Abstract
Gap junctions, composed of proteins from the connexin family, allow for intercellular communication between cells in essentially all tissues. There are 21 connexin genes in the human genome and different tissues express different connexin genes. Most connexins are known to be phosphoproteins. Phosphorylation can regulate connexin assembly into gap junctions, gap junction turnover and channel gating. Given the importance of gap junctions in development, proliferation and carcinogenesis, regulation of gap junction phosphorylation in response to wounding, hypoxia and other tissue insults is proving to be critical for cellular response and return to homeostasis. Connexin43 (Cx43) is the most widely and highly expressed gap junction protein, both in cell culture models and in humans, thus more research has been done on it and more reagents to it are available. In particular, antibodies that can report Cx43 phosphorylation status have been created allowing temporal examination of specific phosphorylation events in vivo. This review is focused on the use of these antibodies in tissue in situ, predominantly looking at Cx43 phosphorylation in brain, heart, endothelium and epithelium with reference to other connexins where data is available. These data allow us to begin to correlate specific phosphorylation events with changes in cell and tissue function.
Keywords: Connexin, Gap Junction, Phosphorylation, Phosphospecific antibodies, Cell Signaling
1. Introduction
The term gap junction refers to a structure consisting of a tightly packed cluster of channels at the plasma membrane of one cell that interacts with a matched set of channels in an opposing cell, effectively connecting the cytoplasm of the two cells. Although different connexins exhibit different permeability, typically molecules of less than 1000 Daltons can transit through these channels including ions, simple sugars, amino acids, nucleotides, short polypeptides and second messengers (e.g., Ca2+, cAMP, cGMP, IP3) [1–4]. Vertebrate gap junctions are composed of proteins from the connexin family, which is comprised of 21 members in humans [5, 6]. Connexin proteins are usually designated with numerical suffixes referring to their calculated molecular weight in kilodaltons (e.g., connexin43 or Cx43). Connexins have a cytoplasmic N-terminus, four hydrophobic membrane-spanning domains, two conserved, extracellular domains, a loop between transmembrane domains 2 and 3, and a cytoplasmic carboxy-terminal tail region which is diverse in size and sequence. Structural studies indicate that the N-terminus plays a role in channel function and gating [7–10] while the extracellular domains are involved in hemichannel docking between cells [11]. In general, the C-termini of connexins can be phosphorylated and mediate interactions with other proteins as discussed below.
Gap junctions play significant regulatory roles in embryonic development, electrical coupling, metabolic transport, apoptosis, differentiation, tissue homeostasis and carcinogenesis [5, 6, 12]. Connexins are differentially expressed in tissues with some being significantly expressed in only a few tissues and some, like Cx43, being more widespread [3, 13]. Given their diverse tissue expression, mutation of different connexins can yield a wide range of diseases including hereditary deafness, cataracts and a variety of skin diseases [5, 6]. Mutation of the Cx43 gene (GJA1) can cause oculodentodigital disease (ODDD), a rare syndrome that can result in small eyes, underdeveloped teeth, and syndactyly [14]. A mouse strain (Gja1Jrt/+) carries the dominant loss-of-function Cx43 mutation, Cx43G60S and displays features of ODDD [15] making it a useful model to probe Cx43 function [16, 17]. Replacement of Cx43 with a truncated version (Cx43K258Stop) lacking the C-terminal, cytoplasmic tail region yielded mice that died shortly after birth due to an epidermal barrier defect [18], not the heart defect that is present in Cx43 deficient mice [19]. The C-terminal region has been shown to have multiple sites of phosphorylation and protein interaction and hence is likely involved in Cx43 regulation [20].
Connexin phosphorylation has been correlated with changes in gap junction assembly, stability and channel properties [20–24]. Although treatment of cultured cells with inhibitors of phosphatases often does not appear to affect gap junction communication, multiple reports indicate that phosphatases may regulate connexin function in certain instances, particularly during dynamic regulation during cardiac ischemia [25–27]. Furthermore, dephosphorylation might be linked to Cx43 relocalization during ischemia [25]. Where examined, most members of the connexin family of proteins have been shown to be substrates for kinases, and, given that tissues can express multiple connexins, a single cell can probably express multiple phosphorylated connexins. Up until the last several years, most connexin phosphorylation-related research was based on cell culture models as detailed in many recent reviews [21–23, 28–30]. In contrast, this review focuses on connexin phosphorylation in tissues and offers speculation on the role that these events play in tissue function. These studies are now possible because phosphorylation-state specific antibodies have become available for several phosphorylation sites in Cx43.
2. Phosphorylation of Cx43
Cx43 has a half-life in the range of 1–3 h in cultured cells or in tissues [31–35]. This fast turnover rate presumably implies a need for a high level of post-translational regulation of gap junction/connexin function. The early work of Musil and Goodenough, the Lau laboratory and several other investigators have shown that Cx43 is differentially phosphorylated throughout its life cycle [31–33, 36–40]. Cx43 typically demonstrates multiple electrophoretic isoforms when analyzed by polyacrylamide gel electrophoresis that can be collapsed by phosphatases to a faster migrating non-phosphorylated isoform. Phosphoamino acid analysis indicates the majority of the phosphorylation events in homeostatic cells occur on serines [39, 41–43], although tyrosine phosphorylation has been observed when pp60src is activated [32, 44]. In Cx43, only the C-terminal region of Cx43 appears to be phosphorylated but Cx36 and Cx46 can be phosphorylated in the intracellular loop region [45, 46]. Cx43 does not contain serine residues in its intracellular loop region, and we are not aware of any reports of phosphorylation near the N-terminus of connexins.
Activation of different kinase systems has been shown to regulate connexin biology. Reports indicate that Cx43 can be phosphorylated on at least 14 of the 21 serines and 2 of the tyrosines in the cytoplasmic tail region (amino acids 245–382). Low levels of threonine phosphorylation have been observed in connexins, including Cx43 [47] and Cx37 [48], but, to our knowledge, have not been investigated further. PKA activation leads to upregulation of several connexins including Cx43 and Cx50 [46, 49, 50], although Cx43 is a poor direct PKA substrate [51, 52], so other kinase systems are likely involved. Several residues in tandem serine repeat regions near the C-terminus of Cx43 can be phosphorylated (i.e., serines 365, 368, 369 and 373) in response to follicle-stimulating hormone, probably at least partially through a PKA-mediated mechanism [53]. PKC has been shown to phosphorylate Cx43 at S368 and S372 in vitro and increased phosphorylation of S262 and S368 is seen in response to TPA [52, 54, 55]. S262 phosphorylation has been linked to increased cellular proliferation through an unknown mechanism [56] while S368 phosphorylation has been shown to underlie the TPA-induced reduction in intercellular communication [54]. Cx43 phosphorylation at S368 results in a reduction in unitary channel conductance with 50pS channels favored over 100pS channels [57]. PKC-mediated phosphorylation of purified recombinant Cx43 abolished sucrose and Lucifer Yellow permeability and led to a conformational change in Cx43 [58]. PKCα and ε were found to associate with Cx43 in cardiomyocytes [59]. PKCγ interacts with Cx43 in lens epithelial cells [60]. Fibroblast growth factor-2, which decreases cardiomyocyte gap junctional permeability and increases Cx43 phosphorylation, increased colocalization of PKCε with Cx43 [61].
