Abstract
Recent evidences have highlighted an influence of micronutrients in the maintenance of telomere length (TL). In order to explore whether diet-related telomere shortening had any physiological relevance and was accompanied by significant damage in the genome, in the present study, TL was assessed by terminal restriction fragment (TRF) analysis in peripheral blood lymphocytes of 56 healthy subjects for which detailed information on dietary habits was available and data were compared \with the incidence of nucleoplasmic bridges (NPBs), a marker of chromosomal instability related to telomere dysfunction visualised with the cytokinesis-blocked micronucleus assay. To increase the capability to detect even slight impairment of telomere function, the incidence of NPBs was also evaluated on cells exposed in vitro to ionising radiation. Care was taken to control for potential confounding factors that might influence TL, viz. age, hTERT genotype and smoking status. Data showed that higher consumption of vegetables was related with significantly higher mean TL (P = 0.013); in particular, the analysis of the association between micronutrients and mean TL highlighted a significant role of antioxidant intake, especially beta-carotene, on telomere maintenance (P = 0.004). However, the diet-related telomere shortening did not result in associated increased spontaneous or radiation-induced NPBs. The distribution of TRFs was also analysed and a slight prevalence of radiation-induced NPBs (P = 0.03) was observed in subjects with higher amount of very short TRFs (<2 kb). The relative incidence of very short TRFs was positively associate with ageing (P = 0.008) but unrelated to vegetables consumption and daily intake of micronutrients, suggesting that the degree of telomere erosion related with low dietary intake of antioxidants observed in this study was not so extensive to lead to chromosome instability.
Introduction
Telomeres are nucleoprotein structures located at the end of chromosomes, consisting of long stretches of TTAGGG repeats associated with specific proteins (1). Telomeres have a pivotal role in maintaining genome stability, preventing chromosome ends from being recognised as double-strand breaks and processed by DNA damage repair machineries (2,3). In most proliferating cells, telomeres shorten progressively because of the inability of DNA polymerases to fully replicate linear molecules—the so-called end-replication problem—and for the action of a 5′- to 3′-specific exonuclease which shorten each telomere by half the overhang length per round of replication (4,5). Mechanisms to maintain chromosome length consist in the addition of new telomere repeats by the cellular reverse transcriptase telomerase (6) and in a specific recombination pathway known as alternative lengthening of telomeres (7). Telomere dysfunction due to accelerated shortening has been recognised as an important cause of chromosomal instability (3) and has been associated with higher mortality and increased risk of age-related diseases, such as heart failure and cancer (8–10). Telomere length (TL) is genetically determined (11,12) but also environmental and lifestyle factors, including smoking, low physical activity or obesity, significantly contribute to accelerate the rate of telomere attrition (13–15). Due to the high content of guanine, telomere DNA is highly sensitive to oxidative damage and, indeed, oxidative stress and inflammation have been identified as major determinants of telomere erosion (16,17). Recent studies have also investigated the impact of dietary factors on TL, probing the hypothesis that inadequate nutrition through the increase of oxidative stress may influence telomere maintenance. In particular, a cross-sectional analysis demonstrated a positive association between multivitamin use and longer TL in leukocytes, pointing to the contribution of dietary antioxidants to telomere maintenance (18); furthermore, a combined effect of raised plasma homocysteine levels and oxidative stress on telomere erosion has been described (19). In a recent epidemiological study, dietary fibre intake was also found to be positively associated with TL (20), supporting the hypothesis that dietary factors can influence TL in leukocytes. The modulation of TL by dietary habits is reasonable in view of the acknowledged role of some micronutrients in DNA synthesis and repair and in the maintenance of DNA methylation, as well as in the protection against oxidative stress (21,22). Uracil misincorporation into DNA due to deficiencies of folate, vitamins B6 or B12 (23) may increase chromosome breaks in the thymidine sequence within the telomeric repeats and cause telomere end fusion; deficiencies of antioxidant vitamins (i.e. vitamins A, C, E) or cofactors of antioxidant enzymes (i.e. Zn) might cause telomere shortening and chromosome instability impairing telomere repair and replication (24); alteration in the methylation of subtelomeric sequences or the CpG island of the promoter of telomerase could also result in telomere dysfunction (25,26). The functional consequences on genome stability of the observed diet-related variation in TL, however, have not yet been elucidated. In order to explore whether diet-related telomere shortening had any physiological relevance and was accompanied by significant damage in the genome, in the present study, the influence of diet on TL was investigated in a healthy human population and data were compared with the incidence of nucleoplasmic bridges (NPBs). Indeed, NPBs represent a convenient marker to assess chromosomal instability related to telomere dysfunction as they can highlight chromosome fusion, visualised with the cytokinesis-blocked micronucleus (CBMN) assay as bridges extending between daughter nuclei. When centromeres of dicentric chromosomes are pulled towards opposite poles during mitosis, in fact, if cytokinesis is inhibited, anaphase bridges do not break and are wrapped with the nuclear membrane forming NPBs. The approach used in this work is fairly in line with the recent recommendation to couple the measurement of TL with the analysis of NPBs in order to get information on the biological correlation between TL and chromosome integrity (27).
Besides, several data suggest that mild telomere shortening may cause subtle alterations only detectable after an external insult [e.g. by ionising radiation (IR) exposure]. For example, in a BALB/c mouse model possessing partial deficiency of DNA-PKcs activity, the telomeric uncapping phenotype is expressed as telomere double-strand break/fusion only after IR treatment and not as spontaneous telomere fusion (28). Similarly, in human primary fibroblasts defective in Artemis, a protein involved in non-homologous end-joining repair, a mild telomere dysfunction phenotype observed in spontaneous conditions was significantly increased only after IR treatment (29). Moreover, the analysis of radiation-induced NPBs may also be convenient on statistical grounds, as it helps overcome the limitations related to the low incidence of spontaneous NPBs in cultured human lymphocytes, which can hinder to appreciate mild interindividual differences in this biomarker. Based on these reasonings, a parallel set of experiments was carried out on cells exposed in vitro to IR.
Materials and methods
Study population
Fifty-six subjects, enrolled in a previous study carried out in Tuscany (30), have been involved in the current project. Detailed information about diet and lifestyle habits and a blood sample were collected for each participant, after they had signed an informed consent form. The analyses were carried out on anonymous coded samples. The study has been approved by the local ethical committee.
