (Figure 3D–G).
Figure 3. β-catenin signaling controls the ability of melanomas to metastasize to lung, bowel, and spleen.
(A) Lungs from a perinatally treated Pten/Braf/Bcat-STA mouse at 27 days of age (left panel) and a perinatally treated Pten/Braf/Bcat-KO mouse at 56 days of age (right panel).
(B) Comparison of number of lung metastases visible on the surface of the lung in perinatally treated litters. Error bars represent SEM.
(C) Lung metastasis from a perinatally treated Pten/Braf/Bcat-STA mouse, scale 100 μM.
(D) Bowel metastasis from a perinatally treated Pten/Braf/Bcat-STA mouse.
(E) Comparison of number of bowel metastases in perinatally treated litters.
(F) H&E stained spleen metastasis from a perinatally treated Pten/Braf/Bcat-STA mouse, scale 200 μM.
(G) Comparison of number of spleen metastases in perinatally treated litters.
See also Figure S2.
Locally-induced melanomas are highly metastatic and can form lethal metastases
The conditional and inducible nature of this mouse model system allows for the generation of small, anatomically restricted melanomas by focal application of a small volume of 4-HT to adult mice (Dankort et al., 2009). Using this approach, rather than the generalized, perinatal inductions used until this point, melanomas were induced on the flank skin of Pten/Braf mice with wildtype, inactivated, or stabilized β-catenin. Melanocytic proliferations generally became grossly apparent approximately 2 weeks after induction of recombination, growing to 1 cm tumors by 4 to 8 weeks, at which point mice were euthanized for analysis (Figure 4A). When β-catenin was stabilized, tumors grew more rapidly to 1 cm (Figure S3A). Melanomas still formed in mice with inactivation of both copies of β-catenin (Figure 4A). PCR combined with Western blotting and immunohistochemistry confirmed recombination of floxed Ctnnb1 alleles as well as the predicted changes to β-catenin at the protein level (Figure S3B–D). When β-catenin was stabilized, it was frequently found in the nucleus of tumor cells and the stabilized protein (lacking exon 3) could be visualized by Western blot. In Pten/Braf melanomas, wildtype β-catenin was generally found within the cytoplasm, but could also be visualized in the nucleus. β-catenin staining was absent in tumor cells from Pten/Braf/Bcat-KO mice.
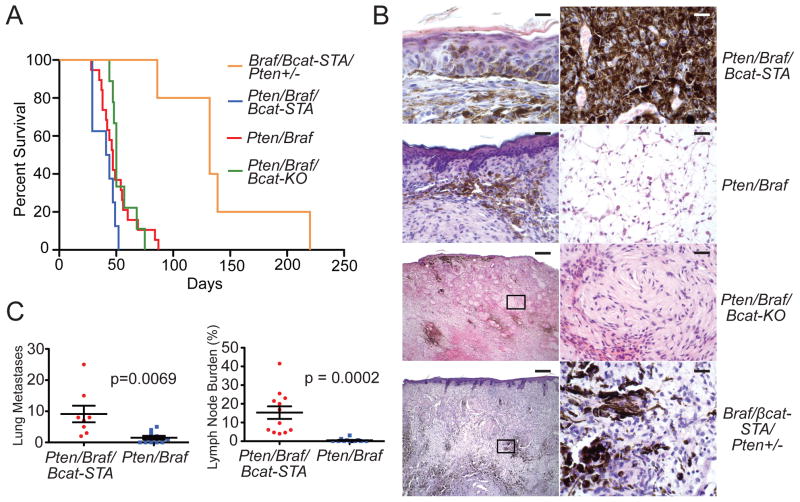
Figure 4. Locally induced melanomas are highly metastatic and can form lethal metastases.
(A) Survival analysis of cohorts after localized tumor induction. Mice were euthanized when tumors reached 1 cm.
(B) H&E stained sections of locally induced tumors from a Pten/Braf/Bcat-STA mouse (top panels, scale 20 μM left, 20 μM right), a Pten/Braf mouse (upper middle panels, scale 100 μM left, 50 μM right), a Pten/Braf/Bcat-KO mouse (lower middle panels, scale 400 μM left, 50 μM right), and a Braf/Bcat-STA/Pten+/− mouse (bottom panels, scale 400 μM left, 50 μM right).
(C) Quantification of lung (left panel) and lymph node (right panel) metastasis in mice with focally induced melanomas. Error bars represent SEM.
In addition to changes in β-catenin, these tumors displayed vastly different histology (Figure 4B). Pten/Braf/Bcat-STA tumors were characterized by large pigmented cells that were present throughout the thickness of the tumor. Pten/Braf tumors were characterized by increased edema in deeper parts of the tumor, where tumor cells were often relatively smaller in size. Similarly to the generalized tumor inductions, Pten/Braf/Bcat-KO melanomas had relatively little pigmentation, bland spindled cytology, and often exhibited areas of nerve sheath-like differentiation. Metastases were infrequent in mice with locally induced Pten/Braf melanomas with either wildtype or inactivated β-catenin. However, when β-catenin was stabilized, lung metastases and expansile lymph node metastases were prevalent (Figure 4C).
In the various iterations of the Pten/Braf model, flank melanomas grow rapidly, leaving little time for metastasis to occur. To circumvent this issue and develop models in which morbidity and mortality were related directly to metastases, rather than the primary tumor, two approaches were taken. First, tumors were induced on the distal tail of Pten/Braf/Bcat-STA mice (Figure S3F). Second, localized tumors were induced on the flank in Braf/Bcat-STA mice that had only one copy of Pten inactivated (Figure 4A–B). Both approaches resulted in slower primary tumor growth, allowing mice to be followed for metastasis for up to 1 year. Clinically symptomatic metastases developed in 2/9 tail melanoma-bearing mice and 1/5 flank melanoma-bearing mice (Figure S3E, S3G, and Table S2). Similar approaches in Pten/Braf mice, without β-catenin stabilization, resulted in primary tumor formation, but not distant metastasis (Table S2).
