Abstract
Aims
In cardiomyocytes, protein kinase D1 (PKD1) plays a central role in the response to stress signals. From a yeast two-hybrid assay, we have identified Enigma Homolog 1 (ENH1) as a new binding partner of PKD1. Since in neurons, ENH1, associated with protein kinase Cε, was shown to modulate the activity of N-type calcium channels, and the pore-forming subunit of the cardiac L-type voltage-gated calcium channel, α1C, possesses a potential phosphorylation site for PKD1, we studied here a possible role of ENH1 and PKD1 in the regulation of the cardiac L-type voltage-gated calcium channel.
Methods and results
PKD1-interacting proteins were searched by yeast two-hybrid screening. In vivo protein interactions in cardiomyocytes isolated from heart ventricles of newborn rats were tested by co-immunoprecipitation. Small interfering RNA and a dominant negative mutant of PKD1 were delivered into cardiomyocytes by use of an adenovirus. Calcium currents were measured by the patch-clamp technique. Both ENH1 and PKD1 interact with α1C in cardiomyocytes. This interaction is increased upon stimulation. Silencing of ENH1 prevented the binding of PKD1 to α1C. Moreover, a dominant negative mutant of PKD1 or the silencing of ENH1 inhibited the α-adrenergic-induced increase of L-type calcium currents.
Conclusion
We found a new binding partner, ENH1, and a new target, α1C, for PKD1 in neonatal rat cardiomyocytes. We propose a model where ENH1 scaffolds PKD1 to α1C in order to form a signalling complex that regulates the activity of cardiac L-type voltage-gated Ca2+ channels.
Keywords: Protein kinases, Ca-channel, Signal transduction
1. Introduction
In heart, neurohumoral factors or mechanical stress activate intracellular signalling pathways that induce increases in contraction frequency and strength and lead ultimately to hypertrophy and heart failure.1,2 The effectors of signalling pathways modulate the activities of many proteins residing at different subcellular localizations, simultaneously or sequentially, to achieve the cardiomyocyte response to the stimuli. Therefore, effectors such as protein kinases have to be selectively translocated to various locations: e.g. protein kinase Cε (PKCε) is localized at transverse tubules after endothelin-1 stimulation or at the Z-discs after arachidonic acid stimulation.3,4 Anchoring and scaffolding proteins, such as A-kinase anchoring proteins (AKAP) and the Receptors for Activated C Kinase, are the proteins that fasten signalling enzymes at specific subcellular locations in close proximity to their targets.5,6 In cardiomyocytes, AKAP15 anchors protein kinase A (PKA) to the L-type voltage-gated calcium channels (L-VCC) after β-adrenergic stimulation, ensuring selective and efficient regulation of L-VCC.7
The cardiac L-VCC activity is regulated by various kinase that phoshorylate the α1C subunit.8–11 It has been suggested that PKD1 might phosphorylate serine 1928 of α1C which is surrounded by a PKD1 consensus sequence.8
PKD1 is activated downstream of PKC in various signalling pathways acting at different subcellular localizations.12 In neonatal rat cardiomyocytes, PKD1 is localized at the Z-discs after α-adrenergic stimulation.13 In heart, under stress signalling, PKD1 contributes to the development of heart hypertrophy.14 PKD1 also phosphorylates different proteins of the myofibrils, such as cardiac troponin I, telethonin, myosin-binding protein C, and myomesin.15
In the initial stage of this study, using a yeast two-hybrid (Y2H) screen, we identified ENH1 as a new binding partner of PKD1. ENH1 is a member of the PDZ-LIM protein family and suspected to play a role in cell signalling.16,17 ENH1 is composed of an N-terminal PDZ domain and a C-terminal domain containing three LIM motifs.17 ENH1 was shown to interact with α-actinin, thus localizing at the Z-discs in neonatal rat cardiomyocytes.18 In neurons, ENH1 recruits PKCε to N-type calcium channels, regulating their activity.19 From these data, we hypothesized that ENH1 could scaffold PKD1 at cardiac L-VCC, leading to the regulation of its activity.
In the present study, we show that ENH1 and PKD1 interact with α1C, the pore subunit of L-VCC in neonatal rat cardiomyocytes. ENH1 silencing with small interfering RNA (siRNA) suppressed the interaction of PKD1 with α1C. Moreover, both ENH1 silencing and expression of a dominant negative mutant of PKD1 strongly inhibited the α-adrenergic, but not the β-adrenergic, up-regulation of L-type Ca2+ currents in neonatal rat cardiomyocytes. Taken together, our results strongly suggest that ENH1, PKD1, and α1C form a signalling complex that regulates the activity of L-VCC in cardiomyocytes.
2. Methods
2.1 Construction and cloning of plasmids and deletion mutants
Constructions of the flag epitope-tagged ENH1, an ENH1 PDZ deletion mutant (dPDZ-FL), and a hemmaglutinin (HA)-tagged PKD1 have been described previously.17,20 α1C (NCBI, Acc. X15539) containing the complete coding sequence of the rabbit cardiac dihydropyridine-sensitive calcium channel fused to a myc-tag was kindly provided by Professor Lutz Birnbaumer (see Supplementary material online). Deletion mutants of the rat ENH1 LIM domains were created by PCR of the full length construct using the following reverse primers: 5′-GTAAAGCTTTCAGAAATTCACAGAATGAGCGTCT GTCTCACAGT-3′ (ENH1 lacking the C-terminal LIM domain, d1LIM-FL), 5′-GTAAAGCTTTCAGAA ATTCACAGAATGAGCA CATAGCT-CACA ATACA-3′ (ENH1 lacking 2 LIM domains at the C-terminus, d2LIM-FL) and 5′-GTAAAGCTTTCAGAAATTCACAGAATGCATGGGAGTC CGCTTCA-3′ (ENH1 lacking the 3 LIM domains, d3LIM-FL). They were all amplified with a forward primer targeting the vector (5′-ATTGGCTGAGCTGCGTTCTA-3′) and cloned into the same vector using NcoI and HindIII sites. A stop codon was fused to the amplified sequence (bold). Sequences of all constructs were verified.
