Abstract
Simian virus 40 (SV40) is a DNA virus isolated in 1960 from contaminated polio vaccines, that induces mesotheliomas, lymphomas, brain and bone tumors, and sarcomas, including osteosarcomas, in hamsters. These same tumor types have been found to contain SV40 DNA and proteins in humans. Mesotheliomas and brain tumors are the two tumor types that have been most consistently associated with SV40, and the range of positivity has varied about from 6 to 60%, although a few reported 100% of positivity and a few reported 0%. It appears unlikely that SV40 infection alone is sufficient to cause human malignancy, as we did not observe an epidemic of cancers following the administration of SV40-contaminated vaccines. However, it seems possible that SV40 may act as a cofactor in the pathogenesis of some tumors. In vitro and animal experiments showing cocarcinogenicity between SV40 and asbestos support this hypothesis.
Keywords: brain tumor, malignant mesothelioma, SV40, Tag, tag, transformation
Simian virus 40
Simian virus 40 (SV40) was first isolated in 1960 from cultures of Rhesus monkey kidney cells used to produce poliovirus vaccines [1] and was assigned to the family of Polyomaviridae, Polyomavirus genus, closely related to human polyomaviruses (BK, JC, KI and WU), based on genomic organization and sequence similarity [2].
Simian virus 40 does not induce disease in its natural host, the Rhesus monkey, or in other monkey species. SV40 transmission among monkeys occurs via urine, feces and bites, and vertical transmission has been documented in monkeys and hamsters [3].
SV40 genomic organization
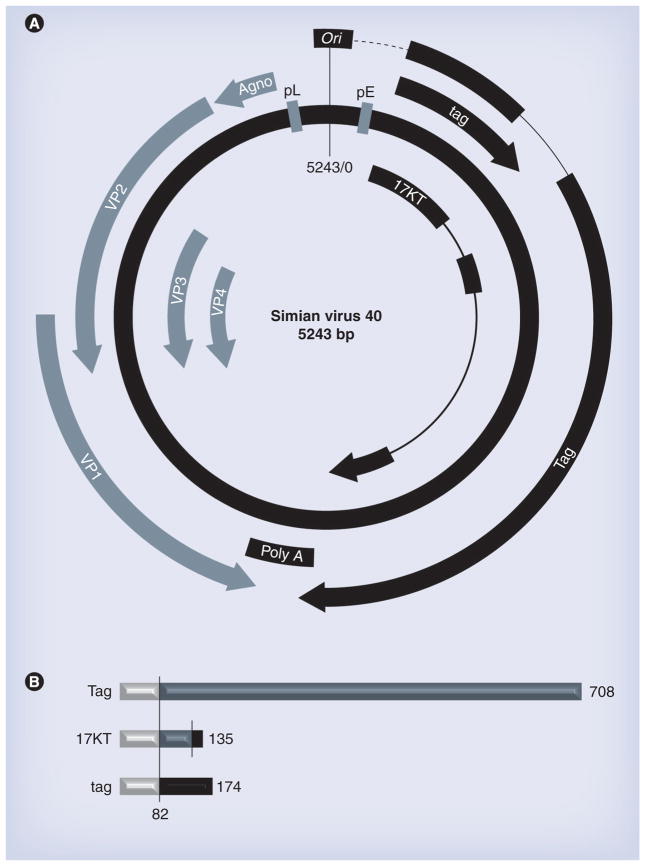
Simian virus 40 is an unenveloped icosahedral virion containing a small, closed circular double-strand DNA genome. The virion is 45–50 nm in diameter [4] and 1.34–1.35 g/cm3 in density. Genome length is approximately 5 kb, depending on various strains. Different regions were identified in the SV40 genome: regulatory region (bidirectional promoter and replication origin); early genes; and late genes (Figure 1A). The terms ‘early’ and ‘late’ reflect the sequential gene transcription and translation events in the host cells. Early and late genes extend in opposite directions. Early genes encode for the large T antigen (Tag), the 17KT and the small t antigen (tag) (Figure 1B). Late genes are transcribed into two classes of late mRNAs: 16S, coding for the major capsid protein VP1; and 19S coding for the VP2, VP3, VP4 and Agnoprotein. To the best of our knowledge, all SV40 strains produce these seven viral proteins. Tag is a nuclear phospho-protein of approximately 94 kDa. Tag induces SV40 DNA replication and, together with tag, promotes cell cycle progression, S-phase entry and DNA synthesis in host cells. VP1, VP2, VP3 and VP4 enable the packaging of replicated viral DNA into transmissible infectious virions, encapsulated by 360 VP1 protein molecules, tightly bound in 72 pentamers. VP2 and VP3 are less abundant, although they also play an essential role in the SV40 packaging process, both in vivo and in vitro [5]. The Agnoprotein controls the perinuclear localization of the viral genome during virion assembly and may contribute to viral late gene transcription and translation, viral release and propagation [6]. VP4, a recently discovered late protein, triggers the lytic release of the virus [7]. Like VP3, the 15-kDa VP4 protein is synthesized starting from the third in-frame AUG codon within the VP2 transcript, and shares the same stop codon with VP3 and VP2. VP4 is expressed in the infected cells 12–24 h later than VP1, VP2 and VP3, and is not incorporated into viral particles. Mutated virus lacking VP4 expression displayed delayed lysis and reduced particle release [7].
Figure 1. Structure and function of simian virus 40.
(A) Physical and functional map of the SV40 genome. The circular DNA genome is 5243 bp. Ori (black). Tag, tag and 17KT (black): ‘Early gene’ mRNAs, controlled by the pE (blue). pL (blue) shares the same sequence with pE, but is oriented in the opposite direction. VP1, VP2, VP3, VP4 and Agno (blue): transcripts of ‘late genes’, are under control of pL and encode capsid proteins VP1, VP2, VP3, and VP4 and Agnoprotein assembly promoting proteins. VP2-4 share the same stop codon. (B) SV40 early proteins: Tag, 17KT and tag share the first 82 amino acid residues at the N-terminal (gray). Tag extends up to 708 residues in length (blue), but skips the tag encoding region (black), owing to differential splicing. 17KT encodes a 135 amino acids protein, sharing the first 131 residues (gray and blue) with Tag, followed by four amino acids encoded by the third intron of the Tag gene, retained by an alternative splicing. SV40 tag is a 174 residue protein.
Ori: Origin of viral DNA replication; pE: Early promoter; pL: Late promoter; SV40: Simian virus 40; Tag: Large T antigen; tag: Small t antigen.
Several different SV40 strains, described below, have been isolated from monkey and human tissues [8]. Strain SV40–776 (wild-type) is the prototype and reference strain. It was isolated from a stock of a contaminated adenovirus vaccine preparation. SV40-B2, the so called ‘Baylor strain’, was isolated from SV40-contaminated polio vaccines (Sabin, attenuated virus). VA45-54 was discovered in a normal cell culture of grivet monkey kidney cells, from which several temperature-sensitive mutants were obtained. SVPML-1 was isolated from progressive multifocal leukoencephalopathy human cells, while SVMEN was isolated from a human meningioma biopsy, an important finding because this virus comes directly from a human tumor biopsy, not from cells in culture. SVCPC, first characterized as an additional strain from a human choroid plexus tumor biopsy, has been shown to be identical to SVMEN. The DNA sequences of SVPML-1, SVMEN/SVCPC are more similar to Baylor than to 776 [9]. It should be noted, however, that these strain differences are very minimal, suggesting that mutations are not tolerated in the small SV40 genome. Most of the differences of SV40 variants were detected in the sequence encoding the carboxyl-terminal variable region of Tag, within the amino acid residues 622 and 708; in addition, some sequence variability was detected in the early transcript intron and the sequences encoding tag, VP1 and Agnoprotein, were affected [10]. Thus, no mutations have been found so far in the critical portions of the SV40 Tag, such as those responsible for binding p53, retinoblastoma protein (pRb) and so on.
SV40 strains are classified as ‘archetypal’ (harboring one copy of the 72-bp enhancer element for early and late viral genes) and ‘nonarchetypal’ SV40 (carrying two copies of this 72-bp enhancer element). VA45-54 and Baylor carry only one 72-bp enhancer element in fresh isolates, but duplication of the 72-bp sequence has occurred during replication in CV-1 monkey infected cells. No other modifications were detected in the remaining sequences, including Tag carboxyl-terminal variable region [8]. This duplication is compatible either with the hypothesis of SV40 adaptation in cell culture, by selection of a rare variant pre-existing in the original sample, or with the generation de novo of the rare duplication of the regulatory region in infected cells [8,10]. The efficiency of virus production in vitro by archetypal SV40 776 was improved by the duplication of the naturally occurring 72-bp enhancer element [8]. Nonarchetypal SV40 stain 776 (commonly known as ‘wild-type’ SV40) and the Baylor strain displayed higher transformation efficiency than archetypal SV40 [11].
SV40 life cycle
Cell infection starts with the binding of SV40 virions (mainly through VP1) to specific receptors on the plasma membrane: GM1 ganglioside [12] and Class I MHC molecules [13]. SV40 enters cells by caveolae-mediated endocytosis. SV40 bypasses the Golgi complex en route to the endoplasmic reticulum, where calcium ions, bridging VP1 capsid proteins, are removed by endoplasmic reticulum absorption, resulting in capsid disassembly [5], which requires low cytosolic calcium concentrations [14]. Afterwards, SV40 genome is translocated to the nuclear compartment via mechanism mediated by the importin α2/β heterodimer and by VP3 [15]. The early promoter of SV40 genome is recognized by host RNA polymerase II leading to early gene transcription soon after nuclear translocation. The primary transcripts are alternatively spliced into two mRNAs encoding Tag and tag, whose ratio varies in different cell types [16]. During the early phase of infection, the late genes transcription does not take place, because of the action of late gene transcriptional repressors [17]. What happens next varies in permissive and nonpermissive cells.