Enhanced metastasis in Pten/Braf/Bcat-STA melanomas is accompanied by an increase in melanocytic differentiation markers
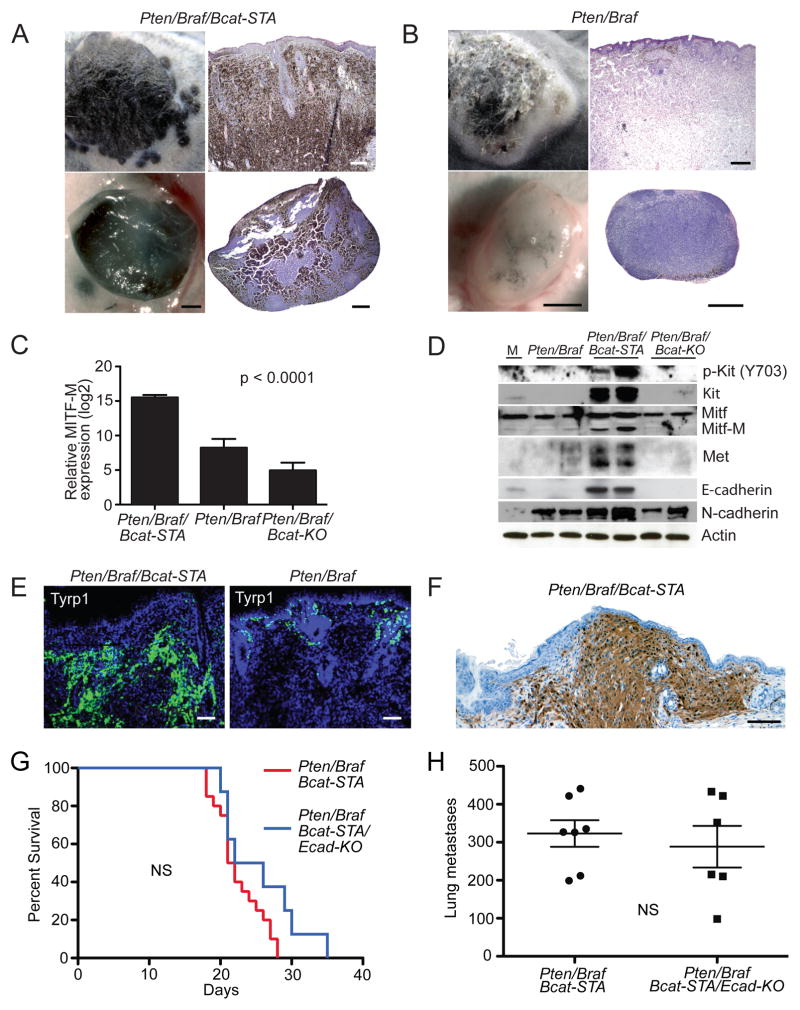
Pten/Braf/Bcat-STA tumors were much more pigmented than Pten/Braf tumors (Figure 5A–B). Pten/Braf tumors showed pigmented melanoma cells at the superficial aspect of the tumor, while deeper parts of the tumor were edematous and amelanotic. In contrast, Pten/Braf/Bcat-STA melanomas were heavily pigmented and remained so even in deeper portions of the tumor. As the expression of many melanocytic pigmentation genes is controlled by MITF-M, a melanocyte-specific isoform of MITF that is a transcriptional target of β-catenin/LEF1 (Widlund et al., 2002; Cheli et al., 2010), we investigated alterations to both Wnt and Mitf target genes in these tumors. qRT-PCR using RNA prepared directly from uncultured tumors showed transcriptional upregulation of both canonical Wnt and Mitf target genes in Pten/Braf/Bcat-STA tumors (Table S3). Conversely, many of these transcripts were downregulated in Pten/Braf/Bcat-KO tumors. Increased or decreased Mitf-M levels were confirmed by qRT-PCR and Western blot analysis (Figure 5C–D). Global expression profiling was also performed on a subset of uncultured melanomas, which more broadly supported these trends in both Wnt and Mitf target gene expression. Together, these models encompass a continuum of β-catenin/Mitf transcriptional activity in the context of Pten loss and Braf activation. Pten/Braf/Bcat-STA melanomas show the highest, Pten/Braf/Bcat-KO, the lowest, and Pten/Braf an intermediate level of β-catenin/Mitf transcriptional activity (Table S3). Activated transcriptional activity of endogenous β-catenin in Pten/Braf models is also supported by the presence of S552 phosphorylation of β-catenin in this model (Figure S3D), a post-translational modification associated with nuclear translocation and transcriptional activation (Zhang et al., 2010)
Figure 5. Enhanced metastasis in Pten/Braf/Bcat-STA melanomas is accompanied by an increase in melanocytic differentiation markers.
(A) Locally induced flank melanoma (upper left panel) with H&E (upper right panel, scale 400 μM) and draining lymph node (lower left panel, scale 500 μM) with H&E (lower right panel, scale 500 μM) in a Pten/Braf/Bcat-STA mouse.
(B) Locally induced flank melanoma (upper left panel) with H&E (upper right panel, scale 400 μM) and draining lymph node (lower left panel, scale 500 μM) with H&E (lower right panel, scale 500 μM) in a Pten/Braf mouse..
(C) Mean expression of Mitf-M determined by qRT-PCR from Pten/Braf/Bcat-STA, Pten/Braf, and Pten/Braf/Bcat-KO melanomas (n=4 per genotype). Expression levels normalized to GAPDH control.
(D) Western blots using protein lysates prepared directly from uncultured flank melanomas. M: normal cultured melanocytes.
(E) Tyrp1 immunofluorescence of locally induced Pten/Braf/Bcat-STA (left panel) and Pten/Braf (right panel) flank melanomas, scale 200 μM.
(F) S100 immunohistochemistry of a locally induced Pten/Braf/Bcat-STA melanoma from an albino mouse, scale 100 μM.
(G) Survival analysis of Pten/Braf/Bcat-STA mice with or without E-cadherin inactivation after perinatal tumor induction, NS: not statistically significantly different.
(H) Quantification of lung metastases in perinatally induced Pten/Braf/Bcat-STA mice with or without E-cadherin inactivation. Error bars represent SEM.