Diet and lifestyle questionnaires
Dietary information on the frequency of consumption of >120 foods and drinks in a 12-month period prior to enrolment was obtained by a self-administered Food Frequency Questionnaire (FFQ), specifically developed for Italian dietary habits and validated in a pilot phase (31), and used in the European Prospective Project Into Cancer and Nutrition (EPIC)—Italy study (32). A detailed description of the EPIC-Italy FFQ has been reported elsewhere (33). Briefly, dietary information on the frequency of consumption of >120 foods and beverages was collected by FFQ, checked and coded by trained dieticians and computerised by optical reading. Then, individual data were transformed into estimates of intake for a series of over 30 nutrients according to specifically developed Italian Food Tables (34). A standardised lifestyle questionnaire (representing the Italian translation of a common EPIC-wide version and specifically printed in two versions for men and women) was also completed by each participant. Detailed information was collected on reproductive history, physical activity, smoking history, alcohol consumption, occupation, educational level and other socio-economic variables.
TL determination
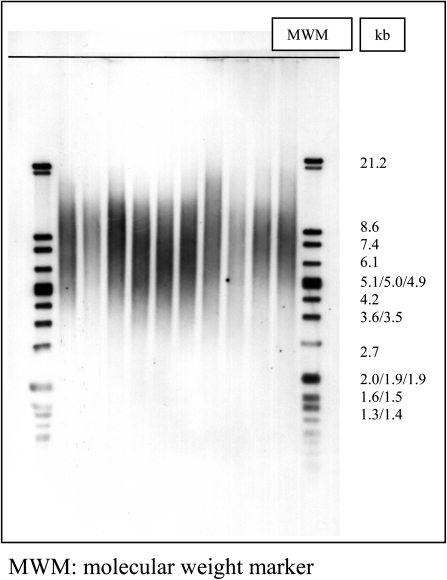
A non-radioactive chemiluminescent assay has been applied to determine mean TL using the TeloTAGGG Telomere Length Assay Kit (Roche), based on terminal restriction fragment (TRF) analysis by Southern hybridisation with telomere repeat containing probes. Briefly, 1 μg of DNA was digested with 20 U of RsaI and HinfI for 2 h at 37°C. The sequence specificity of enzymes has been selected in a way that telomeric DNA and subtelomeric DNA are not cut due to the special sequence characteristics of the repeats, while non-telomeric-DNA is digested to low-molecular weight fragments. After digestion, samples were loaded on a 0.5% agarose gel and run for 21 h at 35 V. Gel was treated with HCl, denaturalised and neutralised and then transferred to a nylon membrane by Southern blotting for 12–18 h. DNA fragments were indirectly visualised by hybridisation with a digoxigenin (DIG)-labelled probe complementary to the telomeric repeat sequence (3 h, 42°C). Finally, images were digitalised using a densitometer. A representative Southern blot is reported in Figure 1 indicative of the methodology used. All lanes were subdivided into intervals of 1–2 mm and mean telomeric length (TL) was determined using the formula: mean TL = Σ (MWi × ODi)/Σ (ODi), where ODi is the densitometer output and MWi is the length of the DNA at position i (35). Sums were calculated over the range 12.2–1.5 kb. To minimise differences arising between gels, a DNA control sample was run on every gel. The distribution of TRFs was also determined in the same gels subdividing each lane into five equal intervals, extending in the following molecular size ranges: 12.2–8.6, 8.6–5, 5–3.5, 3.5–2 and 2–0.9 kb. The percentage of photo-stimulated luminescence (psl) in each molecular weight range was measured using the formula: % psl = intensity of a defined region − background × 100/total lane intensity − background (36).
Fig. 1.
A representative Southern blot showing the distribution of TRF length after hybridisation with the telomeric probe TTAGGG.
hTERT genotyping
DNA was extracted from blood samples using QIAamp-blood Kit (QIAGEN) and normalised working solutions (2.5 ng DNA/μl) were prepared. Allelic discrimination assay was performed by using primers (forward: 5′ CAATTCACAAACACAG-CCCTTTAA 3′; reverse: 5′ GGAGGTTAGCCTCGTCTTGTAAAT 3′) and MGB probes (T allele probe: 5′ FAM-TCTAGAAGAGCGACCTGT 3′; C allele probe: 5′ VIC-CTAGAAGAGCGACCCGT 3′) designed by Primer Express 2.0 software (Applied Biosystems, Foster City, CA, USA). Polymerase chain reaction (PCR) amplification was carried out in a reaction volume of 25 μl containing 900 nM of each primer, 200 nM of each probe, 12.5 μl of TaqMan universal Master Mix (Applied Biosystems) and 10 ng of genomic DNA. PCR was performed on an ABI Prism 7000 Sequence Detection System (Applied Biosystems), according to manufacturer’s instruction. The allelic-specific fluorescence was measured on the ABI prism 7000 Sequence Detection System using the ABI Prism 7000 SDS software. The genotype calls were attributed automatically.
CBMN cytome assay and irradiation
Blood specimens were collected by venipuncture in heparinised tubes (Becton Dickinson, UK) from all study subjects, and within the next 24 h were irradiated with 2 Gy γ-rays from a 137Cs source at a dose rate of 1 Gy/min to perform the mutagen sensitivity assay. For each subject, an aliquot of unirradiated blood served as control. Cultures were set up after 1-h incubation at 37°C, mixing 0.5 ml whole blood with 4.5 ml RPMI 1640 medium (Gibco, UK) supplemented with 20% foetal calf serum (Hyclone, USA), 2% phytohaemagglutinin (PHA; Abbott, USA), 1% penicillin and streptomycin. Lymphocytes were incubated in 5% CO2 atmosphere at 37°C. Forty-four hours after PHA stimulation, cytokinesis was blocked with 6 μg/ml of cytochalasin B (Sigma, Germany), and 22 h later lymphocytes were harvested and fixed as previously described (37); briefly, after a rapid incubation in 0.075 M KCl at room temperature, lymphocytes were gently fixed three times with methanol/acetic acid 5:1. Cells were dropped onto clean slides and stained with 5% Giemsa solution (Carlo Erba, Italy). Scoring criteria for selection of binucleated cells and NPBs were as previously described by Fenech (38). The frequencies of binucleated cells with NPBs (Figure 2) were determined analysing 1000 binucleated lymphocytes with a well-preserved cytoplasm and in which the main nuclei were clearly separated because it is difficult to determine the presence of an NPB when nuclei are touching. Two scorers contributed with 500 cells each from coded slides; the correlation coefficient (r = 0.960) of the two subset of data was statistically significant (P < 0.001).
Fig. 2.
