Abstract
Knowing when and where to express fear is essential to survival. Recent work in fear extinction paradigms reveals that the contextual regulation of fear involves a neural network involving the hippocampus, medial prefrontal cortex, and amygdala. The amygdaloid basal nuclei (BA) receive convergent input from the ventral hippocampus (VH) and prelimbic (PL) prefrontal cortex and may integrate VH and PL input to regulate fear expression. To examine the functional organization of this neural circuit, we used cellular imaging of c-fos expression in anatomically defined neuronal populations and circuit disconnections to identify the pathways involved in the contextual control of extinguished fear. Before behavioral testing, we infused a retrograde tracer into the amygdala to label BA-projecting neurons in VH and PL. Rats then underwent fear conditioning and extinction and were tested for their fear to the extinguished conditioned stimulus (CS) in either the extinction context or in another context; freezing behavior served as the index of conditional fear. CS presentation outside the extinction context renewed conditional freezing and was associated with significantly more c-fos expression in BA-projecting neurons in the VH and PL than that induced by CS presentation in the extinction context. We next examined whether direct or indirect projections of VH to BA mediate fear renewal. Interestingly, disconnections of the VH from either the BA or PL eliminated renewal. These findings suggest that convergent inputs from both the VH and PL in the BA mediate the contextual control of fear after extinction.
Introduction
In recent years, considerable interest has emerged in the extinction of learned fear insofar as it is central to several clinical interventions, including exposure therapy. During extinction, a previously conditioned stimulus (CS) is repeatedly presented without the unconditioned stimulus (US). This results in a gradual decrease in learned fear responses, such as freezing behavior (Maren, 2001). However, extinction does not erase the original fear memory; rather, it yields a new inhibitory memory that reduces fear to the CS (Quirk and Mueller, 2008). Which memory is retrieved depends on the retrieval context; fear to an extinguished CS is suppressed in the extinction context but “renews” when it is presented outside the extinction context (Bouton and Bolles, 1979). The renewal of extinguished fear presents obvious challenges for the efficacy of behavioral interventions for fear and anxiety disorders.
Recently, substantial progress has been made in understanding the neural mechanisms for the context dependence of extinction (Maren, 2005, 2011). This work has revealed that the hippocampus, a structure critical for context processing (Fanselow, 2000), plays an important role in the contextual modulation of fear after extinction. For example, pharmacological inactivation of either the dorsal hippocampus (Corcoran and Maren, 2001) or ventral hippocampus (VH; Hobin et al., 2006) in rats eliminates the renewal of fear to an extinguished CS outside the extinction context. Interestingly, hippocampal inactivation also eliminates the contextual modulation of CS-evoked spike firing in the amygdala after extinction (Hobin et al., 2003; Maren and Hobin, 2007), suggesting that hippocampo–amygdala projections (Canteras and Swanson, 1992; Pitkanen et al., 2000) mediate the context dependence of extinction. Because the VH is the primary source of contextual information to the amygdala (Pitkanen et al., 2000), it is conceivable that this direct projection is necessary for the renewal of fear. Indeed, neurons within the basal amygdala (BA) that are active during renewal receive direct projections from the VH (Herry et al., 2008).
Another route by which the VH can influence BA activity is via the prelimbic cortex (PL). The VH has robust projections to the PL (Vertes, 2006), which in turn has reciprocal connections with the BA (Mcdonald et al., 1996; Vertes, 2004). Prelimbic lesions impair fear expression (Blum et al., 2006; Corcoran and Quirk, 2007), and microstimulation of the PL results in both freezing behavior (Vidal-Gonzalez et al., 2006) and increases in BA firing (Likhtik et al., 2005). Additionally, PL activity during fear conditioning correlates with the expression of freezing (Burgos-Robles et al., 2009). Last, we have found increased c-fos expression in the PL after the renewal of fear (Knapska and Maren, 2009). Hence, it is possible that the hippocampus contributes to the context dependence of extinction through either direct or indirect projections to the BA.
To explore this question, we used functional retrograde tracing to determine whether BA-projecting neurons in the VH and PL are differentially activated (as indexed by c-fos expression) during renewal of fear after extinction. Asymmetric lesions were then used to disconnect the VH from either the BA or PL to determine the necessity of each pathway in renewal (Olton et al., 1982). Collectively, our results indicate that convergent input from the PL and VH within the BA is required for the contextual modulation of fear after extinction.
Materials and Methods
Subjects.
Subjects were male Long–Evans rats (220–224 g; Blue Spruce), obtained from a commercial supplier (Harlan). Animals were individually housed in clear plastic hanging cages and were kept on a 14/10 h light/dark cycle and had access to food and water ad libitum. Rats were handled 15–20 s/d for 5 d before the start of the experiment so as to acclimate the animals to the experimenter. All experimental procedures were performed in accordance with the protocols approved by the University of Michigan Committee on the Use and Care of Animals.
Behavioral apparatus.
All behavioral sessions were performed in eight identical observation chambers (30 × 24 × 21 cm; MED Associates), each located in an individual sound-attenuating cabinet. The observation chambers were constructed of two aluminum sidewalls and a Plexiglas ceiling, back, and door. The floor of each chamber consisted of 19 stainless steel rods (4 mm in diameter) used for delivery of the footshock US. The rods were wired to a shock source and a solid-state shock scrambler (MED Associates). To deliver the acoustic CS, a speaker was mounted on one wall of each chamber. Additionally, ventilation fans and house lights were installed in each chamber to allow for the manipulation of contexts during training, extinction, exposure, and testing. We used a three-context (“ABC”) renewal procedure that allows both fear and c-fos expression evoked by an extinguished CS to be assessed independent of background fear to the context (Corcoran and Maren, 2001; see procedure below). For context A (conditioning context), house lights and room lights were on, ventilation fans (65 dB) were turned on, cabinet doors were left open, and the chambers were cleaned with 1% acetic acid. For context B (extinction and test context), house lights and ventilation fans were turned off, the cabinet doors were closed, and the chambers were cleaned with 1% ammonium hydroxide. Additionally, the room was illuminated by fluorescent red lights. For context C (test context), house lights were on, ventilation fans were off, the room was illuminated with fluorescent red light, and cabinet doors were left open. Black Plexiglas floors were placed on the grid of each chamber, and chambers were cleaned with 10% ethanol. In each context, stainless steel pans were filled with a thin layer of the respective odor of the context and inserted below the grid floor.
During the behavioral sessions, motor activity was measured by recording the displacement of each chamber by a load cell platform located below each chamber. Before the experiment, all load cell amplifiers were calibrated to a fixed chamber displacement, and the output of each amplifier was set to a gain that optimally detected freezing behavior (vernier dial = 8; somatomotor immobility, except that required for breathing). Load-cell amplifier output (−10 to +10 V) was then digitized (5 Hz) and acquired online with Threshold Activity software (MED Associates). Absolute values of load-cell voltages were multiplied by 10, which resulted in an activity score that ranged from 0 to 100. A bout of freezing was scored if at least five contiguous load-cell values fell below the freezing threshold. In other words, activity had to be below threshold for at least 1 s to be scored as freezing behavior. This method of assessing freezing behavior correlates highly with time sampling of behavior by trained observers (Maren, 1998).