Infected cells can be permissive, nonpermissive and semi-permissive to SV40. African green monkey and Rhesus kidney cells are permissive. In these cells, Tag binds as a double hexamer to the viral replication origin (ori), promoting DNA replication. With time, the cellular repressor proteins are titrated-off from the late promoter, while the transcription from the early promoter is repressed. Tag also unwinds the DNA double helix, promoting the recruitment of host cellular DNA polymerase and protein A, which starts transcription. In addition, Tag modulates intracellular signaling by recruiting several cellular proteins involved in cell progression and apoptosis pathways, and Tag transactivates several cellular genes including IGF-1 [18,19], cdc2 [20], hepatocyte growth factor receptor (Met) [21] and Notch-1 [22], thus promoting G2 phase progression (see SV40-mediated human primary mesothelial cells [HM] and astrocyte transformation). These events trigger the transition from early to late phase of SV40 infection. When most of the viral genome is replicated, the capsid proteins are synthesized. The activation of checkpoint kinase Chk1 by ataxia telangiectasia mutated-Rad3-related-dependent phosphorylation blocks the mitosis of the target cells, at the same time late viral proteins accumulate inside the cell [23]. Only the SV40 genome, but not the host’s genome, contains six tandem GC boxes, which represent the viral packaging signal for capsid assembly and viral DNA packaging. The transcriptional factor SP1 binds to the GC box, recruits VP2 and VP3, which in turn bind to VP1 pentamers and start viral assembly [24]. This is followed by the attachment at low affinity of multiple capsomers surrounding the minichromosome. The formation of this immature complex increases the capsomer local concentration and accelerates the assembly in a cooperative manner. The final icosahedron arises either from the progressive addition of single pentamers to the growing shell or from an organized pentamer clustering [25]. The process is followed by VP4-mediated activation of poly (ADP-ribose) polymerase, which leads to cell necrosis. Large amounts of viral particles are released, new cells are infected and a new infection cycle begins. The nuclear factor of activated T-cells (NFATs), specifically NFAT3 and NFAT4, is an important transcriptional factor controlling SV40 infection [26]. In human cells, SV40 Tag increases NFATs activity and NFATs provide a positive feedback loop, transactivating the SV40 promoters.
The tag, inactivates serine/threonine protein phosphatase 2A, thus altering the phosphorylation of several cellular proteins promoting S phase entry. The tag cooperates with Tag supporting virus production in permissive cells [27].
Nonpermissive rodent cells do not support viral DNA replication – or at least support limited viral DNA replication [28]. However, Tag and tag are expressed and promote cell division. Because virus production does not take place in these cells, the cell progeny do not contain SV40 and cells stop growing, a process known as ‘abortive transformation’. Occasionally, the SV40 DNA can integrate into the host chromosomal DNA. When the virus sequences that code Tag and tag are not altered by the integration process, they may be expressed and may cause malignant transformation (a rare event occurring in approximately one in 107–109 infected cells). SV40 integration occurs randomly, often near the nuclear matrix attachment region in tumor cells [29]. It has been suggested that the disruption of human chromosomal interval at 1q21.1, caused by SV40 integration, is an essential step in the process of cellular immortalization [30].
Human cells are called semi-permissive in that they support SV40 replication, although less efficiently than monkey cells. SV40 lyses human fibroblasts and very rarely (less than 1/108 cells) these cells may become transformed, if the viral DNA integrates into the host cell genome [31]. Shein discovered in 1967 that human astrocytes were susceptible to SV40 infection and that, following infection, these cells could become transformed [32]. SV40 transformed astrocytes released infectious SV40 [32], while transformed fibroblasts did not [33]. Whether SV40 enters a true latent state remains to be fully addressed. We discovered that following SV40 infection, 1/103 HM [34] and one in seven human primary astrocytes [11] are transformed and can be established as continuous cell lines (i.e., they do not undergo ‘crisis’). At the same time, in similar experiments conducted on many different fibroblast batches in our laboratory during the past 26 years, we have never developed a single SV40 transformed human fibroblast line. Instead, in parallel experiments we developed several dozen HM and astrocytic cell lines. Some of these HM and astrocytic cell lines release variable amounts of infectious SV40: in a few, the amount of virus released significantly decreased during cell culture, an effect we linked to the production of an antisense mRNA originating in the early region of the SV40 genome that leads to the degradation of late genes mRNAs [11,35] [Carbone M, Unpublished Observations].
SV40-mediated immortalization & transformation
To study SV40 infection of human cells it is necessary to prevent SV40 cell lysis, a normal outcome of SV40 infection of human cells. For this purpose, DNA replication-defective SV40 mutants and SV40 oncogene constructs under the control of an heterologous promoter are often used. The expression of both Tag and tag causes higher transformation efficiency [27]. Following SV40 infection, fibroblasts delay senescence from 40–50 to 60–70 generations, then undergo cell crisis and apoptosis. It has been reported by others that 1/107–1/109 fibroblasts may escape senescence and become immortalized cell lines [36], a phenomenon we have never observed in our laboratory. The expression of Tag and tag following SV40 infection correlates with the induction of telomerase activity in infected HM [34]. Transfection of SV40 constructs does not induce telomerase in fibroblasts unless SV40 is placed under a strong heterologous promoter, such as human cytomegalovirus [34].
It has been proposed that active telomerase mitogenic stimuli (i.e., H-ras pathway) and SV40 early genes, by altering p53, pRb and PP2A activities, are the indispensable genetic requirements to achieve fully malignant transformation of human cells [36]. However, the functions of Tag and tag broadly extend to a number of other host cellular signaling pathways [16].
Some human cell types are more susceptible than others to SV40-mediated transformation. In HM and brain cells, Tag binds and inactivates the tumor suppressor activities of cellular p53, pRb, p130 [37], p300 and p400. At the same time the Tag-p53-multiprotein complex acquires its own oncogenic activity and binds and activates the promoter, which in turn causes IGF-1 secretion and tumor cell growth [18].
Human primary mesothelial cells are an example of human cells that can be transformed solely by SV40 exposure [35]. Approximately one in 5000 of the infected HM develops foci and these foci can be easily propagated in tissue culture. Stable cell lines can be established from about 95% of foci and tumors develop when these cells are injected into immunodeficient mice [38]. In SV40-transformed HM abundant viral DNA persists in episomal form, and a low amount of infectious viral particles are produced and released during passage in tissue culture [35].
SV40 oncogenicity in animals
Hamsters injected with SV40 developed osteosarcomas, sarcomas, ependymomas, mesotheliomas, choroid plexus tumors and true histiocytic lymphomas [16]. The types of tumors induced are influenced by the route of SV40 inoculation and by cell susceptibility, for instance, most cell types are infected, but few can be transformed by SV40 [39]. Over 50% of hamsters injected with SV40 intracardiacally or intraperitoneally, and 100% of those injected intrapleurally, developed mesothelioma [40].
Simian virus 40 transgenic animals have provided excellent models to study the development of various cancers, and to search for possible therapeutic targets and approaches. To date, several transgenic models have been established. The first SV40 transgenic model was established in 1984 by integrating the SV40 early region genes and their own promoter–enhancer into a mouse germ line genome. High percentages of the transgenic mice population developed brain tumors within 3–5 months [41]. Moreover, in a similar model, by using Tag and the SV40 72-bp enhancer element as transgenes, the same group demonstrated that Tag expression is sufficient for tumor induction and that the enhancer region influences tissue tropism, as these animals developed only choroid plexus tumors [42]. Expression of Tag under the control of the glucagon gene 5′ flanking sequences in transgenic mice led to the development of large bowel carcinoma [43]. Tag transgenic mice can produce heritable eye tumors, including ocular tumors and lens tumors [44].
Several different transgenic models have been developed by driving SV40 genes expression under the control of different tissue-specific promoters [45–65]. Table 1 lists a panel of SV40-based transgenic models commonly used to study breast, prostate and other tumors. These transgenic models indicate that the expression of SV40 Tag can cause cancer in any organ, making SV40 Tag the most potent oncogene known to date. The types of cancer induced are determined by the promoter used, which in turn determines the tissue in which Tag is expressed. When Tag is under the control of its own promoter, it causes primarily brain and bone tumors, mesotheliomas and a type of lymphomas knows as true histiocytic lymphomas.
Table 1.
SV40 transgenic animal models.
| Study (year) | Sequence | Promoter | Host | Tumor type | Ref. |
|---|---|---|---|---|---|
| Hanahan (1985) | Tag | Rat insulin II | Mouse | Insulinoma | [45] |
| Wikenheiser et al. (1992) | Tag | Human surfactant protein C | Mouse | Adenocarcinoma | [46] |
| Maroulakou et al. (1994) | Tag, tag | Rat prostatic steroid binding protein C3(1) | Mouse | Adenocarcinoma (only in females) | [47] |
| Greenberg et al. (1995) | Tag, tag | Rat probasin | Mouse | Invasive adenocarcinoma (only in males) | [48] |
| Hurwitz (2001) | Tag, tag | Rat probasin | Mouse | Invasive adenocarcinoma (only in males) | [49] |
| Santarelli et al. (1996) | Tag, tag | Mouse whey acidic milk protein | Mouse | Breast | [50] |
| Perez-Stable et al. (1996) | Tag | Human fetal IgG γ-globin | Mouse | Prostate | [51] |
| Garabedian et al. (1997, 1998) | Tag | Mouse cryptdin-2 | Mouse | Prostate | [52,53] |
| Asamoto et al. (2001) | Tag | Rat probasin | Rat | Prostate carcinoma | [54] |
| Masumori et al. (2001), Gabril et al. (2002) | Tag | Rat probasin | Mouse | Adenocarcinoma | [55,56] |
| Gabril et al. (2002) | Tag | PSP94 | Mouse | Adenocarcinoma | [56] |
| Hicks et al. (2003) | Tag | Clara-cell secretory protein 10 | Mouse | Adenocarcinoma, lung parenchymal | [57] |
| Garson et al. (2003) | Tag | Ovarian-specific promoter 1 | Mouse | Ovarian and brain tumors, abdominal and bone sarcoma | [58] |
| Lou et al. (2005) | Tag, tag | Human antithrombin III gene | Mouse | Hepatocarcinoma | [59] |
| Grippo et al. (2000) | Tag | Cytokeratin 19 | Mouse | Urinary bladder tumors Mesothelioma | [60] |
| Robinson et al. (2006) | Tag | Mesothelin | Mouse | Mesothelioma | [61] |
| Köbbert et al. (2008) | Tag | SM22α | Mouse | Cardiac rhabdomyosarcoma | [62] |
| ter Brugge et al. (2009) | Tag | Immunoglobulin heavy chain, opposite orientation | Mouse | B-cell chronic lymphocytic leukemia | [63] |
| Stahl et al. (2009) | Tag inducible | Hepatocyte-specific albumin | Mouse | Hepatocellular carcinoma | [64] |
| Iwakura et al. (2009) | Tag | Human ghrelin | Mouse | Ghrelinoma | [65] |
Tag: Large T antigen; tag: Small t antigen.
These animal models provide convenient and accurate tools to monitor and observe the events underlying tumor initiation and development, and provide models to test novel preventive and therapeutic approaches.
