Abstract
Achieving efficient cardiac gene transfer in a large animal model has proven to be technically challenging. Prior strategies have employed cardio-pulmonary bypass or dual catheterization with the aid of vasodilators to deliver vectors, such as adenovirus, adeno-associated virus or plasmid DNA. While single stranded adeno-associated virus vectors have shown the greatest promise, they suffer from delayed expression, which might be circumvented by using self-complementary vectors. We sought to optimize cardiac gene transfer using a percutaneous transendocardial injection catheter to deliver adeno-associated virus vectors to the canine myocardium. Four vectors were evaluated—single stranded adeno-associated virus 9, self-complementary adeno-associated virus 9, self-complementary adeno-associated virus 8, self-complementary adeno-associated virus 6—so that comparison could be made between single stranded and self complementary vectors as well as among serotypes 9, 8, and 6. We demonstrate that self-complementary adeno-associated virus is superior to single stranded adeno-associated virus and that adeno-associated virus 6 is superior to other serotypes evaluated. Biodistribution studies revealed that vector genome copies were 15 to 4000 times more abundant in the heart than in any other organ for self-complementary adeno-associated virus 6. Percutaneous transendocardial injection of self-complementary adeno-associated virus 6 is a safe, effective method for achieving efficient cardiac gene transfer.
INTRODUCTION
Gene therapy has great therapeutic potential. Adeno-associated virus (AAV) mediated gene therapy has been described extensively in the literature resulting in efficient cardiac gene transfer in small animal models, including mice,1–8 rats,9 and hamsters,10 and from these reports it appears that serotype 9 (AAV9) is superior to other serotypes in the heart.3, 4, 7, 8 In addition to being efficient, AAV-mediated transgene expression is also stable, an advantage over other commonly used vectors such as adenovirus or plasmid DNA.6 However, AAV vectors have the significant disadvantage of delayed expression, taking nearly one month to reach full expression,11 which would limit their usefulness in a rapidly progressing cardiomyopathy. This limitation might be overcome by using the recently developed self-complementary AAV (scAAV) vectors, which package a double-stranded genome and thus bypass the need for complementary strand synthesis. This offers the advantage of faster onset of expression that may also be more efficient than traditional single-stranded AAV (ssAAV) vectors, albeit at the cost of halving the possible size of the expression cassette.12–16
While cardiac gene transfer has been extremely successful in small animal models, delivery to the heart in large animal models and humans has proven to be technically challenging. Several delivery methods have been investigated with varying degrees of success using AAV, adenovirus, or plasmid DNA as vectors. Pericardial instillation of vector results in gene transfer that is restricted to the epicardium.17, 18 Direct, transepicardial injection of vector following left thoracotomy allows delivery throughout the left ventricular free wall (LVFW) but is highly invasive and cannot target the interventricular septum (IVS).19–23 Infusion of vector into the coronary arteries can lead to efficient gene transfer, but optimal transfer often requires highly invasive cardio-pulmonary bypass24–28 or dual catheterization of a coronary artery and vein29–33 with the use of potentially dangerous pharmacological vasodilators. Promising pre-clinical and Phase I/II results have been obtained using NOGA left ventricular electromechanical mapping to guide transendocardial injections of either plasmid DNA or adenovirus via a percutaneously inserted injection catheter.34–37 Although this method is relatively non-invasive, it requires creation of a 3-D map of the heart using very specific and expensive equipment prior to injection. In addition, the gene transfer vectors used in these studies have been associated with inflammation and unstable expression in the case of adenovirus and low-efficiency, unstable expression in the case of plasmid DNA.38
Our goal in this study was to optimize cardiac gene delivery in a large animal model. We focused on AAV vectors because we believe that their low immunogenicity and stable, efficient expression makes them the ideal gene therapy vector for myocardial diseases. We hypothesized that we could achieve safe, efficient, global cardiac gene transfer in the canine with a novel injection catheter to deliver scAAV vectors under fluoroscopic guidance. We report (i) that percutaneous transendocardial delivery of scAAV6 can be used to achieve highly efficient, global gene transfer to the canine heart with rapid onset of expression and (ii) that AAV6 is superior to other serotypes tested.
RESULTS
Study design
In this canine study, we compared the cardiac transduction efficiency of 4 different AAV vectors 7–10 days following endomyocardial injection via a percutaneously inserted, steerable injection catheter. Forty injections were performed to distribute vector globally throughout the LVFW and IVS. Each AAV vector was designed to express the enhanced GFP reporter gene under control of the chicken β-actin promoter with CMV enhancer (AAV-CB-EGFP). The vectors evaluated included ssAAV9, scAAV9, scAAV8, and scAAV6 (n=4 dogs per group, 16 total dogs). An additional dog received saline only injection to serve as a negative control, and 3 dogs were injected with AAV6 empty capsid for long term (6 months) safety evaluation. By analyzing these vectors, we were able to compare ssAAV vs. scAAV (both AAV9) as well as serotypes 9, 8, and 6 (all scAAV). A subset of canines (n=8), all of whom received parvovirus vaccine, were screened for the presence of pre-existing antibodies for AAV9, 8, and 6. Antibody titer was undetectable for serotypes 8 and 9, and either undetectable (n=2) or borderline detectable [1:20 serum dilution (n=4) or 1:40 serum dilution (n=2)] for AAV6.