2.2 Yeast two-hybrid, β-galactosidase assay
Y2H was performed as described previously.17,21 For the first Y2H screening, the catalytic domain of PKD1 and a Hela cDNA library were inserted into the bait plasmid pGilda and the prey plasmid pB42AD (Clontech), respectively. Secondly, to identify the interacting regions of the two proteins, the PDZ (1–97 amino acid) or LIM1-3 domains (415–585 amino acids) of rat ENH1 and the catalytic or the pleckstrin homology (PH) domain of PKD1 were inserted into the bait plasmid pGilda and the prey plasmid pB42AD, respectively. Relative β-galactosidase activity (U/min/ml-medium) was calculated by using the following equation, U = 1000 × ΔA578/ (t × 0.25 × A600).
2.3 Cell culture
Neonatal rat ventricular myocytes were prepared as described previously.18
Animals were sacrificed in conformity with the Guide for the Care and Use of Laboratory Animals published by the NIH (Publication no. 85–23, 1996). The cardiomyocytes were transferred to culture plates, and fibroblasts were removed by adhesion onto the plates for 1 h. The cardiomyocytes were then plated overnight and maintained in DMEM, containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. The 293T cells were cultured in DMEM, containing 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin, and transfected using Fugene6 (Roche) following the manufacturer recommendations.
2.4 Western blotting and co-immunoprecipitation
Proteins were extracted using a lysis buffer (in mM, 50 Tris, 150 NaCl, 1 EDTA, 1 EGTA, 1 dithiothreitol, 50 NaF, 1 Na3VO4, pH 7.4, 0.5% sodium deoxylate, 1% Nonidet P-40, and a tablet of protease inhibitors, Roche). The lysate was passed through a 25-gauge needle 12 times then centrifuged 20 min at 3000 r.p.m. Total cell lysates were resolved by 10% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) for the detection of ENH1 and PKD1 and 8% SDS–PAGE for the detection of α1C. Proteins were transferred to polyvinylidene difluoride membranes (Millipore) and immunoblotted with the indicated antibody. As secondary antibodies, an anti-mouse or an anti-rabbit IgG linked to horseradish peroxidase (GE Health-care Biosciences Corp.) or anti-rabbit IgG TrueBolt (eBioscience) was used. Proteins were visualized by enhanced chemiluminescence using a Biorad molecular imager gel documentation system, Chemidoc XRS (Biorad Inc.). For immunoprecipitation, 200 μg proteins from cell lysate was incubated with an anti-ENH1 polyclonal antibody,18,19 an anti-PKD1 polyclonal antibody,13 or an anti-α1C polyconal antibody (Alomone labs) on ice for 1 h and then mixed with protein G-Sepharose 4 fast flow beads (30 μL, 50% slurry). The beads were thoroughly washed with the lysis buffer and subjected to western blotting (WB) as described earlier.
2.5 RNA interference
The ‘hairpin strategy’ was used to design siRNA.22 Human U6 promoter sequence was amplified from genomic DNA by nested PCR using the following primers: 5′-CCCGAGTCCAACACCCGTGG-3′ and 5′-GGTGTTTCGTCCTTTCCACA-3′ for the first PCR, then 5′-ATAGAATTCCCGAGTCCAACACCCGTGGG-3′ and 5′-ATAGAATTCGG TGTTTCGTCCTTTCCAC AAG-3′ for the second PCR. The second PCR amplicon was cloned using EcoRI into a shuttle vector. The siRNA was cloned by the standard procedure: a forward primer specific for the vector (5′-CCGATTTCGGCCTATTGGT-3′) and a reverse primer containing a portion that anneals with the 3′ end of the U6 promoter and the specific hairpin sequence of ENH1 corresponding to positions 1698–1718 (ENH1-5) or 1452–1472 (ENH1-6) of cording sequence (ENH1-5; 5′-ATAGCGGCCGCAAAAAAAGTTTGGAA GGGCAGACATGAAGCTTGAAATGTCTGCCCTTCCAAACTCGGTGTTTCG-TCCTTTCCACA-3′, ENH1-6; 5′-ATAGCGGCCGCAAAAAAGTCATCA ATGCTTTGAAGCGAAGCTTGAAGCTTCAAAGCATTGATGACTCGGTGTTT CGTCCTTTCCACA -3′) were used to amplify the vector carrying the U6 promoter. All PCRs were performed using Pfx polymerase (Invitrogen). We selected sequences unique for rat ENH1 in the NCBI cDNA database. The PCR fragments obtained were cut using BglII and XbaI and cloned into a shuttle vector to construct the vector-based ENH1-RNAi.
2.6 Adenovirus vector constructs
Recombinant human adenoviruses-5 encoding a dominant negative mutant of PKD1 tagged with the green fluorescent protein (GFP),23 ENH1-5 RNAi, ENH1-6 RNAi, and the control RNAi were constructed, amplified, and purified as described previously.24
2.7 RNase protection assay
RNase protection assay (RPA) was performed as described previously.25 Total RNA was extracted from neonatal rat cardiomyocytes with an acid phenol–guanidinium reagent (TRI-Reagent, Molecular Research Center, Inc.) according to the manufacturer’s instructions. The riboprobe was prepared from the rat ENH cDNA and subcloned in pGEM-T-easy (Promega). Transcription with T7 RNA polymerase of the BamHI-linearized plasmid yielded a riboprobe of 266 nucleotides (nt), and a 207-nt protected fragment. As an internal control for RNA loading, a rat GAPDH probe was included in the hybridization mixture. The GAPDH plasmid digested with HinfI produced a riboprobe of 185-nt and a 154-nt protected band after RNase digestion. Autoradiographic signals were detected with a PhosphoImager (Molecular Dynamics, Inc.).