Casein kinase 1, particularly the δ isoform, has been shown to interact with and phosphorylate Cx43 [62]. p42/44 MAPK phosphorylates Cx43 at serines 255, 279 and 282 [43] and causes reduced gap junction channel open time [63]. Certainly other kinases phosphorylate Cx43, and it appears to be possible for multiple kinases to phosphorylate the same residue. For example, serine 255 has been reported to be phosphorylated by both MAPK and cdc2 [43, 64]. Thus, considerable evidence indicates that Cx43 is a highly phosphorylated and a highly regulated protein.
3. Phosphorylation-status specific antibodies
While the overall phosphorylation status of connexins within tissue preparations can be probed by SDS-PAGE and analytical techniques such as mass spectrometry, localization of in situ phosphorylation usually requires the use of phosphorylation status specific antibodies. Based on its early availability, a monoclonal antibody that binds primarily to the P0 isoform of Cx43 (Zymed/Invitrogen, 13–8300) [65] has been widely used to probe in vivo Cx43 phosphorylation in a variety of tissue preparations [66–70]. The fact that this antibody binds to predominately to the P0 form indicates that the phosphorylation event(s) that eliminates binding might be a key early regulatory event in Cx43 life cycle. The immunizing peptide that produced this antibody contained residues 360–376, but the specific unphosphorylated serines that comprise the epitope are unknown. The antibody can recognize other minor Cx43 bands in untreated cells and recognizes multiple bands upon TPA treatment [71], due to changes in phosphorylation outside this antibody’s epitope. Another monoclonal antibody specific to a dephosphorylated form of Cx43, termed CT1, has both overlapping and distinct properties and was epitope mapped to non-phosphorylated S364/S365 [72]. Since phosphorylation at both S364 and S365 appear to be important regulatory events [51, 73] and the latter involves phosphorylation that results in SDS-PAGE migration at the P1 position [73], an isoform distinctly not recognized by 13–8300 or CT1 [71, 72]. Thus, either or both of these serines in their unmodified form are probably critical for binding.
Several phosphospecific antibodies to Cx43 have been developed recently and some are commercially available. One of the first commercially available phosphospecific antibodies was to residue S368, a PKC phosphorylation site on Cx43. Now close to a dozen companies sell antibodies to this same site and the adjacent serine at 369. In addition, at least three companies produce phosphospecific antibodies to S262 and one to S255. A few other commercial antibodies have been made to additional sites but for the most part, at least in our hands, these antibodies do not perform well. Several phosphospecific antibodies have been prepared by individual investigators including ones to sites at S279/282 [74, 75], Y247 [76], Y265 [76], S306 [77], and S325/328/330 [78]. These antibodies are beginning to reveal some of the overlapping roles of Cx43 phosphorylation in gap junction function. For example, src-mediated downregulation of gap junctions has been well described, and Cx43 has been shown to be directly phosphorylated by src at Y247 and Y265 [44, 47, 76, 79, 80]. However, data generated in different model systems, using inhibitors of various kinase pathways have led to some controversy as to how src activation actually downregulates gap junction communication. Use of a combination of several phosphospecific antibodies showed that activation of v-src leads not only to phosphorylation on Y247 and Y265, but also phosphorylation at S262, S279/282, and S368 and leads to a decrease in phosphorylation at S364/365 [76]. Thus, oncogenic src activation leads to apparent stimulation of at least src, MAPK and PKC phosphorylation of Cx43 making studies focused on single pathways less conclusive.
To our knowledge, Cx36 is the only other connexin to which phosphospecific antibodies have been prepared. Phosphorylation status dependent antibodies to S110 and S276 have been used to study retinal tissue preparations under different lighting conditions and to examine the role of different kinase systems [46, 81].
4. Connexin Phosphorylation in Brain Tissue
Gap junctions play a critical role in cell-cell communication in the nervous system. They are thought to maintain brain homeostasis by allowing electrical coupling between neurons and regulating neuronal network activity [82, 83]. In brain, Cx43 is the dominant gap junction protein expressed in astrocytes and is abundantly expressed in Bergmann glial cells in the cerebellum [84]. Many diverse functions have been attributed to Cx43 and gap junctional communication in brain, one of which is apparent in Cx43-null [85] and Cx43-C-terminus truncated [86] mutant mice that show delays in neuronal migration.
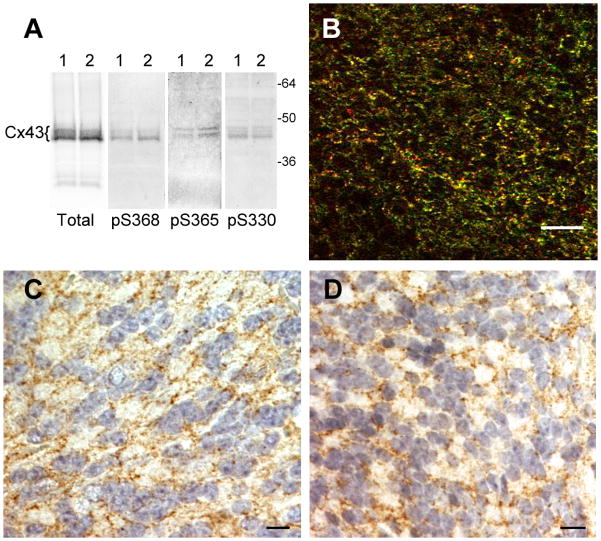
Levels of astrocytic gap junctional intercellular communication are thought to be dynamic and subject to regulation by a variety of factors. However, little published information is present about connexin phosphorylation in intact tissue. We analyzed adult mouse brain for total Cx43 and Cx43 phosphorylated at specific serines residues. Using immunofluorescence and Western blot techniques, examination with antibodies specific for phosphorylation at S365 and S325/S328/S330 showed clear evidence of phosphorylation of Cx43 in brain in apparent typical punctate gap junctional structures and via SDS-PAGE, respectively (Fig. 1). Additionally, using immunohistochemical staining, we also observed that apparent gap junctional staining for phospho-S368 preferentially occurred in cerebellum (Fig. 1D).
Fig. 1. Brain shows phosphorylation at S365, S325/328/330 and S368.
(A) For SDS-PAGE and Western blot, two mouse brains were quickly removed and frozen in liquid nitrogen. The tissue was pulverized in the presence of sample buffer containing protease and phosphatase inhibitors to prevent dephosphorylation during tissue homogenization. Samples from brain 1 and 2 (one in each lane) were run on 10% PAGE, blotted and probed with NT1 antibody to total Cx43 or antibodies to Cx43 phosphorylated at S368 (pS368), S365 (pS365) and S325/328/330 (pS330) [73]. (B) Immunofluorescence of OCT embedded frozen sections of brain using total Cx43 (Cx43IF1 [72] – in green) and S325/328/330 ([78] - in red) antibodies. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue sections stained with Hematoxylin/Eosin showing cerebellar cells staining at opposed plasma membranes (brown) for total Cx43 (C) and phospho-S368 (D). Phospho-S368 levels appeared to be highest in the cerebellar regions of the brain. Bars are 20 μm.