Increased levels of melanocyte differentiation markers in Pten/Braf/Bcat-STA tumors are also present at the protein level. Pten/Braf/Bcat-STA tumors have elevated Tyrp1 staining relative to Pten/Braf tumors (Figure 5E), they also stain strongly for S100 (Figure 5F). Other melanocytic differentiation markers such as Kit, Mitf, Met, and E-cadherin are also increased at the protein level (Figure 5D and S4A–C). N-cadherin levels were also highest in Pten/Braf/Bcat-STA melanomas (Figure 5D). Initially, the finding of increased melanocytic differentiation seemed somewhat counterintuitive as tumor metastasis is often thought to be associated with relative loss of differentiation markers. Along these lines, we decided to determine the effects of conditional inactivation of E-cadherin in the Pten/Braf/Bcat-STA model. E-cadherin loss is thought to result in increased metastasis in some cancers (Kalluri and Weinberg, 2009). Along these lines, E-cadherin loss in Pten/Braf/Bcat-STA melanomas might be predicted to further facilitate metastasis if differentiation in these tumors was functionally restricting metastasis. However, when E-cadherin was inactivated in these tumors cells, neither primary tumor growth nor metastasis was altered (Figure 5G–H). These data support the notion that enhanced differentiation in melanocytic tumors does not functionally restrict metastasis, but rather can be associated with increased metastasis.
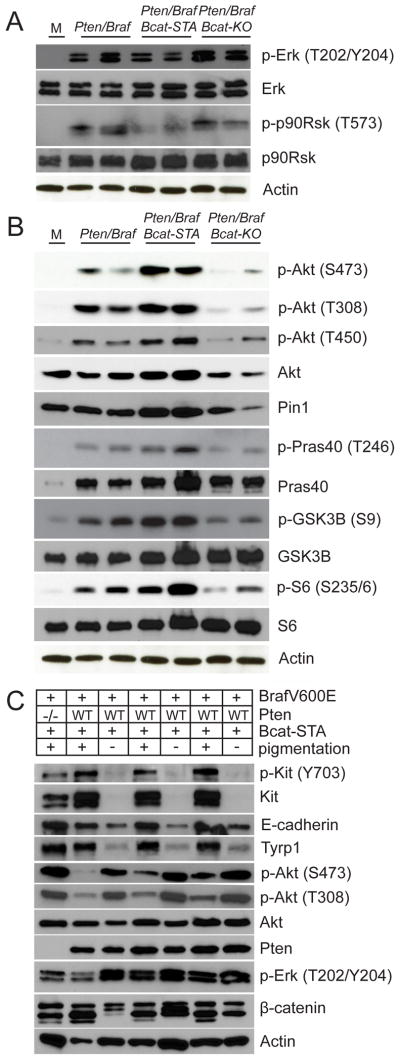
β-catenin stabilization is associated with hyperactivation of PI3K/Akt and MAPK/Erk signaling
As reduced levels of differentiation did not seem to promote or even correlate with enhanced metastasis, we next compared activation of core pathways mediating growth and proliferation: MAPK/Erk and PI3K/Akt. Protein lysates were prepared from uncultured melanomas with either wildtype, stabilized, or inactivated β-catenin. Pten/Braf/Bcat-STA tumors tended to exhibit a relative decrease in phosphorylated Erk and p90Rsk compared to Pten/Braf and Pten/Braf/Bcat-KO tumors (Figure 6A). Previously, it has been proposed that very high levels of Erk phosphorylation may actually impede proliferation (Cheung et al., 2008). Further, it has recently been suggested that an 18-gene transcriptional signature may be the best indicator of MAPK pathway activity in tumor cells (Packer et al., 2009; Dry et al., 2010). In order to complement the phospho-protein analysis and gain a more comprehensive understanding of MAPK pathway activation in these tumors, we also investigated the expression of this gene set. In fact, most of these 18 genes were upregulated in Pten/Braf/Bcat-STA tumors relative to Pten/Braf tumors, suggesting the transcriptional output of the MAPK pathway may actually be elevated despite slightly attenuated phosphorylated Erk levels (Figure S5A). Increased MAPK/Erk signaling in Pten/Braf/Bcat-STA melanomas was accompanied by an increased Ki67 index and mitotic figure count relative to Pten/Braf tumors (Figure S5B). As MITF has been shown to be required for proliferation induced by BrafV600E (Wellbrock et al., 2008), it could be reasonably hypothesized that increased Mitf in Pten/Braf/Bcat-STA tumors may be related to MAPK pathway hyperactivation.
Figure 6. β-catenin stabilization is associated with hyperactivation of PI3K/Akt and MAPK/Erk signaling.
(A–C) Western blots using protein lysates prepared from uncultured flank melanomas. M: Normal cultured melanocytes.
See also Figure S5.
Next, we checked the phosphorylation status of Akt at S473 and T308, two phosphorylation events required for full activation of Akt (Guertin and Sabitini, 2007). Pten/Braf/Bcat-STA tumors showed an increase in both phosphorylation events, in addition to increases in total Akt protein levels (Figure 6B). Pten/Braf/Bcat-KO tumors showed decreased Akt activation and protein levels. The phosphorylation status of Akt substrates (Pras40 and Gsk3β) also supported this trend (Figure 6B). The activation status of mTORC1, which lies downstream of Akt, also seemed highest in Pten/Braf/Bcat-STA melanomas, which had relatively elevated phosphorylation of S6, an indicator of mTORC1 activity (Figure 6B).
At the transcriptional level, Akt gene expression was similar among the models, suggesting that differences in post-transcriptional regulation of Akt might underlie differences in Akt protein levels (Table S3). Pin1, a protein isomerase known to regulate Akt stability and phosphorylation (Liao et al., 2009; Nakatsu et al., 2011) was increased in Pten/Braf/Bcat-STA tumors and decreased in Pten/Braf/Bcat-KO tumors (Figure 6B). Pin1 is transcriptionally upregulated in Pten/Braf/Bcat-STA melanomas (Table S3) and by increasing Akt stability may enable increased levels of activated Akt protein. Further, T450 turn motif phosphorylation of Akt by mTORC2 also promotes Akt stability (Oh et al., 2010) and was highest (independent of increases in total protein level) in Pten/Braf/Bcat-STA tumors (Figure 6B and S5C). Taken together, these observations suggest that Wnt signaling, by promoting Akt stability through Pin1, can enhance PI3K/Akt pathway activty.