scAAV9 vs. ssAAV9 in the canine myocardium
Based on reports that AAV9 is the most efficient AAV serotype for cardiac gene transfer in mice,3, 4,7, 8 we chose first to assess the efficiency of AAV9 mediated cardiac gene transfer in the canine. In addition, since the newly developed scAAV vectors have been used to achieve expression levels 1–2 logs greater than traditional ssAAV vectors,12–15 we compared the gene transfer efficiency of ssAAV9 vs. scAAV9. A lower dose of scAAV9 was used (5×1011 gc/kg) compared to ssAAV9 (2×1013 gc/kg) because we believed that the potentially higher efficiency of this scAAV vector would allow us to achieve significant gene transfer while minimizing viral load in the subject. scAAV9 mediated 50-fold greater cardiac gene transfer expression than ssAAV9 at a 40-fold lower dose (p=0.001), suggesting an efficiency advantage of approximately 3 logs (Figs. 1a–d). However, only approximately 7% of the myocardium was transduced in the scAAV9 group (positive for GFP expression) (Fig. 1e).
Figure 1.
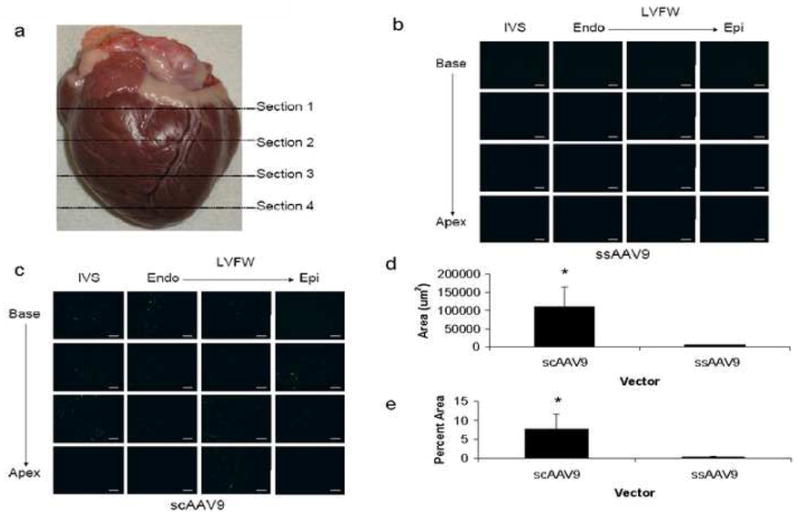
Delivery of ssAAV9 and scAAV9 to the canine heart via needle-tip injection catheter. (a) For analysis, the heart was divided into four equal sections through the short axis from base to apex. GFP expression in representative cryosections of the heart 7–10 days following injection of either (b) 2×1013 gc/kg of ssAAV9-CB-EGFP or (c) 5×1011 gc/kg of scAAV9-CB-EGFP. IVS, interventricular septum; LVFW, left ventricular free wall; Endo, endocardium; Epi, epicardium. Scale bar, 200 μm. Quantitative analysis of GFP expression reported as either (d) total area positive for GFP or (e) percent cardiomyocyte area positive for GFP. Note that scAAV9 is superior to ssAAV9 even at a 40-fold lower dose (*p=0.001). Error bars represent mean + SD.
scAAV6 vs. scAAV8 vs. scAAV9 in the canine myocardium
A vector capable of gene transfer to only 7% of the myocardium would have low therapeutic potential. Therefore, we next evaluated the cardiac gene transfer efficiency of two additional serotypes: 8 and 6. Serotype 8 was chosen because it has relatively high cardiac tropism in the mouse3–5, 8 and rat,9 and serotype 6 was chosen because it has a relatively high cardiac tropism in the pig when compared to serotypes 2 and 5.32, 33 We continued the study with the superior scAAV vector designed to express CB-EGFP at a dose of 5×1011 gc/kg. Although the cardiac gene transfer efficiency of scAAV8 was similar to that of scAAV9, scAAV6 was significantly more efficient than the other vectors evaluated in this study (p=0.0001) (Figs. 2a–d). With scAAV6, high level expression of GFP was evident throughout the LVFW and IVS in approximately 60% of the cardiomyocyte area (Figs. 2b,d). Representative DAPI counterstained sections for each vector and saline control can be found in Figure S1.
Figure 2.