Behavioral procedures.
For the immunohistochemistry experiment, 36 rats were randomly assigned to groups that were to be tested either within the extinction context (SAME) or outside the extinction context (DIFF) and those that were left in the home cages during the test session (HOME). We used a three-context (ABC) renewal procedure in which rats were conditioned in context A, extinguished in context B or C, and then tested in context C (extinction and test contexts were counterbalanced). This yielded groups tested in the extinction context (SAME, ACC) or in another, familiar context that had not hosted extinction (DIFF, ABC). This renewal design is critical because it allows the assessment of fear and c-fos expression to an extinguished CS independent of fear to the context in which the CS is tested (i.e., rats are never tested in the conditioning context as they are in a typical ABA design). Moreover, all rats were tested identically and in the same physical contexts so that any differences in behavior and c-fos expression could be attributed to the meaning of the CS in that context and not the CS or context itself.
One week before all behavioral sessions, rats received unilateral intracranial injections of cholera toxin subunit b (CTb; #104; List Biological) into the BA. The side of the injection was counterbalanced across all behavioral groups. After allowing for 1 week of recovery, rats underwent fear conditioning in context A. Conditioning consisted of five tone (CS; 10 s, 85 dB, 2 kHz)–footshock (US; 1.0 mA, 2.0 s) pairings with 60 s interstimulus intervals (ISIs). The chamber position of each animal was counterbalanced across each experimental group and training squad. Twenty-four hours later, animals underwent extinction in context B or C (counterbalanced across groups) in which they received 45 tone-alone (10 s) presentations with 30 s ISIs. Before this session, the rats were exposed to the alternate context (i.e., they were exposed to context B if they were extinguished in context C) to ensure that the test contexts were equally familiar for all of the rats. Twenty-four hours after extinction, the rats were returned to the observation chambers (context C) for a test session consisting of five tone-alone (10 s) presentations with 30 s ISIs. Rats were killed 90 min after the first tone presentation to assess c-fos expression induced by the test session. In all behavioral sessions, freezing was used as an index of fear.
In the disconnection experiment, 125 rats were randomly assigned to groups that would receive contralateral (C) lesions, ipsilateral (I) lesions, or sham (SH) surgeries in the VH–BA or VH–PL circuits after extinction training. Additionally, these groups were further divided into rats that would be tested outside the extinction context (DIFF) or within the extinction context (SAME). Rats were trained and extinguished, as described previously. Twenty-four to 96 h after extinction, the rats underwent surgery. After 1 week of recovery, the rats were placed back into either context B or context C for the test session [45 tone alone (10 s) presentations with a 30 s ISI]. In this experiment, the extinction and test contexts were counterbalanced across all groups. Renewal was assessed by measuring freezing during the first five trials of the test session in the DIFF context relative to that in the SAME context.
To assess whether the disconnections produced nonspecific impairments in freezing behavior, we ran an additional experiment with another cohort of 59 rats. The rats were conditioned, as described above, but they did not receive extinction training. Rather, they were merely exposed to either context B or context C (no CS presentations) for the same duration as the extinction session in the foregoing experiment. Twenty-four hours later, the rats received either contralateral or ipsilateral lesions placed in the VH and BA or VH and PL or sham surgeries. After 1 week of postoperative recovery, the rats were tested for their retention of fear to the non-extinguished CS in either context B or C. As before, the extinction and test contexts were counterbalanced across lesion groups.
Surgical procedures.
Rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and given atropine sulfate (0.4 mg/kg, i.p.). After being placed into the stereotaxic apparatus (David Kopf Instruments), the scalp was incised and retracted. The head was leveled to ensure that lambda and bregma were in the same horizontal plane. In the tracing/c-fos experiment, one small burr hole was drilled to allow for a 30-gauge injector (Small Parts) to be lowered into the BA [anteroposterior (AP), −3.1 mm; mediolateral (ML), ±4.6–5.1 mm; dorsoventral (DV), −8.1 mm from dura]. The injector was attached to polyethylene tubing, which was connected to Hamilton syringes (10 μl) located on an infusion pump. CTb (#104; List Biological) was unilaterally infused at a rate of 0.1 μl/min for 1 min (0.1 μl total volume; 10 mg/ml). The injector remained in the BA for another 5 min to allow for the diffusion of CTb. The hemisphere of CTb injection was counterbalanced across all behavioral groups (DIFF, SAME, and HOME).
To disconnect the VH and BA or VH and PL, unilateral electrolytic lesions of these structures were placed in opposite hemispheres; ipsilateral lesions served as a control (Fig. 1). We chose to use electrolytic lesions because we were able to produce much more focal damage to the BA and PL than we obtained with excitotoxic lesions. For the VH–BA disconnections, insulated insect pins (size 00; except for 1 mm at the tip; Fine Science Tools) were placed in the VH (AP, −6.7, −6.3, −5.8 mm; ML, +/−5.6, 5.4, 5.2 mm; DV, −5.6, −5.8, −6 mm from dura) and the BA (AP, −2.85, −3.6 mm; ML, +/−5 mm; DV, −9, −9.1 mm from skull). For the VH–PL disconnections, the electrodes were placed in the PL (AP, +3.6, +2.7 mm; ML, ±0.5 mm; DV, −3.7 mm from skull) and VH (AP, −6.7, −6.3, −5.8 mm; ML, ±5.6, 5.4, 5.2 mm; DV, −5.6, −5.8, −6 mm from dura). Lesions were produced by passing anodal current (0.5 mA, 8 s) across the insect pins. Lesion placement in the left or right hemispheres was counterbalanced. The incision was closed with stainless steel wound clips and treated with antibiotic ointment, and the animals were administered an analgesic (carprofen, 5 mg/kg, i.p.). The rats recovered on a heating pad before returning to their home cages and were allowed 1 week for postoperative recovery.
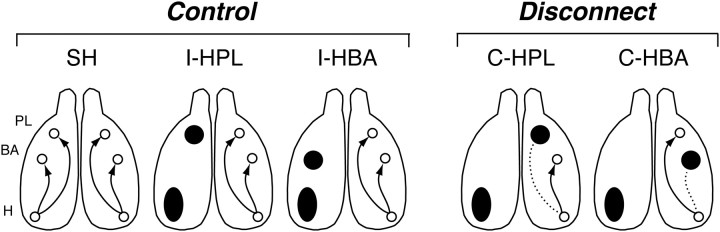
Figure 1.
