Abstract
Trichoscopy performed with a handheld dermoscope or a videodermoscope became an indispensable tool in differential diagnosis of hair and scalp diseases. Current research is focusing on trichoscopy of: 1) non-cicatricial alopecia, 2) cicatricial alopecia, 3) hair shaft disorders, and 4) inflammatory scalp diseases. This review summarizes current knowledge in these four fields of research. In all non-cicatricial alopecias presence of empty follicular openings is a common trichoscopy finding. In alopecia areata black dots and micro-exclamation mark hairs and tapered hairs correlate with disease activity, whereas yellow dots and vellus hairs correlate with disease severity. In androgenic alopecia trichoscopy shows hair shaft thickness heterogeneity, multiple thin and vellus hairs, yellow dots, perifollicular discoloration, and predominance of follicular units with only one hair. These features predominate in the frontal area. In all forms of cicatricial alopecia, trichoscopy shows milky-red or ivory-white areas lacking follicular openings. In classic lichen planopilaris trichoscopy shows perifollicular inflammation, tubular perifollicular scaling, elongated, concentric blood vessels and "classic white dots", which merge to form white areas. Frontal fibrosing alopecia shows mild perifollicular scaling. Folliculitis decalvans is characterized by tufted hairs, large follicular pustules with emerging hair shafts and perifollicular starburst pattern hyperplasia. In dissecting cellulitis characteristic findings are "3D" yellow dots imposed over dystrophic hairs, large, yellow amorphous areas and pinpoint white dots with a whitish halo. Trichoscopy is particularly useful to diagnose hair shaft abnormalities in trichorrhexis nodosa, trichorrhexis invaginata, monilethrix, pili torti, and pili annulati. The method may be also useful in diagnosing inflammatory scalp diseases. In discoid lupus erythematosus trichoscopy shows large arborizing vessels and large hyperkeratotic folliculilar yellow dots. Trichoscopy of scalp psoriasis shows regularly distributed twisted and lacelike blood vessels, whereas in seborroic dermatitis thin arborizing vessels may be observed. In tinea capitis trichoscopy shows comma, corkscrew and zigzag hairs. Examination tinea capitis may be facilitated by UV-light enhanced trichoscopy (UVET). In conclusion, trichoscopy is a non-invasive method which may be applied in differential diagnosis of most hair and scalp diseases.
Keywords: alopecia, dermoscopy, hair, psoriasis, lichen planopilaris, lupus, seborheic dermatitis, trichoscopy, UV, videodermoscopy
Article
Trichoscopy (hair and scalp dermoscopy) may be performed with a handheld dermoscope. This makes it a modern, non-invasive technique, which is well accepted by both, dermatologists and patients. Thus, trichoscopic examination of hair and scalp became an essential method in evaluation of patients with hair loss.
This article reviews current knowledge about structures observed in trichoscopy and about trichoscopy findings in selected diseases.
Trichoscopy structures
Structures which may be visualized by trichoscopy include hair shafts, hair follicle openings, the perifollicular epidermis and cutaneous microvessels.
Hair shafts
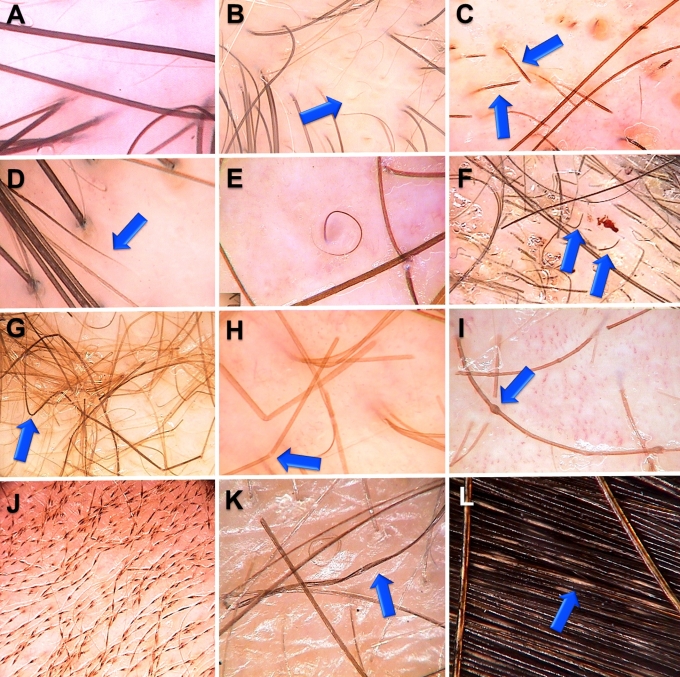
Trichoscopy allows to analyze acquired and congenital hair shaft abnormalities.[1,2,3] Normal hair shafts are uniform in shape and color with continuous, interrupted, fragmented or absent medulla.[4] About 10% of normal human scalp hairs are short, hypopigmented vellus hairs.[2,3] A increased proportion of vellus hairs is characteristic for androgenic alopecia.[5,6] Acquired hair shaft abnormalities, which may be evaluated by trichoscopy include micro-exclamation mark hairs, tapered hairs and tulip hairs[7,8] (in alopecia areata and trichotillomania), regrowing upright or pigtail hairs (in various diseases), comma hair[9] or corckscrew hairs (in tinea capitis).[10,11] We have recently observed zigzag-shaped hairs with transverse structure gaps in a patient with tinea capitis. Trichoscopy also allows to diagnose most genetic hair shaft dystrophies, such as monilethrix, trichorrhexis nodosa, trichorrhexis invaginata, pili torti or pili annulati. See Figure 1 for details.