Malignant mesothelioma
Malignant mesothelioma (MM) is an aggressive cancer, resistant to conventional therapies; about 90% of those diagnosed with this disease die within 2 years [66]. The incidence of MM is increasing in the Western world; in the USA approximately 3000 cases are diagnosed annually, compared with approximately none until 1950 [67]. MM develops from the transformation of the HM of the pleural, pericardial and peritoneal tissues that line the body cavities; about 70% of MM is of pleural origin. Exposure to the mineral fibers asbestos and erionite is the main cause of MM; SV40 infection and radiation are potential cofactors [68]. SV40 is the only agent known to cause malignant transformation of HM in tissue culture. Asbestos does not transform HM in tissue culture; however, when HM are exposed to both SV40 and asbestos, the rate of transformation increases significantly. Thus, SV40 and asbestos are cocarcinogens, and act synergistically in causing malignant transformation of HM in vitro [38,69].
SV40-mediated HM & astrocyte transformation
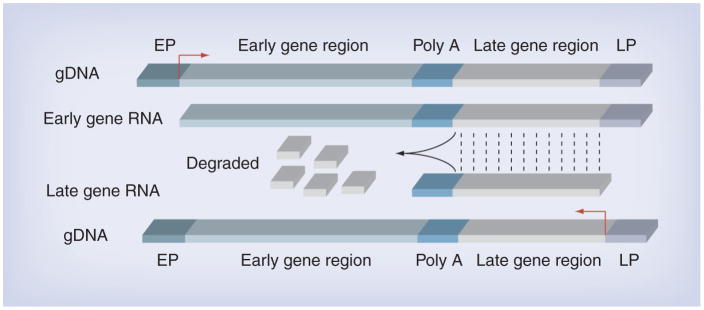
For a long time, we were puzzled by the observation that SV40 remained episomal in human tumor cells. For example, the isolation of infectious SV40 from brain tumors [8] clearly indicated the presence of episomal virus, yet integrated virus was not detected in the same tumors. How could episomal SV40 persist in human cells when, as discussed previously, episomal SV40 should replicate, produce VP4 and cause cell lysis? We discovered that in HM and in human astrocytes, during SV40 late phase infection, late gene expression is suppressed by antisense RNAs that are produced as a result of extension of the early transcripts beyond the early polyadenylation signal into the late region (Figure 2) [35]. The double-stranded RNA molecules, generated by this peculiar mechanism, may either be degraded by Dicer-mediated processing, or they may not be exported into cytoplasm. Experiments are in progress to address this mechanism. Viral late gene silencing allows HM to survive SV40 infection, as viral particles and VP4 are not produced, thus these cells are not lysed. At the same time the continuous expression of Tag causes malignant cell transformation [35]. These findings provided a mechanistic rationale for the unusual susceptibility of HM to SV40-mediated malignant transformation. Consistently, SV40-associated microRNAs were not detected in human MM samples by a highly sensitive reverse transcription PCR survey [70]. Such microRNA could not be produced because of the presence of the antisense RNA discussed previously.
Figure 2. Silencing mechanism of simian virus 40 late gene transcription.
Early genes transcripts extend beyond poly A signal and overlap the late gene region (light gray), leading to late gene antisense RNA. The resulting double-strand RNA is degraded, impairing late gene expression.
EP: Early promoter; gDNA: Genomic DNA; LP: Late promoter.
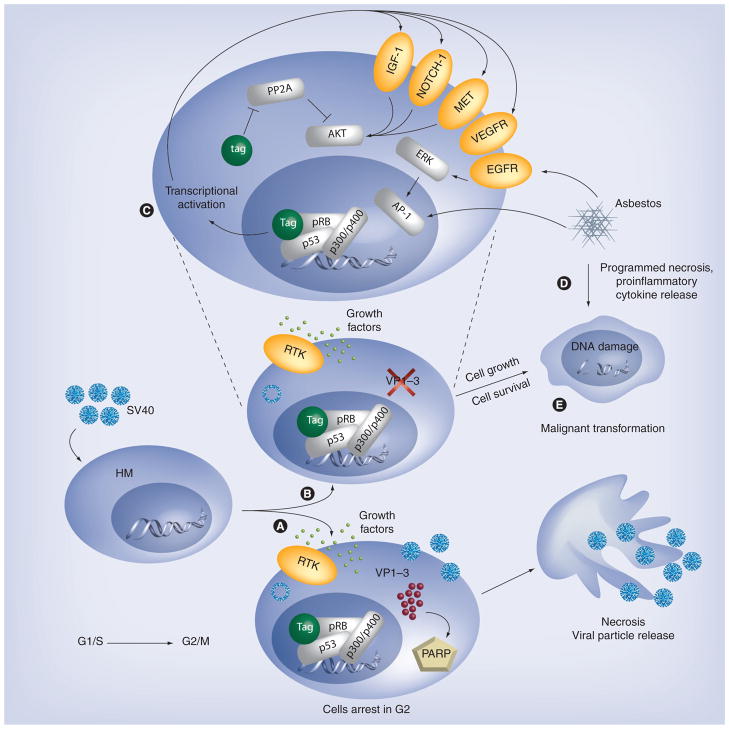
Figure 3 summarizes the molecular events driven by the expression of SV40 Tag in HM that leads to HM transformation and malignant growth. Tag expression also induces a HGF autocrine circuit in Rb-dependent manner in HM, and possibly in other cell types. The resulting activation of HGF receptor Met causes epithelial–mesenchymal transition of HM and S-phase entry, cell cycle progression, virus particle assembling and infection of adjacent cells [21]. VEGF is also expressed and secreted upon SV40 infection of HM, by an autocrine mechanism [71]. VEGF is an important growth factor for MM growth and is a potent angiogenic factor promoting vascularization of MM [72]. Tag and tag are able to upregulate Notch-1 at the transcriptional level, to induce ERK pathway and to induce telomerase activity [22]. Interestingly, Notch-1 and Met are highly expressed in HM and astrocytes transformed by nonarchetypal SV40, while the archetypal SV40 variant can only transform astrocytes [11]. SV40 tag binds and inactivates the PP2A leading to an increase of ERK pathway activity and tumorigenesis [27].
Figure 3. Mechanisms of simian virus 40 infection and cell transformation.
Upon SV40 entry, Tag is expressed and accumulates in the host cells, where it binds to p53, pRb, p300, p400 and possibly other proteins, to form a multiprotein complex that activates transcription of IGF-1. Tag also induces, possibly with similar mechanisms, the expression of other growth factors. (A) In the majority of infected HM, late gene transcription leads to VP1-3 viral capsid protein expression and virion assembly. Host cells undergo necrotic lysis, owing to massive viral release and a PARP-mediated mechanism. (B) In a fraction of infected HM, VP1-3 expression is blocked by the antisense mechanism [35], virions are neither assembled nor released and host cells survive. (C) In these cells, the signaling induced by the autocrine IGF-1/IGF-1R loop (and by other ligand/receptor autocrine circuits) eventually leads to G1/S progression and cell proliferation. Also tag contributes to cell survival inducing Akt activity as a consequence of PP2A inhibition. (D) Exposure to asbestos activates EGFR signaling, leading to ERK activity and AP-1 transcription, and induces programmed cell necrosis with consequent release of HMGB1 and other proinflammatory cytokines [77], leading to chronic inflammation. (E) The signaling induced by asbestos and SV40 infection, either independently or in a cooperative manner, can promote HM transformation and mesothelioma development.
EGFR: EGF receptor; HM: Human primary mesothelial cell; HMGB1: High molecular group binding protein 1; PARP: Poly (ADP-ribose) polymerase; pRb: Retinoblastoma protein; SV40: Simian virus 40; Tag: Large T antigen; tag: Small t antigen; VEGFR: VEGF receptor.
SV40-asbestos: cocarcinogenesis
Asbestos is a mineral fiber that when deposited in sufficient amounts in the lungs can cause progressive fibrosis (i.e., asbestosis), lung cancer and MM. When HM in tissue culture are exposed to both SV40 and asbestos, the rate of malignant transformation increases synergistically [73]. By using the tag-defective SV40 (strain dl883), which does not induce MM in vivo, we demonstrated that pleural and peritoneal injection of crocidolite asbestos, combined with intraventricle administration of dl883 SV40, induced mesothelioma in approximately 90% of hamsters with a shorter tumor latency compared with 20% mesothelioma induction in animals injected with asbestos alone [73]. Therefore, two very different carcinogens, SV40 and asbestos, are cocarcinogens in causing malignant transformation of HM in vitro and in causing MM in animals. This finding was independently reproduced in different laboratories [61,74]. The exact mechanisms underlying cocarcinogenesis are unknown.
Asbestos deposition in tissues causes a chronic inflammatory process. When asbestos fibers are ingested by macrophages and HM, these cells undergo programmed cell necrosis that is a process that leads to the release of high molecular group binding protein 1 (HMGB1) into extracellular space. Extracellular HMGB1 promotes the secretion of TNF-α by surrounding macrophages and HM cells that reduce asbestos-induced cytotoxicity by activating the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) [75–77]. The release of HMGB1 starts and propagates a chronic inflammatory process characterized by the release of reactive oxygen/nitrogen species that cause DNA damage [68]. It appears possible that this inflammatory environment may favor the growth of HM that have accumulated DNA damage owing to the reactive oxygen/nitrogen species released by cells exposed to asbestos, or owing to SV40 Tag expression, or both. Moreover, Tag expression protects HM from asbestos cytotoxicity by activating the PI3K/Akt pathway, thus increasing the percentage of cells that survive asbestos exposure and can therefore undergo malignant transformation [69]. Tag also upregulates calretinin expression [78].
Suggestions of asbestos/SV40 cocarcinogenesis in human MM were reported in some areas in Northeastern Italy [79] and Egypt [80], both with high levels of asbestos exposure and MM incidence. The Italian town of Casale Monferrato (Northwestern Italy) was extensively exposed to asbestos [81]. In this town, 16% of blood specimens from healthy individuals tested positive for SV40 Tag DNA sequences [82]. The unusually high incidence of SV40 in specimens from Italy can be attributed to the inadvertent use of SV40 contaminated vaccines in Italy as recently as 1999, when following a note from the British National Institute for Biological Standards and Control (UK), the Italians finally switched to the SV40-free WHO seed to produce polio vaccines [11] (to the best of our knowledge Italy is the only country in the Western world were SV40 contaminated vaccines have been used until few years ago). Several SV40 strains have been isolated from Italian patients [83].
SV40 association with human cancers
Rhesus monkey cells in culture were extensively used in the preparation of polio vaccines, by using either killed (Salk vaccines) or attenuated virus (Sabin vaccines). In these cultures, indigenous SV40 was not completely killed by the formaldehyde inactivation process used for polio vaccines. Poliovirus vaccines potentially contaminated with SV40 were inadvertently administered to millions of individuals between 1953 and 1963 in the USA and in several other countries [16]. Whether the increased incidence of mesothelioma observed since then in the USA and throughout the world was influenced by these contaminated vaccines continues to be debated. Infectious SV40 particles were also detected in adenovirus vaccines produced between 1957 and 1960, although the distribution of contaminated adenovirus vaccines was much less widespread and largely restricted to military personnel [8]. A seed of polio vaccines from a major eastern European manufacturer that contained infectious SV40 particles was used from 1965 to 1978 throughout the world [84]. In human populations, individuals exposed to SV40 may develop a transient infection, approximately 60% of the male volunteers (ages 21–35 years) developed antibodies after intranasal exposure to SV40 [85]. Whether these people were/are at higher risk of developing cancer remains to be elucidated.