A schematic of the projections between the hippocampus (H), BA, and PL in control rats and rats in which the projections were disconnected. In intact brains (SH), there are ipsilateral projections (arrows) from the VH (H) to both the BA and PL. Control rats with ipsilateral lesions of either the VH and PL (I-HPL) or the VH and BA (I-HBA) maintain connectivity between the hippocampus, amygdala, and PL in the intact hemisphere. However, contralateral lesions of the VH and PL (C-HPL) or VH and BA (C-HBA) disconnect the hippocampus (dashed lines) from the PL and BA, respectively.
Immunohistochemistry.
Ninety minutes after the first tone presentation, rats were overdosed with pentobarbital and transcardially perfused with ice-cold 0.1 m PBS, pH 7.4, and 4% paraformaldehyde (PFA), pH 7.4. Brains were extracted and placed into 4% PFA for 1 h and then transferred into a 30% sucrose/0.1 m PBS solution. Brains were mounted and cut (30 μm) on a cryostat maintained at −20°C. Coronal sections were collected every 150 μm and placed in 0.1 m PBS with 0.2% sodium azide. Additional sections were mounted on subbed slides with 70% ethanol for Nissl staining.
Immunohistochemistry was performed on free-floating sections. Sections were first washed two times in 0.1 m PBS for 10 min, followed by a third wash in 0.1 m PBS with 0.3% Triton X-100 for 10 min. Tissue was then incubated at room temperature in 10% normal donkey serum (NDS) and 0.3% Triton X-100 in 0.1 m PBS for 30 min. Sections were then immediately transferred into the primary antibody solution for a 48 h incubation period [goat anti-c-fos at 1:1000 (sc-52-G; Santa Cruz Biotechnology), rabbit anti-CTb at 1:3000 (C3062; Sigma), in 0.1 m PBS with 10% NDS and 0.3% Triton X-100] at 4°C. Forty-eight hours later, tissue was washed three times in 0.3% Triton X-100 in 0.1 m PBS for 10 min and then incubated in 10% NDS for 30 min. Sections were then incubated in the secondary antibodies [biotinylated donkey anti-goat at 1:200 (sc-2042; Santa Cruz Biotechnology), donkey anti-rabbit conjugated to Alexa Fluor 488 at 1:500 (A-21206; Invitrogen), in 0.3% Triton X-100 and 10% NDS in 0.1 m PBS] for 2 h at room temperature. After being rinsed in 0.1 m PBS with 0.3% Triton X-100, tissue was incubated in streptavidin conjugated to Alexa Fluor 594 (1:200 in 0.3% Triton X-100 and 10% NDS in 0.1 m PBS; S11223; Invitrogen) for 1 h at room temperature. To aid in the fluorescent detection, Signal Enhancer (three drops per 8 ml of solution; Invitrogen) was added to both the secondary antibody and streptavidin solution. After a final wash in 0.1 m PBS, tissue was mounted onto subbed slides in 0.9% saline and coverslipped with Vectashield (H-1200; Vector Laboratories).
Image analysis.
We quantified the number of CTb- and c-fos-positive nuclei in the PL, infralimbic cortex (IL), perirhinal cortex (PRh), auditory cortex (AC), and VH (consisting of the ventral subiculum and ventral CA1 subfield). Multiple images were captured for each region, consistent with other published immunohistochemical reports (Herry and Mons, 2004; Berretta et al., 2005; Kim et al., 2009). Specifically, for the prelimbic region, four images were captured (two sets bilaterally at +3.7 and +2.7 mm anterior to bregma). For the infralimbic region, four images were captured (bilaterally at +3.2 and +2.7 mm anterior to bregma). Three images for the PRh (−3.0, −3.6, and −4.0 mm posterior to bregma), AC (−3.6, −4.0, and −4.6 mm posterior to bregma), and VH (−5.6, −6.3, and −6.8 mm posterior to bregma) were taken on the side of the CTb injection. To verify the borders of cortical areas and the hippocampal layers, adjacent thionin-stained sections were used. All images were taken at 20× magnification (443 × 331 μm; 0.15 mm2) with a Leica DM6000 B microscope outfitted with filters for different excitation/emission wavelengths (Hamlin et al., 2009; Knapska and Maren, 2009). For each region, the number of c-fos-positive, CTb-positive and double-labeled neurons were counted using an image analysis software program (NIH ImageJ). Importantly, double-labeled neurons were readily observable because the resulting stain was visible in a center-surround manner for c-fos and CTb, respectively (Leman et al., 2000; Marchant et al., 2009). Counts for each image of the brain region were averaged across the number of images taken, and group differences in absolute cell counts were analyzed with an ANOVA and Fisher's protected least significant difference (PLSD) post hoc tests. Results are represented as means ± SEM.
Histology.
After behavioral testing, rats in the disconnection studies were overdosed with pentobarbital and perfused across the heart with 0.9% saline followed by 10% Formalin. Brains were extracted and postfixed in 10% Formalin for 2 d, at which time brains were transferred into a solution of 30% sucrose in 10% Formalin. Brains were sectioned (45 μm) on a cryostat maintained at −20°C. Tissue was wet mounted on subbed slides with 70% ethanol and subsequently stained with 0.25% thionin to verify lesion placement and extent.
Behavioral data analysis.
Freezing behavior was measured continuously during all of the behavioral sessions, including the pre-CS “baseline” period as well as during tone presentations and ISI. Freezing was then analyzed and reported for each trial, which consisted of a CS presentation and the subsequent ISI. For each period, the percentage of total observations in which freezing occurred was calculated. These values were submitted to ANOVA and Fisher's PLSD post hoc tests were performed after significant omnibus F ratios were obtained. All data are represented as means ± SEM.
Results
c-fos expression in PL and VH projections to the BA after the renewal of fear
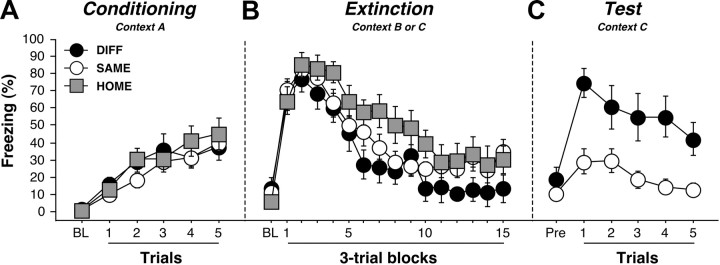
Freezing behavior during conditioning (context A), extinction (contexts B or C), and retrieval testing (context C) is displayed in Figure 2. Freezing behavior during conditioning and extinction was typical and did not differ between the groups [F values <1]. As expected, conditional freezing differed markedly between the groups during the retrieval test. Rats tested outside the extinction context (DIFF) exhibited renewal of conditional freezing to the extinguished CS, whereas those tested within the extinction context (SAME) displayed low levels of fear. This impression was confirmed in an ANOVA performed on the CS-elicited freezing across the test trials (main effect of group, F(1,23) = 24.3, p < 0.01). Importantly, renewal of fear was not attributable to contextual fear because the levels of pre-CS freezing in the two contexts were not statistically different (DIFF, 19.3 ± 6.4%; SAME, 10.9 ± 2.9%; F(1,23) = 1.9, p = 0.2). Moreover, differential freezing among the SAME and DIFF groups was not attributable to physical differences in the test contexts; all testing was conducted in identical contexts with the same CS.