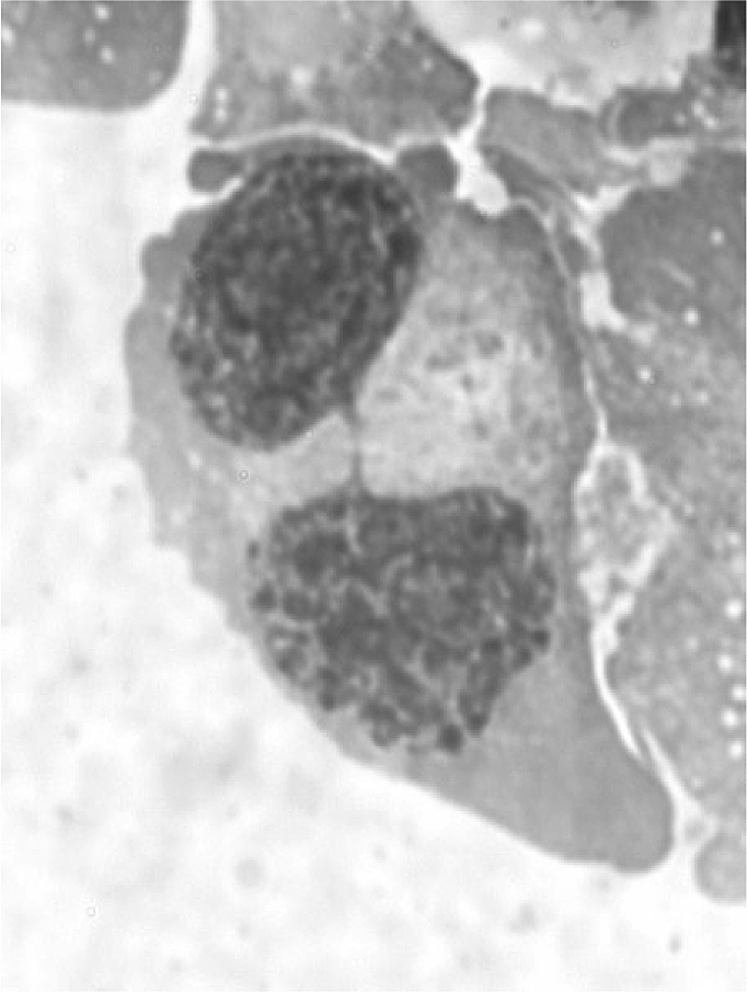
Binucleated cell with a NPB stained with Giemsa.
Statistical analysis
As a first description of the study population, the distribution of 56 subject participating to the study according to baseline characteristics as age, gender, smoking habits and hTERT genotype was obtained. Arithmetic mean and standard deviation were used as descriptive measures of central tendency and variability for TL, and the distributions according to baseline variables were compared by non-parametric tests. Arithmetic mean and standard deviation were also used to summarise food and micronutrient intake levels over the study population. The analyses were based on data reported by the dietary questionnaire administered at the beginning of the study. The influence of each dietary factors (foods and micronutrients) on TL (as outcome) was estimated by logistic multivariate analysis adjusted for age (as continuous variable), gender and energy intake, while multiple regression analysis was performed to evaluate the association of TL or radiation-induced NPBs with independent variables. The correlation between the biomarkers investigated was evaluated by Pearson correlation analysis. The accuracy of study results has been corroborated by evaluating the biological plausibility and internal consistency of data (comparison of estimates with different statistical models). The level of statistical significance was set at P < 0.05. The SPSS/PC statistical software package was used for the analyses.
Results
Subjects characteristics
The demographic characteristics and hTERT genotype of the study population are shown in Table I. As main descriptors of dietary habits, mean values of daily consumption of selected foods or food groups and of daily intake of micronutrients and energy intake, as obtained from the EPIC FFQ, were considered (Table II).
Table I.
Effect of demographic characteristics and hTERT genotype on mean TL in peripheral lymphocytes of study subjects
| Variable | Number of subjects | TL (kb)a (group mean ± SD) |
| All | 56 | 6.0 ± 1.0 |
| Age (years) | ||
| ≤56b | 23 | 6.5 ± 1.0 |
| >56 | 33 | 5.7 ± 0.9** |
| Gender | ||
| Males | 25 | 5.9 ± 1.0 |
| Females | 31 | 6.1 ± 1.0 |
| Smoking habits | ||
| Current | 9 | 6.4 ± 0.6 |
| Ex/never-smokers | 47 | 5.9 ± 1.1 |
| hTERT genotype | ||
| TT | 18 | 6.2 ± 1.2 |
| TC | 28 | 6.0 ± 0.7 |
| CC | 10 | 5.5 ± 1.4 |
TL, individual mean telomere length, as determined by TRF analysis (see Methods).
Mean population age.
**P value = 0.005 (non-parametric Mann–Whitney U test).
Table II.
Descriptors of dietary habits in the study subjects
| Daily intake | Mean ± SD | Range | |
| Energy | Kcal | 2465 ± 698 | 916–5343 |
| Food categories | |||
| Cereals | g/day | 255 ± 106 | 30–557 |
| Vegetables | g/day | 97 ± 60 | 23–319 |
| Fruits | g/day | 290 ± 139 | 19–675 |
| Eggs | g/day | 16 ± 13 | 2–109 |
| Milk | g/day | 163 ± 197 | 0–1120 |
| Yogurt | g/day | 33 ± 104 | 0–875 |
| Cheeses | g/day | 43 ± 25 | 1–153 |
| Olive oil | g/day | 25 ± 12 | 2–73 |
| Seed oil | g/day | 0.3 ± 0.5 | 0–3 |
| Butter | g/day | 2 ± 3 | 0–24 |
| Meat | g/day | 113 ± 56 | 12–333 |
| Fish | g/day | 26 ± 22 | 0–119 |
| Alcohol | g/day | 25 ± 27 | 0–143 |
| Micronutrients | |||
| Vitamin B1 | mg/day | 1 ± 0.3 | 0.3–2 |
| Vitamin B2 (riboflavin) | mg/day | 2 ± 0.6 | 0.5–4 |
| Vitamin B3 (niacin) | mg/day | 20 ± 6 | 6–41 |
| Vitamin B6 | mg/day | 2 ± 0.6 | 1–4 |
| Vitamin B9 (folic acid) | μg/day | 285 ± 83 | 117–634 |
| Vitamin A (retinol) | μg/day | 1162 ± 755 | 191–4494 |
| Vitamin C | mg/day | 122 ± 54 | 28–275 |
| Vitamin D | μg/day | 3 ± 1 | 0.5–7.0 |
| Vitamin E (tocopherol) | mg/day | 7 ± 2 | 2–15 |
| Beta-carotene | μg/day | 2696 ± 1811 | 485–11482 |
Effect of age, gender, smoking habits and hTERT genotype on TL
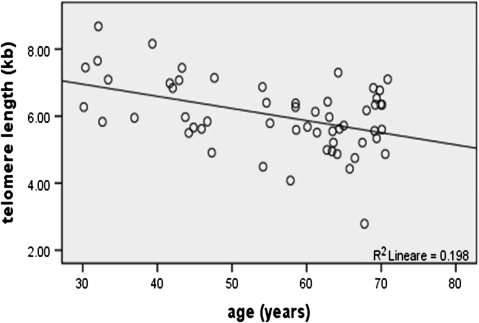
The influence of demographic characteristics on mean TL is displayed in Table I; by univariate analysis, it turned out that mean TL was significantly modulated by age, with shorter mean TL in aged individuals compared to younger subjects (5.7 ± 0.9 kb versus 6.6 ± 1 kb, respectively; P = 0.005). A plot of the distribution of mean TL values as function of age is shown in Figure 3.