Cx43 has been shown to increase in human brain as a result of ischemia [87] and in the hippocampus of patients with epilepsy [88], but at this point phosphorylation of Cx43 has not been analyzed in detail. Since protein phosphorylation has been shown to be involved in a wide variety of neurophysiological events, it will be interesting to see whether the phosphorylation status of Cx43 could be affected and regulate responses under ischemic or inflammatory conditions and in neurological disorders.
5. Connexin phosphorylation in Cardiac Tissue
Impulse propagation in the heart occurs through gap junctions and disruption or dysregulation of gap junctions is a signature of many cardiac pathologies [89]. Normally gap junctions are found in abundance at a distinct and specialized structure at the ends of myocytes referred to as the intercalated disc [90, 91]. This structure also contains a high concentration of desmosomes and adherens junctions that provide mechanical stability to cardiac tissue [92].
Cx43, Cx40, and Cx45 can all be found in heart and are expressed in a spatially distinct manner (reviewed in [93]). Cx43 is the dominant gap junction component in the ventricle and the primary connexin where phosphorylation has been analyzed. Across species, essentially all Cx43 in the ventricle is highly phosphorylated resulting in a conformation that exhibits a migration shift in SDS-PAGE that can be eliminated by phosphatase treatment [25, 67, 94, 95]. Analysis with phosphospecific antibodies show that this migratory isoform of Cx43 is phosphorylated on S365 and S325/S328/S330 and immunohistochemical analysis of mouse heart confirms that phosphorylation of Cx43 at these sites occurs at the intercalated disc [73, 78]. Indeed, phosphorylation of these sites has been shown to be responsible for inducing the conformational change resulting in the migration shift [62, 73, 78, 96]. S306 phosphorylation has also been shown at the intercalated disc in rat heart [77]. Several additional phosphorylation sites in cardiac tissue have been identified via mass spectrometry [97] though the location and prevalence of these events have not yet been elucidated.
Given the integral nature of gap junctions in cardiac function, it is not surprising that changes in connexin expression and gap junction remodeling are a feature of many cardiac pathologies. Typically, this includes a decrease in Cx43 at the intercalated disc and a shift to the lateral edges of the myocyte, a process referred to as lateralization (e.g. [25]). SDS-PAGE analysis of Cx43 from compromised hearts of many etiologies show a shift to the faster migrating forms of the protein, indicating a change in its phosphorylation state [98]. Changes in Cx43 have been implicated in altering susceptibility to arrhythmia under various conditions as well directly impacting damage occurring after myocardial infarction (MI). It is unclear at this point whether these outcomes are related or more likely reflect different roles for Cx43 in maintaining cardiac health.
A mechanistic link demonstrating a role for phosphorylation on S325/328/330 in gap junction remodeling and arrhythmic susceptibility is clearly shown through studies using Cx43 knock-in mice where these sites have been mutated to glutamic acid, to mimic phosphorylation, or alanine, to eliminate phosphorylation, at those sites [96]. Transverse aortic constriction in wild type and S325/328/330A mice resulted in gap junction remodeling while S325/328/330E maintained Cx43 at the intercalated disc. In addition, ventricle tachycardia induction by programmed electrical stimulation showed distinct phenotypes for the mutants where S325/328/330E mutants were resistant to ventricular arrhythmias while the S325/328/330A were more susceptible to arrhythmias than wild type animals. Thus, these mice prove that Cx43 phosphorylation status at these sites is necessary for adaptive responses in cardiac tissue to change in physiologic parameters.
Changes in Cx43 phosphorylation and localization have also been studied during ischemia. This is especially relevant as Cx43 plays a critical role in the phenomenon of preconditioning wherein short bouts of ischemia preceding a longer ischemic interval can actually protect the heart from damage [99]. This is illustrated by the fact that mice that are heterozygous for a null mutation in Cx43 do not respond to preconditioning [100–102]. Though interestingly, these animals exhibit smaller infarcts in response to coronary artery occlusion [103]. Cx43 appears to mediate these types of cardiac effects through a complex interplay of gap junction communication dependent and independent means. While it is clear that preconditioning preserves Cx43 localization and phosphorylation, as evidenced by its continued presence at the intercalated disc and maintenance of the slow migrating isoforms [104], an additional role for Cx43 in the mitochondria and regulation of reactive oxygen species during preconditioning is also emerging [105]. A small fraction of cellular Cx43 has been shown to fractionate with mitochondria and appears to be enriched in the slow migrating isoforms [106], though specific phosphorylation events have not been studied. The role of Cx43 in the preconditioning response appears to be complex, involving the mitochondria and reactive oxygen species regulation, gap junction permeability and Cx43 localization. An interesting example of the complexity involved in these processes is shown in mouse hearts where the Cx43 gene is replaced with Cx32, which likely does not translocate to the mitochondria; this Cx43 heterozygote maintains the ability to be preconditioned through short bouts of ischemia but is not protected by diazoxide, a drug which directly impacts mitochondria function [107].
A few groups have begun to dissect the individual phosphorylation sites and kinase systems involved in these events. The interaction of Cx43 with PKC has received the most attention as PKC has also been shown to be necessary for preconditioning [108, 109], and PKC activation can result in phosphorylation at S368 and S262 [54–56]. Immunoblot analyses from several labs show that phosphorylation on S368 is increased after ischemia [57, 110–113] regardless of preconditioning. However, immunofluorescence analysis of cardiac tissue showed that there was less pS368 at the intercalated disc after preconditioning [110]. Since S368 phosphorylation has clearly been shown to modulate gap junction permeability [54, 57] this site may play a role in mediating gap junction communication dependent aspects of protection. In rat hearts, phosphorylation on S262 was correlated with a preconditioned phenotype [111, 112]. In addition, when a S262A mutant was expressed in cultured cardiomyocytes these cells were prone to cell death upon ischemic-type injury. Signal transduction through MAPK cascades, in particular p38 MAPK and ERK1/2, have also been examined. In rat hearts, ischemia led to an increase in p38 MAPK activity and an associated increase in interaction with Cx43 that was attenuated with preconditioning [114]. However, in pig hearts immunohistochemistry showed increased interaction between Cx43 and p38 MAPK with preconditioning [95]. Interestingly, another group studying terminal differentiation of cardiomyocytes observed a positive feedback loop linking p38 MAPK activation to mitochondrial translocation of Cx43 and ROS generation [115]. ERK1/2 has also been implicated in controlling Cx43 translocation to the mitochondria and promoting survival in stem cells [116]. Thus, the interplay between these various kinase systems seem to be important in regulating multiple aspects of Cx43 biology.