Figure 1.
Hair shaft abnormalities:
A. Normal hair shaft, B. Vellus hairs in androgenetic alopecia, C. Exclamation mark hairs in alopecia areata, D. Tapered hair in alopecia areata, E. Regrowing pigtail hair in alopecia areata, F. Comma hairs in tinea capitis, G. Zigzag hairs in tinea capitis, H. Trichorrhexis nodosa, I. Trichorhexis invaginata in Netherton’s syndrome, J. Monilethrix, K. Pili torti, L. Pili annulati.
Trichoscopy also allows to assess the number of hairs in one follicular unit. In healthy individuals 2-3 hairs emerge from one follicular unit. The number is decreased in non-cicatricial alopecia and increased above 4 in tufted folliculitis, folliculitis decalvans or lichen planopilaris.[2,12]
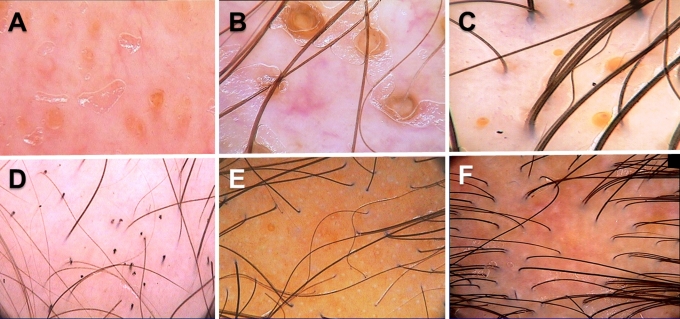
Hair follicle openings: dots
Trichoscopy may distinguish whether hair follicle openings are normal, empty, fibrotic or containing biological material, such as hyperkeratotic plugs or hair residues. "Dots" is a common term for hair follicle openings seen by trichoscopy.[13] Black dots (formerly "cadaverized hairs"), represent pigmented hairs broken or destroyed at scalp level.[13] They are observed in alopecia areata,[14] dissecting cellulitis, tinea capitis, chemotherapy - induced alopecia, and trichotillomania, but may be incidentally observed also in other diseases and after laser depilation or trichogram. Yellow dots are follicular infundibula with keratotic material and/or sebum. They vary in color, shape and size. Yellow dots are present in alopecia areata,[14] discoid lupus erythematosus and androgenic alopecia.[15] The predominance of yellow dots in the frontal area compared to the occipital area favors the diagnosis of (female) androgenic alopecia.[15] Yellow dots, appearing as large "3D" soap bubbles imposed over dark dystrophic hairs are specific for dissecting cellulitis.[16]
The classic, big, irregular white dots represent areas of perifollicular fibrosis and are observed most commonly in lichen planopilaris. Another type of white dots, the small, regular pinpoint white dots are observed in sun exposed areas and in dark skin phototypes regardless of hair loss.[17,18] They correspond to empty hair follicles or to the eccrine sweat duct openings.
Red dots were described in discoid lupus erythematosus and are believed to be a positive prognostic factor.[19]
Regularly distributed brown or brown-gray dots are a characteristic finding in the eyebrow area of patients with frontal fibrosing alopecia. This finding is a favorable prognostic factor for eyebrow regrowth. Common types of abnormalities are presented in Figure 2.
