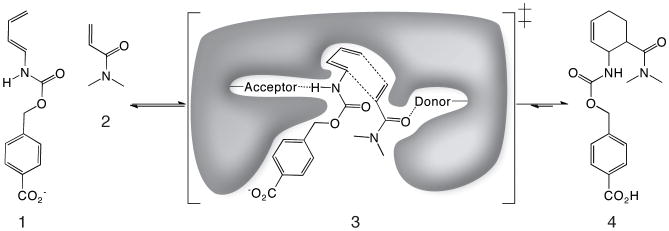
Figure 1. The Diels-Alder reaction.
Diene (1) and dienophile (2) undergo a pericyclic [4+2] cycloaddition (3) to form a chiral cyclohexene ring (4). Also shown in (3) is a schematic of the design target active site, with hydrogen bond acceptor and donor groups activating the diene and dienophile and a complementary binding pocket holding the two substrates in an orientation optimal for catalysis.