Fig. 3.
Relationship between TL and age as determined by correlation analysis (Pearson coefficient = −0.444, P value = 0.001).
Shorter telomeres were also observed in homozygotes for the C allele (CC genotype) of the hTERT−1327T/C polymorphism compared with carriers of the T allele (TT and TC genotypes), but the difference did not reach the level of statistical significance (P = 0.22). No significant influence of gender or smoking habits on mean TL was observed either. However, it has to be noted that only a minority of study subjects were current smokers (9 of 56), thus no firm conclusion on the influence of smoking on TL can be drawn.
Effect of diet on TL
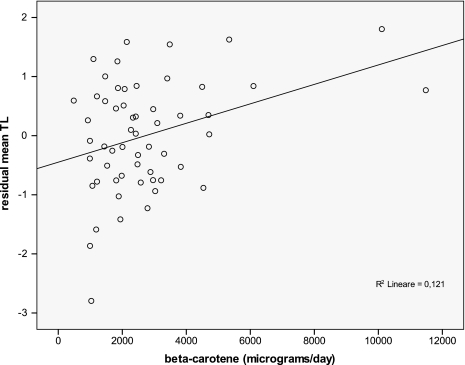
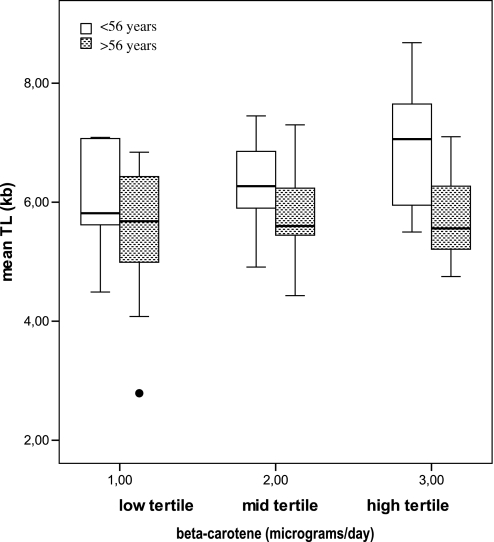
The relationship between TL and selected food groups was investigated by multivariate logistic analysis adjusted by age and energy intake (Table III); the results indicated that vegetables intake was significantly and positively associated with mean TL (P = 0.013, coefficient = 0.504), with root vegetables, pepper and carrots among vegetables the most significantly correlated with TL (Table IV). Besides, the analysis of correlation between mean TL and specific micronutrient intake (Table V) highlighted significant relationships with vitamins A, C, E, folic acid as well as beta-carotene. To further evaluate the strength of the association between mean TL and independent variables, taking into account of any covariate and interactive effects, a multiple regression analysis was performed. Multiple regression modelling of data pointed to beta-carotene as the micronutrient most significantly correlated with TL, being mean TL strongly and positively influenced by beta-carotene intake (P = 0.004, β = 0.178), beyond being inversely associated with age (β = −0.033, P = 0.001) and the hTERT1327CC genotype (β= −0.467, P = 0.006). These three variables explained ∼36% of interindividual variability in TL (R2 of the model = 0.361; Table VI). A plot showing the relationship between mean TL adjusted by age (residual mean TL) and beta-carotene intake is reported in Figure 4. Interestingly, the effect of beta-carotene on mean TL appeared to be modulated by age: in fact, when subjects were dichotomised above and below the mean group age (56 years) and multiple regression analyses repeated in each group, the protective effect of beta-carotene on telomere erosion was only observed among younger individuals (Figure 5).
Table III.
Influence of food intake on mean TL in peripheral lymphocytes of study subjects
| Unita | Coefficientsb | P values | 95% CI | ||
| Cereals | 100 g/day | −0.039 | 0.812 | −0.368 | 0.289 |
| Vegetables | 100 g/day | 0.504 | 0.013 | 0.112 | 0.896 |
| Fruits | 100 g/day | 0.153 | 0.123 | −0.043 | 0.348 |
| Eggs | 10 g/day | 0.241 | 0.140 | −0.082 | 0.563 |
| Dairy | 100 g/day | −0.096 | 0.092 | −0.209 | 0.016 |
| Oils and butter | 10 g/day | 0.163 | 0.117 | −0.042 | 0.368 |
| Meat | 100 g/day | −0.372 | 0.216 | −0.969 | 0.224 |
| Fish | 100 g/day | 0.309 | 0.602 | −0.871 | 1.489 |
Coefficients, P values from Wald test and 95% confidence intervals (95% CI) from multivariate logistic analysis adjusted by age, gender and energy.
Unit change of the exposure variable.
Coefficient associated to the nutrient variables in the models adjusted by age and energy intake. It represents changes in mean TL (kb) for a unit change of the nutrient variable (e.g. 100 g/day increase of cereals intake).
Table IV.
Multivariate logistic analysis for association of TL with vegetable intake
| Main micronutrients | β value | P values | 95% CI | ||
| Leafy vegetables | Folic acid | 0.922 | 0.087 | −0.137 | 1.982 |
| Fruiting vegetables | Vitamins C, E | 0.165 | 0.023 | 0.024 | 0.306 |
| Tomatoes | Beta-carotene | 0.433 | 0.082 | −0.057 | 0.923 |
| Root vegetables | Beta-carotene | 0.161 | 0.014 | 0.034 | 0.288 |
| Cabbages | Vitamin C, calcium | 0.229 | 0.259 | −0.174 | 0.632 |
| Peppers | Vitamin C | 0.173 | 0.022 | 0.026 | 0.320 |
| Carrots | Beta-carotene | 0.161 | 0.022 | 0.024 | 0.298 |
| Spinaches | Folic acid | 0.164 | 0.039 | 0.008 | 0.320 |
Adjusted by age, gender and energy. 95% CI, 95% confidence interval.