Increases in Akt levels and phosphorylation status in the context of Braf activation and β–catenin stabilization seemed to correlate most closely with a metastatic phenotype. To address this further, we reconsidered the Braf/Bcat-STA cohort, which has wildtype Pten and thus reduced Akt activation. These mice form heavily differentiated melanomas that grow slowly and do not metastasize. When Akt is activated via Pten deletion, as in the Pten/Braf/Bcat-STA model, enhanced metastasis is observed in the context of increased melanocytic differentiation, providing strong evidence that activation of Akt through loss of Pten drives metastasis of pigmented melanomas. Interestingly, full Akt activation in Braf/Bcat-STA melanomas was only observed in a small subset of tumors and always in the context of relative decreases in melanocyte differentiation antigen expression (Figure 6C). As neither manifestation of the Braf/Bcat-STA melanomas (low Akt activation with high differentiation or high Akt activation with low differentiation) metastasize, a complex relationship is implied in which both melanocytic differentiation and Akt activation are required for metastasis in the context of Braf/Bcat-STA melanomas.
An alternative explanation to increases in Akt activation status and metastasis in the Pten/Braf/Bcat-STA melanomas could involve increased receptor tyrosine kinase (RTK) expression and/or activation, which are known to signal through this pathway. As the RTKs Kit and Met are both upregulated in Pten/Braf/Bcat-STA melanomas, these receptors emerged as possible mediators of this effect. In vivo functional evaluation of the potential role of each of these receptors in mediating the metastatic phenotype of β-catenin stabilization was evaluated. Overexpression of Met in Pten/Braf melanomas using a doxycycline-inducible mouse strain did not recapitulate the metastatic phenotype induced by β-catenin stabilization (Figure S5D, data not shown). This was not entirely unexpected, as although Met was overexpressed in Pten/Braf/Bcat-STA melanomas, it did not appear to be activated (Figure S5E). Similarly, inhibition of Kit receptor signaling by treating with imatinib in Pten/Braf/Bcat-STA melanomas significantly slowed growth of the primary tumor, but did not alter the frequency of metastasis (Figure S5F–G). These data suggest that increased expression of Met and Kit observed in Pten/Braf/Bcat-STA melanomas is not sufficient to explain the metastatic phenotype.
Pten/Braf/Bcat-STA murine melanomas faithfully recapitulate human melanomas
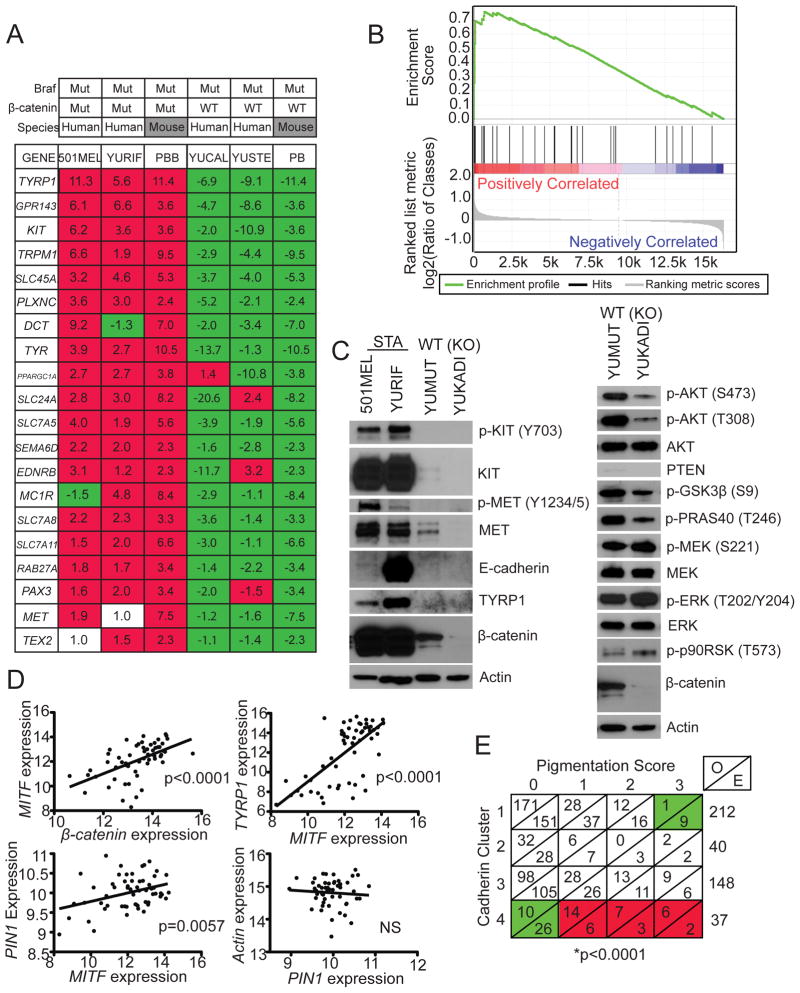
In order to understand the relevance of this model to human melanomas, several approaches were taken. First, human melanoma cell lines with Braf activating mutation and Pten loss/Akt activation were identified. Within this group, we characterized two tumors with β-catenin stabilizing mutations, one with wildtype β-catenin, and one without detectable β-catenin protein levels. These cell lines were characterized at both the mRNA and protein levels. The most differentially expressed transcripts between Pten/Braf/Bcat-STA and Pten/Braf murine melanomas were identified (Figure 7A and Table S4) and used to perform gene set enrichment analysis (GSEA) on the human melanoma expression data (Subramanian et al., 2005). These analyses showed a strong concordance in the expression of this gene subset in human melanomas with β-catenin stabilization (Figure 7B; p<0.001). A similar methodology was also used to identify differentially expressed genes in the human melanoma cell lines with and without β-catenin stabilization and used for GSEA with mouse melanoma expression data (Figure S6A and Table S4). Melanomas with analogous changes showed significant enrichment of these transcripts, while those without the same changes exhibited an inverse relationship for this gene subset (Figure S6B, p<0.001). Together, these analyses suggest that the mouse and human melanomas with the same genetic changes were more similar to each other than were melanomas with different genetic changes, but from the same species.
Figure 7. Pten/Braf/Bcat-STA murine melanomas faithfully recapitulate human melanomas.
(A) Fold-change in expression of individual transcripts relative to mean for each transcript compared between human and murine melanomas. PBB: Pten/Braf/Bcat-STA, PB: Pten/Braf.
