Abstract
Background
Coagulopathic bleeding is a leading cause of in-hospital death after injury. A recently proposed transfusion strategy calls for early and aggressive frozen plasma transfusion to bleeding trauma patients, thus addressing trauma-associated coagulopathy (TAC) by transfusing clotting factors (CF). This strategy may dramatically improve survival of bleeding trauma patients. However, other studies suggest that early TAC occurs by protein C activation and is independent of CF deficiency. The present study investigated whether CF deficiency is associated with early trauma-associated coagulopathy.
Methods
Prospective observational cohort study of severely traumatized patients (ISS ≥16) admitted shortly after injury, receiving minimal fluids and no prehospital blood. Blood was assayed for CF levels, thromboelastography and routine coagulation tests. Critical CF deficiency was defined as ≤30% activity of any CF.
Results
Of 110 patients, 22 (20%) had critical CF deficiency: critically low factor V level was evident in all these patients. INR, aPTT and TEG were abnormal in 32%, 36% and 35% respectively of patients with any critically low CF. Patients with critical CF deficiency suffered more severe injuries, were more acidotic, received more blood transfusions and showed a trend towards higher mortality (32% vs. 18% p=.23). Computational modelling showed coagulopathic patients had pronounced delays and quantitative deficits in generating thrombin.
Conclusions
20% of all severely injured patients had critical clotting factor deficiency on admission, particularly of factor V. The observed factor V deficit aligns with current understanding of the mechanisms underlying early TAC. Critical deficiency of factor V impairs thrombin generation and profoundly affects hemostasis.
Keywords: coagulopathy, clotting factor, factor V, trauma, thromboelastography
Introduction
Hemorrhage accounts for most trauma deaths within the first 24 hours of hospitalization 1-3. The development of coagulopathy remains the key reason why bleeding becomes uncontrollable. The mechanisms leading to coagulopathy following trauma remain poorly understood 4-6. Many classical accounts of trauma-associated coagulopathy (TAC) describe it as a relatively late event, resulting from consumption and loss of clotting elements, as well as their iatrogenic dilution by resuscitation 4,7,8.
Recently, two studies identified the existence of an early trauma-associated coagulopathy (TAC) occurring immediately after injury prior to any substantial fluid resuscitation, and therefore, seemingly independent of the mechanisms just mentioned 9,10. In fact, early clotting factor deficiency was specifically excluded from having any role in the development of early TAC4,11-20. Instead, early TAC appeared to be the result of tissue injury and shock, rather than dilution 4,11-13. The proposed mechanism is that shock and tissue injury lead to systemic anticoagulation by widespread endothelial damage and activation of APC (activated protein C) 11-13. The APC-mediated anticoagulation is compounded by endothelial release of tissue plasminogen activator leading to systemic hyperfibrinolysis and diffuse microvascular “coagulopathic” bleeding 4,11-13.
Concurrently, however, a growing number of retrospective studies have proposed radical changes to the resuscitation of massively bleeding trauma patients 14-19. This new paradigm, Damage Control Resuscitation (DCR), has advocated for early and aggressive clotting factor transfusions in the form of fresh frozen plasma (FFP) in a 1:1 ratio with red cells 3,14,17-20. As well, DCR recommends against large volume crystalloid resuscitation. Most DCR studies report major reductions in mortality compared to traditional resuscitation strategies 14,15,20 and despite methodological limitations 16-18, DCR has been adopted across many trauma centres in North America where thawed FFP is easily available 3,17,18,20.
DCR is primarily a hemostatic resuscitation strategy that targets early coagulopathy, and as such, potentially reverses some of the pathophysiological mechanisms underlying trauma-associated coagulopathy 17,18. However, transfusing clotting factors in the form of FFP implicitly refutes the hypothesis that clotting factor deficiency has no role in early TAC 4,13,18. The lack of studies on the topic precludes any conclusions on this apparent contradiction.
Trauma-associated coagulopathy has many definitions 21. Many papers define trauma-associated coagulopathy in terms of abnormalities in INR (PT) and/or aPTT that exceed some threshold value. 9, 10 INR, PT, and aPTT detect problems with the intrinsic, extrinsic and common pathways of coagulation7,30. Furthermore, the treatment of abnormal INR (PTT) and/or aPTT calls for the administration of clotting factors in the form of fresh frozen plasma. We therefore hypothesize a clotting factor deficiency does occur shortly after injury, and that this clotting factor deficiency does contribute to the development of early trauma-associated coagulopathy. The purpose of this study was to determine whether critical clotting factor deficiency occurs early after injury, and if so, whether it is an underlying mechanism contributing to the development of trauma-associated coagulopathy.
Material and Methods
The present study was a subgroup analysis of a larger prospective observational cohort study on trauma-associated coagulopathy conducted at Sunnybrook Health Sciences Centre, a level 1 Canadian trauma centre. In the larger study, all consecutive adult (age ≥16) trauma patients assessed at our institution between February 5 and October 23, 2007 were enrolled. As part of the initial evaluation of any trauma patient at our institution, blood samples are routinely collected for routine coagulation assays, including INR, aPTT, platelets, and fibrinogen. If admitted to the intensive care unit, routine coagulation assays are also routinely performed on admission and every morning. Subsequent tests are collected depending on the trauma severity and the clinician’s judgement. For our larger, prospective study, two extra blood tubes were collected on every trauma patient for special coagulation assays and for thromboelastography, each time routine coagulation assays were ordered during the first 48 hours after admission.