2.8 Patch-clamp
The activity of L-VCC in a single neonatal rat cardiomyocyte was recorded by patch-clamp in the voltage-clamp mode and the whole cell configuration.26 The bath solution contained (in mM) 125 N-methyl-D-glucamine, 5 4-aminopyridine, 20 tetraethylammonium chloride, 2 CaCl2, 2 MgCl2, 10 D-glucose, and 10 Hepes, pH 7.4. The patch pipettes solution contained (in mM) 130 CsCl, 10 EGTA, 3 MgATP, 0.4 LiGTP, and 25 Hepes, pH 7.2. The reference electrode was placed in a KCl solution linked to the bath with an agar bridge, reducing the liquid junction potential to negligible values. The cell was voltage-clamped using an Axopatch 200B amplifier (Axon CNS, Molecular Devices) at a holding potential of −40 mV to avoid T-type Ca2+ currents contamination and depolarized as indicated in the figure legends. The currents were filtered at 1–2 kHz and sampled at 5 kHz using a Digidata 1322A (Axon CNS). The leak was subtracted automatically by a P/4 protocol (pclamp9, Axon CNS). Cardiomyocytes were stimulated with either Isoproterenol (Iso) or Phenylephrine (PE) (Sigma-Aldrich).
3. Results
3.1 PKD1 interacts with the LIM2 domain of ENH1
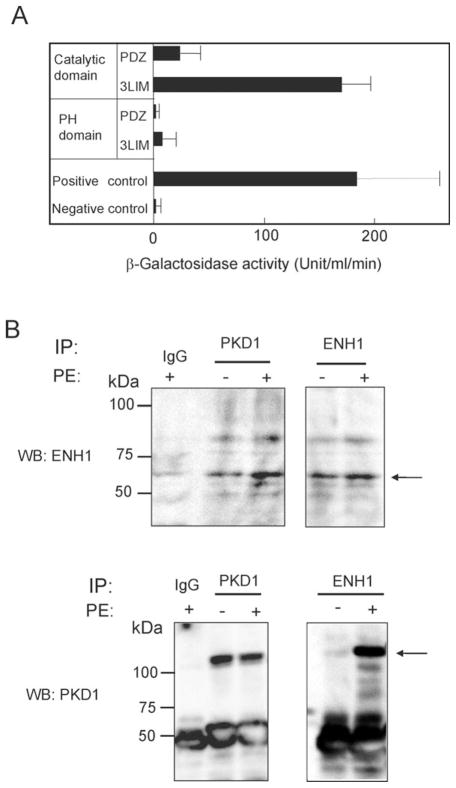
In the initial stage of this study, we aimed at identifying proteins interacting with PKD1. A first Y2H screening of a Hela cell cDNA library using the catalytic domain of PKD1 as bait could identify ENH1 (data not shown). To further specify the interacting regions of the two proteins, we performed the second Y2H and found that the interaction between the PKD1 catalytic domain and ENH1 LIM1-3 domains was as strong as our positive control (Figure 1A). As for other protein motifs in PKD1, the PH domain neither interacted with the PDZ domain nor with the LIM1-3 domains of ENH1. This result suggests that ENH1 and PKD1 may interact with each other in cardiomyocytes, where both proteins are expressed.13,17,18,27 We tested the interaction between ENH1 and PKD1 in neonatal rat cardiomyocytes by immunoprecipitation. Both ENH1 and PKD1 were detected by immunoblotting in PKD1 and ENH1 immunoprecipitates, respectively, the interaction being stronger after PE stimulation than without any stimulation (Figure 1B). PE, an α-adrenergic agonist is known to activate PKD1.13,27 Thus these results strongly suggest that these proteins interact in vivo, and that α-adrenergic stimulation enhanced this interaction.
Figure 1.
PKD1 binds to ENH1 in vitro and in vivo. (A) The PDZ domain and the LIM domains of ENH1 were used as prey in the Y2H assay. The catalytic and the pleckstrin homology domains of PKD1 were used as bait. The β-galactosidase activity in yeast cells was measured as described in Methods. Error bars indicate the standard deviation. (B) ENH1 and PKD1 were immunoprecipitated from the cell extract prepared from the control or PE-stimulated neonatal rat cardiomyocytes. Immunoprecipitated proteins were analysed by WB using an anti-ENH1 (WB:ENH1) antibody or an anti-PKD1 (WB:PKD1) antibody. Immunoblots are representative of three independent experiments.