Table V.
Correlations between mean TL in peripheral blood lymphocytes and intake of selected nutrients in the study population
| Variables | ra (P value) |
| Age | −0.444 (0.001) |
| Smoke (cigarettes/day) | 0.413 (0.269) |
| Proteins (g/day) | 0.104 (0.444) |
| Lipids (g/day) | 0.238 (0.077) |
| Glucids (g/day) | 0.077 (0.574) |
| Alcohol (g/day) | −0.006 (0.962) |
| Caloric intake (kcal/day) | 0.148 (0.278) |
| Vitamin A (μg/day) | 0.321 (0.016) |
| Vitamin B1 (mg/day) | 0.195 (0.150) |
| Vitamin B2 (mg/day) | 0.132 (0.333) |
| Vitamin B3 (mg/day) | 0.116 (0.393) |
| Vitamin B6 (mg/day) | 0.127 (0.349) |
| Vitamin B9 (folic acid) (μg/day) | 0.312 (0.019) |
| Vitamin C (mg/day) | 0.320 (0.016) |
| Vitamin D (μg/day) | 0.208 (0.124) |
| Vitamin E (mg/day) | 0.326 (0.014) |
| Beta-carotene (μg/day) | 0.422 (0.001) |
Pearson correlation.
Table VI.
Modulation of mean TL by diet and individual characteristics of study subjects
| Included variables | Ba | SEb | P valuec |
| Costantd | 7.785 | 0.616 | <0.001 |
| Agee | −0.033 | 0.009 | 0.001 |
| Beta-carotenef | 0.178 | 0.059 | 0.004 |
| hTERT genotypeg | −0.467 | 0.163 | 0.006 |
Multiple regression analysis. Significance of the model: P < 0.001, F = 11.376, R2 = 0.361. Variables not included in the model: gender, smoke, food categories and micronutrients described in Table II.
Slope of the regression line.
Standard error of the regression line.
Significance of the variable.
Estimated intercept value.
Age of subjects in years (continuous variable).
Daily beta-carotene intake (continuous variable).
hTERT (TT, TC and CC).
Fig. 4.
Relationship between TL and daily beta-carotene intake; correlation analysis performed on residual mean TL values after removing the effect of age (Pearson correlation coefficient = 0.348, P value = 0.009).
Fig. 5.
Effect of beta-carotene daily intake on mean TL in younger (<56 years) and older (>56 years) individuals. Low tertile < 1841 μg/day; mid-tertile: 1841–2863 μg/day; high tertile > 2863 μg/day beta-carotene intake. Correlation of mean TL and beta-carotene daily intake in younger subjects (Pearson coefficient = 0.567, P value = 0.005) and older subjects (Pearson coefficient = 0.168, P value = 0.351).
Mean TL and NPBs
To assess if the diet-related variations in TL observed in the study population were related to differences in chromosome stability, the association between mean TL and the incidence of NPBs was investigated. In view of the low frequency of spontaneous NPBs generally found in human lymphocytes, which could hamper to detect mild differences among individuals, the occurrence of radiation-induced NPBs was also evaluated. In first instance, subjects were dichotomised below and above the mean group TL (6.01 kb) and data on NPB frequencies were compared by univariate analysis. No significant difference could be observed (Table VII).
Table VII.
Frequencies of spontaneous and radiation-induced NPBs in subjects dichotomised by mean group TL
| Number of subjects | Baseline NPBs‰ (mean ± SD) | Ranges (minimum–maximum) | γ-ray-induced NPBs‰ (mean ± SD) | Ranges (minimum–maximum) | |
| TL | |||||
| ≤6.01 kba | 30 | 0.24 ± 0.4 | 0–1 | 3.57 ± 4.2 | 0–21 |
| >6.01 kb | 26 | 0.15 ± 0.5 | 0–2 | 2.84 ± 2.7 | 0–11 |
Population mean.
Additionally, the frequency of NPBs was analysed keeping into account the distribution of TRFs in size classes of different molecular weight. As the analysis of TL by Southern blotting is an hybridisation-based technique in which the shorter telomeric repeats are associated with the lower hybridisation signal, it is likely that critically short telomeres be below the threshold of detection of the method; however, the examination of changes in the distribution of TRFs would help overcome this technical limit (39); in particular, the shortest TRFs are expected to represent the fraction of telomeres with lowest size, in the range of the natural heterogeneity of TLs (36,40,41).
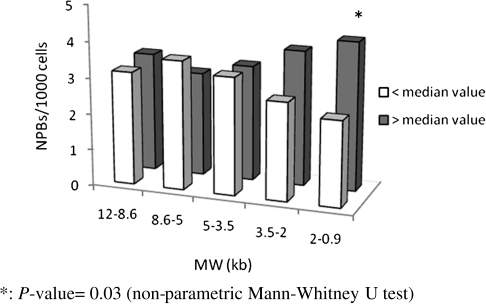
In this analysis, subjects were dichotomised based on the relative incidence (above and below the median) of TRFs in each of the five size classes, and data on spontaneous and radiation-induced NPBs were contrasted and evaluated by univariate analysis (Figure 6). No significant difference could be detected for spontaneous NPBs (data not shown), while a significant prevalence of radiation-induced NPBs (P = 0.03) was observed in subjects with an incidence of shortest TRFs (<2 kb) above the median. A multiple regression analysis was carried out to verify the strength of this observation while controlling for any bias due to interactive effects among related variables. The results confirmed a slight statistically significant association (P = 0.044) between the incidence of shortest TRFs and radiation-induced NPBs (Table VIII).
Fig. 6.
Comparison of the frequencies of radiation-induced NPBs in subjects with the percentage of TRFs above (grey) and below (white) median values in different molecular weight (MW) ranges. *P value = 0.03 (non-parametric Mann–Whitney U test).
Table VIII.
Modulation of radiation-induced NPBs by study variables
Multiple regression analysis. Significance of the model: P < 0.044, F = 4.264, R2 = 0.073. Independent variables not included in the model: age, sex, beta-carotene, %psl 12–8.6, %psl 8.6–5, %psl 5–3.5, %psl 3.5–2 kb.
Slope of the regression line.