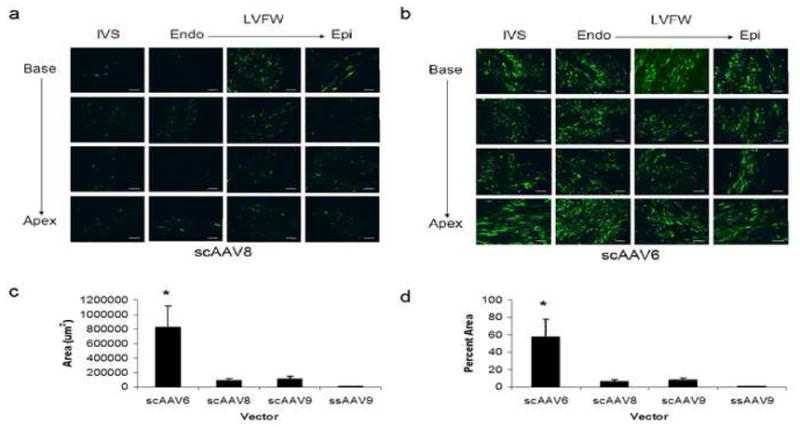
Delivery of scAAV8 and scAAV6 to the canine heart via needle-tip injection catheter. GFP expression in representative cryosections of the heart 7–10 days following injection of either 5×1011 gc/kg of (a) scAAV8-CB-EGFP or (b) scAAV6-CB-EGFP. IVS, interventricular septum; LVFW, left ventricular free wall; Endo, endocardium; Epi, epicardium. Scale bar, 200 μm. Quantitative analysis of GFP expression reported as either (c) total area positive for GFP or (d) percent cardiomyocyte area positive for GFP. Note that scAAV6 is superior to the other serotypes examined (*p=0.0001). Error bars represent mean + SD.
Biodistribution of AAV vectors following transendocardial injection
AAV vector genomes were detected in all tissues examined (Fig. 3a); however, the ratio of genomes detected in the heart to genomes detected in other tissues varied significantly from vector to vector (Fig. 3b). Of the four vectors examined, scAAV6 had the highest cardiac specificity with heart to tissue ratios ranging from 15 in the liver to approximately 100 in the spleen and up to 4000 in other tissues. In contrast, the heart to organ ratios for the other vectors evaluated were approximately 2 or less for the liver and 10 or less in the spleen (Fig. 3b). GFP fluorescence was also minimal in liver sections from animals treated with scAAV6 compared to other sc vectors (p=0.002) (Figs. 3c,d).
Figure 3.
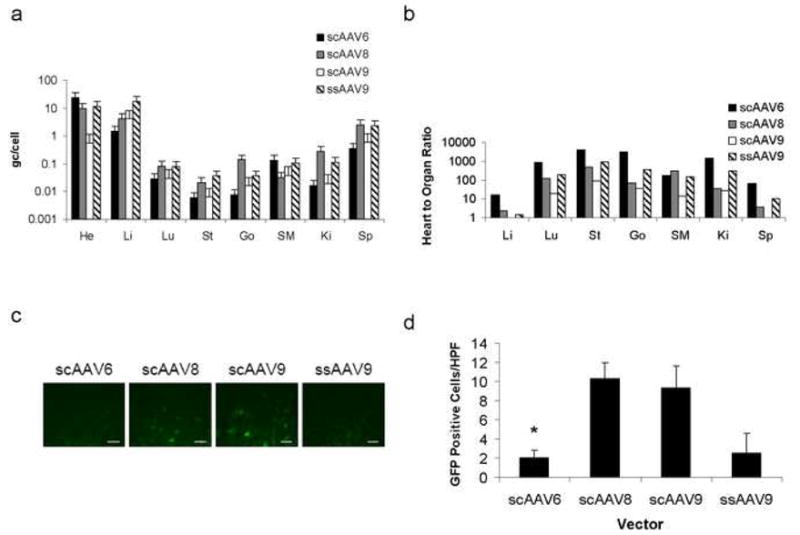
Biodistribution of AAV vectors 7–10 days following delivery to the canine heart via needle-tip injection catheter. (a) Biodistribution in several organs examined. He, heart; Li, liver; Lu, lung; St, stomach; Go, gonad; SM, skeletal muscle; Ki, kidney; Sp, spleen. (b) Tissue tropism of vectors evaluated reported as ratio of vector detected in heart to vector detected in organ of choice. Note that scAAV6 is highly tropic for cardiac tissue when compared to the other vectors. (c) GFP expression in cryosections of the liver 7–10 days following treatment. Scale bar, 40 μm. (d) Quantitative analysis of GFP expression in liver. Note that there are significantly fewer positive cells in the scAAV6 group compared to other scAAV serotypes (*p=0.002). HPF, high power field (40×). Error bars represent mean + SD.
Safety of percutaneous transendocardial delivery of AAV vectors
There was no mortality associated with our protocol. However, ventricular ectopy with secondary hypotension was noted in 14 of 16 dogs at the time of AAV injection. The dog treated with saline only also experienced ventricular ectopy. These arrhythmias were amenable to lidocaine infusion in all 14, and lidocaine was discontinued uneventfully once the injections were completed. Ventricular arrhythmias had resolved in all dogs by the time they had recovered from anesthesia. Color flow Doppler of the aortic valve, which was performed in a subset of animals (n=6 dogs) pre and post procedure, revealed no change in valve leakage secondary to transaortic catheterization in all dogs examined. Histologic analysis of H&E and Trichrome stained cardiac sections revealed minimal amounts of fibrosis and mononuclear cell infiltration at the sites of injection (Figure S2).