Study Group
To be consistent with previous studies on early trauma-associated coagulopathy, for this substudy, we focused on the subgroup of patients with the same characteristics as those described in the two original retrospective studies on, early-TAC 9,10. Thus, only severely injured patients (Injury Severity Score ≥ 16), who were admitted directly from the scene of injury within 3h were included. We excluded patients receiving >1L of crystalloid or any blood transfusion prior to arrival, those patients whose blood samples were not collected within 30min of arrival in the trauma room. We also excluded patients known to have a congenital coagulopathy or taking anticoagulants.
Primary Outcome – Clotting Factor Activity Levels and Critical Deficiency
The primary outcome of this study was to determine whether or not any critical clotting factor deficiency exists on arrival in the trauma room. After literature review, we defined a critical clotting factor deficiency level as any clotting factor activity level ≤30%, because this is the minimum level required of any individual clotting factor for normal in vivo hemostasis 18,22,23. Furthermore, It is broadly accepted as indicative of a major hemostatic defect and has been used by haematologists as the threshold for normal blood clotting in congenital coagulopathies 8,15,17,22-24. For example, in congenital coagulopathies such as haemophilia, guidelines recommend treatment for clotting factor levels below 30% for patients that are bleeding, undergoing surgery or suffered a major trauma 8,23-25. No such studies have been done in the trauma population. Finally, in diverse clinical situations where the transfusion of FFP is indicated, the aim of such transfusion is to increase the clotting factor activity level to above 30% 18,23,24,26.
Secondary Outcomes
1. We determined if critical clotting factor deficiency has correlational validity as a measure of trauma-associated coagulopathy by analyzing its relationship to important baseline injury characteristics and patient outcomes. Particularly, we analyzed for its relationship to injury severity, injury mechanism and degree of shock on presentation. For outcomes, we analyzed for transfusion requirements, and mortality.
2. We also determined the sensitivity and specificity of INR and aPTT for detecting critical clotting factor deficiency. The literature does not previously report critical clotting factor deficiency as the gold standard for assessing INR and aPTT in trauma patients; therefore, reporting sensitivity and specificity of INR and aPTT (instead of a correlational coefficient) for critical clotting factor deficiency may be controversial. However, we think there is an a priori argument to do so. INR is derived from prothrombin time (PT) and measures deficiencies of Factors II, V, VII, X and fibrinogen 7,23. aPTT measures deficiencies of Factors VIII, IX, XI and XII (intrinsic CF) and V, X, fibrinogen and prothrombin (common pathway) 7,23. Therefore, INR and aPTT are really screening tests for detecting critical clotting factor deficits that impair hemostasis. To this end, we used the following thresholds for these routine laboratory assays – assays were abnormal if: INR≥1.5 and aPTT≥45 seconds, which are the levels used in the original TAC studies 9,10 and by our institutional guidelines to mandate treatment.
3. As another secondary outcome, we determined whether or not any measured clotting factor deficiencies observed would have any effects on hemostasis. To do this, we used a computational simulating model to evaluate the combined effect of multiple clotting factor defects on thrombin generation. This model has previously been described and validated 27. In brief, the simulation is initiated by “exposing” picomolar concentrations of Tissue Factor (TF) to an electronic milieu consisting of factors II, IX, X, VII, VIIa, V, and VIII, and the anticoagulants Tissue Factor Pathway Inhibitor (TFPI) and Antithrombin-III (AT-III) at concentrations found in normal plasma or associated with coagulation pathology. The reaction followed in terms of thrombin generation, proceeds through phases that can be operationally defined as initiation, propagation, and termination.
Clotting Factor Levels
All blood samples were promptly transferred to the Hospital Laboratory after collection and centrifuged (1700g ×15min) at 4°C. Plasma was removed and transferred to plastic tubes, centrifuged (1700g) for another 5min at 4°C to ensure platelet free plasma (<10×109/L), transferred to cryo-vials (500μL minimum each) and frozen (−70°C) until analysis. CF activity was done by the Haemostasis Reference Laboratory, McMaster University (Hamilton, Canada) by mixing plasma and deficient controls (Precision BioLogics, Dartmouth, Canada) and the degree of PT/aPTT correction (Dade-Behring Innovin, Marburg, Germany) correlated with plasma CF level. When plasma volumes were insufficient, sequence priority was to test VIII; VII; V; II; IX; XI; XII; X. Normal range for CF is 0.5-1.5 U/ml (50-150% activity level).
Thromboelastography
Thromboelastography was done by qualified technologists trained by the manufacturer in the TEG® 5000 Hemostasis Analyser (Haemoscope Corporation, Illinois, US). Maintenance and quality controls were done as recommended by Haemoscope. Citrated blood samples were kept stationary at room temperature until 40min. from collection (as per owner’s manual recommendations), 500μL was transferred to a vial containing buffered stabilizers and phospholipids (Kaolin®); mixed by vial inversion (5x); 340μL was then transferred to 37°C pre-warmed cups containing 20μL calcium chloride and measurement started and continued for no less than 40min. The manufacturer’s normal ranges were used, as well as Kauffmann’s proposal that defines clinically relevant coagulopathy when ≥2 TEG parameters are abnormal 28.