Figure 2.
Conditioned freezing behavior in rats previously infused with CTb. A, Mean ± SEM percentage of freezing during fear conditioning, which consisted of a 3 min baseline period followed by five tone–shock pairings. Freezing was averaged across the pre-CS baseline (BL) as well as during each of the five conditioning trials; each trial consisted of the average of freezing during each CS presentation and the subsequent ISI. B, Mean ± SEM percentage of freezing during the 45 tone-alone extinction session. Freezing was averaged across the BL period as well as during the 45 extinction trials; as with conditioning, each trial consisted of the average of freezing during each CS presentation and subsequent ISI (data were binned into 15 blocks of three-trial averages). C, Mean ± SEM percentage of freezing during the test session that consisted of five tone-alone presentations with 30 s ISIs. Freezing was measured during the BL period and during the five trials, each of which consisted of a CS presentation and the subsequent ISI. Data are shown for rats that were tested outside the extinction context (DIFF; black circles), tested within the extinction context (SAME; white circles), or not tested at all (HOME; gray squares).
After retrieval testing, rats were killed to assess c-fos expression in BA afferents. A representative CTb injection site in the BA is shown in Figure 3, A and B, along with a schematic illustration of maximal and minimal infusions (Fig. 3C). Only rats for which CTb labeling was confined to the BA were included in the analysis. Four rats were excluded because their CTb injections were not confined to BA. Another 12 rats were excluded because the CTb infusions were too small, resulting in weak staining. The final group sizes were DIFF (n = 9), SAME (n = 16), and HOME (n = 11).
Figure 3.
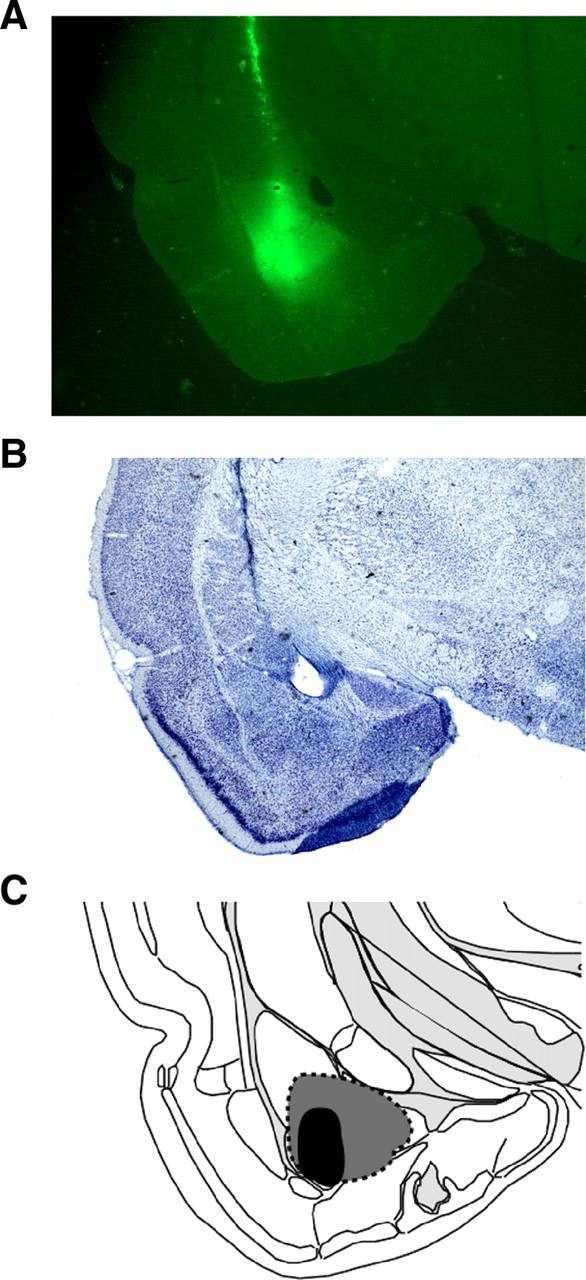
Illustrations of the CTb injection site and CTb spread within the BA. A, A representative CTb-stained coronal section displaying the site of the CTb injection. B, Adjacent thionin sections were used to ensure that CTb spread did not extend beyond the boundaries of the BA. C, Schematic of CTb spread; gray indicates rats with maximal CTb spread, and black represents rats with the smallest injections of CTb. Images were adapted from Swanson (2004).
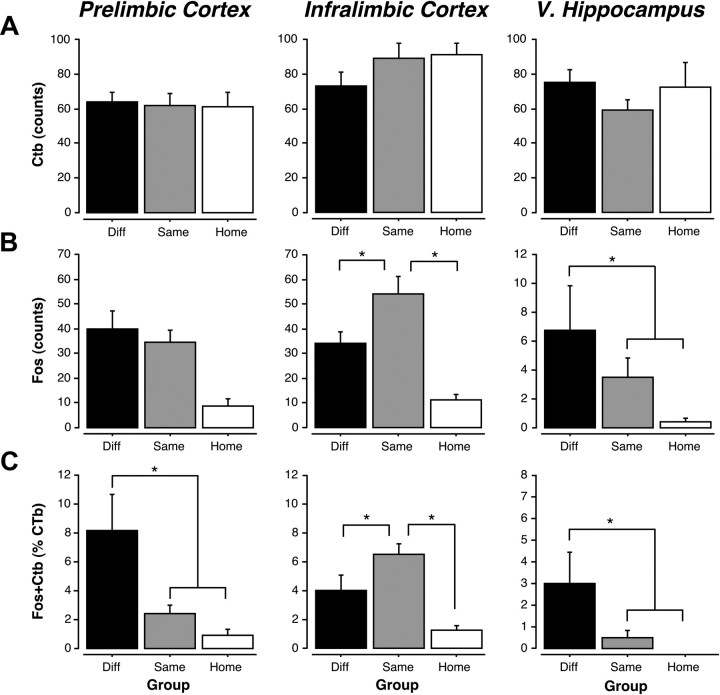
Retrieval testing induced c-fos expression in the PL, IL, and VH, and many c-fos-positive nuclei were colocalized with CTb-positive cells (Fig. 4). The absolute counts of CTb- and c-fos-positive nuclei as well as the percentage of double-labeled [(double-labeled cells/total CTb cells) × 100] neurons for the PL, IL, and VH are shown in Figure 5A–C. Two-way ANOVAs with a within-subjects factor of brain region and between-subjects factor of group were performed for each dependent variable. The extent of CTb labeling in the groups did not differ in any of the brain regions (Fig. 5A; p values >0.05), although there was a main effect of brain region (F(4,132) = 18.6, p < 0.001). Post hoc comparisons revealed that there were significantly more CTb-positive neurons in the IL than the PL (p < 0.001) and the VH (p < 0.01). Additionally, the AC had significantly less CTb-positive neurons than all other brain regions (p < 0.001), whereas the PRh had significantly more CTb-positive neurons than all other brain regions (p < 0.01), with the exception of the IL. This pattern of retrograde labeling is generally consistent with the distribution and density of projections from these regions to the BA (Romanski and LeDoux, 1993; Pitkanen et al., 2000; Vertes, 2004).