DISCUSSION
Cardiac gene therapy has great therapeutic potential, but its application has been hindered due to the lack of a safe and effective delivery method in a large animal model. We report here a simple, relatively non-invasive protocol in the canine which results in highly efficient, global cardiac gene transfer mediated by scAAV6 following delivery via a percutaneous injection catheter. This procedure is superior to previously reported large animal cardiac gene transfer techniques because it combines the advantages of direct injection with those of a non-invasive percutaneous delivery system.17–38 Because it results in high-level transgene expression throughout the left ventricular free wall and interventricular septum, this technique could be useful to treat diseases which affect the heart globally, such as cardiomyopathy. Furthermore, localized delivery is also possible, and importantly can be achieved in poorly perfused areas. This offers a tremendous advantage over vascular approaches in treating regional ischemia, which is important considering that ischemic cardiomyopathy accounts for approximately 40% of all heart failure cases.39
Under fluoroscopic guidance, the catheter is steerable and needle tip length is adjustable so that multiple injections can be targeted throughout the left ventricle from base to apex and from endocardium to epicardium. Fluoroscopy images recorded during the procedure demonstrate how the catheter tip can be deformed to target a specific region of the heart and how contrast media is used to track injections (Figure S3). Therefore, the technique lends itself to global gene delivery or targeted gene delivery for addressing a regional defect.
Moreover, our delivery method is simple, safe, and relatively non-invasive, suggesting it would be better tolerated in patients with significant heart disease than procedures requiring thoracotomy, cardiopulmonary bypass, or electromechanical mapping prior to injection. The catheter has been safely employed to deliver skeletal myoblasts to large animals and humans,40–43 but to our knowledge this is the first report of its use for gene delivery. The only adverse event associated with the injection procedure was ventricular ectopy that was amenable to lidocaine infusion. Since ectopy was noted in the saline-injected animal as well, it is likely a result the intramyocardial injections themselves and not the AAV vector. Pathology at 7–10 days revealed only small fields of fibrosis at the injection sites with minimal mononuclear infiltration, which is similar to what was observed following direct myocardial injection in a porcine model at 2 weeks.23 Finally, our technology does not require the use of expensive and sometimes dangerous vasodilators, such as VEGF, for effective delivery.
Furthermore, we have identified an ideal gene transfer vector in a large animal model. While many investigators have relied on unstable vectors such as adenovirus and plasmid DNA in their large animal cardiac models, we have focused on the stable scAAV vector with the goal of achieving long term expression with rapid onset. The scAAV vectors contain a mutation in one of the replication termination sequences that allows synthesis and packaging of a dimeric inverted repeat.15 As a result, these vectors bypass the rate-limiting requirement for second strand synthesis and have been reported to be more efficient than the traditional ssAAV vectors in several species and tissues.13–15 Our findings confirm these previous reports for the first time in the canine heart. We demonstrate that scAAV is several logs more potent than ssAAV in the canine heart, at least within the short time frame of these experiments (7–10 days). It may be that the ssAAV expression would approach that of the scAAV over weeks to months;44 however this would still limit its possible therapeutic applications as compared to scAAV vectors since many myocardial diseases, such as ischemia/infarction would demand rapid onset of expression for ideal therapeutic efficacy. Although we did not look for expression earlier than one week, small animal data suggest that transgene expression mediated by AAV can initiate as early as 1–4 days.44, 45 Further studies are needed in this canine model to determine a time course of expression.
We also observed that AAV6 is approximately one log more potent than AAV8 and AAV9 in the canine heart. This result was unanticipated based on a review of the literature. Although data from porcine hearts suggest that AAV6 is superior to 2 and 5,32, 33 a study in rats that excluded serotype 9 demonstrated the superiority of AAV8 over serotypes 1–7 in the heart,9 and three reports in mice demonstrated that AAV9 is superior to 1 and 8 in the heart.3, 4, 7 This led us to hypothesize serotype 9 would be superior to 8, which would be superior to 6. However, the small animal data did not accurately predict the large animal outcome since we observed quite the opposite result. Based on our data, scAAV6 is approximately 1 log more potent than the other scAAV vectors evaluated in this study (Fig. 2d). We screened a susbset of our dogs for the presence of pre-existing neutralizing antibodies (NAb) against AAV9, 8, and 6 and detected extremely low titer antibodies (<1:20 to 1:40 serum dilution) only for AAV6. Since we observed that AAV6 was the most efficient cardiac gene transfer vector, it is unlikely that differential titers of pre-existing NAb were responsible for the differential cardiac transduction reported in this study.
A recent study examined the mechanism of high-efficiency cardiac gene transfer by AAV6 and found that serotype 6 displays both enhanced cellular internalization and nuclear uncoating in cardiomyocytes compared to serotype 2.16 Perhaps, the canine heart contains a higher density of the AAV6 receptor compared to other species, and this fact, combined with its high efficiency of nuclear uncoating and self-complementary DNA structure, may explain the superior performance of scAAV6 in this study. Further investigation is needed to confirm this hypothesis. It will also be important to conduct future studies to determine the time course of expression since this may vary depending on the serotype used.