Figure 2.
Follicular openings (dots): A. Yellow dots in alopecia areata, B. Yellow dots in discoid lupus erythematosus, C. Yellow dots in female androgenetic alopecia, D. Black dots in alopecia areata, E. Pinpoint white dots. Empty follicular openings and eccrine gland openings in sun exposed skin. F. Classic white dots resulting from fibrosis in lichen planopilaris.
Perifollicular epidermis
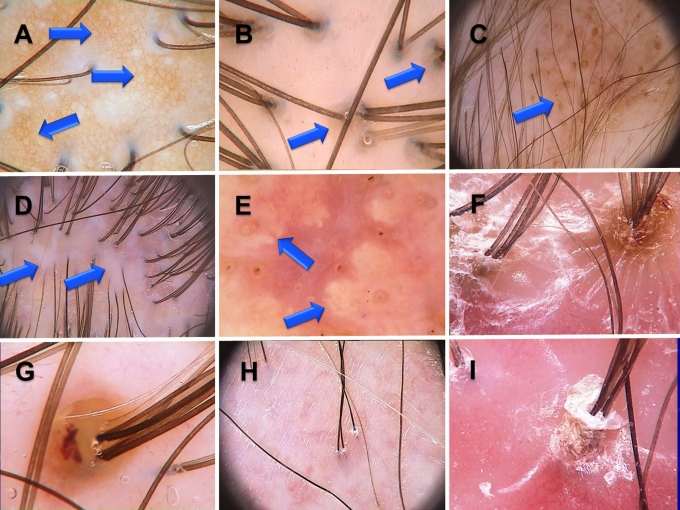
Abnormalities of scalp skin color/structure which may be visualized by trichoscopy include hyperpigmentation, perifollicular discoloration (hyperpigmentation) predominant in androgenic alopecia and perifollicular fibrosis, characteristic for some form of fibrosing alopecia.[6] Common types of abnormalities are presented in Figure 3.
Figure 3.
A. Honeycomb hyperpigmentation, B. Perifollicular hyperpigmentation in female androgenetic alopecia, C. Scattered hiperpigmentation in discoid lupus erythematosus, D. White areas in cicatricial alopecia, E. Amorphous yellow areas in dissecting folliculitis, F. Starburst hyperplasia in folliculitis decalvans, G. Follicular pustule in folliculitis decalvans, H. Mild perifollicular scaling in frontal fibrosing alopecia. Few hairs separated from the hair bearing margin by fibrotic skin. I. Tubular scaling in folliculitis decalvans. Scales fold away from the shaft and form a collar-like structure.
Microvessels
Appearance of cutaneous microvessls in trichoscopy may vary in type and number depending on disease and activity of the process. More detailed information is presented in descriptions of particular diseases.
Selected hair and scalp diseases
Alopecia areata
Trichoscopy features of alopecia areata are: micro-eclamation mark hairs, tapered hairs, black dots, yellow dots, upright regrowing hairs, pigtail regrowing hairs, vellus hairs and broken hairs. The broadest studies on trichoscopy of alopecia areata were performed by Lacarrubba et al.[20], Ross et al.[13], Inui et al.[8,14], and most recently Mane et al.[21]. The results of Inui et al.[8,14] and our observations indicate that black dots and micro-exclamation mark hairs are marker of high disease acitvity, whereas yellow dots predominate in long-lasting alopecia.
It has to be emphasized that exclamation mark hairs can easily lead to misdiagnosis of alopecia areata. Exclamation mark hairs are also observed in trichotillomania.
Androgenetic alopecia
Androgenic alopecia, including female androgenetic alopecia is characterized by predominance of trichoscopy abnormalities in the frontal area compared to the occipital area.[15] These abnormalities include increased proportion of thin and vellus hairs, hair shaft thickness heterogeneity, perifollicular discoloration (hiperpigmentation), and presence of variable number of yellow dots.[5,13,15,20] The character of yellow dots in female androgenetic alopecia is predominantly oily (sebaceous), which differs these structures from yellow dots in other diseases, where they consist mainly of keratotic material. These yellow correspond to the presence of intact sebaceous glands adjacent to miniaturized hair follicles.[15]
Trichoscopy criteria, developed by Rakowska et al.[15] allow to diagnose female androgenic alopecia with a 98% specificity and 72% sensitivity.