Statistical analysis
Continuous data was reported as means and standard deviations, or medians and interquartile range (IQR) and compared using t-test or Wilcoxon rank-sum test as appropriate. Discrete variables were summarized using frequencies and percentages and the differences were tested by chi-square or Fisher’s exact test. Sunnybrook Research Ethics Boards approved the study. Informed consent was obtained from all patients or substitute decision makers for participation and continuation in the study. For patients unable to consent and without substitute decision makers, the consent was delayed in accordance with the Tri-Council Policy Agreement for Research in Emergency Health Situations (Article 2.8). Written informed consent was obtained directly from all patients that recovered. Demographics, co-morbidities, medications, injury data, intravenous fluids, blood transfusions, drugs used and outcomes were prospectively collected by trained research coordinators.
Results
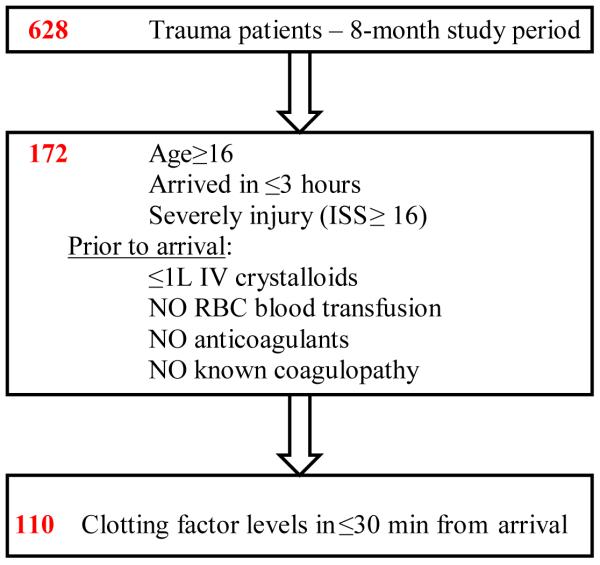
During the study period, a total of 628 patients were enrolled into the large observational trial over the 8 month study period. 110 (17.5%) met our substudy inclusion criteria for the present analysis. See Figure 1. These 110 patients constitute the cohort analyzed and their baseline characteristics are in Table 1.
Figure 1.
Patient enrolment
ISS = injury severity score; L = litre; IV = intravenous; min = minutes
Table 1.
Baseline demographic and Injury Characteristics
| Critical Clotting Factor Deficiency (n=22) |
No Critical Clotting Factor Deficiency (n=88) |
P-value | |
|---|---|---|---|
| Demographic | |||
| Gender (female) (n=110) | 5 (22.7%) | 24 (27.3%) | 0.66 |
| Age (years) (n=110) | 40.5 (21-66) | 37 (24-50.5) | 0.89 |
| Mechanism (blunt) (n=110) | 21 (95.5%) | 74 (84.1%) | 0.29 |
| Time to arrive (min) (n=110) | 67 (50-80) | 55.2 (40-70) | 0.10 |
| Injury Characteristics | |||
| ISS (n=110) | 34 (24-42) | 26 (22-33) | 0.02 |
| AIS head (n=110) | 3 (2-5) | 3 (0-5) | 0.43 |
| GCS on arrival (n=109) | 13 (2-15) | 14 (6-15) | 0.18 |
| GCS at time point 1 (n=104) | 13.5 (3-15) | 14 (5-15) | 0.13 |
| Physiologic Characteristics | |||
| Hg on arrival (g/L) (n=105) | 130 (120-142) | 137.5 (127.5- 149.5) |
0.10 |
| pH on arrival (n=62) | 7.26 (7.17-7.34) | 7.35 (7.29-7.38) | 0.01 |
| Temp arrival (Celsius) (n=72) | 36 (34-36) | 36 (35.0-36) | 0.48 |
| Outcomes | |||
|
NS/RL pre-hospital
(median; IQR) (n=86) |
575 (150-850)
(n=18) |
200 (100-425)
(n=68) |
0.03 |
| NS/RL TR (median; IQR) (n=92) |
1950 (1100- 3850) |
1600 (1150- 2200) |
0.14 |
| NS/RL 24h (median; IQR) in hospital after ER (n=100) |
4400(2800-6875) (n=17) |
4170 (2000- 6150) (n=83) |
0.40 |
| RBC transfusion pre-hospital | none | none | |
|
# RBC U transfused in TR
(n=12) |
7 (2-8)
(n=6) |
1 (1-2)
(n=6) |
0.03 |
|
# RBC U transfused first
24h (n=33) |
6.5 (3-18)
(n=10) |
3 (1-6)
(n=23) |
0.03 |
| FFP transfusion pre-hospital | none | none | |
|
# FFP U transfused in TR
(n=2) |
1 & 2 units | 0 | |
| # FFP U transfused first 24h (n=12) |
11 (5-27) (n=4) |
4 (3-6.5) (n=8) |
0.25 |
| Mortality (n=110) | 7 (31.8%) | 16 (18.2%) | 0.23 |
Coagulopathy=patients with critical clotting factor deficit (≤30%); n = number of patients with data; min = minute; ISS = injury severity score; head injury = head AIS≥1; AIS = abbreviated injury score; # = number of; GCS = Glasgow coma scale; Hg = hemoglobin; temp = temperature; NS/RL = crystalloid (Normal Saline - NaCl 0.9%, Ringer Lactate); Volume NS/RL is in mL; TR = trauma room; RBC = red blood cells; U = units; FFP = fresh frozen plasma.