In addition to being highly efficient, scAAV6 is also relatively cardiac specific compared to the other serotypes evaluated, which further increases its utility as a cardiac gene therapy vector. In terms of vector biodistribution, the heart to liver ratio was 15 for scAAV6 compared to 2 or less for the other serotypes. The heart to organ ratio of scAAV6 was also very favorable in other organs and ranged from 100 in the spleen up to 4000 for the gonads. This preferential cardiac transduction of AAV6 has also been reported in the mouse8 and pig.32 Although the transduced cell types in the spleen and other organs were not investigated, another report has identified the ability of AAV to transduce monocytes in the spleens of non-human primates.46
It should be noted, however, that although our canine data suggest that AAV6 may be the vector of choice for clinical trials of cardiac gene transfer, the human heart may display a different tropism for AAV serotypes. Indeed, a study in non-human primates showed that AAV9 was able to provide 4-fold greater expression than AAV1 in the heart.4 Although AAV1 and AAV6 share >95% sequence homology in their capsids,11 a recent report noted that the relative tissue tropisms of AAV1 and 6 were substantially different in several organs examined, including an almost 2 log advantage for AAV6 in the heart.8 Therefore, despite high sequence homology, AAV1 and 6 may not necessarily perform similarly in the heart. In addition, the non-human primates were injected within 1 hour of birth, and since protein expression profiles change throughout development, the receptors for different AAV serotypes may be differentially expressed in the neonate vs. the adult. A comprehensive comparison of AAV serotypes in adult non-human primate hearts, which would be the large animal model phylogenetically closest to humans, may be necessary to identify the ideal vector for human clinical trials.
This novel protocol has great therapeutic potential because it can be safely used to transfer a vector capable of rapid onset, efficient, long-term expression to the heart via a simple, relatively non-invasive procedure. Its potential applications are numerous, and include most forms of acquired and genetic cardiomyopathy in both the veterinary and human setting. Limitations of our study include a short follow up period post procedure and lack of experience with specific therapeutic transgenes. This period was necessitated by the reporter transgene used in our study. GFP expression was analyzed 7–10 days post-injection so that we could appreciate true transfer efficiency before a T-cell response against the cells expressing the foreign reporter protein was mounted.21 However, injection of empty capsid (AAV6) in three dogs did not result in any clinical or myocardial change for 6 months post procedure, and no T cells reactive to AAV6 capsid were detected via ELISpot (Figure S4). This suggests that AAV6 has potential as a long-term expression vector in the heart, which is important in light of a recent report which described a cellular immune response against the capsids of AAV2 and 6 following direct injection into canine skeletal muscle.47 In this model, immunosuppression was necessary for long-term expression.48 It may be that the immune response to AAV varies from tissue to tissue, and long term expression in the heart may be possible without immune modulation. It is also possible that differences in vector preparation could alter the immune response. Further studies with non-immunogenic transgenes are necessary in our model to confirm this hypothesis.
Our protocol could be optimized in several ways to meet specific clinical needs. The DNA packaging capacity of scAAV is limited to half that of the traditional ssAAV vectors due to the fact that a dimeric repeat is encapsidated.15 If lower expression levels and/or delayed expression could be tolerated, ssAAV6 could be used to deliver a much larger therapeutic gene at the cost of less efficient and/or delayed expression. For safety, in order to restrict expression outside of the heart, transcriptional and/or transductional targeting could be employed.49 In patients with heart disease, considering the frequency of ventricular ectopy in our healthy dogs, a lidocaine constant rate infusion should probably be initiated at the time of anesthetic induction. Finally, increased expression efficiency may be achieved by increasing the number and/or volume of injections, and delivery could easily be directed to the right ventricle following a femoral or jugular vein catheterization. Investigation is underway to evaluate the feasibility of these approaches.
METHODS
Vector design and production
Each vector was designed to express the enhanced green fluorescent protein (EGFP) reporter gene under control of the constitutive chicken beta actin promoter with CMV enhancer (CB promoter). Vectors were produced according to the previously described pseudotyping protocol by the Vector Core of the University of Pennsylvania.11 Briefly, recombinant AAV genomes containing AAV2 inverted terminal repeats (ITR’s) were packaged by triple transfection of 293 cells with a cis-plasmid containing the EGFP transgene, an adenovirus helper plasmid, and a chimeric trans-plasmid containing the AAV2 rep gene fused to the capsid gene of the AAV serotype of interest. Self-complementary vectors contained a mutation in the termination sequence of the 5’ ITR to allow synthesis and encapsidation of a dimeric inverted repeat of the transgene cassette.15
Animal use and vector delivery protocol
All animals were handled in compliance with National Institute of Health and institutional guidelines that were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Twenty mongrel dogs (5–10 kg, 3–6 months old) were used in this study. Sixteen mongrel dogs were randomized to receive 1 of 4 vectors: ssAAV9, scAAV9, scAAV8 or scAAV6 (n=4 per group). An additional dog received saline only injection to serve as a negative control for GFP fluorescence and biodistribution studies, and 3 dogs were treated with AAV6 empty capsid for long term (6 months) safety evaluation. Pre-existing, neutralizing antibody titer for AAV9, 8, and 6 was determined in a subset of canines (n=8) from the colony, all of whom were vaccinated against parvovirus. Titer was determined by incubating Huh7 cells with serial dilutions of canine serum and AAV-CMV-EGFP of the serotype in question and observing the dilution at which the number of GFP positive cells was reduced by 50% compared to control wells.