No significant differences in trichoscopy are observed between female and male androgenic alopecia.
Telogen effluvium
There are no specific trichoscopy findings in telogen effluvium. However, presence of upright regrowing hairs and predominance of hair follicle openings with only one emerging hair shaft may be indicative of telogen effluvium in absence of features characteristic for other causes of hair loss. Thus, according to current knowledge, telogen effluvium is rather a diagnosis of exclusion and the diagnosis should not rely solely on trichoscopy.
Classic lichen planopilaris and Graham Little syndrome
Trichoscopy features of lichen planopilaris differ, depending on disease stage and activity. In active lichen planopilaris trichoscopy shows silver-white perifollicular scaling with scales entangling hair shafts up to few milimeters above scalp surface, perifollicular inflamation and elongated, concentrically oriented blood vessels. After this phase, violaceous- blue interfollicular areas, corresponding to pigment incontinence may be visible in trichoscopy.[16]
In the fibrotic stage of lichen planopilaris the dominating features are big, irregular (classic) white dots, which merge into milky-red (strawberry icecream color) or white areas.[16,22,23]
In patients with Graham Little syndrome trichoscopy of the scalp is comparable to classic lichen planopilaris. In the axillae and pubic area hairs become thinned and eventually disappear.
Frontal fibrosing alopecia
Trichoscopy findings in active frontal fibrosing alopecia include minor perifollicular scaling and a strong predominance of follicular ostia with only one hair. Arborizing vessels were described in one study,[23] but not confirmed by other authors.[24,25]
In contrast to classic lichen planopilaris, where the background may be milky-red, in the early fibrotic phase of disease, the background in patients with frontal fibrosing alopecia is always ivory-white.[24,25] Late frontal fibrosing alopecia is characterized by lack of follicular ostia.
In the eyebrow area trichoscopy shows regularly distributed red or grey dots throughout the course disease with some tendency to loss of follicular openings in very late and/or advanced disease.[16]
Pseudopelade Brocq
Trichoscopy features of classic pseudopelade of Brocq are nonspecific and include loss of follicular ostia, ivory-white areas and occasionally solitary dystrophic hairs at the periphery of the lesion. Trichoscopy of eyebrows is normal. Unlike other authors, we have not observed white dots in pseudopelade Brocq. Pseudopelade Brocq is a diagnosis of exclusion both clinically and by trichoscopy.
Discoid lupus erythematosus
Most characteristic trichoscopy features of discoid lupus erythematosus of the scalp are: large yellow dots, thick arborizing vessels and scattered dark-brown discoloration of the skin.[16] Large yellow dots with radial, thin arborizing vessels emerging from the dot are considered characteristic for DLE. This feature is sometimes referred to, as "red spider in yellow dot".[16]
Follicular red dots, described by Tosti et al.[19] as a characteristic feature of active DLE and a good prognostic factor for hair regrowth, were rarely observed in our patients. Longlasting, inactive DLE lesions differ from active lesions by presence of structureless milky-red areas, and lack of follicular orfices.
Folliculitis decalvans and tufted folliculitis
The hallmark of folliculitis decalvans is presence of multiple hairs emerging from a single dilated follicular opening. These follicular tufts usually consist of 5-20 hairs.[26] This feature may be observed both clinically and by trichoscopy. Additional trichoscopy findings include perifollicular hyperplasia arranged in a starburst pattern,[16] yellowish tubular scaling (with a collar-like widening at the distal end), crusting, and follicular pustules.[27] In long-lasting lesions white and milkyred (strawberry icecream color) areas lacking follicular openings predominate in trichoscopy images.[16] White dots are rare, present in less than 20% of patients.[28] There seems to be no characteristic vascular pattern, although some authors observed increased numbers of interfollicular twisted red loops.[28]
Dissecting cellulitis
In dissecting cellulitis (dissecting folliculitis, perifolliculitis capitis abscedens et suffodiens) trichoscopy shows yellow, structureless areas and yellow dots with "3D" structure imposed over dystrophic hair shafts as most characteristic findings. Black dots are occasionally present.[16] Pinpoint-like vessels with whitish halo, were described in patients with dissecting folliculitis, but they are not uncommon in other scalp diseases.[16] End-stage disease with scarring lesions is characterized by confluent ivory-white areas lacking follicular ostia and is indistinguishable from scarring alopecia of other origin.