Standard error of the regression line.
Significance of the variable.
Estimated intercept value.
%photo-stimulated fluorescence in the range of terminal restriction fragments from 2 to 0.9 kb.
Finally, a correlation analysis was performed to investigate the influence of selected dietary components on the shortest TRFs. Apart from age, none of the variables considered was significantly correlated with TRFs of the shortest size range (Table IX).
Table IX.
Coefficients of correlation between the percentage of TRFs in the range from 2 to 0.9 kb and the intake of selected nutrients in the study population
| Variables | ra (P value) |
| Age | 0.352 (0.008) |
| Smoke (cigarettes/day) | −0.012 (0.976) |
| Proteins (g/day) | −0.059 (0.666) |
| Lipids (g/day) | −0.102 (0.454) |
| Glucids (g/day) | 0.023 (0.869) |
| Alcohol (g/day) | 0.259 (0.054) |
| Caloric intake (kcal/day) | 0.017 (0.900) |
| Vitamin A (μg/day) | −0176 (0194) |
| Vitamin B1 (mg/day) | −0.002 (0.990) |
| Vitamin B2 (mg/day) | 0.054 (0.691) |
| Vitamin B3 (mg/day) | 0.000 (1.000) |
| Vitamin B6 (mg/day) | −0.003 (0.985) |
| Vitamin B9 (folic acid) (μg/day) | −0.164 (0.227) |
| Vitamin C (mg/day) | −0.151 (0.265) |
| Vitamin D (μg/day) | −0.150 (0.270) |
| Vitamin E (mg/day) | −0.119 (0.383) |
| Beta-carotene (μg/day) | −0.191 (0.159) |
Pearson correlation.
Discussion
In the present study, the influence of dietary habits on TL and the functional consequences of diet-related shortening of telomeres on chromosome stability were investigated. To this aim, TL was determined by TRF analysis in a well-characterised study population and data were compared with dietary habits as well as with the incidence of spontaneous and radiation-induced NPBs, a marker of chromosome end-fusions associated with chromosome instability. Both mean TRF length and the percentage distribution of TRFs in size classes were considered. Care was taken to control for potential confounding factors that might influence TL, viz. age, hTERT genotype and smoking status.
Previous investigations demonstrated that telomeres shorten by 20–200 b.p.s. per cell division (42,43), and indeed, leucocyte TL has been proposed as a biomarker of human ageing (16). The results of this study confirm the recognised association between ageing and telomere erosion since a significant age-related decrease of mean TRF length was observed in the population investigated.
Telomerase activity has a pivotal role in preventing accelerated telomere erosion and represents another factor that can modulate individual TL. Telomerase activity can be regulated by the expression of the catalytic reverse transcriptase subunit (hTERT), and functional polymorphisms in its promoter region have been identified (44). The hTERT−1327 T/C polymorphism, in particular, was shown to affect TL as an association between the C/C genotype and shorter telomeres in peripheral leucocytes has been reported (45). Also in this work, a tendency to lower mean TRF length in hTERT−1327 C/C homozygotes was observed, even though such association did not reach statistical significance, possibly because of the limited number of C/C homozygotes. For the same reason, i.e. the low number of relevant study subjects, no firm conclusion on the effect of smoking can be drawn. On the other hand, previous studies indicate that smoking only marginally affects TL, being detected as a significant modifier only in large population studies (10,20,46–48).
To evaluate the effect of diet on TL, firstly, detailed information on dietary habits was retrieved for all subjects from the EPIC questionnaire. The latter provided quantitative information on daily intake of main food categories and micronutrients, which was previously shown to be significantly correlated with blood micronutrient levels (49). A initial inspection of data showed that higher consumption of vegetables, mainly tomatoes, peppers, roots, was related with higher mean TRF length, while the analysis of the association between micronutrients and mean TRF length highlighted a significant role of antioxidant intake, especially beta-carotene, on telomere maintenance. These results add to the indications provided by recent studies on the influence of lifestyle-related factors on TL and support the view that diets rich in fruits and vegetables tend to be associated with longer telomeres (18–20).
A protective role of dietary antioxidants on telomere integrity is plausible, as telomeres are known to be very sensitive to oxidative stress (50), due to the positioning of three guanines next to one another (51); besides, numerous studies demonstrated that antioxidant treatments or overexpression of antioxidant enzymes prevent telomere attrition (52). The exact mechanism(s) of oxidation-induced telomere erosion has not yet been elucidated: accumulation of base damage on telomeric DNA has been proposed as a possible mechanism since the presence of damaged bases might interfere with the progress of replication fork at telomeres (50) or attenuate telomere binding by the shelterin components TRF1 and TRF2, thus altering the maintenance of telomeres (53,54). The incomplete repair of oxidised bases, due to the decreased DNA repair capacity at telomeres (35,55), may also result in a high density of single-strand breaks (55) which promote telomere erosion (56). In addition to the direct effect on telomere sequence, reactive oxygens species (ROS) have also been proposed to affect TL interacting with telomerase activity (57) and with several regulatory factors involved in cell signalling pathways (57,58).
In the present investigation, the protective effect of beta-carotene on TL was mainly observed in younger individuals. This suggests that the balance between oxidative damage and antioxidant defense capacity, a well-known determinant of TL (50), can be critically influenced by dietary supplements in young subjects, while in older individuals, the increased endogenous ROS generation and the physiological decrease of antioxidant defenses lead to a severe unbalance between pro- and antioxidant processes which cannot easily be restored by the supply of antioxidants with the diet. In addition, the intervention in the process of ageing of mechanisms different from the accumulation of oxidative damage could be considered (59).
The influence of diet components on TL has been pointed out also by recent studies in healthy subjects; however, the biological consequences of this effect at genome level have not yet been investigated. The decreased mean TFR length observed herein can provide an indication of telomere shortening, which may eventually result in chromosome instability. In principle, telomere shortening can lead to the loss of the end-capping function with subsequent telomere fusion, an event triggering breakage–fusion–bridge cycles, which have been implicated in tumorigenesis and cancer. Telomere fusions can be visualised in mitotic cells as sticky chromosomes, dicentrics, sister chromatid fusions, anaphase bridges (53), while in interphase cells, they may be detected as NPBs (60), structures extending between the two daughter nuclei at the end of mitosis. Actually the analysis of NPBs does not allow alone to discriminate between chromosome end-to-end fusion (resulting from critical telomere shortening or loss) and telomere fusion (in which telomere sequence remains at breakpoint), unless telomere-specific probes are used. Anyway both events indicate the loss of telomere function, and indeed, the joint analysis of TL and NPBs has recently been recommended as a convenient approach to assess the influence of telomere shortening on chromosome integrity (27).