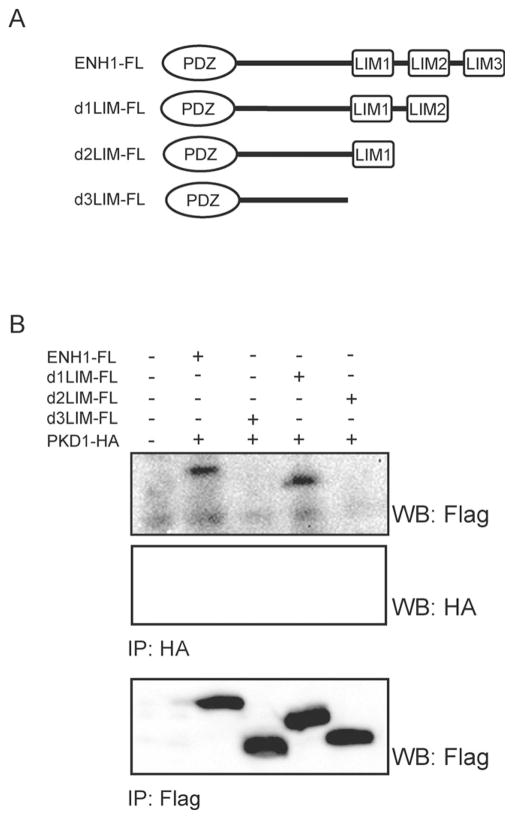
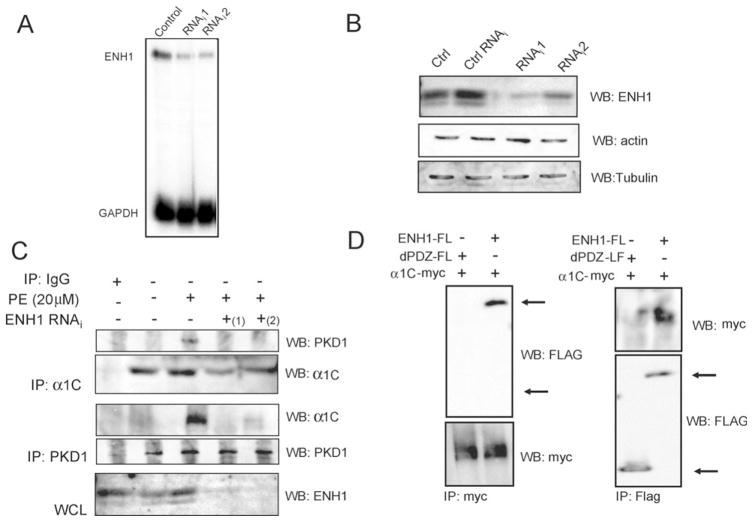
In order to identify which one of the three ENH1 LIM1-3 motifs mediates the interaction with PKD1, we constructed deletion mutants with a flag tag, as depicted in Figure 2A. We then co-expressed them in 293 cells with the full-length HA-tagged PKD1 (HA-PKD1). Immunoprecipitation experiments showed that d2LIM-FL and d3LIM-FL could not be detected in HA-PKD1 immunoprecipitates. These results show that the LIM2 motif of ENH1 is responsible for the interaction with PKD1. Thus, the catalytic domain of PKD1 and the LIM2 motif of ENH1 are involved in the interaction between the two proteins.
Figure 2.
PKD1 is bound to the second LIM domain of ENH1. Flag-tagged ENH1 (ENH1-Fl) or LIM deletion mutants (d1LIM-FL, d2LIM-FL, and d3LIM-FL) schematically presented in (A) were co-expressed with an HA-tagged PKD1 in 293T cells by transient transfection. From the 293T cell extract, either ENH1-FL or PKD1-HA was immunoprecipitated using anti-Flag (IP:Flag) or anti-HA (IP:HA) monoclonal antibodies, respectively. Immunoprecipitated proteins were analysed by WB using an HRP conjugated anti-Flag (WB:Flag) or anti-HA monoclonal antibodies (WB:HA).
3.2 ENH1 and PKD1 interact with the α1C subunit of the L-VCC
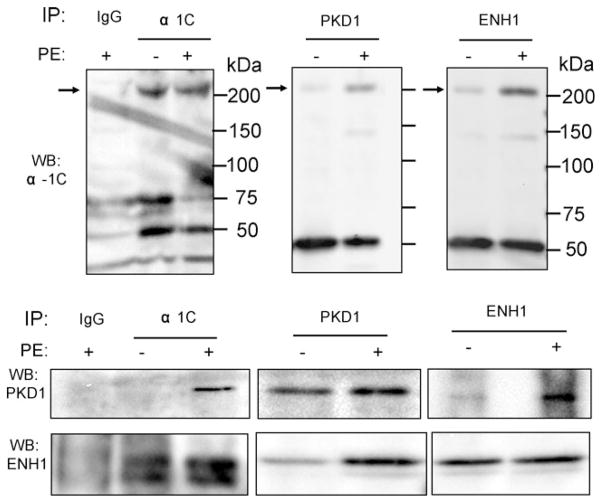
In neurons, ENH1 recruits PKCε to the N-type voltage-gated Ca2+ channel.20 We therefore hypothesized that PKD1 bound to ENH1 could similarly be recruited to LVCC in neonatal rat cardiomyocytes. In order to test this, cardiomyocytes were stimulated or not with PE for 20 min and total proteins were extracted and split in three for parallel immunoprecipitations. Each binding partner was immunoprecipitated and the complex formation was examined by WB analysis. Upon PE stimulation PKD1 and ENH1 were detected by immuno-blotting in the α1C immunoprecipitates (Figure 3). Concomitantly, PKD1 and α1C, or ENH1 and α1C were detected in the ENH1 and PKD1 immunoprecipitates, respectively. Interestingly, under non-stimulated conditions these proteins also co-immunoprecipitated, but to a much lower extent (Figure 3). However, in non-stimulated conditions, PKD1 was detected in immunoprecipitation from cell lysates that were not split (data not shown). Immunofluorescence showed colocalization between ENH1 and α1C in cardiomyocytes (see Supplementary material online, Figure S1). This suggests that ENH1, PKD1, and α1C complex formation is enhanced upon PE stimulation.
Figure 3.
PKD1 and ENH1 interact with L-VCC. Neonatal rat cardiomyocytes were stimulated or not with PE for 20 min and protein were extracted. PKD1, α1C, and ENH1 were immunoprecipitated using their respective specific antibodies. Immunoprecipitates were analysed by WB (n = 3–5) using anti-PKD1 (WB:PKD1), anti-ENH1 (WB:ENH1), and anti-α1C (WB:α1C) antibodies. Each experiment was repeated three to five times.