SV40 possible transmission route among human populations
Humans have been exposed to both archetypal and nonarchetypal SV40. SV40 virus with an archetypal configuration of the regulatory region, are the most common viruses present in monkeys, therefore, these were the strains most commonly detected in polio vaccines [8,84]. Following oral administration of SV40-contaminated polio vaccine, SV40 was detected in the feces for several weeks, indicating that the virus was replicating in some of the cells of the GI tract [86]. SV40 was detected in the urine of immunocompromised patients and patients with collapsing focal segmental glomerulosclerosis [87], and in the tonsils of immunocompetent children [88]. Polyomaviruses (BK, JC or SV40) were detected in 46% of stool samples from hospitalized children [89] and 20 out of 110 stool specimens from healthy adults [90]. Among the 20 positive samples, one of the stool samples contained both SV40 and JC virus [90]. These findings indicate that SV40 infects and replicates in humans, and that the GI tract may be a site of polyomavirus persistence. It appears possible that human to human SV40 infection occurs as observed for other viruses. More research is needed to address this question.
SV40 & MM
The association of SV40 and human MM has been extensively explored. Table 2 summarizes most of the reports published since 2002; previous work has been well summarized by Klein et al. [91]. The presence of SV40 in human MM was first reported in approximately 60% of US specimens in 1994, by using PCR, western blotting, immunoprecipitation and immunohistochemical (IHC) staining [92]. It was confirmed by a multi-institutional study [93], and by several other research teams [79,80,94–98]. Microdissection experiments confirmed IHC findings, indicating that SV40 was present in tumor cells but not in the surrounding stroma [95]. However, some research groups did not detect SV40 in MM biopsies [99–102].
Table 2.
Simian virus 40 incidence in malignant mesothelioma.
| Study (year) | SV40 (%) | Asbestos (%) | SV40 + asbestos (%) | Source† | Techniques | Ref. |
|---|---|---|---|---|---|---|
| Carbone et al. (1994) | 60 | 76 | 38 | USA | PCR, IHC, WB, IP | [92] |
| Testa et al. (1998) | 83 | 71 | 71 | USA | PCR | [93] |
| Ramael et al. (1999) | 60 | 100 | 60 | Belgium | PRINS and IHC | [94] |
| Shivapurkar et al. (1999) | 57 | NA | NA | Germany, North America | PCR and DNA seq | [95] |
| De Rienzo et al. (2002) | 36/0‡ | NA | NA | USA, Turkey | PCR and DNA seq | [96] |
| Priftakis et al. (2002) | 10 | NA | NA | Sweden | PCR and DNA seq | [97] |
| Cristaudo et al. (2005)§ | 42 | 68 | 88 | Italy | PCR, DNA seq, F-Hybri | [98] |
| Comar et al. (2007)¶ | 16 | 90 | 29 | Italy | PCR and DNA seq | [79] |
| Zekri et al. (2007) | 50 | 78 | 58 | Egypt | qRT-PCR | [80] |
| Gordon et al. (2002) | 6 | NA | NA | USA | qRT-PCR | [125] |
| Lopez-Rios et al. (2004) | 6 | NA | NA | USA | PCR and DNA seq | [124] |
| Jin et al. (2004) | 44/0# | NA | NA | Japan | PCR, DNA seq, IHC | [126] |
| Aoe et al. (2006) | 6/0†† | NA | NA | Japan | qRT-PCR | [127] |
| Hmeljak et al. (2010)‡‡ | 4/0§§ | NA | NA | Slovenia | qRT-PCR and IHC | [128] |
| Leithner et al. (2002) | 0 | NA | NA | Austria | PCR | [99] |
| Hubner et al. (2002) | 0 | NA | NA | Belgium | PCR | [100] |
| Mayall et al. (2003) | 0 | NA | NA | New Zealand | qRT-PCR | [101] |
| Brousset et al. (2005) | 0 | NA | NA | France | IHC | [102] |
Source: country of origin of the specimens tested in the study.
36% of US samples and 0% of Turkish samples are SV40 DNA positive.
33% SV40-postive DNA in bladder urotheliomas.
16.7% normal liver tissues and 12.5% kidney tissues tested positive for SV40.
44% positive specimens by PCR and 0% by IHC.
6% positive specimens by qRT-PCR and 0% by IHC.
Croatia, South Africa, UK and USA.
4% positive specimens by qRT-PCR, 0% by IHC.
DNA seq: DNA sequence analysis; F-Hybri: Filter hybridization; IHC: Immunohistochemistry; IP: Immunoprecipitation; NA: The presence of asbestos was not investigated in these studies; PRINS: Primed in situ; qRT-PCR: Quantitative real time PCR; SV40: Simian virus 40; WB: Western blotting.
SV40 & brain tumors
Tag expression was first detected in a meningioma in 1975 [103], and infectious SV40 was isolated from specimens of this tumor [104]. Between 1975 and 2002, 13 studies reported an overall prevalence of about 18% of brain tumors containing SV40 sequences (reviewed in [105]). High frequencies of SV40 DNA sequence were found in choroid plexus tumors, ependymomas, low-grade astrocytomas, anaplastic astrocytomas and secondary glioblastomas, and lower frequencies were detected in gemistocytic astrocytomas, oligodendrogliomas, glioma and medulloblastoma (Table 3) [106–108]. Some research teams did not detect SV40 in brain tumors (Table 3) [109–111].
Table 3.
Simian virus 40 incidence in brain tumors.
| Study (year) | Brain tumors (%) | Source† | Techniques | Ref. |
|---|---|---|---|---|
| Weiss et al. (1976) | 38 ME | Germany | IHC | [104] |
| Wang et al. (1998) | 50 CP; 90 EM | USA | PCR and DNA seq | [106] |
| Huang et al. (1999) | 25 OL; 32 GA 59 LA, AA, SG; | France | PCR, Southern blotting | [107] |
| Vilchez et al. (2003) | 21 | Different‡ | PCR, IHC | [105] |
| Rollison et al. (2005) | 1.8 GL, MD | USA | PCR, Southern blotting, qRT-PCR | [108] |
| Kim et al. (2002) | 0 | USA | PCR | [109] |
| Montesions-Rongen et al. (2004) | 0 | German | PCR | [110] |
| Sabatier et al. (2005) | 0 CNS | France | IHC | [111] |
Source: country of origin of the specimens tested in the study.
Assessment of 13 different reports on primary brain tumors from different sources.
AA: Anaplastic astrocytoma; CNS: CNS-related tumor; CP: Choroid plexus tumor; DNA seq: DNA sequence analysis; EM: Ependymoma; GA: Gemistocytic astrocytoma; GL: Glioma; IHC: Immunohistochemistry; LA: Low-grade astrocytoma; MD: Medulloblastoma; ME: Meningioma; OL: Oligodendroglioma; qRT-PCR: Quantitative real-time PCR; SG: Secondary glioblastoma.
At least three reports showed the presence of SV40 in Li-Fraumeni Syndrome (LFS) [106,112,113]. A total of 18 out of 151 LFS patient specimens were SV40 positive, and 11 of these were osteosarcoma biopsies from LFS patients [106]. SV40 DNA sequences and Tag protein were detected in the choroid plexus carcinoma (CPC) sample from one LFS patient. This patient also had an embryonal rhabdomyosarcoma; SV40 was detected only in the CPC. In a second LFS patient that had developed CPC, osteosarcoma and renal cell carcinoma, SV40 DNA and Tag were detected in both CPC and renal cell carcinoma specimens [113].
SV40 & other tumors
SV40 has been detected in several other tumor types, including lymphomas [114–117], osteosarcomas [118,119], breast carcinomas [120] and, more recently, colon cancer [121]. The significance of these findings remains to be established.
Geographic differences in SV40-positive tumors
It has been suggested that SV40 may be transmitted in humans by both horizontal and vertical infection [16]. In the USA, about 90% of children and 60% of adults were immunized with polio vaccines between 1955 and 1961, are, therefore, potentially infected [16]. In the former USSR, and in countries under its influence, polio vaccines containing infectious SV40 were administered until 1978 [84], and possibly later. Moreover, at least one polio seed virus from which vaccines were prepared in Italy until 1999 was SV40 contaminated [122] [Phil Minor, Pers. Comm.]. Some other countries, such as China, still produce polio vaccines using monkey cells, raising the possibility that their vaccines remain contaminated with SV40 [84].
Simian virus 40-positive tumors were found in the USA, Canada, China, Japan, Europe and New Zealand [123], but not in Finland, Turkey, Yugoslavia and Austria, countries that did not use SV40-contaminated vaccines [16]. Human exposure to SV40 through contaminated polio vaccines has been extensive, and has been influenced by geographical differences associated with the use of contaminated or noncontaminated vaccines [16]. The reasons that some countries did not use contaminated vaccines vary, some of them started polio vaccination in the 1970s, when the vaccines had been cleaned from infections SV40 (Turkey); others made their own vaccines and they made them using SV40-free substrates (Yugoslavia).
The incidence of SV40 positive MM shows dramatic geographical differences, whether these differences are related to the use of contaminated vaccines remains a controversial issue because of potential liability and litigation. For example, tumor tissue samples from the USA and Europe were found positive in 20–83% of cases [92,93,95–98], however, we and others did not detect SV40 sequences in samples from Turkey [96].
Geographical differences were reported for the SV40 incidence in bone tumors as well. In 143 out of 277 (42%) osteosarcoma tissue samples from Germany and Hungary, SV40 DNA sequences were detected by quantitative real-time PCR. However, differences in the distribution of SV40 were observed between the two countries. Approximately 74% of Hungarian osteosarcoma samples harbored high copy numbers of SV40 genomic DNA (>100 copies), whereas 24% of the German osteosarcoma samples contained low copy numbers of SV40 genomic DNA (about ten copies) [119]. On the other hand, no SV40 sequences were detected in ten osteosarcomas and 14 giant cell tumors from Austrian patients [99]. These results support geographical differences associated with the detection of SV40 in human specimens [16].
Expert commentary
The presence of SV40 sequences in several human cancers, including MM, bone and brain tumors, and lymphomas has raised concerns about the possible pathogenic role of SV40 in humans. Unfortunately, the controversy over the percent of tumor specimens containing SV40 DNA and proteins has paralyzed this research field. This controversy was magnified by the legal implications of associating the production and distribution of contaminated poliovaccines to the development of human mesotheliomas and brain tumors. Study sections reviewers have been unwilling to support SV40 research citing the need to first address the ‘controversy’, yet without funding it is impossible to conduct studies to address controversial findings.