Accordingly, in this study, the incidence of both spontaneous and radiation-induced NPBs was compared with mean TRF length to investigate the possible association between the two end points and evaluate whether the influence of diet on TL had adverse effects on chromosome stability. The results obtained suggest that the shortening of TL, consequent to poor intake of vegetables and antioxidants, does not lead to a significant impairment of chromosome stability as indicated by the lack of association between mean TRF length and spontaneous or radiation-induced NPBs. This experimental observation can be explained taking into account that shorter telomeres are not necessarily dysfunctional telomeres (61) and that a critical TL has to be reached before chromosome ends become fusogenic (62). Thus, it can be hypothesised that the mild degree of telomere erosion associated with low dietary intake of antioxidants observed herein is not so extensive to result in telomere dysfunction.
The absence of correlation between mean TRF length and NPBs can be also explained on the light of recent evidences showing that it is the shortest telomere and not the average TL, which affects chromosome stability (63). It could be hypothesised that a marker associated with the presence of short telomeres could be a more suitable measure than average TL to be compared with NPBs. In the present study to test this idea, the distribution of TRFs in various molecular size regions has been determined following the rationale that this end point may better describe the presence of short telomeres compared to mean TL. Indeed, the distribution of TRFs has previously been used to detect small changes in telomere size that can be masked when using the mean TRF conventional method. It has been shown, in fact, that the mean TRF length method could be unable to visualise the length of individual short telomeres in a distribution of TRFs, as the TRFs with few telomeric repeats could be obscured by the strong signal from other TRFs with long telomeres (36,39). Besides, it has been demonstrated the ability of telomere percentage profile to detect small changes in TRF distribution, while the analysis of the same Southern blots based on the mean TRF length did not show any difference (36,40,41). The results obtained in the present study highlighted a positive association between very low-molecular weight TRFs and increased radiation-induced NPBs. These finding, however, should be interpreted with caution because of the marginal significance of the association, which needs confirmation in independent studies. It is worth considering that even though this size range (i.e. 2–0.9 kb) may exceed the threshold reported for telomere dysfunction (63), because of TRFs content of a sizeable amount of subtelomeric DNA (64), this low-molecular weight range can also include critically short telomeres (65). For the time being, it can be observed that the frequency of small size TRFs was apparently unrelated to dietary habits such as vegetables and antioxidants intake, confirming that no functional consequences on chromosome integrity of diet-related variations in TL could be detected in this study population. This may indicate the involvement of mechanisms different from oxidative damage in their generation. In this respect, it can be noted that several alternative mechanisms, based on unequal recombination or replication slippage, have been implicated in sporadic telomere deletion or as responsible of significant changes in TL (66,67).
Overall, the results obtained in this study indicate that high dietary intake of vegetables and beta-carotene is positively associated with mean TL in blood lymphocytes, confirming the implication of oxidative damage in telomere erosion (17). This investigation also evaluated for the first time whether the diet-related variation in TL had any detectable effect on chromosome stability in a healthy population. The results obtained suggest that the degree of telomere erosion associated with low dietary intake of antioxidants may be not so extensive to result in telomere dysfunction and related chromosome instability. However, further investigation is needed to elucidate whether more severe antioxidant deficit, or malnourishment, can negatively affect telomere function beyond TL.
Funding
Italian National Institute of Health.
Acknowledgments
Conflict of interest statement: None declared.
References
- 1.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 2.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell. Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666. doi: 10.1016/s0092-8674(00)81908-x. [DOI] [PubMed] [Google Scholar]
- 5.Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:273–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 6.Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci. Am. 1996;274:92–97. doi: 10.1038/scientificamerican0296-92. [DOI] [PubMed] [Google Scholar]
- 7.Reddel RR, Bryan TM. Alternative lengthening of telomeres: dangerous road less travelled. Lancet. 2003;361:1840–1841. doi: 10.1016/S0140-6736(03)13538-6. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, Luo S, Hong WK, Spitz MR. Telomere dysfunction: a potential cancer predisposition factor. J. Natl. Cancer Inst. 2003;95:1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 11.Vasa-Nicotera M, Brouilette S, Mangino M, et al. Mapping of a major locus that determines telomere length in humans. Am. J. Hum. Genet. 2005;76:147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrew T, Aviv A, Falchi M, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am. J. Hum. Genet. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 14.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 15.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. U. S. A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr. Mol. Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 17.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic. Biol. Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. Am. J. Clin. Nutr. 2009;89:1857–1863. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bull CF, O'Callaghan NJ, Mayrhofer G, Fenech MF. Telomere length in lymphocytes of older South Australian men may be inversely associated with plasma homocysteine. Rejuvenation Res. 2009;12:341–349. doi: 10.1089/rej.2009.0868. [DOI] [PubMed] [Google Scholar]
- 20.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010;91:1273–1280. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl Acad. Sci. U. S. A. 2006;103:17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bull C, Fenech M. Genome-health nutrigenomics and nutrigenetics: nutritional requirements or 'nutriomes' for chromosomal stability and telomere maintenance at the individual level. Proc. Nutr. Soc. 2008;67:146–156. doi: 10.1017/S0029665108006988. [DOI] [PubMed] [Google Scholar]
- 23.Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc. Natl Acad. Sci. U. S. A. 1997;94:3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maret W, Krezel A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007;13:371–375. doi: 10.2119/2007-00036.Maret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalo S, Jaco I, Fraga MF, Chen T, Li E, Esteller M, Blasco MA. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat. Cell Biol. 2006;8:416–424. doi: 10.1038/ncb1386. [DOI] [PubMed] [Google Scholar]