3.3 Dominant negative PKD1 mutant prevents the α-adrenergic-induced increase of L-type Ca2+ currents
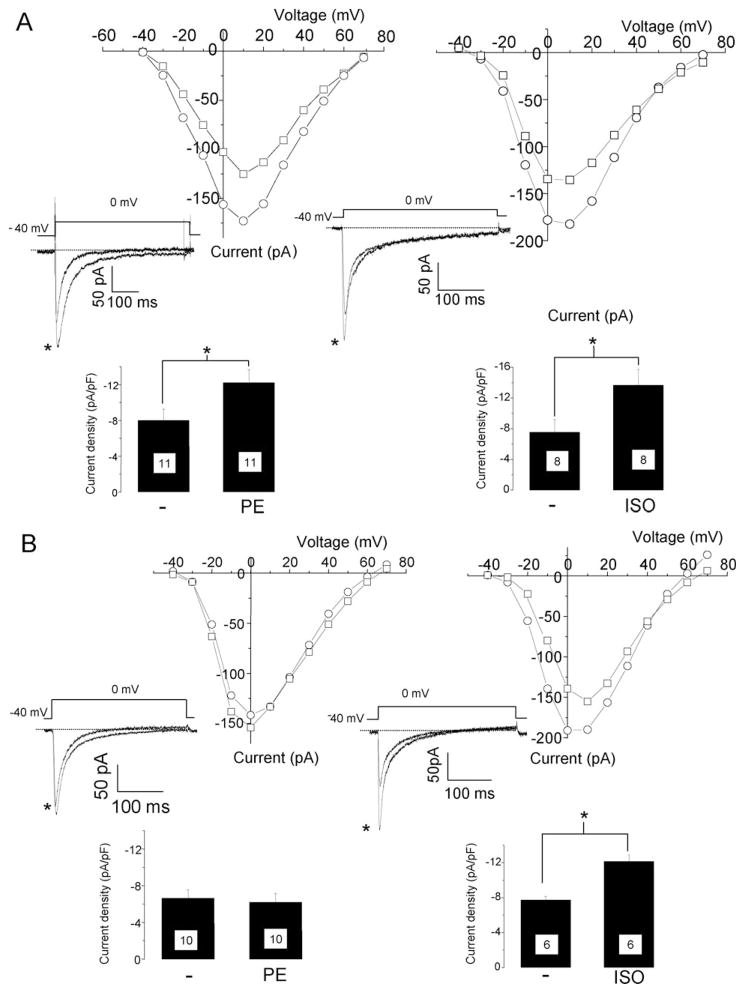
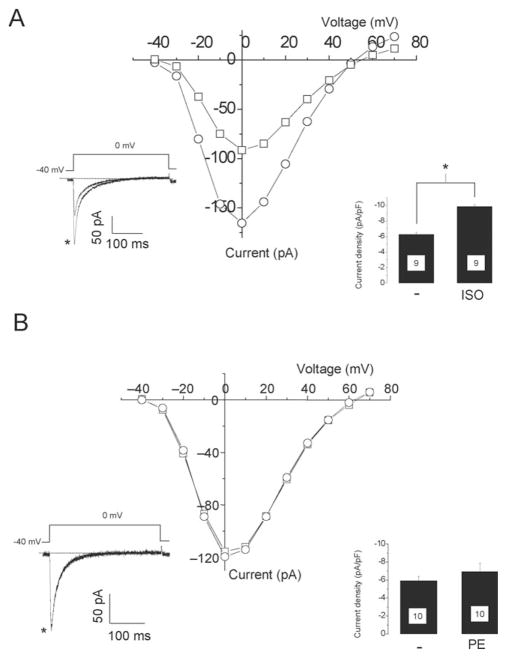
To test if PKD1 can modulate the activity of L-VCC, we measured the L-type Ca2+ currents in neonatal rat cardiomyocytes by patch-clamp. The application of 20 μM PE or 1 μM ISO induced a significant increase of L-type Ca2+ current density (81% for ISO and 53% for PE, Figure 4A) as previously reported.28 Because no specific inhibitors for PKD1 are currently available, a dominant negative mutant of PKD1 tagged with GFP (PKD1-DN-GFP) was expressed in neonatal rat cardiomyocytes using an adenoviral vector. Currents were measured at 24–36 h after the infection. The spontaneous contractions and the morphology of cardiomyocytes were not affected by the expression of PKD1-DN-GFP (data not shown). The membrane capacitance of cardiomyocyte, reflecting the membrane surface, was also not significantly changed (control: 17.00 ± 2.08pF, n = 19, PKD1-DN-GFP: 18.60 ± 1.13pF, n = 20, values are mean and s.e.m). In the PKD1-DN-GFP cardiomyocytes, PE 20 μM did not induce an increase of the L-type Ca2+ current density as observed in the control cells (Figure 4B). However, 1 μM ISO caused an increase in the L-type Ca2+ current density similar to the control cardiomyocytes (Figure 4B).
Figure 4.
A dominant negative mutant of PKD1 inhibits α-adrenergic enhancement of L-type Ca2+ currents in neonatal rat cardiomyocytes. The cells were cultured on glass bottom dishes and L-type Ca2+ currents were measured by patch-clamp. Membrane potential was held at −40 mV to avoid contamination by T-type Ca2+ currents. Current–voltage (IV) relationship before (square) and 10 min after addition of 20 μM PE (left) or 1 μM ISO (right) measured on a particular cardiomyocyte in control condition (A) or infected with a GFP-tagged PKD1 dominant negative mutant adenovirus (B). Insets are traces obtained upon depolarization from −40 mV to 0 mV before and after PE (left,*) or ISO (right,*) addition. Lower bar graph of the current density (pA/pF) with mean and standard error of the mean (s.e.m.) measured at 0 mV. Number of measured cells is indicated in the bar graph. *P < 0.02.
PKC is assumed to mediate the activation of L-VCC and PKD1 in cardiac cell.10,28 Inhibition of PKC using specific inhibitors, Chelerythrine and GF109203X, completely blocked the increase of calcium currents in response to PE (see Supplementary material online, Figure S2), suggesting that the PKD1 modulation of L-VCC activity is mediated by PKC.