Regardless of its pathogenic role, the presence of SV40 in some tumors provides a potential target for therapy. However, until the existing controversy over the presence of SV40 in human tumors is addressed and funding becomes available, it appears unlikely that progress will be made to develop therapies aimed at tumor cells carrying SV40 and or expressing SV40 antigens. So, the reader may ask, is SV40 really present in some human biopsies?
Over 50 laboratories, using different techniques, have detected SV40 in human tumors (mostly in MM and brain tumors). The techniques used to detect SV40 included PCR, quantitative PCR, Southern blotting, IHC staining for Tag, DNA sequencing, in situ hybridization et cetera. It is unlikely that so many researchers using different technical approaches would consistently be wrong. A critical review of the papers reporting SV40 in human specimens show that some were carefully planned and executed, yet others lacked appropriate controls, and were of poor quality. Therefore, it is possible that some of these ‘positive’ studies were flawed and overestimated the presence of SV40 in human tumors. At the same time, many other papers, for example those from the laboratories of J Butel, J Lednicky, A Gazdar, R Garcea and J Melinck, to cite a few, were carefully controlled and appear to have been well executed. On the other hand, several reports were negative, or better the titles claimed negative results, but the data contained in those papers reported a low incidence, 5–6% positivity of SV40 in the specimens tested, and it is the low percentage of positivity that was interpreted as insignificant by the authors (Table 2) [124,125]. This is a critical point of the controversy: most ‘negative’ papers were actually not negative at all. These papers instead reported a low incidence of SV40 in human specimens. In addition, some authors detected SV40 DNA but did not detect SV40 proteins, raising the question of whether finding SV40 DNA was of any pathogenic significance (Table 2) [126–128]. These are valid questions (i.e., if SV40 proteins are not expressed, is the presence of SV40 DNA alone of any pathogenic significance?) that should be carefully studied, as suggested by a review of the Institute of Medicine in 2002 [129]. The problem is that analyzing the so-called ‘hit and run mechanism’ or ‘indirect carcinogenesis’ is not an easy task, as the investigator is faced with the outcome of the carcinogenic process and must reconstruct the events that led to cancer. There is documented evidence in the literature for a ‘hit and run’ carcinogenesis by SV40 in causing tumors in transgenic animals [130], but we do not know how to study this phenomenon in humans.
It is of course impossible to provide a comprehensive satisfactory explanation for controversial findings, as multiple factors may have been at play. At times, technical reasons may have caused some of the discrepancies in the literature. We experienced this problem in our own laboratory, when Maurizio Bocchetta told one of the authors of this review (Carbone) that there was no Tag in MM biopsies [131]. Previously, Carbone had performed the experiments using 125I-labeled antibodies [132], but as lab techniques evolved, Bocchetta was using enhanced chemiluminescence (ECL). Eventually, after much discussion and repeated attempts, we discovered that the results were influenced by the type of ECL kit used for detection, the least sensitive one gave 0% positivity. By using an intermediate sensitivity method, which allowed us to test different exposure times, at least seven out of 20 MM samples tested Tag positive. Instead, the most sensitive ECL detected only two out of 20 positive samples, because the reaction was so rapid that we had to interrupt it after 1–2 sec before the whole membrane became black, and we could not test different exposure times [131]. If we had not previously done these same experiments, most likely we would not have taken the time to repeat multiple times the experiments to address the discrepancy between present and past results, and we would have erroneously published ‘negative’ results.
The discrepancies in the results may have also been influenced by geographical differences linked to the distribution of contaminated poliovaccines [84]. For example, the high incidence of SV40 reported in Italian specimens correlates with the finding that Italy is the only country in the Western world that used SV40 contaminated vaccines until 1999 [84].
Some authors reported that positive results were due to contamination by laboratory plasmids containing SV40 sequences [124]. Lopez-Rios et al. found that the high incidence of SV40 in human MM they tested (52–62%) was obtained with primers annealing to sequences present in common laboratory plasmids used in their own laboratory [124]. When the results were repeated with different primers, only 6% of the MM biopsies were confirmed as SV40 positive. It is possible that the high incidence of SV40 reported by some studies may have suffered from similar technical pitfalls.
The presence of SV40 in some human specimens is supported by serological studies showing widespread human exposure to SV40. Recent studies using ELISA assays that reliably distinguish among SV40, and the human poliomaviruses JCV and BKV revealed that 1% of those tested had anti-SV40 antibodies, suggesting widespread human exposure to a virus (SV40) that should be confined to monkeys [133].
In summary, the data suggest that at least 5% of human mesotheliomas and brain tumors – according to the so-called negative papers, or more according to the ‘positive papers’ – contain SV40 DNA, the possible pathogenic role of SV40 in these tumors should be further investigated to address causality issues.
It is unfortunate that the controversy about the presence of SV40 in human tumors has been too heated to allow an unbiased analysis of the results. As noted by a panel of the Institute of Medicine that reviewed this issue, this area of investigation deserves further attention [134]. At the same time, the conflicting arguments and lack of funding caused many researchers to leave this field. Progress in the near future remains unclear because of lack of funding. We are pleased to note, however, that regardless of the ‘controversy’ over the role of SV40 in human carcinogenesis, this research has led to important discoveries about mechanisms of SV40 infection, carcinogenesis and cocarcinogenesis that are important to the whole field of cancer. In addition, this research has uncovered the extent of human exposure to SV40, and has and will continue to influence future regulatory decisions related to vaccine production and safety.
Five-year view
Simian virus 40 remains an invaluable tool to study mechanisms of viral carcinogenesis and regardless of the controversial issues related to the possible pathogenic role of SV40 in humans, SV40 will continue to be studied to address basic mechanisms of carcinogenesis. The precise mechanisms by which SV40 either lyses or transforms different human cell types are becoming more evident. Recent work has indicated that an antisense mechanism that appears unique to HM and brain cells suppresses late viral gene expression, allowing these cell types to survive infection, maintain SV40 as episomal with expression of Tag and tag but not of the VP1–4 proteins and become transformed. It is hoped that these mechanisms will be fully elucidated in the next 5 years, and that the information may help us understand the process and possible biological significance of SV40 infection in humans, and more generally advance our understanding of viral carcinogenesis. Future study will have to elucidate the cellular factors that appear unique to HM and brain cells, and that regulate the expression of this antisense RNA. Researchers are starting to address the puzzle of why archetypal SV40 DNA sequences are detectable in human brain tumors, while the SV40 DNA sequence in MM samples are most often nonarchetypal. A recent paper showed that these findings reflect the tissue tropism of SV40 [11] and that host cell signaling contributes to the transformation process [135].
Key issues.
Simian virus 40 (SV40) is a small DNA tumor virus, transmitted from monkeys to humans through contaminated polio vaccines. It has been postulated that SV40 is transmitted among humans both vertically and horizontally.
The early gene product SV40 large T antigen promotes viral DNA replication and is the main SV40 oncogene. SV40 Tag interacts with and transactivates multiple intracellular signaling proteins. Tag-p53 complex recruits retinoblastoma protein, p300, p400 and CREB-binding protein to induce specific gene transcription, resulting in the activation of IGF-1, Met and Notch-1 pathways.
Recent studies using ELISA assays that reliably distinguish among SV40, JC and BK viruses revealed that 1% of those tested had anti-SV40 antibodies.
SV40 and asbestos are cocarcinogens in causing mesothelioma in hamster and mice, and in causing malignant transformation of human primary mesothelial cells in tissue culture.
Many laboratories reported the presence of SV40 DNA, RNA or proteins in human tumor biopsies, other laboratories reported negative results, or more often reported a low incidence of SV40 in these specimens (6%). These conflicting findings have generated controversy that has paralyzed this research field.
The conflicting results can be at least in part attributed to technical discrepancies, laboratory contamination with SV40 plasmids, inadequate technical approaches and geographical differences in the prevalence of SV40 linked to the distribution of contaminated poliovaccines.