Figure 4.
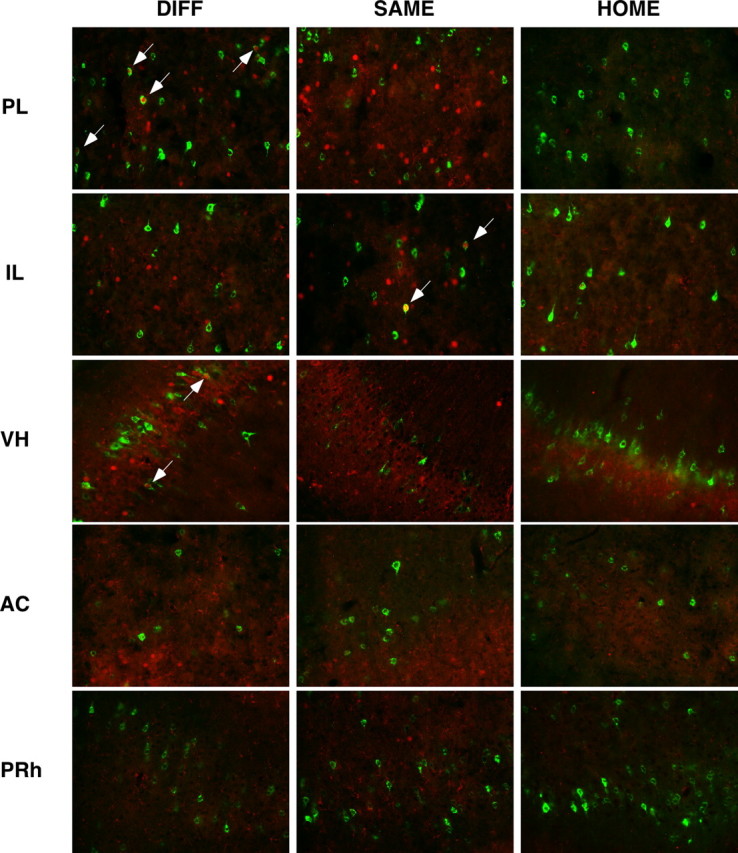
Photomicrographs of representative double-labeled neurons in the PL, IL, VH, AC, and PRh for each behavioral group (DIFF, SAME, HOME). White arrows indicate double-labeled neurons; CTb-positive neurons are stained green, and c-fos-positive neurons are stained red.
Figure 5.
Quantification of CTb-positive, c-fos positive, and double-labeled neurons in the PL, IL, and VH. A, Mean ± SEM cell counts for CTb-positive neurons in the PL, IL, and VH. B, Mean ± SEM cell counts for c-fos-positive neurons in the PL, IL, and VH. C, Mean ± SEM percentage of double-labeled neurons (counts of CTB + c-fos-positive neurons divided by counts of CTB alone) in the PL, IL, and VH.
As we have previously reported, either the renewal or suppression of fear was associated with different patterns of c-fos expression in the PL, IL, and VH (Fig. 5B). This was confirmed by significant main effects of group (F(2,33) = 17. 5, p < 0.001) and brain region (F(4,132) = 54.0, p < 0.001) and a significant group × region interaction (F(8,132) = 8.3, p < 0.001) in the ANOVA. Within the PL, post hoc comparisons revealed that rats in both the SAME and DIFF groups exhibited significant increases in c-fos expression relative to rats in the HOME group (p < 0.001). In the IL, c-fos expression was greater in the SAME group relative to both the DIFF and HOME groups (p < 0.05); c-fos expression was not significantly different between the DIFF and HOME groups (p > 0.05). In the VH, rats in the DIFF group exhibited significantly more c-fos expression than rats in the HOME group (p < 0.05). There were no significant group differences for c-fos expression in the AC and PRh (p > 0.05). This suggests that the PL, IL, and VH are differentially engaged by the renewal or suppression of fear to an extinguished CS.
Of considerable interest is the proportion of retrogradely labeled neurons in each area that express c-fos during retrieval testing (Fig. 5C). We found significant group differences in the number of double-labeled neurons in the PL, IL, and VH. In particular, the VH and PL exhibited significantly more double-labeled neurons in the DIFF condition relative to the SAME and HOME conditions, whereas the number of double-labeled cells in the IL was greatest in the SAME condition. These impressions were confirmed in the ANOVA by significant main effects of group (F(2,32) = 9.4, p < 0.001) and brain region (F(4,128) = 22.2, p < 0.001) as well as a group × brain region interaction (F(8,128) = 8.1, p < 0.001). Within the PL, post hoc comparisons revealed that there were significantly more double-labeled neurons in the DIFF group than the SAME (p < 0.001) and the HOME (p < 0.001) groups. Within the IL, however, there were significantly more double-labeled neurons in the SAME group relative to the DIFF (p < 0.05) and HOME (p < 0.001) groups. Additionally, there were more double-labeled neurons in the IL in the DIFF group than the HOME group (p < 0.05). In the VH, there were significantly more double-labeled neurons in the DIFF group relative to SAME (p < 0.01) and HOME (p < 0.005) groups. Within the VH, the SAME and HOME groups were not significantly different from one another, and there were no significant group differences in the AC or PRh. These data reveal that BA-projecting neurons in the VH and PL are preferentially engaged during fear renewal, whereas those in IL are engaged by the suppression of fear during the expression of extinction. Importantly, these patterns of c-fos expression are related not to the specific sensory properties of the CS or the test context (both of which are the same for the SAME and DIFF groups) but rather the meaning of the CS in a particular context.
Interestingly, the proportion of double-labeled neurons across the PL, IL, and VH within a retrieval condition was significantly different. Within the DIFF condition, post hoc comparisons showed that the PL had the highest proportion of double-labeled neurons relative to all of the other brain regions (p < 0.05). Hence, although BA-projecting neurons in the VH exhibited greater c-fos expression in the DIFF relative to SAME conditions, the proportion of BA-projecting neurons exhibiting c-fos was much lower than that in the PL. In the SAME condition, however, the IL exhibited the highest proportion of double-labeled cells relative to the other groups (p < 0.05). Together, these results indicate that a greater number of BA-projecting neurons in the PL are engaged during renewal than BA-projecting neurons in the VH. Conversely, a greater number of BA-projecting neurons in the IL are engaged during the retrieval of extinction relative to the PL and VH.