As emphasized above, dephosphorylation of Cx43 has repeatedly been shown to correlate with cardiac pathogenesis across species. In addition to the migration shift, the two monoclonal antibodies have been used to detect “non-phosphorylated” Cx43, Zymed/Invitrogen 13–8300 and CT1 [72] in ischemic tissues. The CT1 antibody begins to detect this dephosphorylation event within 5 minutes of ischemia, well before the levels are sufficient to detect the migration shift by an antibody to total Cx43. This same antibody also shows increased signal for Cx43 after 30 minutes of ischemia at both the intercalated disc and in lateralized regions of the myocytes (Fig. 2). Similar data has been seen using the 13–8300 antibody [25]. Signal from this antibody is also diminished when rat hearts are treated with FGF-2 before ischemia, a situation that mimics preconditioning [111]. Interestingly, this study included a reperfusion step, during which 13–8300 signal was completely lost with a concomitant increase in the slower migrating phosphoform. Similarly, another ischemia-reperfusion study indicated that the ability of a heart to recover contractile activity during reperfusion was strongly correlated with this same loss of 13–8300 and gain of the slower migrating phosphoform recognized by an antibody to total Cx43 [25]. These data indicate a potential but important role for a phosphatase in Cx43 regulation during ischemia and are consistent with a report showing that an inhibitor of PP1 (protein phosphatase1)-like phosphatases could inhibit ischemia-induced dephosphorylation of Cx43 [26]. These data, in combination with the knock-in mouse data, indicate that the phosphorylation sites and the kinases responsible for homeostatic regulation of Cx43 could be major players in cardiac pathogenesis.
Fig. 2. Gap junction remodeling in heart occurs in response to ischemia.
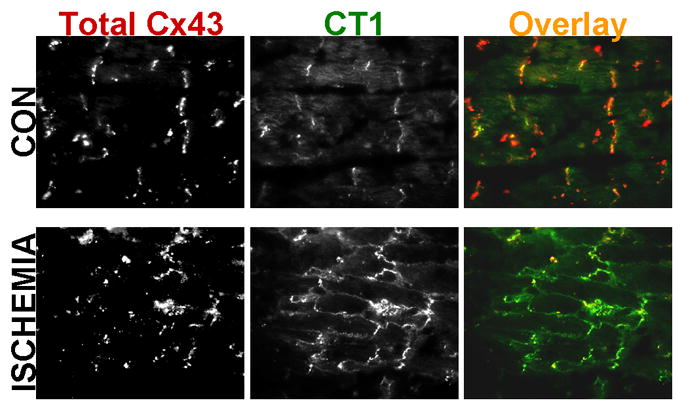
Mouse hearts were excised and immediately fixed in formalin (CON) or first incubated without perfusion in non-oxygenated PBS for 30 min (ISCHEMIA). Paraffin embedded sections were co-labeled with a rabbit antibody to Total Cx43 (Sigma-Aldrich, St. Louis, MO) and a mouse antibody, CT1, which recognizes Cx43 unphosphorylated on S364/365 (FHCRC, Seattle, WA). Overlay panel shows the Total Cx43 antibody labeled with Alexa 546-conjugated secondary antibody in red and CT1 labeled with Alexa 488-conjugated secondary antibody in green.
6. Connexin Phosphorylation in Endothelial Tissue
Connexins expressed within the vasculature include Cx37, 40, 43 and 45 [117]. Cx43 phosphorylation has been implicated in affecting vessel biology through both gap junction dependent and independent means. For example, in resistance vessels, heterocellular communication between vascular smooth muscle cells (VSMC) and endothelial cells (ECs) are thought to occur through a specialized actin based structure called the myoendothelial junction (MEJ) [118, 119]. Electrical and dye coupling through the MEJ can be differentially regulated in different tissues and two complementary studies have shown that this can be regulated via Cx43 phosphorylation [118, 120]. Isakson, et al. showed the presence of Cx43 phosphorylated at S368 at the MEJ in mouse cremaster arterioles where there is little to no coupling. This is in contrast to rat mesentery where coupling occurs at the MEJ and Cx43 is found without S368 phosphorylation, consistent with the role S368 phosphorylation plays in channel gating. This finding was explored furthered through in vivo dye transfer assays performed on mouse cremasters in the presence or absence of pCPT, a cAMP analog [120]. cAMP has long been known for its ability to increase gap junctions and intercellular communication [49, 50]. In this case, activation of cAMP on cremaster arterioles led to a loss of S368 phosphorylation and a significant increase in the ability of VSMCs to transfer dye to ECs. This relationship between phosphorylation on S368 and heterocellular communication was further corroborated in vitro using a vascular cell co-culture model that also looked at the movement of second messengers, inositol tri-phosphate (IP3) and calcium. In this case phosphorylation on S368 could inhibit IP3 transfer while allowing calcium movement, which is, again, consistent with a role for S368 in modulating channel gating.
Cx43 phosphorylation has also been shown to change during atherogenesis where minimally modified low density lipoproteins, the oxidative products of low-density proteins, accumulate in vessel walls forming a plaque [121]. Components of these complexes are thought to mediate changes in VSMCs, including increased proliferation, and contribute to disease. In the ApoE−/− mouse, a model of atherogenesis that is prone to artherogenic plaques, total Cx43 expression was diminished in carotid arteries [122], while phosphorylation on S368 and S279/282 were significantly increased. Using specific biologically active derivatives of minimally modified low density lipoprotein embedded in a pluronic gel, Johnstone et al. were able to distinctly induce these phosphorylation events in the carotids of normal mice. Application of 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine (POVPC) was able to induce S279/282 phosphorylation while 1-palmitoyl-2-glutaroyl-sn-glycero-3-phophorylcholine (PGPC) induced S368 phosphorylation. Notably, POVPC, and not PGPC, was able to induce proliferation in VSMCs, as well. As the authors point out, it is interesting to speculate that phosphorylation on S279/282 could help mediate this proliferative phenotype, but further work will be required to explore this hypothesis.
7. Connexin Phosphorylation in Epithelial Tissue
Epithelium is an avascular tissue defined by a polarized cell morphology. Epithelial tissues encapsulate many of the structures and organs in vertebrates. Cx43 is expressed in many epithelial tissues [13]. Phosphorylation of Cx43 at S368 has been detected in intestinal epithelial cells of mice deficient in the MDR1α gene [123]. MDR1α−/− mice develop spontaneous chronic colitis, a chronic inflammatory injury of the intestinal epithelial cell barrier resembling ulcerative colitis. Ey et al. detected elevated pS368 by Western blotting of colon tissue from MDR1α−/− mice sacrificed on day 67 postpartum, relative to age matched wild type control mice. The observed hyperphosphorylation of Cx43 at S368 could be prevented by treating MDR1α−/− mice with a TLR2 agonist.