The high incidence of SV40 sequences in Italian specimens, for example, is probably linked to the fact that Italy is the only country in the Western world that used contaminated vaccines as late as 1999.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work presented here was supported by the NCI R01 and P01 and the UH Foundation (to Michele Carbone), the Mesothelioma Applied Research Foundation and the Riviera United-4 a CURE (to Haining Yang) and the Hawaii Community Foundation (to Haining Yang and to Giovanni Gaudino). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Sweet BH, Hilleman MR. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- 2.Poulin DL, DeCaprio JA. Is there a role for SV40 in human cancer? J Clin Oncol. 2006;24(26):4356–4365. doi: 10.1200/JCO.2005.03.7101. [DOI] [PubMed] [Google Scholar]
- 3.Patel NC, Halvorson SJ, Sroller V, et al. Viral regulatory region effects on vertical transmission of polyomavirus SV40 in hamsters. Virology. 2009;386(1):94–101. doi: 10.1016/j.virol.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakanishi A, Itoh N, Li PP, Handa H, Liddington RC, Kasamatsu H. Minor capsid proteins of simian virus 40 are dispensable for nucleocapsid assembly and cell entry but are required for nuclear entry of the viral genome. J Virol. 2007;81(8):3778–3785. doi: 10.1128/JVI.02664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano MA, Inoue T, Tsukamoto H, et al. The VP2/VP3 minor capsid protein of simian virus 40 promotes the in vitro assembly of the major capsid protein VP1 into particles. J Biol Chem. 2006;281(15):10164–10173. doi: 10.1074/jbc.M511261200. [DOI] [PubMed] [Google Scholar]
- 6.Carswell S, Alwine JC. Simian virus 40 agnoprotein facilitates perinuclear-nuclear localization of VP1, the major capsid protein. J Virol. 1986;60(3):1055–1061. doi: 10.1128/jvi.60.3.1055-1061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels R, Sadowicz D, Hebert DN. A very late viral protein triggers the lytic release of SV40. PLoS Pathog. 2007;3(7):e98. doi: 10.1371/journal.ppat.0030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Butel JS, Lednicky JA. Cell and molecular biology of simian virus 40: implications for human infections and disease. J Natl Cancer Inst. 1999;91(2):119–134. doi: 10.1093/jnci/91.2.119. This paper describes multiple infectious simian virus 40 (SV40) strains, isolated from human brain tumor biopsies. [DOI] [PubMed] [Google Scholar]
- 9.Stewart AR, Lednicky JA, Butel JS. Sequence analyses of human tumor-associated SV40 DNAs and SV40 viral isolates from monkeys and humans. J Neurovirol. 1998;4(2):182–193. doi: 10.3109/13550289809114518. [DOI] [PubMed] [Google Scholar]
- 10.Peden K, Sheng L, Omeir R, et al. Recovery of strains of the polyomavirus SV40 from rhesus monkey kidney cells dating from the 1950s to the early 1960s. Virology. 2008;370(1):63–76. doi: 10.1016/j.virol.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Qi F, Gaudino G, et al. Tissue tropism of SV40 transformation of human cells: role of the viral regulatory region and of cellular oncogenes. Genes Cancer. 2011;1(10):1008–1020. doi: 10.1177/1947601910395580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewers H, Romer W, Smith AE, et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat Cell Biol. 2010;12(1):11–18. doi: 10.1038/ncb1999. suppl 11–12. [DOI] [PubMed] [Google Scholar]
- 13.Atwood WJ, Norkin LC. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J Virol. 1989;63(10):4474–4477. doi: 10.1128/jvi.63.10.4474-4477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schelhaas M, Malmstrom J, Pelkmans L, et al. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131(3):516–529. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi A, Li PP, Qu Q, Jafri QH, Kasamatsu H. Molecular dissection of nuclear entry-competent SV40 during infection. Virus Res. 2007;124(1–2):226–230. doi: 10.1016/j.virusres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Gazdar AF, Butel JS, Carbone M. SV40 and human tumours: myth, association or causality? Nat Rev Cancer. 2002;2(12):957–964. doi: 10.1038/nrc947. A comprehensive overview of the historical issues accompanying the discovery of SV40 in human tumors and of the finding on the involvement of SV40 in human cancer pathogenesis. [DOI] [PubMed] [Google Scholar]
- 17.Wiley SR, Kraus RJ, Zuo F, Murray EE, Loritz K, Mertz JE. SV40 early-to-late switch involves titration of cellular transcriptional repressors. Genes Dev. 1993;7(11):2206–2219. doi: 10.1101/gad.7.11.2206. [DOI] [PubMed] [Google Scholar]
- 18••.Bocchetta M, Eliasz S, De Marco MA, Rudzinski J, Zhang L, Carbone M. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008;68(4):1022–1029. doi: 10.1158/0008-5472.CAN-07-5203. Introduces a new concept on the function of the SV40 large T antigen (Tag)/p53 complex, no longer restricted to p53 neutralization. Tag and p53 cooperate to the induction of the transcription of genes involved in cell proliferation and survival. [DOI] [PubMed] [Google Scholar]
- 19.Porcu P, Grana X, Li S, et al. An E2F binding sequence negatively regulates the response of the insulin-like growth factor 1 (IGF-I) promoter to simian virus 40T antigen and to serum. Oncogene. 1994;9(8):2125–2134. [PubMed] [Google Scholar]
- 20.Chen H, Campisi J, Padmanabhan R. SV40 large T antigen transactivates the human cdc2 promoter by inducing a CCAAT box binding factor. J Biol Chem. 1996;271(24):13959–13967. [PubMed] [Google Scholar]
- 21.Cacciotti P, Libener R, Betta P, et al. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma. Proc Natl Acad Sci USA. 2001;98(21):12032–12037. doi: 10.1073/pnas.211026798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bocchetta M, Miele L, Pass HI, Carbone M. Notch-1 induction, a novel activity of SV40 required for growth of SV40-transformed human mesothelial cells. Oncogene. 2003;22(1):81–89. doi: 10.1038/sj.onc.1206097. [DOI] [PubMed] [Google Scholar]
- 23.Okubo E, Lehman JM, Friedrich TD. Negative regulation of mitotic promoting factor by the checkpoint kinase chk1 in simian virus 40 lytic infection. J Virol. 2003;77(2):1257–1267. doi: 10.1128/JVI.77.2.1257-1267.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon-Shaag A, Ben-Nun-Shaul O, Roitman V, Yosef Y, Oppenheim A. Cellular transcription factor Sp1 recruits simian virus 40 capsid proteins to the viral packaging signal, ses. J Virol. 2002;76(12):5915–5924. doi: 10.1128/JVI.76.12.5915-5924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oppenheim A, Ben-Nun-Shaul O, Mukherjee S, Abd-El-Latif M. SV40 assembly in vivo and in vitro. Comput Math Methods Med. 2008;9(3–4):265–276. [Google Scholar]
- 26.Manley K, O’Hara BA, Atwood WJ. Nuclear factor of activated T-cells (NFAT) plays a role in SV40 infection. Virology. 2008;372(1):48–55. doi: 10.1016/j.virol.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalili K, Sariyer IK, Safak M. Small tumor antigen of polyomaviruses: role in viral life cycle and cell transformation. J Cell Physiol. 2008;215(2):309–319. doi: 10.1002/jcp.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriegler M, Perez CF, Hardy C, Botchan M. Transformation mediated by the SV40 T antigens: separation of the overlapping SV40 early genes with a retroviral vector. Cell. 1984;38(2):483–491. doi: 10.1016/0092-8674(84)90503-8. [DOI] [PubMed] [Google Scholar]
- 29.Shera KA, Shera CA, McDougall JK. Small tumor virus genomes are integrated near nuclear matrix attachment regions in transformed cells. J Virol. 2001;75(24):12339–12346. doi: 10.1128/JVI.75.24.12339-12346.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Liu J, Kaur G, Zhawar VK, et al. Role of SV40 integration site at chromosomal interval 1q21.1 in immortalized CRL2504 cells. Cancer Res. 2009;69(19):7819–7825. doi: 10.1158/0008-5472.CAN-09-1003. Reveals the importance of SV40 integration into a specific chromosome interval, for suppression of apoptosis and senescence, leading to human cell immmortalization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen F, Koprowski H, Ponten JA. Rapid transformation of human fibroblast cultures by simian virus. Proc Natl Acad Sci USA. 1963;50:343–348. doi: 10.1073/pnas.50.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shein HM. Transformation of astrocytes and destruction of spongioblasts induced by a simian tumor virus (SV40) in cultures of human fetal neuroglia. J Neuropathol Exp Neurol. 1967;26(1):60–76. doi: 10.1097/00005072-196701000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Ponten J, Jensen F, Koprowski H. Morphological and virological investigation of human tissue cultures transformed with SV40. J Cell Comp Physiol. 1963;61:145–163. doi: 10.1002/jcp.1030610206. [DOI] [PubMed] [Google Scholar]
- 34.Foddis R, De Rienzo A, Broccoli D, et al. SV40 infection induces telomerase activity in human mesothelial cells. Oncogene. 2002;21(9):1434–1442. doi: 10.1038/sj.onc.1205203. [DOI] [PubMed] [Google Scholar]
- 35•.Carbone M, Pannuti A, Zhang L, Testa JR, Bocchetta M. A novel mechanism of late gene silencing drives SV40 transformation of human mesothelial cells. Cancer Res. 2008;68(22):9488–9496. doi: 10.1158/0008-5472.CAN-08-2332. This work reveals a novel mechanism of human primary mesothelial cell (HM) transformation, based on an antisense-mediated silencing of SV40 late gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bocchetta M, Carbone M. SV40-mediated oncogenesis. In: Pass H, Vogelzan N, Carbone M, editors. Malignant Mesothelioma: Advances in Pathogenesis, Diagnosis, and Translational Therapies. Springer; New York, NY, USA: 2005. pp. 34–59. [Google Scholar]
- 37.De Luca A, Baldi A, Esposito V, et al. The retinoblastoma gene family pRb/p105, p107, pRb2/p130 and simian virus-40 large T-antigen in human mesotheliomas. Nat Med. 1997;3(8):913–916. doi: 10.1038/nm0897-913. [DOI] [PubMed] [Google Scholar]
- 38.Bocchetta M, Di Resta I, Powers A, et al. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci USA. 2000;97(18):10214–10219. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sroller V, Vilchez RA, Stewart AR, Wong C, Butel JS. Influence of the viral regulatory region on tumor induction by simian virus 40 in hamsters. J Virol. 2008;82(2):871–879. doi: 10.1128/JVI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cicala C, Pompetti F, Carbone M. SV40 induces mesotheliomas in hamsters. Am J Pathol. 1993;142(5):1524–1533. [PMC free article] [PubMed] [Google Scholar]
- 41.Brinster RL, Chen HY, Messing A, van Dyke T, Levine AJ, Palmiter RD. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmiter RD, Chen HY, Messing A, Brinster RL. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature. 1985;316(6027):457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- 43.Lee YC, Asa SL, Drucker DJ. Glucagon gene 5′-flanking sequences direct expression of simian virus 40 large T antigen to the intestine, producing carcinoma of the large bowel in transgenic mice. J Biol Chem. 1992;267(15):10705–10708. [PubMed] [Google Scholar]