Disruption of direct or indirect hippocampal projections to the BA impairs fear renewal
To determine the role of direct and indirect projections of the VH to the BA in the renewal of fear, we disconnected the VH and BA or VH and PL with asymmetric unilateral lesions in each structure (Fig. 1). Control rats received the same lesions, but they were localized in the same hemisphere, leaving connections between the VA, PL, and BA intact in the opposite hemisphere. Representative photomicrographs of the lesions are shown in Figure 6. We used strict criteria when analyzing the histology to ensure that only animals with focal lesions in the targeted brain regions were included in the analysis. For the BA, we excluded animals for which the lesions extended dorsally into the lateral amygdala. Similarly, subjects were excluded from the analysis if lesions of the PL encroached on either the cingulate cortex dorsally or the IL ventrally. Finally, we required that VH lesions were localized to the ventral subiculum and ventral CA1 area. If damage to the entorhinal cortex or dentate gyrus was apparent, then the subject was excluded from the analyses. In all cases, lesions had to encompass at least two-thirds of the intended target to be considered a “hit” and included in the statistical analyses. Based on these criteria, we excluded 68 rats, which left a total of 125 animals in the analysis. The final group sizes were as follows: SH-SAME, n = 20; SH-DIFF, n = 22; I-HPL-SAME, n = 8; I-HPL-DIFF, n = 6; I-HBA-SAME, n = 14; I-HBA-DIFF, n = 14; C-HPL-SAME, n = 8; C-HPL-DIFF, n = 8; C-HBA-SAME, n = 12; C-HBA-DIFF, n = 13. It is important to note that the relatively large number of rats in the sham groups is a consequence of the exclusion of many rats with lesions that did not meet our histological criteria for inclusion in the analysis.
Figure 6.
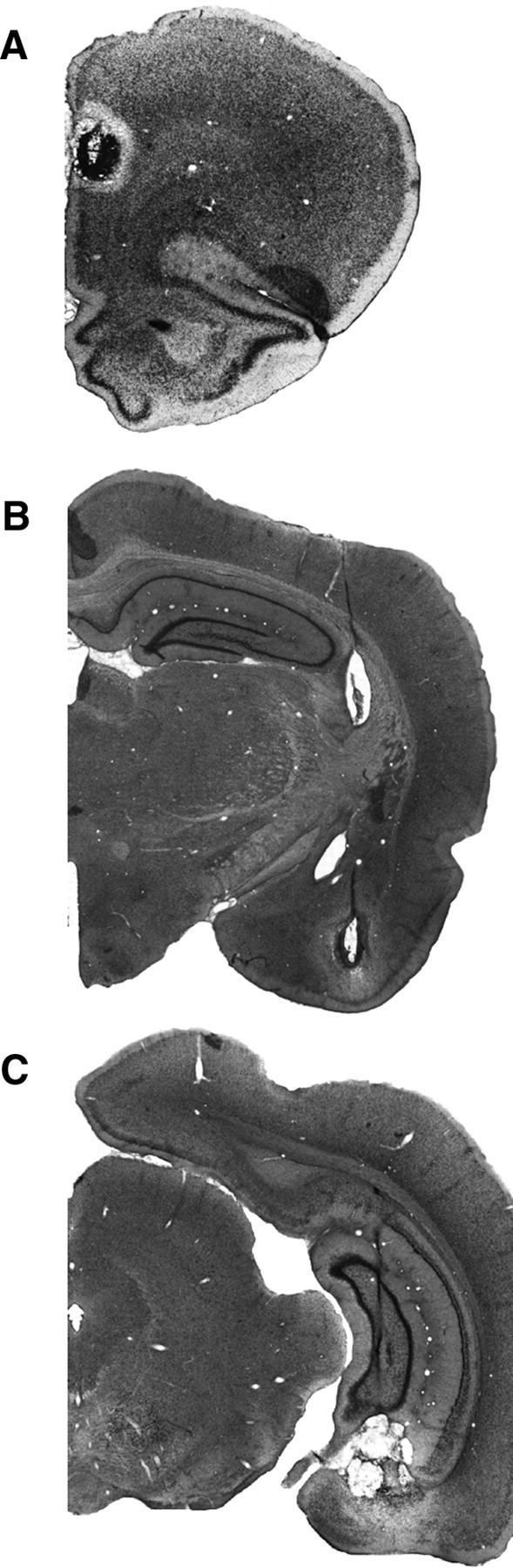
Photomicrographs of thionin-stained coronal sections showing representative lesions in the PL (A), BA (B), and VH (C) for three different rats in the disconnection experiment.
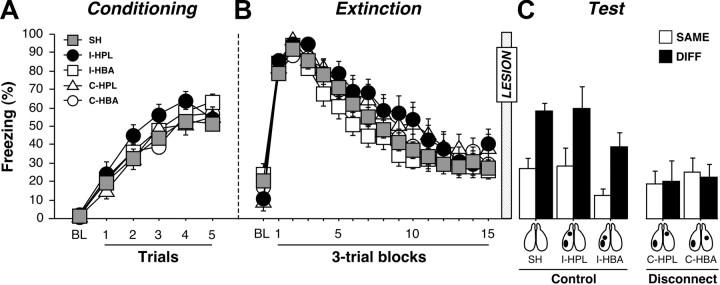
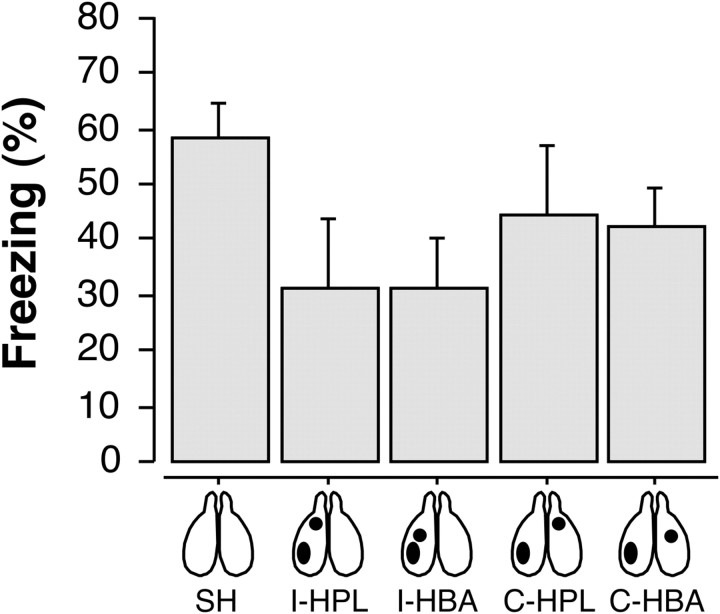
Freezing behavior during the conditioning, extinction, and test session is displayed in Figure 7. Before surgery, there were no differences in freezing behavior during the conditioning and extinction sessions (p values >0.1). During the test session, pre-CS freezing in the SAME and DIFF conditions for each group did not differ from one another (F(1,115) = 2.4, p = 0.1), nor did pre-CS freezing in any of groups differ from one another (F(4,115) = 0.9, p = 0.4; data not shown). We therefore normalized the average CS-elicited freezing (freezing during the CS and ISIs averaged across each test trial) to the pre-CS baseline for each rat. As shown in Figure 7, the groups exhibited marked differences in the degree of fear renewal during the test session. This was indicated by a main effect of lesion (F(4,115) = 5.2, p < 0.001) and significant interaction of lesion and test context in the ANOVA (F(4,115) = 2.9, p < 0.05). Post hoc comparisons revealed that both sham animals and rats with ipsilateral lesions (I-HPL and I-HBA) renewed their fear to the CS outside the extinction context (p values <0.05). However, rats with contralateral lesions (C-HPL and C-HBA) failed to exhibit renewal (p values >0.05). Hence, either VH-BA or VH-PL disconnections eliminated the contextual retrieval of fear after extinction.