(B) GSEA was performed on human melanomas with β-catenin stabilizing mutation using a subset of differentially regulated transcripts identified using murine melanomas, p<0.001.
(C) Western blot analysis using protein lysates prepared from human melanoma cell lines. β-catenin status indicated for each sample.
(D) Regression analysis comparing MITF, TYRP1, and PIN1 expression in a large human melanoma expression data set.
(E) Contingency table of pigmentation status in human melanomas based on cadherin clusters (Kreizenbeck et al. 2008). O: observed, E: expected. Green: less than expected by chance, red: greater than expected by chance.
Western blotting using protein lysates from these human melanoma cell lines showed similar changes to mouse melanomas with analogous genetic changes. Melanomas with β-catenin stabilization showed much higher expression of melanocytic differentiation markers relative to tumors with wildtype or no beta-catenin (Figure 7C). Also, human melanomas with wildtype β-catenin compared to those without β-catenin staining, mirrored changes to MAPK and PI3K/Akt signaling identified by comparing Pten/Braf and Pten/Braf/Bcat-KO mouse melanomas (Figures 7C and S6C). Lastly, analysis of a large expression dataset from a genetically diverse collection of cultured human melanomas, supported the finding that increased β-catenin expression is associated with increased MITF, which was in turn associated with increased TYRP1 and PIN1 expression (Figure 7D). Analysis of the human PIN1 promoter revealed a CATNNG motif E box (-457CACATG-452), a promoter element to which MITF is specifically known to bind (Khaled et al., 2010). Together, these data support the hypothesis that alterations in β-catenin levels in human melanomas result in similar phenotypic changes to those observed in the murine melanoma models.
In order to assess how frequently human melanomas exhibit characteristics seen in mouse models with Braf, Akt, and Wnt signaling activation, we employed a previously published data set (Kreizenbeck et al., 2008). Here, primary and metastatic melanoma biopsies were stained in order to assess cadherin and catenin expression patterns, which formed the basis for clustering into four groups. One of these four clusters (cluster 4) was characterized by relatively high levels of N-cadherin, E-cadherin, with several cases also showing relatively elevated β-catenin. This phenotype is very similar to the Pten/Braf/Bcat-STA mouse melanomas, which exhibit increased E-cadherin, β-catenin, and high N-cadherin levels, in addition to being heavily pigmented. To follow up on these observations, we conducted additional analyses of this human melanoma data set, which revealed that melanomas in cluster 4 were significantly more pigmented than would be expected by chance, with the largest proportion of heavily pigmented melanomas compared to other clusters (p<0.0001; Figure 7E). Other clinical features of these melanomas are described in Table S4. These data support the notion that the Pten/Braf/Bcat-STA murine melanomas closely recapitulate an entire class of human melanomas, not just those with Braf activation, Pten loss, and β-catenin stabilization.
DISCUSSION
The MAPK and Akt pathways are nearly universally dysregulated in human melanomas, with specific occurrence of Pten loss together with Braf activation in at least 20% of tumors (Tsao et al., 2004; Curtin et al., 2005). The precise role of Wnt/β-catenin signaling in human melanoma has remained elusive despite extensive in vitro study, mouse modeling efforts, and immunohistochemical analyses of human melanoma specimens. Here, by characterizing mouse models based on genetic alterations commonly observed in human melanoma, a definitive role for β-catenin as a mediator of tumor progression and metastasis in Pten inactivated, Braf activated melanomas has been established. In the Pten/Braf mouse models, metastasis can be either enhanced or repressed by either increasing or decreasing β-catenin levels, respectively.
In recent analytical studies of human melanoma specimens, it has been proposed that reduced β-catenin levels are associated with a relatively worse prognosis (Chien et al., 2009). However, when β-catenin is inactivated in Pten/Braf melanomas, tumor formation is delayed, survival is extended, and metastasis is nearly eliminated. These data demonstrate the requirement for endogenous β-catenin in melanoma formation and progression. The phenotypic alterations in Pten/Braf/Bcat-KO tumors seem to be related to decreased Wnt transcriptional output, as well as decreased Mitf levels and Mitf target gene expression. In a similar vein, when β-catenin is stabilized in the Pten/Braf melanomas, survival is reduced and metastasis to lymph node, lung, bowel, and spleen is enhanced, some of the most common sites of metastasis in human melanoma patients. In addition to being more metastatic, Pten/Braf/Bcat-STA melanomas are also characterized by increased Wnt-related transcription, primarily as an increase in Mitf and Mitf target gene expression. Thus, in the Pten/Braf model, more metastatic tumors are characterized by enhanced melanocyte differentiation, while less metastatic tumors exhibit reduced melanocytic differentiation.
The relationship between melanoma differentiation/pigmentation and metastasis has long been of research interest. 25 years ago it was observed that more pigmented mouse melanoma cell lines had an enhanced ability to metastasize (Bennett et al., 1986). Since this time, other work has suggested that the melanocytic differentiation program may intrinsically predispose melanomas to metastasis after oncogenic transformation (Gupta et al., 2005). The finding of Mitf amplification in >20% of metastatic melanomas, also suggests a powerful oncogenic potential for melanocyte differentiation programs (Garraway et al., 2005). These observations are also supported clinically, as melanomas are known to frequently metastasize early in disease progression, even from relatively thin primary tumors (Bedrosian et al., 2000). These observations are contrasted with observations suggesting that in general melanomas with increased MITF staining (and β-catenin staining) are associated with relatively improved survival in melanoma (Chien et al., 2009; Nazarian et al., 2010).
An improved understanding of human melanoma genetics has begun to allow for classification of melanomas based on fundamental driving mutations, such as mutant NRAS or BRAF. Here, specific evaluation of the consequences of β-catenin stabilization or inactivation in the context of precisely defined genetic changes was possible. When β-catenin is stabilized in the context of Pten loss and Braf activation, melanomas are very metastatic, grow rapidly, and are highly differentiated. However, in the presence of wildtype Pten, Braf activation and β-catenin stabilization have very different effects. Although highly differentiated tumors form, they do not metastasize. Further, Braf/Bcat-STA melanomas grow very slowly and it is only in association with relative loss of melanocytic differentiation, that tumors exhibit more rapid growth. Comparison of Pten/Braf/Bcat-STA melanomas to Braf/Bcat-STA revealed that activation of Akt in the context of Braf activation and β-catenin stabilization is strongly associated with metastasis in the highly differentiated melanomas. Further, when one considers the amelanotic Braf/Bcat-STA melanoma variant, in which accelerated tumor growth occurred only in the context of relative loss of differentiation markers, it becomes clear that in some contexts melanocytic differentiation may actually restrict exuberant melanoma growth. It is notable that in this model loss of Pten has the potential to switch the melanocyte differentiation program from a transforming, but relatively growth restrictive pathway to a pathway that promotes growth and metastasis. It is also notable that increased differentiation, which includes increased E-cadherin expression, rather than preventing metastasis, is associated with an increase in this process.