- 44.Saenz Robles MT, Pipas JM. T antigen transgenic mouse models. Semin Cancer Biol. 2009;19(4):229–235. doi: 10.1016/j.semcancer.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanahan D. Heritable formation of pancreatic β-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 46.Wikenheiser KA, Clark JC, Linnoila RI, Stahlman MT, Whitsett JA. Simian virus 40 large T antigen directed by transcriptional elements of the human surfactant protein C gene produces pulmonary adenocarcinomas in transgenic mice. Cancer Res. 1992;52(19):5342–5352. [PubMed] [Google Scholar]
- 47.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci USA. 1994;91(23):11236–11240. doi: 10.1073/pnas.91.23.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED. The TRAMP mouse as a model for prostate cancer. Curr Protoc Immunol. 2001;Chapter 20(Unit 20.5) doi: 10.1002/0471142735.im2005s45. [DOI] [PubMed] [Google Scholar]
- 50.Santarelli R, Tzeng YJ, Zimmermann C, Guhl E, Graessmann A. SV40 T-antigen induces breast cancer formation with a high efficiency in lactating and virgin WAP-SV-T transgenic animals but with a low efficiency in ovariectomized animals. Oncogene. 1996;12(3):495–505. [PubMed] [Google Scholar]
- 51.Perez-Stable C, Altman NH, Brown J, Harbison M, Cray C, Roos BA. Prostate, adrenocortical, and brown adipose tumors in fetal globin/T antigen transgenic mice. Lab Invest. 1996;74(2):363–373. [PubMed] [Google Scholar]
- 52.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272(38):23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 53.Garabedian EM, Humphrey PA, Gordon JI. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci USA. 1998;95(26):15382–15387. doi: 10.1073/pnas.95.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asamoto M, Hokaiwado N, Cho YM, et al. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res. 2001;61(12):4693–4700. [PubMed] [Google Scholar]
- 55.Masumori N, Thomas TZ, Chaurand P, et al. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61(5):2239–2249. [PubMed] [Google Scholar]
- 56.Gabril MY, Onita T, Ji PG, et al. Prostate targeting: PSP94 gene promoter/enhancer region directed prostate tissue-specific expression in a transgenic mouse prostate cancer model. Gene Ther. 2002;9(23):1589–1599. doi: 10.1038/sj.gt.3301895. [DOI] [PubMed] [Google Scholar]
- 57.Hicks SM, Vassallo JD, Dieter MZ, et al. Immunohistochemical analysis of Clara cell secretory protein expression in a transgenic model of mouse lung carcinogenesis. Toxicology. 2003;187(2–3):217–228. doi: 10.1016/s0300-483x(03)00060-x. [DOI] [PubMed] [Google Scholar]
- 58.Garson K, Macdonald E, Dube M, Bao R, Hamilton TC, Vanderhyden BC. Generation of tumors in transgenic mice expressing the SV40 T antigen under the control of ovarian-specific promoter 1. J Soc Gynecol Investig. 2003;10(4):244–250. doi: 10.1016/s1071-5576(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 59.Lou DQ, Molina T, Bennoun M, et al. Conditional hepatocarcinogenesis in mice expressing SV 40 early sequences. Cancer Lett. 2005;229(1):107–114. doi: 10.1016/j.canlet.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Grippo PJ, Sandgren EP. Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. Am J Pathol. 2000;157(3):805–813. doi: 10.1016/S0002-9440(10)64594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Robinson C, van Bruggen I, Segal A, et al. A novel SV40 TAg transgenic model of asbestos-induced mesothelioma: malignant transformation is dose dependent. Cancer Res. 2006;66(22):10786–10794. doi: 10.1158/0008-5472.CAN-05-4668. A novel and powerful model to study the mechanisms of mesothelioma onset and progression, based on SV40 Tag transgenic mice. This model established that asbestos induced transformation is dose dependent. [DOI] [PubMed] [Google Scholar]
- 62.Köbbert C, Mollmann C, Schafers M, et al. Transgenic model of cardiac rhabdomyosarcoma formation. J Thorac Cardiovasc Surg. 2008;136(5):1178–1186. doi: 10.1016/j.jtcvs.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 63.ter Brugge PJ, Ta VB, de Bruijn MJ, et al. A mouse model for chronic lymphocytic leukemia based on expression of the SV40 large T antigen. Blood. 2009;114(1):119–127. doi: 10.1182/blood-2009-01-198937. [DOI] [PubMed] [Google Scholar]
- 64.Stahl S, Sacher T, Bechtold A, et al. Tumor agonist peptides break tolerance and elicit effective CTL responses in an inducible mouse model of hepatocellular carcinoma. Immunol Lett. 2009;123(1):31–37. doi: 10.1016/j.imlet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Iwakura H, Ariyasu H, Li Y, et al. A mouse model of ghrelinoma exhibited activated growth hormone-insulin-like growth factor I axis and glucose intolerance. Am J Physiol Endocrinol Metab. 2009;297(3):E802–E811. doi: 10.1152/ajpendo.00205.2009. [DOI] [PubMed] [Google Scholar]
- 66.Pass HI, Vogelzang N, Hahn SM, Carbone M. Benign and malignant mesothelioma. In: De Vita VT, Hellmann S, Rosemberg SA, editors. Cancer, Principles & Practice of Oncology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2005. pp. 1687–1715. [Google Scholar]
- 67.Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: Facts, myths and hypotheses. J Cell Physiol. 2011 doi: 10.1002/jcp.22724. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramos-Nino ME, Testa JR, Altomare DA, et al. Cellular and molecular parameters of mesothelioma. J Cell Biochem. 2006;98(4):723–734. doi: 10.1002/jcb.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cacciotti P, Barbone D, Porta C, et al. SV40-dependent AKT activity drives mesothelial cell transformation after asbestos exposure. Cancer Res. 2005;65(12):5256–5262. doi: 10.1158/0008-5472.CAN-05-0127. [DOI] [PubMed] [Google Scholar]
- 70.Gee GV, Stanifer ML, Christensen BC, et al. SV40 associated miRNAs are not detectable in mesotheliomas. Br J Cancer. 2010;103(6):885–888. doi: 10.1038/sj.bjc.6605848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cacciotti P, Strizzi L, Vianale G, et al. The presence of simian-virus 40 sequences in mesothelioma and mesothelial cells is associated with high levels of vascular endothelial growth factor. Am J Respir Cell Mol Biol. 2002;26(2):189–193. doi: 10.1165/ajrcmb.26.2.4673. [DOI] [PubMed] [Google Scholar]
- 72.Strizzi L, Catalano A, Vianale G, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193(4):468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 73•.Kroczynska B, Cutrone R, Bocchetta M, et al. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci USA. 2006;103(38):14128–14133. doi: 10.1073/pnas.0604544103. Provides a rationale for the cooperation between asbestos fibers and SV40 Tag expression in HM transformation, through the induction of Akt activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pietruska JR, Kane AB. SV40 oncoproteins enhance asbestos-induced DNA double-strand breaks and abrogate senescence in murine mesothelial cells. Cancer Res. 2007;67(8):3637–3645. doi: 10.1158/0008-5472.CAN-05-3727. [DOI] [PubMed] [Google Scholar]
- 75.Yang H, Bocchetta M, Kroczynska B, et al. TNF-α inhibits asbestos-induced cytotoxicity via a NF-κB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci USA. 2006;103(27):10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sartore-Bianchi A, Gasparri F, Galvani A, et al. Bortezomib inhibits nuclear factor-κB dependent survival and has potent in vivo activity in mesothelioma. Clin Cancer Res. 2007;13(19):5942–5951. doi: 10.1158/1078-0432.CCR-07-0536. [DOI] [PubMed] [Google Scholar]
- 77.Yang H, Rivera Z, Jube S, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci USA. 2010;107(28):12611–12616. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henzi T, Blum WV, Pfefferli M, Kawecki TJ, Salicio V, Schwaller B. SV40-induced expression of calretinin protects mesothelial cells from asbestos cytotoxicity and may be a key factor contributing to mesothelioma pathogenesis. Am J Pathol. 2009;174(6):2324–2336. doi: 10.2353/ajpath.2009.080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Comar M, Rizzardi C, de Zotti R, et al. SV40 multiple tissue infection and asbestos exposure in a hyperendemic area for malignant mesothelioma. Cancer Res. 2007;67(18):8456–8459. doi: 10.1158/0008-5472.CAN-07-2232. [DOI] [PubMed] [Google Scholar]
- 80.Zekri AR, Bahnassy AA, Mohamed WS, et al. Evaluation of simian virus-40 as a biological prognostic factor in Egyptian patients with malignant pleural mesothelioma. Pathol Int. 2007;57(8):493–501. doi: 10.1111/j.1440-1827.2007.02130.x. [DOI] [PubMed] [Google Scholar]
- 81.Degiovanni D, Pesce B, Pondrano N. Asbestos in Italy. Int J Occup Environ Health. 2004;10(2):193–197. doi: 10.1179/oeh.2004.10.2.193. [DOI] [PubMed] [Google Scholar]
- 82.Pancaldi C, Balatti V, Guaschino R, et al. Simian virus 40 sequences in blood specimens from healthy individuals of Casale Monferrato, an industrial town with a history of asbestos pollution. J Infect. 2009;58(1):53–60. doi: 10.1016/j.jinf.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Barbanti-Brodano G, Sabbioni S, Martini F, Negrini M, Corallini A, Tognon M. Simian virus 40 infection in humans and association with human diseases: results and hypotheses. Virology. 2004;318(1):1–9. doi: 10.1016/j.virol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 84•.Cutrone R, Lednicky J, Dunn G, et al. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res. 2005;65(22):10273–10279. doi: 10.1158/0008-5472.CAN-05-2028. This multilaboratory comprehensive evaluation of poliovaccines around the world, upon request of the WHO, revealed that all vaccines produced in the USSR were heavily infected with SV40 until at least 1978 and that those prepared in the Western world have been SV40-free since 1963, with the exception of the Italian vaccines, which were contaminated until 1999. [DOI] [PubMed] [Google Scholar]
- 85.Morris JA, Johnson KM, Aulisio CG, Chanock RM, Knight V. Clinical and serologic responses in volunteers given vacuolating virus (SV-40) by respiratory route. Proc Soc Exp Biol Med. 1961;108:56–59. doi: 10.3181/00379727-108-26843. [DOI] [PubMed] [Google Scholar]
- 86.Horvath BL, Fornosi F. Excretion of SV-40 virus after oral administration of contaminated polio vaccine. Acta Microbiol Acad Sci Hung. 1964;11:271–275. [PubMed] [Google Scholar]
- 87.Li RM, Branton MH, Tanawattanacharoen S, Falk RA, Jennette JC, Kopp JB. Molecular identification of SV40 infection in human subjects and possible association with kidney disease. J Am Soc Nephrol. 2002;13(9):2320–2330. doi: 10.1097/01.asn.0000028249.06596.cf. [DOI] [PubMed] [Google Scholar]