Procedures were performed under general anesthesia, and dogs were placed in left lateral recumbency. Heart rate, respiratory rate, systolic blood pressure, electrocardiogram, and oxygen saturation were monitored throughout the anesthetic period. In a subset of dogs (n=6), transesophageal echocardiography (TEE) was also performed throughout the procedure to monitor patency of the aortic valve.
A right carotid arterotomy was performed, and a 7 French introducer was placed in the vessel, followed by insertion of the injection catheter. This catheter was a steerable injection catheter with an adjustable length core needle (MyoCath, Bioheart Inc, Sunrise, FL),42 which has previously been employed to deliver skeletal myoblasts to large animals and humans.40–43 The catheter was flushed with heparinized blood prior to vector infusion to prevent inactivation of the virus.50 Next, under fluoroscopic guidance, the catheter was advanced into the left ventricular cavity, and by steering the needle tip and adjusting the needle length, approximately 40 trandsendocardial injections of 250 ul each were performed to target the LVFW and IVS from base to apex and from endocardium to epicardium with AAV vector. Contrast media was added to the vector solution so that injection sites could be visualized. This allowed us to differentiate between injected and uninjected regions of the heart and helped to ensure that the vector solution was distributed globally throughout the myocardium. Still fluoroscopy images recorded during the procedure demonstrate how the catheter tip can be deformed to target a specific region of the heart (Figure S3a) and how contrast media was used to track single (Figure S3a) and multiple (Figure S3b) injections during the same procedure.
A dose of 2×1013 genome copies per kilogram (gc/kg) was used for ssAAV9; however, a lower dose (5×1011 gc/kg) was used for the self complementary vector injections. For each procedure vector was mixed with 2 cc of sterile contrast solution (Omnipaque®) and diluted with sterile saline to produce 10 cc for injection. Lidocaine (2 mg/kg as a bolus followed by a constant rate infusion at 50 μg/kg/min) was initiated if ventricular tachycardia developed during the procedure (14 of 16 dogs). After recovery, dogs were treated with carprofen for two days and amoxicillin-clauvolonic acid for 5 days.
Histologic analysis of transgene expression and pathology
Dogs were euthanized 7 to 10 days following treatment and tissues were harvested for determination of reporter transgene expression and vector biodistribution. For analysis of GFP fluorescence, the heart was divided into four sections along the short axis from the apex to base and fixed in 4% paraformaldehyde overnight at 4°C. The tissue was then washed 3×10 minutes in PBS and dehydrated in 20% sucrose overnight at 4°C. Next, each section was frozen in OCT embedding compound (Tissue Tek, Torrance, CA), and 10μm cryosections were prepared. Slides were mounted with Vectashield DAPI media (Vector Laboratories, Burlingame, CA) and examined for GFP fluorescence using a Leitz DMRBE fluorescent microscope (Leica, Bannockburn, IL) equipped with a Micro Max digital camera (Princeton Instruments, Trenton, NJ) interfaced with Image Pro Plus software (Media Cybernetics, Bethesda, MD). For each animal, four representative photographs were recorded under constant exposure conditions using the 10× objective from the IVS and LVFW from the endocardium to the epicardium in each of the four heart sections, and the area positive for GFP fluorescence was quantified using Open Lab software (Improvison, Waltham, MA). The threshold for detection was set above background levels measured in the negative control, saline treated dog. Immunofluorescent staining for cardiac troponin T (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) was also performed, and the GFP positive area was normalized to the troponin positive area to report the percent cardiomyocyte area positive for GFP. A sample of liver from each animal was also fixed, sectioned, and analyzed as described above to quantify GFP positive hepatocytes. For pathology, slides were stained independently with H&E and Trichrome (Sigma, St. Louis, MO) to identify mononuclear infiltrate and fibrosis, respectively.
Biodistribution analysis
For biodistribution analysis, samples were snap frozen in liquid nitrogen. Following DNA extraction, genome copy titers were quantified by TaqMan PCR (Applied Biosystems, Foster City, CA) using primers and probes designed against the EGFP transgene. 1 ug of template DNA was used per reaction, and several controls were performed to confirm the specificity and accuracy of the PCR. A spike control was performed in which the test sample was spiked with exogenous test assay target to rule out PCR inhibition. The assay was also performed on samples from the negative control, saline injected animal to determine background signal of the assay, which was negligible (<1 gc per 5,000 cells, or >1 log lower than the minimum gc detected in experimental samples). Finally, a control endogenous gene (GAPDH) was run as a loading control and displayed minimal sample to sample variation (<0.25 cycles) with 1 ug template DNA.
Statistical analysis
Mean values from each experimental group were compared using the two-tailed Student’s t test or one-way ANOVA with Student Newman-Keuls post-hoc analysis.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NHLBI (P01-HL059407) (to H.L.S.), from the Parent Project Muscular Dystrophy (to H.L.S.), by a Wellstone Muscular Dystrophy Cooperative Center Grant (U54-AR052646) (to H.L.S.), and by T32-HL-007748 (to L.T.B.). JMW and GPG are inventors on patents that have been licensed to various biopharmaceutical companies. We also thank Roberto Calcedo (University of Pennsylvania) for the determining the titer of pre-existing neutralizing antibodies and Daniel Hui and Katherine High (Children’s Hospital of Philadelphia) for performing the ELISpot assay.