Upon arrival, 22 of the 110 patients (20%) had at least one critically low clotting factor (≤30% activity), meeting the study definition of critical clotting factor deficiency (Table 2). In 100% of these 22 patients, factor V was critically low. In addition, factors VIII and XI were also critically low in four patients (18%) and three patients (15%) patients respectively. Seventeen patients (77%) had a single critical deficit, two patients had two critical deficits, one patient had three and two patients had critical deficits of all 8 clotting factors on arrival (Table 2).
Table 2.
Clotting factor deficit on arrival
| Clotting factors |
Critical deficit ≤30% clotting factor activity 22 patients |
|---|---|
| Factor II (n=105 ) | 2 (9.1%) |
| Factor V (n=105 ) | 22 (100%) |
| Factor VII (n=108) | 1 (4.5%) |
| Factor VIII (n=110) | 4 (18.2%) |
| Factor IX (n=105) | 2 (10%) |
| Factor X (n=96) | 2 (10%) |
| Factor XI (n=99) | 3 (15%) |
| Factor XII (n=97) | 2 (10%) |
n = number of patients with data.
Some differences became apparent when comparing patients with any critical clotting factor deficiency versus patients without any clotting factor deficits (Table 1). Clotting factor deficient patients suffered a higher injury load (ISS 34 vs. 26 p=.02), and were significantly more acidotic (pH 7.26 vs. 7.35 p=.01). As well, patients with any critical CF deficit were more likely to have suffered blunt mechanisms of injury, although this was not a statistically significant finding. With regards to outcomes, patients with any critical CF deficit were more likely to have received significantly more transfusions (7 vs. 1 unit of RBC; 1.5 vs. 0 units of FFP). As well, patients with critical CF deficits had a higher mortality than those without any critical deficits (32% vs. 18%), although the difference was not statistically significant. Also, significantly more patients with a critical CF deficit received RBC transfusion (27% vs. 1% p=.0136) and FFP (9% vs. 0% p=.03) in the trauma room, despite having similar hemoglobin levels (130 vs. 137 g/L p=.1). This finding suggests that patients with critical clotting factor deficiency had clinical evidence of ongoing bleeding. Interestingly, FFP was transfused only to two of the 22 patients with critical CF deficits in the trauma room and only one patient received enough to correct the CF deficit to above 30%.
INR, and aPTT, were significantly worse in patients with critical clotting factor deficiencies noted; however, on average, the mean values of these routine coagulation assays remained within normal ranges even in patients with critical clotting factor deficits (Table 3). Using the pre-defined levels discussed previously, the INR had a sensitivity of about 32% for critical CF deficits and aPTT had a sensitivity of about 36%. Interestingly, patients without critical clotting factor deficiency all had normal INR and aPTT levels, meaning INR and aPTT had a specificity of 100% for critical clotting factor deficits (Table 4).
Table 3.
Laboratorial measurements of coagulopathy
| Critical Clotting Factor Deficiency (n=22) |
No Critical Clotting Factor Deficiency (n=88) |
P-value | |
|---|---|---|---|
| INR on arrival (n=110) | 1.18 (1.07-1.57) | 1.08 (1.02-1.13) | <0.001 |
| % cases INR ≥ 1.5 | 32% | 0% | <0.001 |
| aPTT on arrival (n=109) | 41.8 (30.6-53.9) | 27.5 (25.3-30.9) | <0.001 |
| % cases PTT ≥ 45 | 36% | 0% | <0.001 |
| TEG on arrival (n=99) | |||
| ≥2 abnormal | 35% | 11% | 0.02 |
| abnormal R (>8) | 17.7% | 2.5% | 0.03 |
| abnormal K (K>3) | 35.3% | 12.5% | 0.03 |
| abnormal α angle (α<55) | 35.3% | 18.8% | 0.19 |
| abnormal MA (MA<51) | 35.3% | 5) | 0.001 |
Critical deficit = ≤30% clotting factor activity; # = number of patients with; INR = international normalized ratio; aPTT = activated prothrombin time; platelet count = x109/L; TEG = thromboelastography.
Table 4.