Figure 7.
Conditioned freezing in rats that received post-extinction VH, PL, or BA lesions. A, Mean ± SEM percentage of freezing during fear conditioning that consisted of a 3 min baseline period (BL) followed by five tone–shock presentations. Freezing was averaged across the pre-CS BL as well as during each of the five conditioning trials; each trial consisted of the average of freezing during each CS presentation and subsequent ISI. B, Mean ± SEM percentage of freezing during the 45 tone-alone extinction session. As with conditioning, freezing was averaged across the 3 min BL period as well as during the 45 trials, each of which consisted of a CS presentation and the subsequent ISI (data were binned into 15 blocks of three-trial averages). C, Mean ± SEM percentage of freezing during the first 5 min of the test session. Freezing was measured during the 3 min BL period as well as during the first five trials. Data are shown for rats that received sham surgeries (SH; gray squares), rats that received ipsilateral lesions of the VH and PL (I-HPL; black circles), ipsilateral lesions of the VH and BA (I-HBA; white squares), contralateral lesions of the VH and PL (C-HPL; white triangles), or contralateral lesions of the VH and BA (C-HBA; white circles).
Although control rats with ipsilateral lesions exhibited normal renewal, it is possible that the deficit in rats with contralateral disconnections was attributable to a nonspecific impairment in fear expression to the auditory CS rather than a deficit in renewal per se. To examine this possibility, we conducted an additional experiment examining the consequences of ipsilateral or contralateral lesions in the VH-BA and VH-PL circuits on the expression of conditioned fear to an auditory CS that was not extinguished. The behavioral paradigm was identical to the previous experiment, except that animals did not hear auditory CSs during the postconditioning session in context B (i.e., there was no extinction training). Twenty-one rats were excluded from the analysis because their lesions did not meet our histological criteria; this left a total of 59 rats in the analyses. The final group sizes were as follows: SH, n = 15; I-HPL, n = 8; I-HBA, n = 14; C-HPL, n = 7; C-HBA, n = 15. Freezing behavior during fear conditioning and the context exposure session was not different between any of the groups (p values > 0.05). As shown in Figure 8, the test session reveals that neither VH-BA nor VH-PL disconnections affected the expression of freezing behavior during the retention test (F(4,54) = 1.8, p > 0.1). Nonetheless, there was a nonsignificant trend for reduced freezing in all of the rats with lesions, regardless of whether they were placed ipsilaterally or contralaterally in the VH-BA and VH-PL circuits. However, this general suppression of fear in rats with ipsilateral or contralateral lesions does not account for the selective deficit in renewal in rats with contralateral lesions in the previous experiment. Therefore, deficits in fear renewal in rats with contralateral disconnections in the previous experiment are not merely attributable to deficits in the expression of conditional freezing.
Figure 8.
Conditioned freezing in rats that received VH, PL, or BA lesions after fear conditioning. During the test session, freezing was averaged across the baseline period as well as during the 45 trials, each of which consisted of a CS presentation and the subsequent ISI. The data are represented by the mean ± SEM percentage of freezing during the first 5 min of the test session.
Discussion
The present data reveal that convergent input from both the VH and PL to the BA is required for the renewal of fear after extinction. First, we found that significantly more BA-projecting neurons in the PL and VH exhibited c-fos expression during the renewal of fear compared with the recall of extinction. Conversely, more BA-projecting neurons within the IL exhibited c-fos expression during the recall of extinction than during renewal. Second, we showed that disconnection of either the direct projection from VH to BA or indirect projection via the PL eliminated the renewal of fear. These results reveal that the contextual regulation of extinguished fear memories requires a distributed neural network involving the VH, PL, and BA.
These results replicate and extend recent work from our laboratory that has shown that c-fos expression in the prefrontal cortex and hippocampus is regulated by the context in which an extinguished CS is retrieved (Knapska and Maren, 2009). In both studies, for example, we found that c-fos expression in the IL was significantly greater during extinction recall than during renewal. The present results extend this work by demonstrating that BA-projecting neurons within the IL are strongly recruited during extinction recall. Interestingly, we also found that BA-projecting neurons in the IL are engaged during renewal of fear, albeit to a lesser extent than those in the PL. Although IL activation in the renewal context was weak, it suggests that IL neurons that project to the BA may nonetheless have some role in promoting fear expression during renewal. Collectively, these data support the view that the IL has an important role in the retrieval of extinction memories (Milad and Quirk, 2002; Sierra-Mercado et al., 2006).
The present results also confirm our previous findings showing greater c-fos expression in the VH and PL during renewal of fear to an extinguished CS (Knapska and Maren, 2009). However, unlike Knapska and Maren (2009), we observed an increase in c-fos expression within the PL during both the renewal of fear and during the expression of extinction (at levels similar to that in the IL). One potential reason for this discrepancy may be the differences in renewal paradigms used in each study; we used a three-context renewal design (ABC), whereas Knapska and Maren (2009) used a two-context design (AAB). In addition, different immunohistochemical detection methods for c-fos were used in each study. Regardless of these differences, however, both studies clearly demonstrate that neurons within VH and PL are engaged during fear renewal.
Of course, it is possible that the retrograde labeling we observed in BA afferents is attributable to CTb spread into neighboring amygdala regions. For example, CTb labeling in the IL could be a result of CTb diffusion into the adjacent intercalated cell masses (ITC), clusters of GABAergic interneurons that receive glutamatergic projections from the IL (Berretta et al., 2005). We believe that this possibility is unlikely, however, insofar as we were careful to only include subjects for which we had focal BA CTb injections. In addition, the afferent pathways we chose to investigate have very specific connections with the BA. For example, the BA is the only region of the amygdala that receives robust input from the ventral CA1 area of the hippocampus (Pitkanen et al., 2000). Given that cholera toxin is a selective monosynaptic retrograde tracer (Bruce and Grofova, 1992; Conte et al., 2009), we are confident that the CTb labeling we have observed reflects specific afferent projections of the BA.