References
- 1.Bostick B, Ghosh A, Yue Y, Long C, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007 Nov;14(22):1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 2.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004 Aug;10(8):828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006 Jul;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006 Aug 18;99(4):e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005 Mar;23(3):321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 6.Woo YJ, Zhang JC, Taylor MD, Cohen JE, Hsu VM, Sweeney HL. One year transgene expression with adeno-associated virus cardiac gene transfer. Int J Cardiol. 2005 Apr 28;100(3):421–426. doi: 10.1016/j.ijcard.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Vandendriessche T, Thorrez L, Acosta-Sanchez A, Petrus I, Wang L, Ma L, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 2007 Jan;5(1):16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 8.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008 Jun;16(6):1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 9.Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007 Jul;14(13):989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- 10.Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002 Aug;8(8):864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 11.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi VW, Samulski RJ, McCarty DM. Effects of adeno-associated virus DNA hairpin structure on recombination. J Virol. 2005 Jun;79(11):6801–6807. doi: 10.1128/JVI.79.11.6801-6807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao GP, Lu Y, Sun X, Johnston J, Calcedo R, Grant R, et al. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J Virol. 2006 Jun;80(12):6192–6194. doi: 10.1128/JVI.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003 Dec;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 15.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001 Aug;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 16.Sipo I, Fechner H, Pinkert S, Suckau L, Wang X, Weger S, et al. Differential internalization and nuclear uncoating of self-complementary adeno-associated virus pseudotype vectors as determinants of cardiac cell transduction. Gene Ther. 2007 Sep;14(18):1319–1329. doi: 10.1038/sj.gt.3302987. [DOI] [PubMed] [Google Scholar]
- 17.Lamping KG, Rios CD, Chun JA, Ooboshi H, Davidson BL, Heistad DD. Intrapericardial administration of adenovirus for gene transfer. Am J Physiol. 1997 Jan;272(1 Pt 2):H310–317. doi: 10.1152/ajpheart.1997.272.1.H310. [DOI] [PubMed] [Google Scholar]
- 18.Lazarous DF, Shou M, Stiber JA, Hodge E, Thirumurti V, Goncalves L, et al. Adenoviral-mediated gene transfer induces sustained pericardial VEGF expression in dogs: effect on myocardial angiogenesis. Cardiovasc Res. 1999 Nov;44(2):294–302. doi: 10.1016/s0008-6363(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 19.Ferrarini M, Arsic N, Recchia FA, Zentilin L, Zacchigna S, Xu X, et al. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ Res. 2006 Apr 14;98(7):954–961. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 20.Laguens R, Cabeza Meckert P, Vera Janavel G, De Lorenzi A, Lascano E, Negroni J, et al. Cardiomyocyte hyperplasia after plasmid-mediated vascular endothelial growth factor gene transfer in pigs with chronic myocardial ischemia. J Gene Med. 2004 Feb;6(2):222–227. doi: 10.1002/jgm.478. [DOI] [PubMed] [Google Scholar]
- 21.McTiernan CF, Mathier MA, Zhu X, Xiao X, Klein E, Swan CH, et al. Myocarditis following adeno-associated viral gene expression of human soluble TNF receptor (TNFRII-Fc) in baboon hearts. Gene Ther. 2007 Sep 13; doi: 10.1038/sj.gt.3303020. [DOI] [PubMed] [Google Scholar]
- 22.Vera Janavel G, Crottogini A, Cabeza Meckert P, Cuniberti L, Mele A, Papouchado M, et al. Plasmid-mediated VEGF gene transfer induces cardiomyogenesis and reduces myocardial infarct size in sheep. Gene Ther. 2006 Aug;13(15):1133–1142. doi: 10.1038/sj.gt.3302708. [DOI] [PubMed] [Google Scholar]
- 23.Su H, Yeghiazarians Y, Lee A, Huang Y, Arakawa-Hoyt J, Ye J, et al. AAV serotype 1 mediates more efficient gene transfer to pig myocardium than AAV serotype 2 and plasmid. J Gene Med. 2008 Jan;10(1):33–41. doi: 10.1002/jgm.1129. [DOI] [PubMed] [Google Scholar]
- 24.Bridges CR, Burkman JM, Malekan R, Konig SM, Chen H, Yarnall CB, et al. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann Thorac Surg. 2002 Jun;73(6):1939–1946. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- 25.Bridges CR, Gopal K, Holt DE, Yarnall C, Cole S, Anderson RB, et al. Efficient myocyte gene delivery with complete cardiac surgical isolation in situ. J Thorac Cardiovasc Surg. 2005 Nov;130(5):1364. doi: 10.1016/j.jtcvs.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Davidson MJ, Jones JM, Emani SM, Wilson KH, Jaggers J, Koch WJ, et al. Cardiac gene delivery with cardiopulmonary bypass. Circulation. 2001 Jul 10;104(2):131–133. doi: 10.1161/01.cir.104.2.131. [DOI] [PubMed] [Google Scholar]