INR, aPTT and TEG sensitivity and specificity analyzing the 110 patients that had clotting factor activity measured upon arrival
| INR ( ≥1.5) |
aPTT (≥45) |
TEG (≥2 abnormal parameters) |
|
|---|---|---|---|
| Sensitivity |
31.8% (13.9%, 54.9%) |
36.4% (17.2%, 59.3%) |
35.3% (14.2%, 61.7%) |
| Specificity |
100% (95.9%, 100%) |
100% (95.9%, 100%) |
88.8% (79.7%, 94.7%) |
| PPV |
100% (59.0%, 100%) |
100% (63.1%, 100%) |
40.0% (16.3%, 67.7%) |
| NPV |
85.4% (77.1%, 91.6%) |
86.1% (77.8%, 92.2%) |
86.6% (77.3%, 93.1%) |
Using Kauffmann’s definition which requires at least two TEG values to be abnormal, TEG parameters had a similar sensitivity as INR and aPTT for critical clotting factor deficiencies: its sensitivity was about 35%. However, the specificity of TEG for critical clotting factor deficits was inferior to either INR or aPTT: TEG was abnormal in up to 19% of patients without critical clotting factor deficiencies (Table 4).
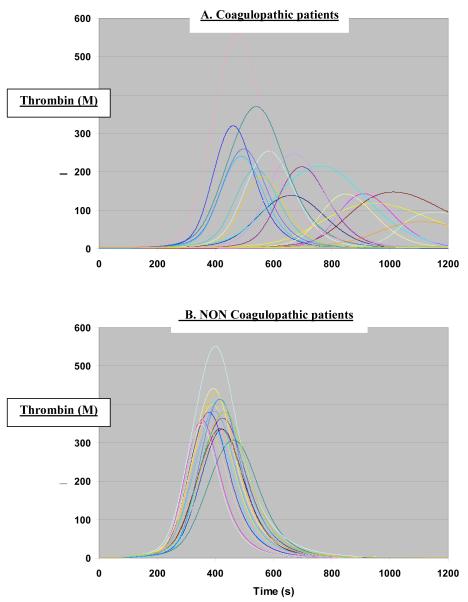
The critical clotting factor deficiencies that we measured in this study were likely to have impacted on in vivo hemostasis. Our computational simulating model revealed that when the clotting factor activity levels were inputted and analyzed in the model for patients with critical deficits, these deficits would result in marked delay and quantitative decreases in active thrombin generation (Fig 2A), in comparison to a control group of twenty patients who had normal range clotting factor activity levels (Fig 2B). Therefore, we presume that these critical clotting factor deficiencies that we observed would result in marked abnormalities in in vivo hemostasis.
Figure 2.
Computational model with concentration of thrombin as a function of time in a reaction initiated by tissue factor 27. A = coagulopathic patients. B = non coagulopathic patients with all clotting factor levels >30%
Discussion
The mechanisms underlying trauma-associated coagulopathy (TAC) are still poorly understood, and therefore, haemorrhage remains a common cause of death after injury 4. The major finding of this study is the description of a critical clotting factor deficit that occurs early after injury, particularly of Factor V. This critical deficit occurs in 20% of the severely traumatized patients on arrival to the Trauma Room.
Twenty two patients had critical clotting factor deficiencies, and factor V was critically low in all of them. This finding has some possible explanations. The first explanation is that a critical deficit of factor V is part of early trauma coagulopathy, a hypothesis that aligns with the activated protein C (APC) mechanism recently proposed by Brohi and others 4,10-13. The initiation phase of hemostasis produces trace amounts of thrombin that activates the 330 KDa single chain factor V molecule (residues 1-2196) to the heavy (1-708) and light (1545-2196) chains of activated factor V. On platelet surfaces, factor Va binds to factor Xa forming the prothrombinase complex, which in turn leads to massive thrombin generation 29,30. This procoagulant process is inhibited by anticoagulant enzymes including APC and plasmin 30. Plasma protein C is activated to APC by the thrombin-thrombomodulin complex that down-regulates factor Va by proteolytic cleavage at Arg506 and Arg307 30,31. Thus, a drop in factor V activity levels is expected in a pathological process mediated by the activation of APC, such as believed to occur in TAC.
Considering the key role of factor Va and of the prothrombinase complex (factor Xa-factor Va) which is 300,000 fold more active than factor Xa alone 30, an early and supra physiological inactivation of factor Va by APC would cripple the hemostatic response to trauma with major clinical consequences. This crippling effect was observed when the CF deficits were analyzed in a computational simulating model that has been previously validated34. This computational model demonstrated delays and marked reduction in the amount of active thrombin generated in patients with critical clotting factor deficiencies, strengthening the argument that an early factor V deficit could be central in the pathogenesis of early trauma-associated coagulopathy.
An alternative explanation accounting for the factor V deficit might be that factor V degradation occurred during the sample manipulation or storage phase of this study 23,24,32. Factor V is a labile factor, and among the first to decline 5,30,32. However, Zurcher in 2008 demonstrated that at room temperature, factor V activity remains unchanged for up to 12 hours in citrated blood (as used in this study). Only after 24 and 48 hours did factor V levels decline 12% and 27%, respectively 33. When frozen (−70°C ) within eight hours of collection, factor V activity remains unchanged for 18 months 32,34,35. Of note, Zurcher’s study was done using blood from healthy individuals. All blood samples in the present study were transferred immediately to the Laboratory where they were centrifuged at −4°C, frozen in less than one hour and stored at −70°C. While it is impossible to completely exclude the possibility of FV degradation during manipulation or storage, the authors find no evidence to support this argument.