Phosphorylated Cx43 has also been reported in mammary tissue. Gould et al. developed an antiserum (SA226P) shown to react with S279/282 of Cx43 when phosphorylated by MAPK [124]. In normal adult human breast, Cx43 phosphorylated at S279/S282 is detected in myoepithelial cells. In a mouse model of the human Cx43-related disease occulodental digital dysplasia, Plante and Laird [125] observed a decrease in the highly phosphorylated forms of Cx43 in the mammary glands of Gja1Jrt/+ mice when compared to wild type littermate controls. Additionally, Plante and Laird observed delayed development of the mammary ducts and a defect in milk ejection in these mice.
Epidermis is a stratified surface epithelium and functions as a dynamic organ capable of responding to injury and environmental insults. Several connexins are expressed in epidermis with Cx43 predominating and Cx26, Cx30, Cx30.3, Cx31, Cx40 and Cx45 expressed at different levels in different regions and in response to different stimuli [126, 127]. In addition to providing a barrier function and protection from ultraviolet light, epidermis is capable of responding to and repairing injury to the organ. Connexin proteins are known to play a role in this repair process during which they presumably undergo phosphorylation by a diverse array of kinases. Phosphorylated S368 was observed to be elevated in embryonic mouse skin, whereas adult mouse epidermis exhibited low to moderate levels localized to the suprabasal cell layers [128]. This expression pattern of low to moderate levels of phosphorylated S368 is also found in human adult epidermis. However, upon wounding, Cx43 phosphorylation at S368 is elevated specifically in the basal cell compartment proximal to the wound margin [129]. The observed increase in S368 phosphorylation reaches maximal levels in skin at 24 hours post wounding and returns to baseline by 72 hours [129].
8. Connexin Phosphorylation in other Tissues and during Carcinogenesis
A physiological role for in vivo Cx43 phosphorylation has been demonstrated in mouse ovarian follicles. Cx43 is the most prevalent connexin in the ovary [130], connecting granulosa cells within each follicle. In response to luteinizing hormone (LH) from the pituitary, which signals meiosis to resume in oocytes, Cx43 in granulosa cells becomes phosphorylated [131], through a MAPK-dependent mechanism [132] on serines 255, 262, and 279/282 [75]. These events cause a decrease in gap junction permeability between granulosa cells, contributing to the resumption of meiosis in the oocyte. Phosphorylation at these sites is transient, and by five hours after LH treatment, serines 255, 262, and 279/282 are no longer phosphorylated [75]. In ovarian follicles that have not yet been exposed to LH, there are several phospho-isoforms of Cx43 [75, 131]. The significance of this phosphorylation is not clear, but it will be interesting to investigate further.
Cx50 is expressed in fiber cells of the lens of the eye and has been reported to be phosphorylated in vivo on S395 [133]. This study showed that PKA is likely the kinase that phosphorylates this site in vivo and that phosphorylation enhances gap junction and hemichannel function [133]. The level of Cx43 phosphorylation in the cornea at S368 appears to be developmentally regulated with distinct differences in embryonic (high) and adult (low) tissue [128].
Immunohistochemical investigation of mouse skin neoplasia for total Cx43 and Cx43 phosphorylated at S368 in DMBA/TPA-induced mouse skin showed dramatic changes during progression. An increase in total Cx43 and phospho-S368 antibody reactivity was observed in junctional structures in hyperplastic skin compared with more aggressive tumors which exhibited decreased levels and nonfunctional, cytoplasmic localization of Cx43 [128]. Upregulation of Cx43 phosphorylated at S279/282 was observed in breast hyperplasias and carcinomas [124].
9. Concluding remarks
Studies performed in tissue culture systems have clearly shown that connexins and gap junction biology are affected by phosphorylation. However, until recently, little was known about whether these phosphorylation events actually occur in tissue and whether they affect tissue function. Phosphorylation status specific antibodies have revolutionized the field of cell biology, and the few commercially available phosphospecific antibodies have already shown that Cx43 phosphorylation is regulated in vivo. More antibodies will begin to give us an overall view of how different signaling pathways interact to affect tissue biology. In addition, generation of knock-in mice expressing phosphorylation site mutants will allow us to see how connexin phosphorylation impacts tissue function. As these tools allow a better understanding of the consequences of connexin phosphorylation and an increased appreciation of the consequences of connexin phosphorylation on its interactions with other proteins, we expect future studies will show that connexin phosphorylation plays key biological roles critical for proper tissue function.
Connexin phosphorylation regulates gap junction assembly and degradation
Connexin phosphorylation is dynamic and changes during development
Connexin phosphorylation changes in tissue in response to wounding and ischemia
Acknowledgments
Practical considerations have led us to utilize reviews or limit citations of original work, and we apologize for these deficiencies. The work in the authors’ laboratory was supported in part by grants from the National Institutes Health (GM55632 and CA149554 to PDL). RPN was supported by T32CA80416.
Abbreviations
- Cx
connexin
- MAPK
Mitogen-activated protein kinase
- PKC
protein kinase C
- PKA
protein kinase A
- MEJ
myoendothelial junction
- SDS-PAGE
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- VSMC
vascular smooth muscle cell
- EC
endothelial cell
- LH
luteinizing hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 2.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 3.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 4.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Dobrowolski R, Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid Redox Signal. 2009;11:283–295. doi: 10.1089/ars.2008.2128. [DOI] [PubMed] [Google Scholar]
- 7.Kalmatsky BD, Bhagan S, Tang Q, Bargiello TA, Dowd TL. Structural studies of the N-terminus of Connexin 32 using 1H NMR spectroscopy. Arch Biochem Biophys. 2009;490:9–16. doi: 10.1016/j.abb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle JW, Minogue PJ, Thomas BC, Domowicz DA, Berthoud VM, Hanck DA, Beyer EC. An intact connexin N-terminus is required for function but not gap junction formation. J Cell Sci. 2008;121:2744–2750. doi: 10.1242/jcs.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Projection structure of a N-terminal deletion mutant of connexin 26 channel with decreased central pore density. Cell Commun Adhes. 2008;15:85–93. doi: 10.1080/15419060802013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima A, Tani K, Toloue MM, Hiroaki Y, Smock A, Inukai S, Cone A, Nicholson BJ, Sosinsky GE, Fujiyoshi Y. Asymmetric configurations and N-terminal rearrangements in connexin26 gap junction channels. J Mol Biol. 405:724–735. doi: 10.1016/j.jmb.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer RA, Laird DW, Revel J-P, Johnson RG. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol. 1992;119:179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- 13.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, Nieman BJ, Peterson K, Qu D, Roscoe W, Shao Q, Tong D, Veitch GI, Voronina I, Vukobradovic I, Wood GA, Zhu Y, Zirngibl RA, Aubin JE, Bai D, Bruneau BG, Grynpas M, Henderson JE, Henkelman RM, McKerlie C, Sled JG, Stanford WL, Laird DW, Kidder GM, Adamson SL, Rossant J. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132:4375–4386. doi: 10.1242/dev.02011. [DOI] [PubMed] [Google Scholar]