- 27.Jones JM, Wilson KH, Koch WJ, Milano CA. Adenoviral gene transfer to the heart during cardiopulmonary bypass: effect of myocardial protection technique on transgene expression. Eur J Cardiothorac Surg. 2002 May;21(5):847–852. doi: 10.1016/s1010-7940(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 28.Oi K, Davies WR, Tazelaar HD, Bailey KR, Federspeil MJ, Russell SJ, et al. Ex vivo hypothermic recirculatory adenoviral gene transfer to the transplanted pig heart. J Gene Med. 2006 Jul;8(7):795–803. doi: 10.1002/jgm.913. [DOI] [PubMed] [Google Scholar]
- 29.Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000 Feb;7(3):232–240. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 30.Hayase M, Del Monte F, Kawase Y, Macneill BD, McGregor J, Yoneyama R, et al. Catheter-based antegrade intracoronary viral gene delivery with coronary venous blockade. Am J Physiol Heart Circ Physiol. 2005 Jun;288(6):H2995–3000. doi: 10.1152/ajpheart.00703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, et al. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007 Jul 17;50(3):253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 32.Raake PW, Hinkel R, Muller S, Delker S, Kreuzpointer R, Kupatt C, et al. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2007 Oct 18; doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- 33.Sasano T, Kikuchi K, McDonald AD, Lai S, Donahue JK. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007 May;42(5):954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs S, Battler A, Kornowski R. Catheter-based stem cell and gene therapy for refractory myocardial ischemia. Nat Clin Pract Cardiovasc Med. 2007 Feb;4(Suppl 1):S89–95. doi: 10.1038/ncpcardio0762. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs S, Dib N, Cohen BM, Okubagzi P, Diethrich EB, Campbell A, et al. A randomized, double-blind, placebo-controlled, multicenter, pilot study of the safety and feasibility of catheter-based intramyocardial injection of AdVEGF121 in patients with refractory advanced coronary artery disease. Catheter Cardiovasc Interv. 2006 Sep;68(3):372–378. doi: 10.1002/ccd.20859. [DOI] [PubMed] [Google Scholar]
- 36.Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002 Apr 30;105(17):2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 37.Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, Curry CM, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001 May 1;103(17):2138–2143. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 38.Li JJ, Ueno H, Pan Y, Tomita H, Yamamoto H, Kanegae Y, et al. Percutaneous transluminal gene transfer into canine myocardium in vivo by replication-defective adenovirus. Cardiovasc Res. 1995 Jul;30(1):97–105. [PubMed] [Google Scholar]
- 39.Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002 Mar;143(3):398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 40.Gavira JJ, Perez-Ilzarbe M, Abizanda G, Garcia-Rodriguez A, Orbe J, Paramo JA, et al. A comparison between percutaneous and surgical transplantation of autologous skeletal myoblasts in a swine model of chronic myocardial infarction. Cardiovasc Res. 2006 Sep 1;71(4):744–753. doi: 10.1016/j.cardiores.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 41.He KL, Yi GH, Sherman W, Zhou H, Zhang GP, Gu A, et al. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J Heart Lung Transplant. 2005 Nov;24(11):1940–1949. doi: 10.1016/j.healun.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Meliga E, Duckers HJ, Spencer R, Serruys PW. Rationale and interim analysis data from the SEISMIC study. EuroIntervention Supplement. 2007;2(Supplement B):B84–B88. [Google Scholar]
- 43.Saeed M, Lee R, Martin A, Weber O, Krombach GA, Schalla S, et al. Transendocardial delivery of extracellular myocardial markers by using combination X-ray/MR fluoroscopic guidance: feasibility study in dogs. Radiology. 2004 Jun;231(3):689–696. doi: 10.1148/radiol.2313030683. [DOI] [PubMed] [Google Scholar]
- 44.Andino LM, Conlon TJ, Porvasnik SL, Boye SL, Hauswirth WW, Lewin AS. Rapid, widespread transduction of the murine myocardium using self-complementary Adeno-associated virus. Genet Vaccines Ther. 2007;5:13. doi: 10.1186/1479-0556-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su H, Huang Y, Takagawa J, Barcena A, Arakawa-Hoyt J, Ye J, et al. AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene Ther. 2006 Nov;13(21):1495–1502. doi: 10.1038/sj.gt.3302787. [DOI] [PubMed] [Google Scholar]
- 46.Lai L, Davison BB, Veazey RS, Fisher KJ, Baskin GB. A preliminary evaluation of recombinant adeno-associated virus biodistribution in rhesus monkeys after intrahepatic inoculation in utero. Hum Gene Ther. 2002 Nov 20;13(17):2027–2039. doi: 10.1089/10430340260395884. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007 Jan;18(1):18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007 Jun;15(6):1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- 49.Muller OJ, Leuchs B, Pleger ST, Grimm D, Franz WM, Katus HA, et al. Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors. Cardiovasc Res. 2006 Apr 1;70(1):70–78. doi: 10.1016/j.cardiores.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Marshall DJ, Palasis M, Lepore JJ, Leiden JM. Biocompatibility of cardiovascular gene delivery catheters with adenovirus vectors: an important determinant of the efficiency of cardiovascular gene transfer. Mol Ther. 2000 May;1(5 Pt 1):423–429. doi: 10.1006/mthe.2000.0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