Factor V is essential for normal hemostasis, as are all other individual clotting factors except for factor XII 5. Clotting factor deficits impair thrombin generation, causing unstable fibrin clots and a severe bleeding disorder 30,36. While normal hemostasis requires about 30% of activity of each individual clotting factor 23,24, the levels needed after injury or when multiple deficiencies coexist are not known and are possibly significantly higher than the critical thresholds used in this study 32,37. For haemophilia patients with single CF defects, the recommendation is to raise the deficient CF levels to 100% if undergoing surgery or after major trauma 34.
The role of clotting factor deficiency in trauma coagulopathy was a topic of interest decades ago. Earlier studies focused on a “late” coagulopathy that followed massive blood transfusions and dilution. An example is the 1985 study in which hemorrhagic shock reduced all clotting factor levels by 25-35% in a dog model, a drop aggravated by fluid resuscitation (dilution) 38. Boyan in 1962 was among the first to propose that a deficit of factor V was central in post-massive transfusion coagulopathy 39. Ciavarella in 1987 described clotting factor deficits (<30%) in a third of all massively transfused patients. In 92% of the patients the deficit was of factor V 25 and 80% had obvious clinical evidence of coagulopathic bleeding 25. Later studies by Lucas & Ledgerwood consolidated the concept of “late” TAC resulting from platelet dysfunction and clotting factor deficits 37,40,41.
The role of clotting factor deficit in the development of “early” coagulopathy is controversial. Previously, clotting factor deficits were only thought to occur as a late development after injury, and as the result of dilution, hypothermia and consumption 3,4,11,17. The prevalent hypothesis is that early TAC results from the combination of shock (acidosis) and tissue destruction, independent of dilution or CF deficit 4,11,13. The present study shows that critical clotting factor deficiencies do occur immediately after injury, prior to substantial fluid resuscitation and that these deficiencies are associated with poorer outcomes. As well, these deficiencies are sufficient to impair thrombin generation, when analyzed using a computational model. Our finding may resolve the apparent conflict between the contention that clotting factor deficits play no role in the development of early TAC and numerous clinical reports that large FFP transfusions (main source of clotting factors) increases survival of patients with early coagulopathy. We found that factor V is often critically low, whereas previous studies examining clotting factor deficiency investigated either a single or a few clotting factors, but not factor V 4,11,13.
Early TAC has been most commonly defined by routine laboratorial tests such as INR and aPTT, which are known to have shortcomings, particularly when used in trauma 4,9,10,42. In contrast, the present study compared abnormalities in INR and aPTT with critical clotting factor deficiencies, and used a threshold for clotting factor level that is widely accepted as indicative of major hemostatic defects 8,23-25,32,37. Considering that both tests measure the activity of some CF 23,24, directly measuring CF is intuitively superior. As such, we decided to use clotting factor level as the gold standards to evaluate the sensitivity and specificity of INR, aPTT and TEG. Using a threshold of 30% for critically low clotting factor activity, we found that 20% of all severely traumatized patients were coagulopathic, less than one hour after trauma, a figure similar to the 25% reported by others 9,10. Had INR been used to define coagulopathy, it would have underestimated the incidence of early coagulopathy, and diagnosed only a third of those with critical CF deficits. The reasons for the disparity between INR/aPTT and the CF levels they are supposed to measure include their nonlinear relationship and the fact that they were designed to diagnose hemostatic defect in known bleeders but not in-vivo hemostasis, thus under or overestimating hemostatic defects 23,24. Ciavarella showed that 30-35% of the PT variability and 15-20% of aPTT was unrelated to clotting factor levels 25 while Chowdhury reported that 45% of the patients with INR>1.8 had no CF deficit and that the FFP transfused to these patients, based on the prolonged INR was inappropriate 26. Using direct CF measurements we found that INR/aPTT had sensitivities below 40% in diagnosing critical deficits of clotting factors, but each had 100% specificity.
TEG has been proposed as a better alternative to INR/aPTT in trauma. While TEG and the similar ROTEM (rotational thromboelastometry) are commonly used in some countries, their role in trauma remains poorly defined 43-45. In the present study, TEG was not superior to INR/aPTT in diagnosing clotting factor deficits (Table 4). Experimental TEG studies on factor V deficient plasma report prolonged R (with celite activation) and decreased α values (with tissue factor activation) 46. In the present study using kaolin activation, neither R nor α angle was better than MA, nor was Kauffmann’s proposal of ≥2 abnormal TEG parameters. Using direct CF measurements we found that TEG also has a low sensitivity for critical clotting factor deficiency, and was actually inferior to INR and aPTT with regards to specificity for critical clotting factor deficits.
Our data suggests that coagulopathic patients had more clinical evidence of bleeding since they were transfused more RBC and FFP. FFP however, was not transfused to 91% of the coagulopathic patients, supporting the argument that current resuscitation standards often fail to address early TAC 3,14,15. At least one study showed that larger FFP doses than those dictated by approved guidelines, are needed to correct CF deficits in critically ill patients 26. There is however, surprisingly little evidence that FFP corrects coagulopathy 23,24,47. In dilutional coagulopathy, Schols reported that bleeding stopped only in patients that after FFP were able to increase thrombin generation, while others continued to bleed 48. On the other hand, FFP consumption is increasing worldwide 24 and a recent audit found that near half of the FFP transfusions in Canada do not follow current guidelines 49. Thus, a better understanding of the role of clotting factor activity levels in trauma associated coagulopathy is clinically relevant and may help to improve guidelines for FFP (clotting factors) transfusions.