The regulation of L-VCC by PKD1 might be mediated by the phosphorylation of serine residues on α1C. The C-terminal domain of α1C contains two serine residues occurring in a consensus site specific for PKD1 (LXRXXS).11 In vitro phosphorylation assay showed that recombinant active PKD1 could phosphorylate the C-terminal domain of α1C (see Supplementary material online, Figure S3). Thus PKD1 could directly phosphorylate α1C to modulate the activity of L-VCC. Taken together, these results suggest that PKD1 modulates the activity of L-VCC, in a PKC-dependent manner, selectively in response to the α-adrenergic- but not to β-adrenergic-stimulation.
3.4 ENH1 is necessary for PDK1 binding to the α1C subunit of L-VCC
Anchoring proteins specifically hook kinases to their targets.5,6 Therefore, we aimed at testing if the binding of PKD1 to L-VCC was direct or if it required ENH1, serving as a scaffold protein for PKD1. Although we have shown above that the proteins constitute a part of the same complex (Figures 2 and 3), no indication has been given on which partners directly interact. To test this, ENH1 expression was silenced using siRNA delivered by an adenoviral vector in neonatal rat cardiomyocytes and the complex formation was analysed. First, both ENH1 messenger RNA and protein were efficiently reduced by two different siRNAs 48 h after the infection (Figure 5A and B). We then proceeded to the immunoprecipitation of PKD1 and α1C upon ENH1 silencing (Figure 5C). The presence of the binding partner after PE stimulation was used as a positive control. PKD1 or α1C could not be detected in the α1C and PKD1 immunoprecipitates obtained from the lysate of the ENH1-RNAi-treated cardiomyocytes stimulated with 20 μM PE. Thus, these results demonstrate that ENH1 was necessary for the anchoring of PKD1 to the α1C subunit of the L-VCC and suggest that this anchoring was related to a specific stimulation of L-VCC.
Figure 5.
ENH1 siRNA inhibits PKD1 interaction with α1C in neonatal rat cardiomyocytes. Cells were infected 48 h with the ENH1-RNAi adenovirus. Then total RNA and proteins were extracted. (A) WB analyses for ENH1, actin, and tubulin as control (Ctrl), under the unrelated siRNA (Ctrl RNAi), ENH1 RNAi (RNAi1, RNAi2) conditions. (B) Detection of ENH1 and GAPDH RNA messenger by RPA from control, RNAi1, and RNAi2 conditions. ENH1 siRNA efficiency was tested independently by WB (n = 3) or RPA (n = 3). (C) Protein extracts from control or infected with ENH1 RNAi adenovirus conditions were immunoprecipitated using an anti-PKD1 antibody or an anti-α1C antibody (n = 3). Precipitates were analysed by WB. The lowest panel, WB shows ENH1 expression. (D) Flag-tagged ENH1 (ENH1-FL) and PDZ deletion mutant ENH1 (dPDZ-FL) were co-transfected with myc-tagged α1C (α1C-myc) in 293T cells. ENH1 or PDZ deletion mutants-Fl or α1C-myc were immunoprecipitated from cell extracts using an anti-Flag (IP:Flag) or an anti-myc (IP:myc) monoclonal antibodies. Immunoprecipitates were analysed by WB using monoclonal HRP conjugated anti-Flag (WB:Flag) or anti-myc antibodies (WB:myc).
In order to identify which domain of ENH1 mediates its interaction with α1C, deletion mutants of ENH1 were co-expressed with the myc-tagged α1C cDNA in 293 cells. Deletion of the PDZ domain suppressed the interaction between ENH1 and α1C (Figure 5D). However, deletion of LIM motifs had no effect on the interaction between both proteins (data not shown). Thus, the interaction between ENH1 and α1C involves the PDZ domain of ENH1.
3.5 ENH1 is required for α-adrenergic induced increase of L-type Ca21 currents
We found that ENH1 was necessary for anchoring PKD1 to L-VCC. Thus, ENH1 might also participate in the regulation of L-VCC activity. To test this hypothesis, ENH1 was silenced by RNAi in neonatal rat cardiomyocytes and L-type Ca2+ currents were measured. ENH1-RNAi adenovirus infection did not significantly change the cell capacitance, 17.00 ± 2.08pF vs. 16.54 ± 0.85pF, mean and s.e.m, for the control (n = 19) and ENH1 RNAi (n = 21), respectively. Stimulation with 1 μM ISO induced a significant increase of the L-type Ca2+ currents as measured in control conditions (Figure 6A). However, the increase of L-type Ca2+ currents elicited with 20 μM PE was strongly reduced upon ENH1 silencing (Figure 6B). A slight increase (16%) of the L-type Ca2+ current density after the application of PE was not significant (P = 0.2).
Figure 6.
ENH1 siRNA inhibits PE-induced L-type Ca2+ currents increase in neonatal cardiomyocytes. The cells were infected with the ENH1-RNAi adenovirus and L-type Ca2+ currents were measured 48 h after infection. IV relationship of a particular cardiomyocyte recorded before (square) and after (circle) addition of (A) 1 μM ISO or (B) 20 μM PE. Left inset shows traces recorded at 0 mV depolarization step from a −40 mV holding potential before and after (*) stimulation. Right insets are mean and s.e.m. of the current density. The number of tested cells is indicated in the bar graph. *P < 0.02.
These data are in good agreement with those using the PKD1 mutant (Figure 4B) and support our proposal for ENH1 being necessary for the recruitment of the signalling machinery and the regulation, upon α-adrenergic- but not β-adrenergic-stimulation, of L-VCC in neonatal rat cardiomyocytes.
4. Discussion
Our study identified ENH1 as a new interacting partner for PKD1 in neonatal rat cardiomyocytes. To our knowledge, this is the first report of the interaction of PKD1 with an LIM domain. Furthermore, we have found that both ENH1 and PKD1 interacted with α1C, with ENH1 playing the role of scaffold protein. We have further shown that LIM2 motif of ENH1 bound to PKD1 (Figure 2B) and its PDZ domain anchors α1C (Figure 5D). Thus, ENH1 is the scaffold that links PKD1 and α1C in a functional complex. This conclusion was supported by the data obtained using ENH1 siRNA: when the ENH1 was silenced, the complex formation was abolished. Finally, both silencing of ENH1 and expression of a PKD1 dominant negative mutant inhibited the increase of the L-type Ca2+ currents induced by the α-adrenergic- but not β-adrenergic- stimulation. We therefore concluded that ENH1 scaffolds PKD1 to α1C which in turn is regulated by the kinase.