- 16.Churko JM, Chan J, Shao Q, Laird DW. The G60S Connexin43 Mutant Regulates Hair Growth and Hair Fiber Morphology in a Mouse Model of Human Oculodentodigital Dysplasia. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.183. [DOI] [PubMed] [Google Scholar]
- 17.Manias JL, Plante I, Gong XQ, Shao Q, Churko J, Bai D, Laird DW. Fate of connexin43 in cardiac tissue harbouring a disease-linked connexin43 mutant. Cardiovasc Res. 2008;80:385–395. doi: 10.1093/cvr/cvn203. [DOI] [PubMed] [Google Scholar]
- 18.Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, Grummer R, Kretz M, Lewalter T, Tiemann K, Winterhager E, Herzog V, Willecke K. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 20.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J of Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Moreno AP, Lau AF. Gap junction channel gating modulated through protein phosphorylation. Prog Biophys Mol Biol. 2007;94:107–119. doi: 10.1016/j.pbiomolbio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 26.Jeyaraman M, Tanguy S, Fandrich RR, Lukas A, Kardami E. Ischemia-induced dephosphorylation of cardiomyocyte connexin-43 is reduced by okadaic acid and calyculin A but not fostriecin. Mol Cell Biochem. 2003;242:129–134. [PubMed] [Google Scholar]
- 27.Turner MS, Haywood GA, Andreka P, You L, Martin PE, Evans WH, Webster KA, Bishopric NH. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ Res. 2004;95:726–733. doi: 10.1161/01.RES.0000144805.11519.1e. [DOI] [PubMed] [Google Scholar]
- 28.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 29.Stagg RB, Fletcher WH. The hormone-induced regulation of contact-dependent cell-cell communication by phosphorylation. Endocr Rev. 1990;11:302–325. doi: 10.1210/edrv-11-2-302. [DOI] [PubMed] [Google Scholar]
- 30.Moreno AP. Connexin phosphorylation as a regulatory event linked to channel gating. Biochim Biophys Acta. 2005;1711:164–171. doi: 10.1016/j.bbamem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: Molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- 32.Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273:67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampe PD. Analyzing phorbol ester effects on gap junction communication: A dramatic inhibition of assembly. J Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beardslee M, Laing J, Beyer E, Saffitz J. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 36.Berthoud VM, Ledbetter MLS, Hertzberg EL, Saez JC. Connexin43 in MDCK cells: Regulation by a tumor-promoting phorbol ester and calcium. Eur J Cell Biol. 1992;57:40–50. [PubMed] [Google Scholar]
- 37.Brissette JL, Kumar NM, Gilula NB, Dotto GP. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol Cell Biol. 1991;11:5364–5371. doi: 10.1128/mcb.11.10.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadle R, Zhang JT, Nicholson BJ. Tissue-specific distribution of differentially phosphorylated forms of Cx43. Mol Cell Biol. 1991;11:363–369. doi: 10.1128/mcb.11.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of gap junction protein connexin43 in junctional communication-competent and deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lampe PD, Kurata WE, Warn-Cramer B, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J Cell Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- 42.Laird DL, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in Brefeldin A-treated rat mammary tumor cells. J Cell Biol. 1995;131:1193–1203. doi: 10.1083/jcb.131.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- 44.Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berthoud VM, Beyer EC, Kurata WE, Lau AF, Lampe PD. The gap junction protein connexin 56 is phosphorylated in the intracellular loop and the carboxy-terminal region. Eur J Biochem. 1997;244:89–97. doi: 10.1111/j.1432-1033.1997.00089.x. [DOI] [PubMed] [Google Scholar]
- 46.Kothmann WW, Li X, Burr GS, O’Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J Biol Chem. 1997;272:22824–22831. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- 48.Traub O, Hertlein B, Kasper M, Eckert R, Krisciukaitis A, Hulser D, Willecke K. Characterization of the gap junction protein connexin37 in murine endothelium, respiratory epithelium, and after transfection in human HeLa cells. Eur J Cell Biol. 1998;77:313–322. doi: 10.1016/S0171-9335(98)80090-3. [DOI] [PubMed] [Google Scholar]
- 49.Atkinson MM, Lampe PD, Lin HH, Kollander R, Li XR, Kiang DT. Cyclic AMP modifies the cellular distribution of connexin 43 and induces a persistent increase in the junctional permeability of mouse mammary tumor cells. J Cell Sci. 1995;108:3079–3090. doi: 10.1242/jcs.108.9.3079. [DOI] [PubMed] [Google Scholar]
- 50.Burghardt RC, Barhoumi R, Sewall TC, Bowen JA. Cyclic AMP induces rapid increases in gap junction permeability and changes in the cellular distribution of connexin43. J Membr Biol. 1995;148:243–253. doi: 10.1007/BF00235042. [DOI] [PubMed] [Google Scholar]
- 51.TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol. 2001;155:1307–1318. doi: 10.1083/jcb.200102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah MM, Martinez AM, Fletcher WH. The connexin43 gap junction protein is phosphorylated by protein kinase A and protein kinase C: In vivo and in vtitro studies. Mol Cell Biochem. 2002;238:57–68. doi: 10.1023/a:1019902920693. [DOI] [PubMed] [Google Scholar]
- 53.Yogo K, Ogawa T, Akiyama M, Ishida N, Takeya T. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulosa cells. FEBS Lett. 2002;531:132–136. doi: 10.1016/s0014-5793(02)03441-5. [DOI] [PubMed] [Google Scholar]
- 54.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- 56.Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci. 2004;117:507–514. doi: 10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- 57.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of Connexin 43 Channels Is Regulated Through Protein Kinase C-Dependent Phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 59.Bowling N, Huang X, Sandusky GE, Fouts RL, Mintze K, Esterman M, Allen PD, Maddi R, McCall E, Vlahos CJ. Protein kinase C-alpha and -epsilon modulate connexin-43 phosphorylation in human heart. J Mol Cell Cardiol. 2001;33:789–798. doi: 10.1006/jmcc.2000.1349. [DOI] [PubMed] [Google Scholar]
- 60.Lin D, Zhou J, Zelenka PS, Takemoto DJ. Protein kinase Cgamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci. 2003;44:5259–5268. doi: 10.1167/iovs.03-0296. [DOI] [PubMed] [Google Scholar]