The study has limitations. It did not perform all assays in all patients, mostly due to difficulties in obtaining sufficient blood samples in all patients. This issue was partially addressed during the study, and the sample volume increased. Another major limitation was the lack of standardized definitions for early trauma associated coagulopathy. There is no universally acknowledged threshold value of INR or aPTT that defines coagulopathy. Similarly, there are many problems with using a threshold value of 30% for defining a critical clotting factor deficiency – this definition has not previously been used in the trauma context, for one. Actual, observed microvascular bleeding is a definition of coagulopathy; however, there are too many logistical challenges to operationalize this definition for a study.
In conclusion, this observational cohort study found that 20% of all severely traumatized patients have at least one critical clotting factor deficiency (particularly factor V), soon after injury and on arrival to hospital. These patients are more severely injured, more acidotic, more likely to have defects in thrombin generation and have worse outcomes which supports the contention that critical clotting factor deficiency is actually an underlying mechanism behind early trauma associated coagulopathy. Our finding is consistent with they the hypothesis of an APC-mediated process in early TAC. Our study also represent biological evidence to support the paradigm of damage control resuscitation, which calls for early and aggressive clotting factor transfusions in the form of FFP34. Trauma-associated coagulopathy is among the most complex physiological processes with multiple causes and can not be fully explained by a critical deficit of factor V alone. The present findings however, add to a vastly unknown process that better understood can be targeted therapeutically and lead to a reduction of its death toll.
Acknowledgements
The authors are deeply in debt with Matthew Whelihan (University of Vermont) for the outstanding technical work done with computational simulating model.
The authors also want to thank those involved in the Steering Committee of this observational study including Ms Vanessa Speers, Dr Shawn Rhind, Dr Sue Bello, Dr Jennifer Pacher Hoffmann and Dr Mohammed Al Mahroos. We also thank the support with data provided by Ms Cyndy Rogers and Ms Andrea Phillips from the Tory Trauma Center – Sunnybrook Health Sciences Centre.
This study was supported by a research contract from Defense Research and Development Canada (DRDC) and by the Canadian Forces Health Services.
Footnotes
Disclosure of other funding: SBR received a salary award from the Canadian Institute of Health Research/NovoNordisk, honoraria and speaking fees from NovoNordisk. KGM received HL46703 Core B and HL34575
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoyt DB, Bulger EM, Knudson MM, et al. Death in the operating room: an analysis of a multicenter experience. J Trauma. 1994;37:426–432. [PubMed] [Google Scholar]
- 2.Tien HC, Spencer F, Tremblay LN, et al. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62:142–146. doi: 10.1097/01.ta.0000251558.38388.47. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 4.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 5.Lasne D, Jude B, Susen S. From normal to pathological hemostasis. Can J Anaesth. 2006;53:S2–11. doi: 10.1007/BF03022247. [DOI] [PubMed] [Google Scholar]
- 6.Stansbury LG, Dutton RP, Stein DM, et al. Controversy in trauma resuscitation: do ratios of plasma to red blood cells matter? Transfus Med Rev. 2009;23:255–265. doi: 10.1016/j.tmrv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Kozek-Langenecker S. Management of massive operative blood loss. Minerva Anestesiol. 2007;73:401–415. [PubMed] [Google Scholar]
- 8.Erber WN, Perry DJ. Plasma and plasma products in the treatment of massive haemorrhage. Best Pract Res Clin Haematol. 2006;19:97–112. doi: 10.1016/j.beha.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 10.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 11.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 12.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 13.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zink KA, Sambasivan CN, Holcomb JB, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–570. doi: 10.1016/j.amjsurg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira PG, Inaba K, Shulman I, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66:693–697. doi: 10.1097/TA.0b013e31817e5c77. [DOI] [PubMed] [Google Scholar]
- 16.Snyder CW, Weinberg JA, McGwin G, Jr., et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66:358–362. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 17.Stansbury LG, Dutton RP, Stein DM, et al. Controversy in trauma resuscitation: do ratios of plasma to red blood cells matter? Transfus Med Rev. 2009;23:255–265. doi: 10.1016/j.tmrv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento B, Callum J, Rubenfeld G, Neto JB Rezende, Lin Y, Rizoli S. Fresh Frozen Plasma in Massive Bleedings: More Questions than Answers. Crit Care. 2010;14:202–209. doi: 10.1186/cc8205. Ref Type: Journal (Full) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phan HH, Wisner DH. Should we increase the ratio of plasma/platelets to red blood cells in massive transfusion: what is the evidence? Vox Sanguinis. 2009:1–8. doi: 10.1111/j.1423-0410.2009.01265.x. Epub ahead of print. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 20.Moore FA, Nelson T, McKinley BA, et al. Is there a role for aggressive use of fresh frozen plasma in massive transfusion of civilian trauma patients? Am J Surg. 2008;196:948–958. doi: 10.1016/j.amjsurg.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 21.Rizoli SB, Boffard KD, Riou B, et al. Recombinant activated factor VII as an adjunctive therapy for bleeding control in severe trauma patients with coagulopathy: subgroup analysis from two randomized trials. Crit Care. 2006;10:R178. doi: 10.1186/cc5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg. 2009;108:1433–1446. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- 23.Dzik WH. Component Therapy Before Bedside Procedures. In: Mintz PD, editor. Transfusion Therapy: Clinical Principles and Practice. AABB Press; Bethesda: 2005. pp. 1–26. [Google Scholar]