Monilethrix
In monilethrix trichoscopy shows uniform elliptical nodes and intermittent constrictions of the hair shaft, causing variation in hair shaft thickness. Internodes are regularly distributed in constant intervals in one patient, but may differ even in one family. Hairs are bended regularly at multiple locations and have a tendency to fracture at constriction sites.[29–32] The term "regularly bended ribbon sign" was suggested to differentiate monilethrix from pseudomonilethrix and other causes of hair loss.[29] Monilethrix has to be differentiated from monilethrix-like congenital hypotrichosis and from monilethrix-like hairs.
Trichorrhexis invaginata
In trichorrhexis invaginata (bamboo hair) trichoscopy shows a hair shaft telescopes into itself (invaginates) at several points along the shaft. At lower magnifications (i.e. handheld dermoscope) this may appear as nodular structures located along the hair shaft. When the hair fractures at the site of invagination, the proximal end will appear cupped. This type of fractured hairs are also called "golf-tee hairs".[33–35] Eyebrows are a preferential site to visualize this abnormality.
Trichorrhexis nodosa
In trichorrhexis nodosa the shaft splits longitudinally into numerous small fibers. These fibers have a high tendency to break, producing an appearance suggestive of the ends of two brushes aligned in opposition. Eventually the hair shaft breaks at these points leaving hair shafts with brushlike ends. High magnification trichoscopy shows these fibers in detail, while at low magnification these structures appear as light colored nodules or gaps located along the hair shaft.
Pili annulati
In pili annulati trichoscopy shows hair shafts with alternating light and dark bands.[30] The borders of the light bands are not clear-cut, but rather misty and the bands are shorter than the remaining, dark portion of the hair shaft. Usually about 20-80% of hairs are affected.
Psoriasis and seborrheic dermatitis
Kim et al.[36] performed the biggest study focused on differentiating scalp psoriasis from seborrheic dermatitis. Their results indicate that most significant findings scalp psoriasis are: red dots and globules, twisted red loops, and glomerular vessels. Seborrheic dermatitis is characterized by presence of thin arborizing vessels and atypical red vessels. Scaling is observed commonly in both diseases. Our unpublished data show that the scales are silver-white in psoriasis and yellowish in seborrheic dermatitis. This is another feature, which differentiates these two diseases.
Tinea capitis
Comma hairs[9], and corckscrew hairs[11] were found to be characteristic for tinea capitis. They have to be distinguished from corckscrew hairs observed in ectodermal dysplasias. Additional findings in tinea capitis are broken hairs, damaged hairs and black dots. Our unpublished findings show also zigzag hairs and interrupted (Morse code-like) hairs in patients with tinea capitis. Examination of microsporum canis-induced tinea capitis may be facilitated by UV-light enhanced trichoscopy.
UV-enhanced trichoscopy (UVET)
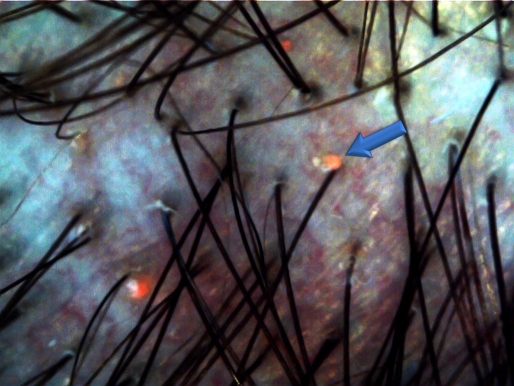
New equipment available on the market allows performing trichoscopy with UV light, at a wavelength covering the spectrum of a Wood's lamp.[37] This feature enhances the diagnostic potential of trichoscopy in tinea capitis, pityrosporum folliculitis and various types of porphyria. Figure 4 shows perifollicular orange glow under UVET.
Figure 4.
UV-enhanced trichoscopy (UVET) in a patient with pityrosporum folliculitis shows a perifollicular orange glow.
Trichoscopy in general medicine
Case reports and conference presentations indicate that possible usefulness of trichoscopy beyond dermatology is an interesting area of exploration. This includes possible application of trichoscopy in identifying follicular spicules in multiple myeloma,[38] follicular mucinosis in lymphoproliferative disorders,[39] scalp lesions in Langerhans histiocytosis or altered interfollicular microvessels in dermatomyositis and scleroderma.