- 61.Doble BW, Ping P, Kardami E. The epsilon subtype of protein kinase C is required for cardiomyocyte connexin-43 phosphorylation. Circulation Research. 2000;86:293–301. doi: 10.1161/01.res.86.3.293. [DOI] [PubMed] [Google Scholar]
- 62.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 63.Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol. 2003;284:C511–520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanemitsu MY, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ. 1998;9:13–21. [PubMed] [Google Scholar]
- 65.Nagy JI, Li WEI, Roy C, Doble BW, Gilchrist JS, Kardami E, Hertzberg EL. Selective monoclonal antibody recognition and cellular localization of an unphosporylated form of connexin43. Exper Cell Res. 1997;236:127–136. doi: 10.1006/excr.1997.3716. [DOI] [PubMed] [Google Scholar]
- 66.Li WE, Ochalski PA, Hertzberg EL, Nagy JI. Immunorecognition, ultrastructure and phosphorylation status of astrocytic gap junctions and connexin43 in rat brain after cerebral focal ischaemia. Eur J Neurosci. 1998;10:2444–2463. doi: 10.1046/j.1460-9568.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 67.Jain SK, Schuessler RB, Saffitz JE. Mechanisms of delayed electrical uncoupling induced by ischemic preconditioning. Circ Res. 2003;92:1138–1144. doi: 10.1161/01.RES.0000074883.66422.C5. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Levin MD, Xiong Y, Petrenko N, Patel VV, Radice GL. N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility. J Mol Cell Cardiol. 2008;44:597–606. doi: 10.1016/j.yjmcc.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Sundset R, Ytrehus K, Zhang Y, Saffitz JE, Yamada KA. Repeated simulated ischemia and protection against gap junctional uncoupling. Cell Commun Adhes. 2007;14:239–249. doi: 10.1080/15419060701821149. [DOI] [PubMed] [Google Scholar]
- 71.Cruciani V, Mikalsen SO. Stimulated phosphorylation of intracellular connexin43. Exp Cell Res. 1999;251:285–298. doi: 10.1006/excr.1999.4574. [DOI] [PubMed] [Google Scholar]
- 72.Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Lee GJ, Mackey MR, Lampe PD. The C-terminus of Connexin43 adopts different conformations in the golgi and gap junction as detected with structure specific antibodies. Biochem J. 2007;408:375–385. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation of Cx43 at S365 is a gatekeeper event that changes the structure of Cx43 and prevents downregulation by PKC. J Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leykauf K, Durst M, Alonso A. Phosphorylation and subcellular distribution of connexin43 in normal and stressed cells. Cell Tissue Res. 2003;311:23–30. doi: 10.1007/s00441-002-0645-5. [DOI] [PubMed] [Google Scholar]
- 75.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solan JL, Lampe PD. Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun Adhes. 2008;15:75–84. doi: 10.1080/15419060802014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Procida K, Jorgensen L, Schmitt N, Delmar M, Taffet SM, Holstein-Rathlou NH, Nielsen MS, Braunstein TH. Phosphorylation of connexin43 on serine 306 regulates electrical coupling. Heart Rhythm. 2009;6:1632–1638. doi: 10.1016/j.hrthm.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci. 2006;119:3435–3442. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol. 2001;154:815–827. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin R, Martyn KD, Guyette CV, Lau AF, Warn-Cramer BJ. v-Src tyrosine phosphorylation of connexin43: regulation of gap junction communication and effects on cell transformation. Cell Commun Adhes. 2006;13:199–216. doi: 10.1080/15419060600848516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- 82.Collignon F, Wetjen NM, Cohen-Gadol AA, Cascino GD, Parisi J, Meyer FB, Marsh WR, Roche P, Weigand SD. Altered expression of connexin subtypes in mesial temporal lobe epilepsy in humans. J Neurosurg. 2006;105:77–87. doi: 10.3171/jns.2006.105.1.77. [DOI] [PubMed] [Google Scholar]
- 83.Nakase T, Fushiki S, Naus CC. Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke. 2003;34:1987–1993. doi: 10.1161/01.STR.0000079814.72027.34. [DOI] [PubMed] [Google Scholar]
- 84.De Maio A, Vega VL, Contreras JE. Gap junctions, homeostasis, and injury. J Cell Physiol. 2002;191:269–282. doi: 10.1002/jcp.10108. [DOI] [PubMed] [Google Scholar]
- 85.Fushiki S, Perez Velazquez JL, Zhang L, Bechberger JF, Carlen PL, Naus CC. Changes in neuronal migration in neocortex of connexin43 null mutant mice. J Neuropathol Exp Neurol. 2003;62:304–314. doi: 10.1093/jnen/62.3.304. [DOI] [PubMed] [Google Scholar]
- 86.Cina C, Maass K, Theis M, Willecke K, Bechberger JF, Naus CC. Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. J Neurosci. 2009;29:2009–2021. doi: 10.1523/JNEUROSCI.5025-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montoro RJ, Yuste R. Gap junctions in developing neocortex: a review. Brain Res Brain Res Rev. 2004;47:216–226. doi: 10.1016/j.brainresrev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka M, Yamaguchi K, Tatsukawa T, Nishioka C, Nishiyama H, Theis M, Willecke K, Itohara S. Lack of Connexin43-mediated bergmann glial gap junctional coupling does not affect cerebellar long-term depression, motor coordination, or eyeblink conditioning. Front Behav Neurosci. 2008;2:1. doi: 10.3389/neuro.08.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hesketh GG, Van Eyk JE, Tomaselli GF. Mechanisms of gap junction traffic in health and disease. J Cardiovasc Pharmacol. 2009;54:263–272. doi: 10.1097/FJC.0b013e3181ba0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Severs NJ. The cardiac gap junction and intercalated disc. Int J Cardiol. 1990;26:137–173. doi: 10.1016/0167-5273(90)90030-9. [DOI] [PubMed] [Google Scholar]
- 92.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res. 1997;80:88–94. doi: 10.1161/01.res.80.1.88. [DOI] [PubMed] [Google Scholar]
- 93.Severs NJ. Connexins in the Heart. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; New York: 2009. pp. 435–456. [Google Scholar]
- 94.Miura T, Ohnuma Y, Kuno A, Tanno M, Ichikawa Y, Nakamura Y, Yano T, Miki T, Sakamoto J, Shimamoto K. Protective role of gap junctions in preconditioning against myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;286:H214–221. doi: 10.1152/ajpheart.00441.2003. [DOI] [PubMed] [Google Scholar]
- 95.Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003;17:1355–1357. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- 96.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, Liu FY, Zhang J, Lent DS, Morley GE, Fishman GI. Phosphatase-Resistant Gap Junctions Inhibit Pathological Remodeling and Prevent Arrhythmias. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Axelsen L, Stahlhut M, Mohammed S, Larsen B, Nielsen M, Holstein-Rathlou N, Andersen S, Jensen O, Hennan J, Kjølbye A. J Mol Cell Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 98.Saffitz JE, Hames KY, Kanno S. Remodeling of gap junctions in ischemic and nonischemic forms of heart disease. J Membr Biol. 2007;218:65–71. doi: 10.1007/s00232-007-9031-2. [DOI] [PubMed] [Google Scholar]