Immunoprecipitation results suggest that ENH1, PKD1, and α1C are pre-assembled under basal conditions (Figure 3), but this complex is probably not functional because neither the dominant negative mutant of PKD1 nor the ENH1 silencing inhibited the basal L-type Ca2+ current. The pre-assembled complex PKD1–ENH1–α1C in the basal condition might contribute to a rapid and specific regulation of L-VCC upon stimulation. The binding between ENH1 and α1C in non-stimulated conditions was obvious, suggesting that the scaffold protein might constitutively be bound to the channel. Since upon stimulation, both ENH1 and PKD1 levels are increased, it is tempting to speculate that conformational changes might also occur: the affinity between ENH1 and PKD1 becoming more important. Other players might take part of the channel regulation, such as PKC. Upon α-adrenergic activation PKC might bind one of the LIM domains of ENH1 after translocation.17 PKC phosphorylation of ENH117 could be responsible for the enhanced interactions within the PKD1-ENH1-α1C complex. Simultaneously, PKC might activate PKD1 being already bound to ENH1. Further experiments will be required to validate these hypotheses.
To investigate the role of PKD1 in the modulation of the L-VCC, we measured L-type Ca2+ currents in neonatal rat cardiomyocytes that expressed a dominant negative mutant of PKD1. The increase of L-type Ca2+ currents induced by α-adrenergic stimuli was inhibited by the expression of the PKD1 mutant (Figure 4). In contrast, the β-adrenergic enhancement was not affected by the PKD1 mutant. Furthermore, PKC inhibition prevents the current increase after PE stimulation (see Supplementary material online, Figure S2). This result suggests that α-adrenergic regulation of the L-VCC involves both PKC, as reported previously,27 but also PKD1, whose modulation of the L-VCC depends on PKC activity upon PE stimulation.26 Our study provides the first evidence that PKD1 functionally regulates the activity of the L-VCC in neonatal rat cardiomyocytes. However, as the regulation of L-VCC by α-adrenergic stimulation depends on the animal species and the patch-clamp configuration,10,28 further experiments will be performed to verify if the regulation of L-VCC by PKD1 is a general or particular mechanism.
In vitro phosphorylation assay showed that PKD1 can directly phosphorylate the C-terminal domain of α1C. The C-terminal domain of α1C contains two serine residues occurring in a consensus site specific Ser1928 (SASLGRRAS1928) and the Ser1971 (LQRSHS1971).11 Both serine residues and their context are conserved in the α1C subunit of various species,28 suggesting that they might be important for the regulation of the channel. Further experiments will be necessary to verify if either putative or other serine residues on α1C are phosphorylated by PKD1. Ser1928 is phosphorylated by the PKA and also by various PKC isoforms.8,29 Nevertheless, the functional role of Ser1928 phosphorylation by PKA is still controversial. The expression in cardiomyocytes of a dihydropyridine resistant L-VCC, in which the Ser1928 has been mutated, showed that the L-type Ca2+ current increase stimulated by β-adrenergic was not affected by the mutation.30 However, Ser1928 might not be the only phosphorylation site necessary to regulate the activity of L-VCC.9 Three PKC isoforms (PKCα, PKCε, and PKCζ) also phosphorylate the Ser1928 in vitro and in vivo.8 Functional evidences for the role of PKCε and PKCβII in the modulation of the L-VCC has been previously presented.31,32 The approach described by Ganesan et al.30 will be useful to clarify the role of functional phosphorylation sites α1C subunit of the L-VCC.
Similar to the overexpression of the dominant negative PKD1 mutant, the silencing of EHN1 expression inhibited the α-adrenergic increase of L-type Ca2+ currents in neonatal rat cardiomyocytes but not the β-adrenergic-stimulated increase, suggesting that ENH1 is involved in the α-adrenergic signalling pathway regulating the L-VCC. Co-expression of ENH1 and α1B, the pore subunit of the N-type voltage-gated Ca2+ channels, in oocytes enhanced and accelerated the response of the N-type Ca2+ currents to phorbolester.19 The interaction of the N-type Ca2+ channels with ENH1 requires its LIM2 domain.33 Our results showed that ENH1 interacts with the L-VCC through the PDZ domain of ENH1. The C-terminal domain of α1C contains a PDZ interacting motifs (VSXL).34 This motif has been shown to bind of the PDZ domain of Shank in neurons.34 The PDZ domain of ENH might also bind the same motif on α1C. The PDZ interaction motif is not present in the α1B subunit of the N-type Ca2+ channels, thus explaining the difference in the anchoring mechanism of ENH1 with the L- and N-type Ca2+ channels.
In conclusion, we have shown that ENH1 is a new binding partner of PKD1. ENH1 scaffolds PKD1 to α1C forming a signalling complex that regulates the activity of L-VCC in response to α-adrenergic stimulation in cardiomyocytes.
Supplementary Material
Acknowledgments
We thank S. Skånland, Drs M. Frieden and M. Rossier for critical reading of the manuscript.
Funding
This work was supported by Grants-in-Aid for the 21st Century Center of Excellence Program ‘Towards Creating New Industries Based on Inter-Nanoscience’, ‘Structural and Functional Proteomics Consortium for Research on the Proteins Working in Brain and Nervous System’, the Scientific Research in Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#13041036). A.D.M. was a recipient of a Japan Society for Promotion of Science fellowship grant (P03146).
Footnotes
Supplementary Material is available at Cardiovascular Research Online.
Conflict of interest: none declared.