References
- Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651–654. [PubMed] [Google Scholar]
- Rakowska A. Trichoscopy (hair and scalp videodermoscopy) in the healthy female. Method standardization and norms for measurable parameters. J Dermatol Case Rep. 2009;3:14–19. doi: 10.3315/jdcr.2008.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A, McElwee KJ, Blume-Peytavi U. Biology of the hair follicle. In: Blume-Peytavi U, Tosti A, Whiting D, Trüeb R (Eds), Hair; from basic science to clinical application. Berlin, Germany: Springer-Verlag; 2008. pp. 1–22. [Google Scholar]
- Wagner R, Joekes I. Hair medulla morphology and mechanical properties. J Cosmet Sci. 2007;58:359–368. [PubMed] [Google Scholar]
- Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009;36:82–85. doi: 10.1111/j.1346-8138.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- Van Neste D. Natural scalp hair regression in preclinical stages of male androgenetic alopecia and its reversal by finasteride. Skin Pharmacol Physiol. 2006;19:168–176. doi: 10.1159/000093051. [DOI] [PubMed] [Google Scholar]
- Shuster S. The coudability sign of alopecia areata: the real story. Clin Exp Dermatol. 2011;36:554–555. doi: 10.1111/j.1365-2230.2010.04010.x. [DOI] [PubMed] [Google Scholar]
- Inui S, Nakajima T, Itami S. Coudability hairs: a revisited sign of alopecia areata assessed by trichoscopy. Clin Exp Dermatol. 2010;35:361–365. doi: 10.1111/j.1365-2230.2009.03510.x. [DOI] [PubMed] [Google Scholar]
- Slowinska M, Rudnicka L, Schwartz RA, Kowalska-Oledzka E, Rakowska A, Sicinska J, Lukomska M, Olszewska M, Szymanska E. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. J Am Acad Dermatol. 2008;59 (5 Suppl):S77–79. doi: 10.1016/j.jaad.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Sandoval AB, Ortiz JA, Rodriguez JM, Vargas AG, Quintero DG. Dermoscopic pattern in tinea capitis. Rev Iberoam Micol. 2010;27:151–152. doi: 10.1016/j.riam.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Hughes R, Chiaverini C, Bahadoran P, Lacour JP. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355–356. doi: 10.1001/archdermatol.2011.31. [DOI] [PubMed] [Google Scholar]
- Yazdabadi A, Magee J, Harrison S, Sinclair R. The Ludwig pattern of androgenetic alopecia is due to a hierarchy of androgen sensitivity within follicular units that leads to selective miniaturization and a reduction in the number of terminal hairs per follicular unit. Br J Dermatol. 2008;159:1300–1302. doi: 10.1111/j.1365-2133.2008.08820.x. [DOI] [PubMed] [Google Scholar]
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int J Dermatol. 2008;47:688–693. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1:123–130. doi: 10.4103/0974-7753.58555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowska A Slowinska M Kowalska-Oledzka E Warszawik O Czuwara J Olszewska M Rudnicka L Trichoscopy of cicatricial alopecia 2011. (in press) [PubMed] [Google Scholar]
- de Moura LH, Duque-Estrada B, Abraham LS, Barcaui CB, Sodre CT. Dermoscopy findings of alopecia areata in an African-American patient. J Dermatol Case Rep. 2008;2:52–54. doi: 10.3315/jdcr.2008.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham LS, Piñeiro-Maceira J, Duque-Estrada B, Barcaui CB, Sodré CT. Pinpoint white dots in the scalp: dermoscopic and histopathologic correlation. J Am Acad Dermatol. 2010;63:721–722. doi: 10.1016/j.jaad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Tosti A, Torres F, Misciali C, Vincenzi C, Starace M, Miteva M, Romanelli P. Follicular red dots: a novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch Dermatol. 2009;145:1406–1409. doi: 10.1001/archdermatol.2009.277. [DOI] [PubMed] [Google Scholar]
- Lacarrubba F, Dall'Oglio F, Rita Nasca M, Micali G. Videodermatoscopy enhances diagnostic capability in some forms of hair loss. Am J Clin Dermatol. 2004;5:205–208. doi: 10.2165/00128071-200405030-00009. [DOI] [PubMed] [Google Scholar]
- Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011;56:407–411. doi: 10.4103/0019-5154.84768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossard S, Zagarella S. Spotted cicatricial alopecia in dark skin. A dermoscopic clue to fibrous tracts. Australas J Dermatol. 1993;34:49–51. doi: 10.1111/j.1440-0960.1993.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Duque-Estrada B, Tamler C, Sodré CT, Barcaui CB, Pereira FB. Dermoscopy patterns of cicatricial alopecia resulting from discoid lupus erythematosus and lichen planopilaris. An Bras Dermatol. 2010;85:179–183. doi: 10.1590/s0365-05962010000200008. [DOI] [PubMed] [Google Scholar]
- Inui S, Nakajima T, Shono F, Itami S. Dermoscopic findings in frontal fibrosing alopecia: report of four cases. Int J Dermatol. 2008;47:796–799. doi: 10.1111/j.1365-4632.2008.03681.x. [DOI] [PubMed] [Google Scholar]
- Rubegni P, Mandato F, Fimiani M. Frontal Fibrosing Alopecia: Role of Dermoscopy in Differential Diagnosis. Case Rep Dermatol. 2010;2:40–45. doi: 10.1159/000298283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Alzolibani AA, Otberg N, Shapiro J. Lichen planopilaris. Dermatol Ther. 2008;21:249–256. doi: 10.1111/j.1529-8019.2008.00206.x. [DOI] [PubMed] [Google Scholar]
- Trüeb RM. Systematic approach to hair loss in women. J Dtsch Dermatol Ges. 2010;8:284–298. doi: 10.1111/j.1610-0387.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- Baroni A, Romano F. Tufted hair folliculitis in a patient affected by pachydermoperiostosis: case report and videodermoscopic features. Skinmed. 2011;9:186–188. [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Czuwara J, Olszewska M, Rudnicka L. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222–224. [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Rudnicka L. Trichoscopy in genetic hair shaft abnormalities. J Dermatol Case Rep. 2008;2:14–20. doi: 10.3315/jdcr.2008.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MP, de Berker DA. Hair diagnoses and signs: the use of dermatoscopy. Clin Exp Dermatol. 2010;35:41–46. doi: 10.1111/j.1365-2230.2009.03383.x. [DOI] [PubMed] [Google Scholar]
- Jain N, Khopkar U. Monilethrix in pattern distribution in siblings: diagnosis by trichoscopy. Int J Trichology. 2010;2:56–59. doi: 10.4103/0974-7753.66918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Berker DA, Paige DG, Ferguson DJ, Dawber RP. Golf tee hairs in Netherton disease. Pediatr Dermatol. 1995;12:7–11. doi: 10.1111/j.1525-1470.1995.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Kowalska-Oledzka E, Slowinska M, Rosinska D, Rudnicka L. Hair shaft videodermoscopy in Netherton syndrome. Pediatr Dermatol. 2009;26:320–322. doi: 10.1111/j.1525-1470.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- Burk C, Hu S, Lee C, Connelly EA. Netherton syndrome and trichorrhexis invaginata--a novel diagnostic approach. Pediatr Dermatol. 2008;25:287–288. doi: 10.1111/j.1525-1470.2008.00663.x. [DOI] [PubMed] [Google Scholar]
- Kim GW, Jung HJ, Ko HC, Kim MB, Lee WJ, Lee SJ, Kim DW, Kim BS. Dermoscopy can be useful in differentiating scalp psoriasis from seborrhoeic dermatitis. Br J Dermatol. 2011;164:652–656. doi: 10.1111/j.1365-2133.2010.10180.x. [DOI] [PubMed] [Google Scholar]
- Asawanonda P, Taylor CR. Wood's light in dermatology. Int J Dermatol. 1999;38:801–807. doi: 10.1046/j.1365-4362.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- Tay LK, Lim FL, Ng HJ, Lee HY, Pang SM, Thirumoorthy T. Cutaneous follicular hyperkeratotic spicules--the first clinical sign of multiple myeloma progression or relapse. Int J Dermatol. 2010;49:934–936. doi: 10.1111/j.1365-4632.2009.04369.x. [DOI] [PubMed] [Google Scholar]
- LeBoit PE. Alopecia mucinosa, inflammatory disease or mycosis fungoides: must we choose? And are there other choices? Am J Dermatopathol. 2004;26:167–170. doi: 10.1097/00000372-200404000-00040. [DOI] [PubMed] [Google Scholar]