- 99.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 100.Li X, Heinzel FR, Boengler K, Schulz R, Heusch G. Role of connexin 43 in ischemic preconditioning does not involve intercellular communication through gap junctions. J Mol Cell Cardiol. 2004;36:161–163. doi: 10.1016/j.yjmcc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 101.Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740–1742. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- 102.Schwanke U, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin 43-deficient mice--a further in vivo study. Basic Res Cardiol. 2003;98:181–182. doi: 10.1007/s003950300002. [DOI] [PubMed] [Google Scholar]
- 103.Kanno S, Kovacs A, Yamada KA, Saffitz JE. Connexin43 as a determinant of myocardial infarct size following coronary occlusion in mice. J Am Coll Cardiol. 2003;41:681–686. doi: 10.1016/s0735-1097(02)02893-0. [DOI] [PubMed] [Google Scholar]
- 104.Schulz R, Heusch G. Connexin 43 and ischemic preconditioning. Cardiovasc Res. 2004;62:335–344. doi: 10.1016/j.cardiores.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 105.Ruiz-Meana M, Rodriguez-Sinovas A, Cabestrero A, Boengler K, Heusch G, Garcia-Dorado D. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2008;77:325–333. doi: 10.1093/cvr/cvm062. [DOI] [PubMed] [Google Scholar]
- 106.Salameh A, Schneider P, Muhlberg K, Hagendorff A, Dhein S, Pfeiffer D. Chronic regulation of the expression of gap junction proteins connexin40, connexin43, and connexin45 in neonatal rat cardiomyocytes. Eur J Pharmacol. 2004;503:9–16. doi: 10.1016/j.ejphar.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 107.Lopez D, Rodriguez-Sinovas A, Agullo E, Garcia A, Sanchez JA, Garcia-Dorado D. Replacement of connexin 43 by connexin 32 in a knock-in mice model attenuates aortic endothelium-derived hyperpolarizing factor-mediated relaxation. Exp Physiol. 2009;94:1088–1097. doi: 10.1113/expphysiol.2009.048413. [DOI] [PubMed] [Google Scholar]
- 108.Gray MO, Zhou HZ, Schafhalter-Zoppoth I, Zhu P, Mochly-Rosen D, Messing RO. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J Biol Chem. 2004;279:3596–3604. doi: 10.1074/jbc.M311459200. [DOI] [PubMed] [Google Scholar]
- 109.Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS. Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc Res. 2002;55:672–680. doi: 10.1016/s0008-6363(02)00325-5. [DOI] [PubMed] [Google Scholar]
- 110.Hund TJ, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Protein kinase Cepsilon mediates salutary effects on electrical coupling induced by ischemic preconditioning. Heart Rhythm. 2007;4:1183–1193. doi: 10.1016/j.hrthm.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Srisakuldee W, Jeyaraman MM, Nickel BE, Tanguy S, Jiang ZS, Kardami E. Phosphorylation of connexin-43 at serine 262 promotes a cardiac injury-resistant state. Cardiovasc Res. 2009;83:672–681. doi: 10.1093/cvr/cvp142. [DOI] [PubMed] [Google Scholar]
- 112.Srisakuldee W, Nickel BE, Fandrich RR, Jiang ZS, Kardami E. Administration of FGF-2 to the heart stimulates connexin-43 phosphorylation at protein kinase C target sites. Cell Commun Adhes. 2006;13:13–19. doi: 10.1080/15419060600631326. [DOI] [PubMed] [Google Scholar]
- 113.Miura T, Yano T, Naitoh K, Nishihara M, Miki T, Tanno M, Shimamoto K. Delta-opioid receptor activation before ischemia reduces gap junction permeability in ischemic myocardium by PKC-epsilon-mediated phosphorylation of connexin 43. Am J Physiol Heart Circ Physiol. 2007;293:H1425–1431. doi: 10.1152/ajpheart.01115.2006. [DOI] [PubMed] [Google Scholar]
- 114.Naitoh K, Yano T, Miura T, Itoh T, Miki T, Tanno M, Sato T, Hotta H, Terashima Y, Shimamoto K. Roles of Cx43-associated protein kinases in suppression of gap junction-mediated chemical coupling by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2009;296:H396–403. doi: 10.1152/ajpheart.00448.2008. [DOI] [PubMed] [Google Scholar]
- 115.Matsuyama D, Kawahara K. Oxidative stress-induced formation of a positive-feedback loop for the sustained activation of p38 MAPK leading to the loss of cell division in cardiomyocytes soon after birth. Basic Res Cardiol. 2011 doi: 10.1007/s00395-011-0178-8. [DOI] [PubMed] [Google Scholar]
- 116.Lu G, Haider H, Porollo A, Ashraf M. Mitochondria-specific transgenic overexpression of connexin-43 simulates preconditioning-induced cytoprotection of stem cells. Cardiovasc Res. 2010;88:277–286. doi: 10.1093/cvr/cvq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Wit C, Wolfe SE. Connexins in the Vasculature. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; New York: 2009. pp. 457–468. [Google Scholar]
- 118.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 119.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 120.Straub AC, Johnstone SR, Heberlein KR, Rizzo MJ, Best AK, Boitano S, Isakson BE. Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res. 2010;47:277–286. doi: 10.1159/000265562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, Binder BR, Weber C, Leitinger N. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–638. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- 122.Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe PD, Leitinger N, Isakson BE. Oxidized phospholipid species promote in vivo differential cx43 phosphorylation and vascular smooth muscle cell proliferation. Am J Pathol. 2009;175:916–924. doi: 10.2353/ajpath.2009.090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem. 2009;284:22332–22343. doi: 10.1074/jbc.M901619200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gould VE, Mosquera JM, Leykauf K, Gattuso P, Durst M, Alonso A. The phosphorylated form of connexin43 is up-regulated in breast hyperplasias and carcinomas and in their neoformed capillaries. Hum Pathol. 2005;36:536–545. doi: 10.1016/j.humpath.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 125.Plante I, Laird DW. Decreased levels of connexin43 result in impaired development of the mammary gland in a mouse model of oculodentodigital dysplasia. Dev Biol. 2008;318:312–322. doi: 10.1016/j.ydbio.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 126.Kretz M, Maass K, Willecke K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur J Cell Biol. 2004;83:647–654. doi: 10.1078/0171-9335-00422. [DOI] [PubMed] [Google Scholar]
- 127.Aasen T, Kelsell DP. Connexins in Skin Biology. In: Harris A, Locke D, editors. Connexins: A Guide. Humana; New York: 2009. pp. 307–322. [Google Scholar]
- 128.King TJ, Lampe PD. Temporal regulation of connexin phosphorylation in embryonic and adult tissues. Biochim Biophys Acta. 2005;1719:24–35. doi: 10.1016/j.bbamem.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Richards TS, Dunn CA, Carter WG, Usui ML, Olerud JE, Lampe PD. Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J Cell Biol. 2004;167:555–562. doi: 10.1083/jcb.200404142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kidder GM, Winterhager E. Connexins in the female reproductive system. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; New York: 2009. pp. 481–494. [Google Scholar]
- 131.Granot I, Dekel N. Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem. 1994;269:30502–30509. [PubMed] [Google Scholar]
- 132.Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- 133.Liu J, Ek Vitorin JF, Weintraub ST, Gu S, Shi Q, Burt JM, Jiang JX. Phosphorylation of connexin 50 by protein kinase a enhances gap junction and hemichannel function. J Biol Chem. 2011 doi: 10.1074/jbc.M111.218735. [DOI] [PMC free article] [PubMed] [Google Scholar]