- 88.Patel NC, Vilchez RA, Killen DE, et al. Detection of polyomavirus SV40 in tonsils from immunocompetent children. J Clin Virol. 2008;43(1):66–72. doi: 10.1016/j.jcv.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanchiere JA, Nicome RK, Greer JM, Demmler GJ, Butel JS. Frequent detection of polyomaviruses in stool samples from hospitalized children. J Infect Dis. 2005;192(4):658–664. doi: 10.1086/432076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanchiere JA, Abudayyeh S, Copeland CM, Lu LB, Graham DY, Butel JS. Polyomavirus shedding in the stool of healthy adults. J Clin Microbiol. 2009;47(8):2388–2391. doi: 10.1128/JCM.02472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klein G, Powers A, Croce C. Association of SV40 with human tumors. Oncogene. 2002;21(8):1141–1149. doi: 10.1038/sj.onc.1205173. [DOI] [PubMed] [Google Scholar]
- 92.Carbone M, Pass HI, Rizzo P, et al. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9(6):1781–1790. [PubMed] [Google Scholar]
- 93.Testa JR, Carbone M, Hirvonen A, et al. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res. 1998;58(20):4505–4509. [PubMed] [Google Scholar]
- 94.Ramael M, Nagels J, Heylen H, et al. Detection of SV40 like viral DNA and viral antigens in malignant pleural mesothelioma. Eur Respir J. 1999;14(6):1381–1386. doi: 10.1183/09031936.99.14613819. [DOI] [PubMed] [Google Scholar]
- 95.Shivapurkar N, Wiethege T, Wistuba II, et al. Presence of simian virus 40 sequences in malignant mesotheliomas and mesothelial cell proliferations. J Cell Biochem. 1999;76(2):181–188. doi: 10.1002/(sici)1097-4644(20000201)76:2<181::aid-jcb2>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 96.De Rienzo A, Tor M, Sterman DH, Aksoy F, Albelda SM, Testa JR. Detection of SV40 DNA sequences in malignant mesothelioma specimens from the United States, but not from Turkey. J Cell Biochem. 2002;84(3):455–459. [PubMed] [Google Scholar]
- 97.Priftakis P, Bogdanovic G, Hjerpe A, Dalianis T. Presence of simian virus 40 (SV40) is not frequent in Swedish malignant mesotheliomas. Anticancer Res. 2002;22(3):1357–1360. [PubMed] [Google Scholar]
- 98.Cristaudo A, Foddis R, Vivaldi A, et al. SV40 enhances the risk of malignant mesothelioma among people exposed to asbestos: a molecular epidemiologic case-control study. Cancer Res. 2005;65(8):3049–3052. doi: 10.1158/0008-5472.CAN-04-2219. [DOI] [PubMed] [Google Scholar]
- 99.Leithner A, Weinhaeusel A, Windhager R, et al. Absence of SV40 in Austrian tumors correlates with low incidence of mesotheliomas. Cancer Biol Ther. 2002;1(4):375–379. [PubMed] [Google Scholar]
- 100.Hubner R, Van Marck E. Reappraisal of the strong association between simian virus 40 and human malignant mesothelioma of the pleura (Belgium) Cancer Biol Ther. 2002;13(2):121–129. doi: 10.1023/a:1014321729038. [DOI] [PubMed] [Google Scholar]
- 101.Mayall F, Barratt K, Shanks J. The detection of Simian virus 40 in mesotheliomas from New Zealand and England using real time FRET probe PCR protocols. J Clin Pathol. 2003;56(10):728–730. doi: 10.1136/jcp.56.10.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brousset P, Sabatier J, Galateau-Salle F. SV40 infection in human cancers. Ann Oncol. 2005;16(7):1212–1213. doi: 10.1093/annonc/mdi202. [DOI] [PubMed] [Google Scholar]
- 103.Weiss AF, Portmann R, Fischer H, Simon J, Zang KD. Simian virus 40-related antigens in three human meningiomas with defined chromosome loss. Proc Natl Acad Sci USA. 1975;72(2):609–613. doi: 10.1073/pnas.72.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weiss AF, Zang KD, Birkmayer GD, Miller F. SV 40 related papova-viruses in human meningiomas. Acta Neuropathol. 1976;34(2):171–174. doi: 10.1007/BF00684667. [DOI] [PubMed] [Google Scholar]
- 105.Vilchez RA, Butel JS. SV40 in human brain cancers and non-Hodgkin’s lymphoma. Oncogene. 2003;22(33):5164–5172. doi: 10.1038/sj.onc.1206547. [DOI] [PubMed] [Google Scholar]
- 106.Wang J, Garcea RL. Simian virus 40 DNA sequences in human brain and bone tumours. Dev Biol Stand. 1998;94:13–21. [PubMed] [Google Scholar]
- 107.Huang H, Reis R, Yonekawa Y, Lopes JM, Kleihues P, Ohgaki H. Identification in human brain tumors of DNA sequences specific for SV40 large T antigen. Brain Pathol. 1999;9(1):33–42. doi: 10.1111/j.1750-3639.1999.tb00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rollison DE, Utaipat U, Ryschkewitsch C, et al. Investigation of human brain tumors for the presence of polyomavirus genome sequences by two independent laboratories. Int J Cancer. 2005;113(5):769–774. doi: 10.1002/ijc.20641. [DOI] [PubMed] [Google Scholar]
- 109.Kim JY, Koralnik IJ, LeFave M, Segal RA, Pfister LA, Pomeroy SL. Medulloblastomas and primitive neuroectodermal tumors rarely contain polyomavirus DNA sequences. Neuro Oncol. 2002;4(3):165–170. doi: 10.1093/neuonc/4.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Montesinos-Rongen M, Besleaga R, Heinsohn S, et al. Absence of simian virus 40 DNA sequences in primary central nervous system lymphoma in HIV-negative patients. Virchows Arch. 2004;444(5):436–438. doi: 10.1007/s00428-004-1001-9. [DOI] [PubMed] [Google Scholar]
- 111.Sabatier J, Uro-Coste E, Benouaich A, et al. Immunodetection of SV40 large T antigen in human central nervous system tumours. J Clin Pathol. 2005;58(4):429–431. doi: 10.1136/jcp.2004.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bergsagel DJ, Finegold MJ, Butel JS, Kupsky WJ, Garcea RL. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992;326(15):988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- 113•.Malkin D, Chilton-MacNeill S, Meister LA, Sexsmith E, Diller L, Garcea RL. Tissue-specific expression of SV40 in tumors associated with the Li-Fraumeni syndrome. Oncogene. 2001;20(33):4441–4449. doi: 10.1038/sj.onc.1204583. Extends the tissue tropism of SV40 and links SV40 Tag expression to the Li–Fraumeni syndrome and to p53 mutations. [DOI] [PubMed] [Google Scholar]
- 114.Meneses A, Lopez-Terrada D, Zanwar P, et al. Lymphoproliferative disorders in Costa Rica and simian virus 40. Haematologica. 2005;90(12):1635–1642. [PubMed] [Google Scholar]
- 115.Zekri AR, Mohamed W, Bahnassy A, et al. Detection of simian virus 40 DNA sequences in Egyptian patients with different hematological malignancies. Leuk Lymphoma. 2007;48(9):1828–1834. doi: 10.1080/10428190701534408. [DOI] [PubMed] [Google Scholar]
- 116.Amara K, Trimeche M, Ziadi S, et al. Presence of simian virus 40 DNA sequences in diffuse large B-cell lymphomas in Tunisia correlates with aberrant promoter hypermethylation of multiple tumor suppressor genes. Int J Cancer. 2007;121(12):2693–2702. doi: 10.1002/ijc.23038. [DOI] [PubMed] [Google Scholar]
- 117.Heinsohn S, Scholz R, Kabisch H. SV40 and p53 as team players in childhood lymphoproliferative disorders. Int J Oncol. 2011;38(5):1307–1317. doi: 10.3892/ijo.2011.967. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto H, Nakayama T, Murakami H, et al. High incidence of SV40-like sequences detection in tumour and peripheral blood cells of Japanese osteosarcoma patients. Br J Cancer. 2000;82(10):1677–1681. doi: 10.1054/bjoc.2000.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heinsohn S, Szendroi M, Bielack S, Stadt UZ, Kabisch H. Evaluation of SV40 in osteosarcoma and healthy population: a Hungarian-German study. Oncol Rep. 2009;21(2):289–297. [PubMed] [Google Scholar]
- 120.Hachana M, Trimeche M, Ziadi S, Amara K, Korbi S. Evidence for a role of the Simian virus 40 in human breast carcinomas. Breast Cancer Res Treat. 2009;113(1):43–58. doi: 10.1007/s10549-008-9901-z. [DOI] [PubMed] [Google Scholar]
- 121.Campello C, Comar M, Zanotta N, Minicozzi A, Rodella L, Poli A. Detection of SV40 in colon cancer: a molecular case-control study from northeast Italy. J Med Virol. 2010;82(7):1197–1200. doi: 10.1002/jmv.21798. [DOI] [PubMed] [Google Scholar]
- 122.Sangar D, Pipkin PA, Wood DJ, Minor PD. Examination of poliovirus vaccine preparations for SV40 sequences. Biologicals. 1999;27(1):1–10. doi: 10.1006/biol.1998.0170. [DOI] [PubMed] [Google Scholar]
- 123.Pershouse MA, Heivly S, Girtsman T. The role of SV40 in malignant mesothelioma and other human malignancies. Inhal Toxicol. 2006;18(12):995–1000. doi: 10.1080/08958370600835377. [DOI] [PubMed] [Google Scholar]
- 124.Lopez-Rios F, Illei PB, Rusch V, Ladanyi M. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet. 2004;364(9440):1157–1166. doi: 10.1016/S0140-6736(04)17102-X. [DOI] [PubMed] [Google Scholar]
- 125.Gordon GJ, Chen CJ, Jaklitsch MT, Richards WG, Sugarbaker DJ, Bueno R. Detection and quantification of SV40 large T-antigen DNA in mesothelioma tissues and cell lines. Oncol Rep. 2002;9(3):631–634. [PubMed] [Google Scholar]
- 126.Jin M, Sawa H, Suzuki T, et al. Investigation of simian virus 40 large T antigen in 18 autopsied malignant mesothelioma patients in Japan. J Med Virol. 2004;74(4):668–676. doi: 10.1002/jmv.20219. [DOI] [PubMed] [Google Scholar]
- 127.Aoe K, Hiraki A, Murakami T, et al. Infrequent existence of simian virus 40 large T antigen DNA in malignant mesothelioma in Japan. Cancer Sci. 2006;97(4):292–295. doi: 10.1111/j.1349-7006.2006.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hmeljak J, Kern I, Cor A. No implication of Simian virus 40 in pathogenesis of malignant pleural mesothelioma in Slovenia. Tumori. 2010;96(5):667–673. doi: 10.1177/030089161009600504. [DOI] [PubMed] [Google Scholar]
- 129.Stratton K, Almario DA IOM. Institute of Medicine (IOM) The National Academy of Sciences; Washington, DC, USA: 2002. Immunization safety review: SV40 contamination of polio vaccines and cancer. [PubMed] [Google Scholar]
- 130.Liu J, Li H, Nomura K, Dofuku R, Kitagawa T. Cytogenetic analysis of hepatic cell lines derived from SV40-T antigen gene-harboring transgenic mice. Cancer Genet Cytogenet. 1991;55(2):207–216. doi: 10.1016/0165-4608(91)90079-a. [DOI] [PubMed] [Google Scholar]
- 131.Carbone M, Rdzanek MA, Rudzinski JJ, et al. SV40 detection in human tumor specimens. Cancer Res. 2005;65(21):10120–10121. doi: 10.1158/0008-5472.CAN-05-1911. [DOI] [PubMed] [Google Scholar]
- 132.Carbone M, Rizzo P, Procopio A, et al. SV40-like sequences in human bone tumors. Oncogene. 1996;13(3):527–535. [PubMed] [Google Scholar]
- 133•.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5(3):e1000363. doi: 10.1371/journal.ppat.1000363. Shows that approximately 1% of SV40 seroprevalence occurs in healthy donors and suggests that primary exposure likely occurs in the childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferber D. Public health. Creeping consensus on SV40 and polio vaccine. Science. 2002;298(5594):725–727. doi: 10.1126/science.298.5594.725b. [DOI] [PubMed] [Google Scholar]
- 135.Cantalupo PG, Saenz-Robles MT, Rathi AV, et al. Cell-type specific regulation of gene expression by simian virus 40 T antigens. Virology. 2009;386(1):183–191. doi: 10.1016/j.virol.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]