These findings raise the possibility that factors promoting metastasis in one melanoma may not in related, but genetically distinct melanomas. These considerations suggest that broadly defining the biological effects of pathways such as Wnt signaling and MITF in melanoma may not be possible out of context of other mutational changes, and help to explain the relative inconsistency of previous studies in this regard. Such context-specific roles of mutated proteins in cancer are likely to emerge as a paradigm as we learn better ways to classify melanomas into biologically meaningful groups.
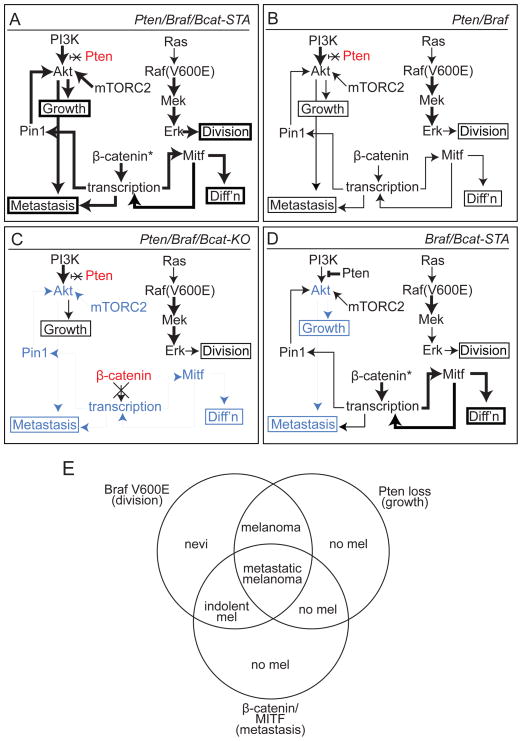
In the Pten/Braf melanomas, simultaneous activation of core signaling pathways mediating growth and survival correlate very closely with the ability to metastasize. Concurrent activation of MAPK, PI3K/Akt, and Wnt/Mitf pathways are all required for the full metastatic melanoma phenotype, as loss of any component abrogates the effect (summarized in Figure 8). Further, changes to Wnt signaling not only alter Wnt output and the melanoma phenotype, but also impact signaling through other core pathways regulating cellular growth and survival. Pten/Braf/Bcat-STA melanomas show enhanced MAPK and PI3K/Akt activation, while Pten/Braf/Bcat-KO melanomas show decreased PI3K/Akt signaling. It is likely that MAPK pathway activation in Pten/Braf/Bcat-STA tumors is at least in part related to increased Kit and/or Mitf levels, especially given the growth inhibitory effect of imatinib treatment, however other possible mediators of this effect are not excluded. Additionally, increased Mitf may also play a role in the elevated proliferation rates observed in Pten/Braf/Bcat-STA melanomas, as Mitf has been shown to be an important integrator of upstream signaling pathways in regulating proliferation and differentiation the melanocytic lineage. Increased Akt activity, however, is likely due to transcriptional upregulation of Pin1, which enhances Akt stability, leading to an increase in Akt protein levels, and ultimately activated Akt. This link between Wnt signaling and Akt in melanoma is likely also important in human melanomas, where increased PIN1 transcription is associated with increased Wnt/MITF. As PIN1 has also been shown to potentiate the effects of additional oncogenes such as Jun (Han et al., 2011), mutant p53 (Girardini et al., 2011), and Cyclin D1 (Li et al., 2006), PIN1-dependent effects are likely to play a broad role in cancer.
Figure 8. Signaling and phenotypic overview of murine melanoma models.
(A) Pten/Braf/Bcat-STA melanomas are characterized by rapid growth, high degree of melanocytic differentiation, and extensive metastasis. These phenotypes are associated with increased MAPK/Erk and PI3K/Akt signaling as well as elevated Mitf and Pin1 levels. Bold text: relatively increased levels and/or activity compared to other models, blue shading: relatively decreased compared to other models, red shading: genetically inactivated, *: stabilized.
(B) Pten/Braf melanomas exhibit rapid tumor growth, but only intermediate melanocytic differentiation and metastasis. Signaling pathway activation, Mitf, and Pin1 are intermediate in this model.
(C) Pten/Braf/Bcat-KO melanomas exhibit tumor growth, but significantly reduced melanocytic differentiation and almost no metastasis. Inactivation of β-catenin in this model is associated with reduced PI3K/Akt signaling and Mitf levels.
(D) Braf/Bcat-STA melanomas are characterized by long latency and slow tumor growth, as well as lack of metastases. These tumors are differentiated and heavily pigmented due to high Mitf levels, but show reduced PI3K/Akt pathway activation.
(E) A three-pathway synergy is observed when MAPK/Erk, PI3K/Akt, and β-catenin/Mitf signaling pathways are simultaneously activated in melanoma. If any of these three changes is not present, the metastatic melanoma phenotype is abrogated.
Components of the MAPK and also the PI3K/Akt pathways are central targets for new cancer therapeutics. For example, vemurafenib (PLX4032), a mutant Braf inhibitor has been shown to improve survival in late stage melanoma patients with Braf mutations (Chapman et al., 2011). Along these lines, understanding how simultaneous activation of additional signaling pathways in a given tumor can impact inhibitor-targeted pathways is essential and may assist in understanding why some patients with a given driver mutation respond differentially to treatment with the same inhibitor. In this context, it is particularly noteworthy that changes to β-catenin can have such profound effects to both the MAPK and PI3K/Akt signaling pathways.