- 24.Dzik WH. The James Blundell Award Lecture 2006: transfusion and the treatment of haemorrhage: past, present and future. Transfus Med. 2007;17:367–374. doi: 10.1111/j.1365-3148.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 25.Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol. 1987;67:365–368. doi: 10.1111/j.1365-2141.1987.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 27.Hockin MF, Jones KC, Everse SJ, et al. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann CR, Dwyer KM, Crews JD, et al. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997;42:716–720. doi: 10.1097/00005373-199704000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Mann KG, Brummel-Ziedins K, Orfeo T, et al. Models of blood coagulation. Blood Cells Mol Dis. 2006;36:108–117. doi: 10.1016/j.bcmd.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Mann KG, Kalafatis M. Factor V: a combination of Dr Jekyll and Mr Hyde. Blood. 2003;101:20–30. doi: 10.1182/blood-2002-01-0290. [DOI] [PubMed] [Google Scholar]
- 31.Tran S, Norstrom E, Dahlback B. Effects of prothrombin on the individual activated protein C-mediated cleavages of coagulation factor Va. J Biol Chem. 2008;283:6648–6655. doi: 10.1074/jbc.M708036200. [DOI] [PubMed] [Google Scholar]
- 32.AGGELER PM. Physiological basis for transfusion therapy in hemorrhagic disorders: a critical review. Transfusion. 1961;1:71–86. doi: 10.1111/j.1537-2995.1961.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 33.Zurcher M, Sulzer I, Barizzi G, et al. Stability of coagulation assays performed in plasma from citrated whole blood transported at ambient temperature. Thromb Haemost. 2008;99:416–426. doi: 10.1160/TH07-07-0448. [DOI] [PubMed] [Google Scholar]
- 34.O’Shaughnessy DF, Atterbury C, Bolton MP, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 35.Woodhams B, Girardot O, Blanco MJ, et al. Stability of coagulation proteins in frozen plasma. Blood Coagul Fibrinolysis. 2001;12:229–236. doi: 10.1097/00001721-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Tracy PB, Giles AR, Mann KG, et al. Factor V (Quebec): a bleeding diathesis associated with a qualitative platelet Factor V deficiency. J Clin Invest. 1984;74:1221–1228. doi: 10.1172/JCI111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledgerwood AM, Lucas CE. A review of studies on the effects of hemorrhagic shock and resuscitation on the coagulation profile. J Trauma. 2003;54:S68–S74. doi: 10.1097/01.TA.0000064513.59253.70. [DOI] [PubMed] [Google Scholar]
- 38.Martin DJ, Lucas CE, Ledgerwood AM, et al. Fresh frozen plasma supplement to massive red blood cell transfusion. Ann Surg. 1985;202:505–511. doi: 10.1097/00000658-198510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.BOYAN CP, HOWLAND WS. Problems related to massive blood replacement. Anesth Analg. 1962;41:497–508. [PubMed] [Google Scholar]
- 40.Harrigan C, Lucas CE, Ledgerwood AM. The effect of hemorrhagic shock on the clotting cascade in injured patients. J Trauma. 1989;29:1416–1421. doi: 10.1097/00005373-198910000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Lucas CE, Ledgerwood AM, Mammen EF. Altered coagulation protein content after albumin resuscitation. Ann Surg. 1982;196:198–202. doi: 10.1097/00000658-198208000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 43.Kheirabadi BS, Crissey JM, Deguzman R, et al. In vivo bleeding time and in vitro thrombelastography measurements are better indicators of dilutional hypothermic coagulopathy than prothrombin time. J Trauma. 2007;62:1352–1359. doi: 10.1097/TA.0b013e318047b805. [DOI] [PubMed] [Google Scholar]
- 44.Carroll RC, Craft RM, Langdon RJ, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009;154:34–39. doi: 10.1016/j.trsl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Scarpelini S, Rhind SG, Nascimento B, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–1217. doi: 10.1590/s0100-879x2009001200015. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen VG, Cohen BM, Cohen E. Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastography: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiol Scand. 2005;49:222–231. doi: 10.1111/j.1399-6576.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 47.Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–152. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 48.Schols SE, van der Meijden PE, van OR, et al. Increased thrombin generation and fibrinogen level after therapeutic plasma transfusion: relation to bleeding. Thromb Haemost. 2008;99:64–70. doi: 10.1160/TH07-07-0438. [DOI] [PubMed] [Google Scholar]
- 49.Luk C, Eckert KM, Barr RM, et al. Prospective audit of the use of fresh-frozen plasma, based on Canadian Medical Association transfusion guidelines. CMAJ. 2002;166:1539–1540. [PMC free article] [PubMed] [Google Scholar]