- 26.Renaud S, Loukinov D, Abdullaev Z, Guilleret I, Bosman FT, Lobanenkov V, Benhattar J. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007;35:1245–1256. doi: 10.1093/nar/gkl1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenech MF. Dietary reference values of individual micronutrients and nutriomes for genome damage prevention: current status and a road map to the future. Am. J. Clin. Nutr. 2010;91:1438S–1454S. doi: 10.3945/ajcn.2010.28674D. [DOI] [PubMed] [Google Scholar]
- 28.Williams ES, Klingler R, Ponnaiya B, Hardt T, Schrock E, Lees-Miller SP, Meek K, Ullrich RL, Bailey SM. Telomere dysfunction and DNA-PKcs deficiency: characterization and consequence. Cancer Res. 2009;69:2100–2107. doi: 10.1158/0008-5472.CAN-08-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasaei H, Slijepcevic P. Defective Artemis causes mild telomere dysfunction. Genome Integr. 2010;1:3. doi: 10.1186/2041-9414-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcon F, Palli D, Zufferli A, Mazzoli E, Siniscalchi E, Sera F, Saieva C, Crebelli R. Evaluation of radiation-induced chromosome instability in subjects with a family history of gastric cancer. Biomarkers. 2009;14:226–234. doi: 10.1080/13547500902968538. [DOI] [PubMed] [Google Scholar]
- 31.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997;26(Suppl. 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 32.Palli D, Berrino F, Vineis P, et al. A molecular epidemiology project on diet and cancer: the EPIC-Italy Prospective Study. Design and baseline characteristics of participants. Tumori. 2003;89:586–593. doi: 10.1177/030089160308900602. [DOI] [PubMed] [Google Scholar]
- 33.Pala V, Sieri S, Palli D, et al. Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori. 2003;89:594–607. doi: 10.1177/030089160308900603. [DOI] [PubMed] [Google Scholar]
- 34.Salvini S. A food composition database for epidemiological studies in Italy. Cancer Lett. 1997;114:299–300. doi: 10.1016/s0304-3835(97)04686-7. [DOI] [PubMed] [Google Scholar]
- 35.Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc. Natl Acad. Sci. U. S. A. 1995;92:258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cherif H, Tarry JL, Ozanne SE, Hales CN. Ageing and telomeres: a study into organ-and gender-specific telomere shortening. Nucleic Acids Res. 2003;31:1576–1583. doi: 10.1093/nar/gkg208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carere A, Antoccia A, Crebelli R, et al. Genetic effects of petroleum fuels: cytogenetic monitoring of gasoline station attendants. Mutat. Res. 1995;332:17–26. doi: 10.1016/0027-5107(95)00081-9. [DOI] [PubMed] [Google Scholar]
- 38.Fenech M. The in vitro micronucleus technique. Mutat. Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 39.Baird DM. New developments in telomere length analysis. Exp. Gerontol. 2005;40:363–368. doi: 10.1016/j.exger.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, Ozanne SE. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009;23:1521–1528. doi: 10.1096/fj.08-122796. [DOI] [PubMed] [Google Scholar]
- 41.Guan JZ, Maeda T, Sugano M, Oyama J, Higuchi Y, Makino N. Change in the telomere length distribution with age in the Japanese population. Mol. Cell Biochem. 2007;304:353–360. doi: 10.1007/s11010-007-9518-2. [DOI] [PubMed] [Google Scholar]
- 42.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 43.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat. Rev. Mol. Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 44.Hsu CP, Hsu NY, Lee LW, Ko JL. Ets2 binding site single nucleotide polymorphism at the hTERT gene promoter–effect on telomerase expression and telomere length maintenance in non-small cell lung cancer. Eur. J. Cancer. 2006;42:1466–1474. doi: 10.1016/j.ejca.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Matsubara Y, Murata M, Yoshida T, Watanabe K, Saito I, Miyaki K, Omae K, Ikeda Y. Telomere length of normal leukocytes is affected by a functional polymorphism of hTERT. Biochem. Biophys. Res. Commun. 2006;341:128–131. doi: 10.1016/j.bbrc.2005.12.163. [DOI] [PubMed] [Google Scholar]
- 46.Jang JS, Choi YY, Lee WK, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99:1385–1389. doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol. Biomarkers Prev. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 48.Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, Crawford ED, De Vivo I, Hayes RB, Savage SA. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8:405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ocké MC, Bueno-de-Mesquita HB, Pols MA, Smit HA, van Staveren WA, Kromhout D. The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int. J. Epidemiol. 1997;26(Suppl. 1):S49–S58. doi: 10.1093/ije/26.suppl_1.s49. [DOI] [PubMed] [Google Scholar]
- 50.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 51.Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 52.Serra V, von Zglinicki T, Lorenz M, Saretzki G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J. Biol. Chem. 2003;278:6824–6830. doi: 10.1074/jbc.M207939200. [DOI] [PubMed] [Google Scholar]
- 53.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 54.Opresko PL, Fan J, Danzy S, Wilson DM, 3rd, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petersen S, Saretzki G, von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp. Cell Res. 1998;239:152160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 56.Stewart SA, Ben-Porath I, Carey VJ, O'Connor BF, Hahn WC, Weinberg RA. Erosion of the telomeric single-strand overhang at replicative senescence. Nat. Genet. 2003;33:492–496. doi: 10.1038/ng1127. [DOI] [PubMed] [Google Scholar]
- 57.Makpol S, Zainuddin A, Rahim NA, Yusof YA, Ngah WZ. Alphatocopherol modulates hydrogen peroxide-induced DNA damage and telomere shortening of human skin fibroblasts derived from differently aged individuals. Planta Med. 2010;76:869–875. doi: 10.1055/s-0029-1240812. [DOI] [PubMed] [Google Scholar]
- 58.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Caballero A, Ugidos A, Liu B, et al. Absence of mitochondrial translation control proteins extends life span by activating sirtuin-dependent silencing. Mol. Cell. 2011;42:390–400. doi: 10.1016/j.molcel.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 60.Fenech M, Kirsch-Volders M, Natarajan AT, et al. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26:125–132. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- 61.Saltman D, Morgan R, Cleary ML, de Lange T. Telomeric structure in cells with chromosome end associations. Chromosoma. 1993;102:121–128. doi: 10.1007/BF00356029. [DOI] [PubMed] [Google Scholar]
- 62.Capper R, Britt-Compton B, Tankimanova M, Rowson J, Letsolo B, Man S, Haughton M, Baird DM. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 64.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimura M, Barbieri M, Gardner JP, Skurnick J, Cao X, van Riel N, Rizzo MR, Paoliso G, Aviv A. Leukocytes of exceptionally old persons display ultra-short telomeres. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R2210–R2217. doi: 10.1152/ajpregu.00615.2007. [DOI] [PubMed] [Google Scholar]
- 66.Baird DM. Mechanisms of telomeric instability. Cytogenet. Genome Res. 2008;122:308–314. doi: 10.1159/000167817. [DOI] [PubMed] [Google Scholar]
- 67.Letsolo BT, Rowson J, Baird DM. Fusion of short telomeres in human cells is characterized by extensive deletion and microhomology, and can result in complex rearrangements. Nucleic Acids Res. 2010;38:1841–1852. doi: 10.1093/nar/gkp1183. [DOI] [PMC free article] [PubMed] [Google Scholar]