Here we have described several mouse models of melanoma that have provided fundamental insight into mechanisms by which β-catenin/Wnt signaling alterations can impact melanoma formation and progression in vivo. Extensive analyses were conducted in order to ensure that these models closely resemble human melanomas with analogous genetic changes and cellular phenotypes. Faithful recapitulation of human melanoma, combined with features such as rapid tumor growth and reproducible metastasis to lymph nodes and lung, make this model attractive for future studies in melanoma.
EXPERIMENTAL PROCEDURES
Mouse strains and in vivo experiments
The Ctnnb1lox, E-cadherinlox, and TRE::Met strains were obtained from Jackson Labs. These mice were genotyped and assayed for recombination using PCR as described previously (Brault et al., 2001; Wang et al., 2001; Boussadia et al., 2002). The Tyr-CreER, Ptenlox, BrafCA, and Ctnnb1loxex3 alleles have also been described previously and were genotyped and assayed for recombination using PCR (Harada et al., 1999; Dankort et al., 2009). The Tyr::rtTA strain was obtained from MMHCC, and genotyped as described previously (Chin et al., 1999). All strains were maintained on a mixed C57BL/6, FVB, 129 background. For imatinib experiments, Pten/Braf/Bcat-STA mice were treated daily with 200mg/kg imatinib (LC Laboratories) in water by oral gavage. All experiments involving animals were reviewed and approved by the Yale Institutional Animal Care and Use Committee (IACUC).
Immunohistochemistry, immunofluoresence, and Western blotting
Immunofluoresence and immunohistochemistry were performed on formalin-fixed paraffin-embedded and frozen tumor sections. Western blotting was performed using standard methods on uncultured, macrodissected tumor protein lysates or lysates from cultured human melanoma cell lines.
RNA purification and qRT-PCR
Total RNA was isolated from homogenous portions of macrodissected, uncultured tumors. cDNA was prepared for qRT-PCR analysis using SYBR green detection methods and ΔΔCt quantification relative to an endogenous GAPDH control.
Statistical Analyses
Kaplan-Meyer survival curves were compiled using Prism statistical analysis software. Significance was assessed using the Log-rank (Mantel-Cox) test. For comparison of pooled data between two different groups, unpaired t-tests were used to determine significance. For comparison of data among three groups, one-way ANOVA was used to determine significance. R was used for hierarchical clustering and Fisher’s Exact test was used to determine significance for contingency table analysis.
Supplementary Material
HIGHLIGHTS.
β-catenin loss in Pten/Braf melanomas improves survival and inhibits metastasis.
β-catenin stabilization in Pten/Braf melanomas enhances metastasis.
Highly differentiated melanomas can be very metastatic in vivo.
β-catenin status in melanoma regulates PI3K/Akt and MAPK/Erk signaling.
SIGNIFICANCE.
Melanoma has a predilection for early and extensive metastasis. Despite an improved understanding of genetic changes found in human melanoma, how these changes relate to the metastatic process is unclear. Here, using an inducible genetically engineered mouse model of melanoma driven by some of the most common alterations found in human melanoma, we identify β-catenin as a central mediator of the metastatic process in vivo. In this study, the relationship between β-catenin, MITF, tumor differentiation, metastasis, and signaling through core pathways that regulate proliferation and survival are evaluated. This study also introduces a mouse model characterized by inducible and rapid primary tumor growth, high melanocytic antigen expression, and numerous metastases.
Acknowledgments
The authors would like to thank all members of the Bosenberg lab, past and present. Special thanks is due to R. Halaban and the Yale SPORE in skin cancer for providing human melanoma cell lines and microarray expression data. We would also like to thank V. Hearing for providing the Tyrp1 (Pep1) antisera; S. Tighe, J. Bond, and J. Dragon for microarray analyses; K. Tworkoski and D. Stern for the phospho-Met control lysate; G. Lyon for FACS sort; the Yale Dermatopathology lab for tissue processing; and Q. Yan for useful suggestions regarding the manuscript. This work was supported by grants from the National Cancer Institute (R01 CA112054, P50 CA121974, UVM NIEHS T32) and the Joanna M. Nicolay Melanoma Foundation. David Rimm is a consultant to and stockholder in HistoRx, the exclusive licensee of the Yale held patent on the AQUA technology.
Footnotes
ACCESSION NUMBERS
Microarray datasets were deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus database with the accession number GSE32907.
Supplemental Information includes six figures, four tables, and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedrosian I, Faries MB, Guerry D, 4th, Elenitsas R, Schuchter L, Mick R, Spitz FR, Bucky LP, Alavi A, Elder DE, et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (< or = 1mm) with vertical growth phase. Ann Surg Oncol. 2000;7:262–267. doi: 10.1007/s10434-000-0262-z. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Dexter TJ, Ormerod EJ, Hart IR. Increased experimental metastatic capability of a murine melanoma following induction of differentiation. Cancer Res. 1986;46:3239–3244. [PubMed] [Google Scholar]
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli Y, Ohanna M, Ballotti R, Bertolotto C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2010;23:27–40. doi: 10.1111/j.1755-148X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–3439. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated Wnt/β-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci USA. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagan R, Pantginis J, Zhou H, et al. Essential role for oncogenic Ras in tumor maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas V, Beermann F, Martinozzi S, Carreira S, Ackermann J, Kumasaka M, Denat L, Goodall J, Luciani F, Viros A, et al. B-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–2925. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demunter A, Libbrecht L, Degreef H, De Wolf-Peeters C, van den Oord JJ. Loss of membranous expression of β-catenin is associated with tumor progression in cutaneous melanoma and rarely caused by exon 3 mutations. Mod Pathol. 2002;15:454–461. doi: 10.1038/modpathol.3880546. [DOI] [PubMed] [Google Scholar]