Although considerable data indicate an important role for the hippocampus in the renewal of extinguished fear (Corcoran and Maren, 2001; Corcoran et al., 2005; Hobin et al., 2006; Maren, 2011), we now show for the first time that the PL has an important role in this process. We found significantly greater numbers of BA-projecting neurons in the PL relative to the VH during the renewal of fear, and disconnection of projections from the VH to PL eliminated fear renewal. The involvement of the PL in renewal is consistent with the emerging view that it has an important role in the expression of conditioned fear. For example, previous work has shown that CS-evoked neuronal activity in the PL parallels freezing behavior during conditioning and extinction (Burgos-Robles et al., 2009), and inactivation of the PL disrupts the expression of learned fear (Corcoran and Quirk, 2007; Sierra-Mercado et al., 2011). It should be noted that the PL is not required for fear expression because PL inactivation spares unconditioned freezing (Corcoran and Quirk, 2007). Moreover, we have found that the expression of fear to a non-extinguished CS does not induce c-fos in PL neurons (Knapska and Maren, 2009). These data suggest that the PL, like the hippocampus, has an important role in contextual memory retrieval rather than in fear expression per se.
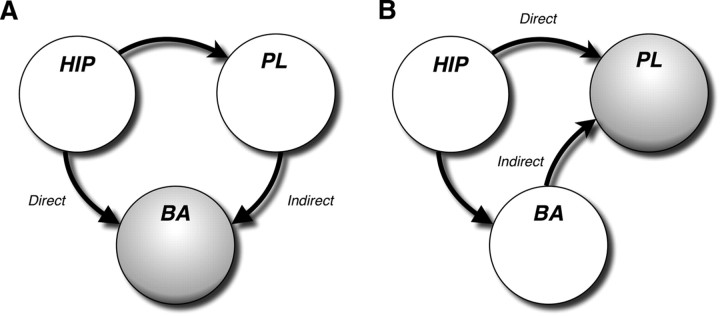
Because both the VH and PL are important routes by which contextual information reaches the BA to regulate fear output, we propose that the convergence of hippocampal and prefrontal input in the BA is essential for the contextual regulation of extinction memory (Fig. 9). In support of this model, interrupting hippocampal input to either the BA or PL eliminates the renewal of fear. In the former case, VH and BA disconnections spare BA–PL interconnection in one hemisphere but isolate this circuit from hippocampal input. In the latter case, VH and PL disconnections spare VH–BA connections in one hemisphere but isolate this circuit from prelimbic input. By this view, convergent excitatory input from the VH and PL may be required to overcome inhibitory networks in the amygdala that suppress fear responses after extinction. Extinction-related inhibition might come from either local inhibitory interneurons in BA (Chhatwal et al., 2005) or through IL-gated inhibitory networks in ITC clusters (Paré et al., 2004; Amano et al., 2010). Although neurons in the BA that respond to CSs during fear renewal receive hippocampal input, it is not clear that they also receive convergent prelimbic input (Herry et al., 2008). However, it has been reported that single VH neurons project to both PL and BA (Ishikawa and Nakamura, 2006). This raises the possibility that VH neurons projecting to PL and BA form a common anatomical hub to regulate excitability in both the PL and BA, as well as encouraging coherence in PL–BA activity (Seidenbecher et al., 2003; Pape et al., 2005; Adhikari et al., 2010). Nonetheless, our data do not address whether single BA neurons receive convergent VH and PL input or whether VH and PL projections that converge on the BA do so on different populations of BA neurons. Dual anterograde tracing of VH and PL projections to the amygdala, as well as simultaneous neural recordings in this network, will be important steps to assess this anatomical question as well as the validity of this model.
Figure 9.
Circuit model of hippocampal–prefrontal–amygdaloid interactions in the renewal of fear. A, In this scenario, both direct and indirect projections of the VH to the BA are required for the renewal of fear after extinction. Disconnection of either the direct or indirect pathways deprives the BA of convergent input from the VH and PL. B, Another possibility is that convergence of direct and indirect projections from the VH to the PL mediate the renewal of fear. Indeed, disconnection of either the VH–PL or VH–BA projection prevents convergence of VH and BA input in the PL.
Another possibility is that the convergence of VH and BA information in the PL, rather than VH–PL convergence in BA, is required for fear renewal. It is well known, for example, that the BA has robust projections to both the medial prefrontal cortex and hippocampus (Pitkanen et al., 2000; Hoover and Vertes, 2007) and that BA inactivation impairs fear renewal (Herry et al., 2008). It is also apparent from our circuit model (Fig. 9) that VH–BA and VH–PL disconnections eliminate not only VH and PL convergence in BA but also the convergence of VH and BA input in PL. Consistent with the possibility that BA and VH convergence in PL is important for fear renewal, Herry et al. (2008) report that neurons in the BA that are active during fear renewal project to the medial prefrontal cortex. Indeed, PL neurons receive convergent hippocampal and amygdala input (Ishikawa and Nakamura, 2003). Hence, this alternative model predicts that BA inactivation, which impairs fear renewal (Herry et al., 2008), would also eliminate renewal-induced c-fos signals in the PL. Experiments to test this hypothesis are underway.
In conclusion, our results provide new insight into the neural circuitry involved in the contextual regulation of fear memories after extinction. Specifically, we found that BA-projecting neurons in both the PL and VH are preferentially active during the renewal of fear to an extinguished CS. Moreover, disconnection of either the direct or indirect routes by which the VH projects to the BA impaired the renewal of fear memories after extinction. Because fear renewal poses a challenge to patients and clinicians, it is critical to understand how dysfunction in hippocampal–prefrontal–amygdaloid connectivity underlies psychopathology. Insights into the neural mechanisms of fear and extinction promise to facilitate the development of more effective therapeutic interventions for individuals suffering from fear and anxiety disorders, such as posttraumatic stress disorder.
Footnotes
This work was supported by National Institute of Mental Health Grants R01MH065961 (S.M.) and F31MH091822 (C.A.O.) and the American Psychological Association (C.A.O.).
References
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bruce K, Grofova I. Notes on a light and electron microscopic double-labeling method combining anterograde tracing with Phaseolus vulgaris leucoagglutinin and retrograde tracing with cholera toxin subunit b. J Neurosci Methods. 1992;45:23–33. doi: 10.1016/0165-0270(92)90040-k. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte WL, Kamishina H, Reep RL. The efficacy of the fluorescent conjugates of cholera toxin subunit B for multiple retrograde tract tracing in the central nervous system. Brain Struct Funct. 2009;213:367–373. doi: 10.1007/s00429-009-0212-x. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunihistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leman S, Viltart O, Sequeira H. Double immunocytochemistry for the detection of Fos protein in retrogradely identified neurons using cholera toxin B subunit. Brain Res Brain Res Protoc. 2000;5:298–304. doi: 10.1016/s1385-299x(00)00026-x. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Wolf WA. A disconnection analysis of hippocampal function. Brain Res. 1982;233:241–253. doi: 10.1016/0006-8993(82)91200-8. [DOI] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–880. doi: 10.1002/hipo.20120. [DOI] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebrón-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Ed 3. San Diego: Elsevier Academic; 2004. [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]