- Dry JR, Pavey S, Pratilas CA, Harbron C, Runswick S, Hodgson D, Chresta C, McCormack R, Byrne N, Cockerill M, et al. Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244) Cancer Res. 2010;70:2264–2273. doi: 10.1158/0008-5472.CAN-09-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Tang G, Bindal N, Bamford S, Dawson E, Cole C, Kok CY, Jia M, Ewing R, Menzies A, et al. COSMIC (the Catalogue of Somatic Mutations in Cancer): a resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2010;38:D652–D657. doi: 10.1093/nar/gkp995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- Girardini JE, Napoli M, Piazza S, Rustighi A, Marotta C, Radaelli E, Capaci V, Jordan L, Quinlan P, Thompson A, et al. A Pin1/mutant p53 axis promotes aggressiveness in breast cancer. Cancer Cell. 2011;20:79–91. doi: 10.1016/j.ccr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabitini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37:1047– 1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CY, Hien TT, Lim SC, Kang KW. Role of Pin1 in UVA-induced cell proliferation and malignant transformation in epidermal cells. Biochem Biophys Res Commun. 2011;410:68–74. doi: 10.1016/j.bbrc.2011.05.106. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of β-catenin in neural crest development. J Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageshita T, Hamby CV, Ishihara T, Matsumoto K, Saida T, Ono T. Loss of β-catenin expression associated with disease progression in malignant melanoma. Br J Dermatol. 2001;145:210–216. doi: 10.1046/j.1365-2133.2001.04336.x. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled M, Levy C, Fisher DE. Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 2010;24:2276–2281. doi: 10.1101/gad.1937710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielhorn E, Provost E, Olsen D, D’Aquila TG, Smith BL, Campl RL, Rimm DL. Tissue microarray-based analysis shows phospho-β-catenin expression in malignant melanoma is associated with poor outcome. Int J Cancer. 2003;103:652–656. doi: 10.1002/ijc.10893. [DOI] [PubMed] [Google Scholar]
- Kreizenbeck GM, Berger AJ, Subtil A, Rimm DL, Gould Rothberg BE. Prognostic significance of cadherin-based adhesion molecules in cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2008;17:949–958. doi: 10.1158/1055-9965.EPI-07-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Delmas V. The WNT/β-catenin pathway in melanoma. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- Li H, Wang S, Zhu T, Zhou J, Xu Q, Lu Y, Ma D. Pin1 contributes to cervical tumorigenesis by regulating cyclin D1 expression. Oncol Rep. 2006;16:491–496. [PubMed] [Google Scholar]
- Liao Y, Wei Y, Zhou X, Yang JY, Dai C, Chen YJ, Agarwal NK, Sarbassov D, Shi D, Yu D, et al. Peptidyl-prolyl cis/trans isomerase Pin1 is critical for the regulation of PKB/Akt stability and activation phosphorylation. Oncogene. 2009;28:2436–2445. doi: 10.1038/onc.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu Y, Sakoda H, Kushiyama A, Zhang J, Ono H, Fujishrio M, Kikuchi T, Fukushima T, Yoneda M, Ohno H, et al. Pepitdyl-prolyl cis/tras isomerase NIMA-interacting 1 associates with insulin receptor substrate-1 and ehances insulin actions and adipogenesis. J Biol Chem. 2011;286:20812–20822. doi: 10.1074/jbc.M110.206904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian RM, Prieto VG, Elder DE, Duncan LM. Melanoma biomarker expression in melanocytic tumor progression: a tissue microarray study. J Cutan Pathol. 2010;37:41–47. doi: 10.1111/j.1600-0560.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- Oh WJ, Wu CC, Kim SJ, Facchinetti V, Julien LA, Finlan M, Roux PP, Su B, Jacinto E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 2010 doi: 10.1038/emboj.2010.271. Published online: 2010 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omholt K, Platz A, Ringborg U, Hansson J. Cytoplasmic and nuclear accumulation of β-catenin is rarely caused by CTNNB1 exon 3 mutations in cutaneous malignant melanoma. Int J Cancer. 2001;92:839–842. doi: 10.1002/ijc.1270. [DOI] [PubMed] [Google Scholar]
- Packer LM, East P, Reis-Filho JS, Marais R. Identification of direct transcriptional targets of (V600E)BRAF/MEK signaling in melanoma. Pigment Cell Melanoma Res. 2009;22:785–793. doi: 10.1111/j.1755-148X.2009.00618.x. [DOI] [PubMed] [Google Scholar]
- Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Derm. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberger J, Knobbe CB, Wolter M, Blaschke B, Schulte KW, Pietsch T, Ruzicka T, Reifenberger G. Molecular genetic analysis of malignant melanomas for aberrations of the Wnt signaling pathway genes CTNNB1, APC, ICAT, and BTRC. Int J Cancer. 2002;100:549–556. doi: 10.1002/ijc.10512. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of β-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Sanders DS, Blessing K, Hassan GA, Bruton R, Marsden JR, Jankowski J. Alterations in cadherin and catenin expression during the biological progression of melanocytic tumours. Mod Pathol. 1999;52:151–157. doi: 10.1136/mp.52.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silye R, Karayiannakis AJ, Syrigos KN, Poole S, van Noorden S, Batchelor W, Regele H, Sega W, Boesmueller H, Krausz T, et al. E-cadherin/catenin complex in benign and malignant melanocytic lesions. J Pathol. 1998;186:350–355. doi: 10.1002/(SICI)1096-9896(199812)186:4<350::AID-PATH181>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci MG, Lucarini G, Brancorsini D, Zizzi A, Pugnaloni A, Giacchetti A, Ricotti G, Biagini G. Involvement of E-cadherin, β-catenin, Cdc42, and CXCR4 in the progression and prognosis of cutaneous melanoma. Br J Dermatol. 2007;157:1212–1216. doi: 10.1111/j.1365-2133.2007.08246.x. [DOI] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurel S, Houben R, Schrama D, Voigt H, Zapatka M, Schadendorf D, Brocker EB, Becker JC. Microphthalmia-associated transcription factor gene amplification in metastatic melanoma is a prognostic marker for patient survival, but not a predictive marker for chemosensitivity and chemotherapy response. Clin Cancer Res. 2007;13:6344–6350. doi: 10.1158/1078-0432.CCR-06-2682. [DOI] [PubMed] [Google Scholar]
- Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellbrock C, Rana S, Paterson H, Pickersgill H, Brummelkamp T, Marais R. Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS One. 2008;3:e2734. doi: 10.1371/journal.pone.0002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlund HR, Horstmann MA, Price ER, Cui J, Lessnick SL, Wu M, He X, Fisher DE. β-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, O’neil MF, Cunningham MT, Fan F, Olyaee M, Li L. Abnormal Wnt signaling and stem cell activation in reactive lymphoid tissue and low-grade marginal zone lymphoma. Leuk Lymphoma. 2010;51:906–910. doi: 10.3109/10428191003695645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.