References
- 1.Molkentin JD, Dorn GW., II Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 2.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 3.Robu VG, Pfeiffer ES, Robia SL, Balijepalli RC, Pi Y, Kamp TJ, et al. Localization of functional endothelin receptor signaling complexes in cardiac transverse tubules. J Biol Chem. 2003;278:48154–48161. doi: 10.1074/jbc.M304396200. [DOI] [PubMed] [Google Scholar]
- 4.Huang XP, Pi Y, Lokuta AJ, Greaser ML, Walker JW. Arachidonic acid stimulates protein kinase C-epsilon redistribution in heart cells. J Cell Sci. 1997;110:1625–1634. doi: 10.1242/jcs.110.14.1625. [DOI] [PubMed] [Google Scholar]
- 5.Wong W, Scott JD. AKAP signaling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 6.Mackay K, Mochly-Rosen D. Localization, anchoring, and functions of protein kinase C isozymes in the heart. J Mol Cell Cardiol. 2001;33:1301–1307. doi: 10.1006/jmcc.2001.1400. [DOI] [PubMed] [Google Scholar]
- 7.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1. 2 channels via a leucine zipper interaction with a kinase-anchoring protein 15. Proc Natl Acad Sci USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, et al. Ser1928 is a common site for Cav1. 2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 9.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1. 2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O-Uchi J, Komukai K, Kusakari Y, Obata T, Hongo K, Sasaki H, et al. alpha1-adrenoceptor stimulation potentiates L-type Ca2+ current through Ca2+/calmodulin-dependent PK II (CaMKII) activation in rat ventricular myocytes. Proc Natl Acad Sci USA. 2005;102:9400–9405. doi: 10.1073/pnas.0503569102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 12.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 13.Iwata M, Maturana A, Hoshijima M, Tatematsu K, Okajima T, Vandenheede JR, et al. PKCepsilon-PKD1 signaling complex at Z-discs plays a pivotal role in the cardiac hypertrophy induced by G-protein coupling receptor agonists. Biochem Biophys Res Commun. 2005;327:1105–11013. doi: 10.1016/j.bbrc.2004.12.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison BC, Kim MS, van Rooij E, Plato CF, Papst PJ, Vega RB, et al. Regulation of cardiac stress signaling by protein kinase d1. Mol Cell Biol. 2006;26:3875–3888. doi: 10.1128/MCB.26.10.3875-3888.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, et al. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 16.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroda S, Tokunaga C, Kiyohara Y, Higuchi O, Konishi H, Mizuno K, et al. Protein-protein interaction of zinc finger LIM domains with protein kinase C. J Biol Chem. 1996;271:31029–31032. doi: 10.1074/jbc.271.49.31029. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa N, Hoshijima M, Oyasu M, Saito N, Tanizawa K, Kuroda S. ENH, containing PDZ and LIM domains, heart/skeletal muscle-specific protein, associates with cytoskeletal proteins through the PDZ domain. Biochem Biophys Res Commun. 2000;272:505–512. doi: 10.1006/bbrc.2000.2787. [DOI] [PubMed] [Google Scholar]
- 19.Maeno-Hikichi Y, Chang S, Matsumura K, Lai M, Lin H, Nakagawa N, et al. A PKCepsilon-ENH-channel complex specifically modulates N-type Ca2+ channels. Nat Neurosci. 2003;6:468–475. doi: 10.1038/nn1041. [DOI] [PubMed] [Google Scholar]
- 20.Van Lint JV, Sinnett-Smith J, Rozengurt E. Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J Biol Chem. 1995;270:1455–1461. doi: 10.1074/jbc.270.3.1455. [DOI] [PubMed] [Google Scholar]
- 21.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 22.Tuschl T. Expanding small RNA interference. Nat Biotechnol. 2002;20:446–448. doi: 10.1038/nbt0502-446. [DOI] [PubMed] [Google Scholar]
- 23.Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y, Gu Y, Iwanaga Y, Hoshijima M, Oh SS, Giordano FJ, et al. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation. 2002;105:502–508. doi: 10.1161/hc0402.102953. [DOI] [PubMed] [Google Scholar]
- 25.Ryser S, Tortola S, van Haasteren G, Muda M, Li S, Schlegel W. MAP kinase phosphatase-1 gene transcription in rat neuroendocrine cells is modulated by a calcium-sensitive block to elongation in the first exon. J Biol Chem. 2001;276:33319–33327. doi: 10.1074/jbc.M102326200. [DOI] [PubMed] [Google Scholar]
- 26.Maturana AD, Casal AJ, Demaurex N, Vallotton MB, Capponi AM, Rossier MF. Angiotensin II negatively modulates L-type calcium channels through a pertussis toxin-sensitive G protein in adrenal glomerulosa cells. J Biol Chem. 1999;274:19943–19948. doi: 10.1074/jbc.274.28.19943. [DOI] [PubMed] [Google Scholar]
- 27.Haworth RS, Goss MW, Rozengurt E, Avkiran M. Expression and activity of protein kinase D/protein kinase C mu in myocardium: evidence for alpha1-adrenergic receptor- and protein kinase C-mediated regulation. J Mol Cell Cardiol. 2000;32:1013–1023. doi: 10.1006/jmcc.2000.1143. [DOI] [PubMed] [Google Scholar]
- 28.van der Heyden MA, Wijnhoven TJ, Opthof T. Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res. 2005;65:28–39. doi: 10.1016/j.cardiores.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 29.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 30.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu K, Mochly-Rosen D, Boutjdir M. Evidence for functional role of epsilonPKC isozyme in the regulation of cardiac Ca2+ channels. Am J Physiol Heart Circ Physiol. 2000;279:H2658–H6264. doi: 10.1152/ajpheart.2000.279.6.H2658. [DOI] [PubMed] [Google Scholar]
- 32.Alden KJ, Goldspink PH, Ruch SW, Buttrick PM, Garcia J. Enhancement of L-type Ca2+ current from neonatal mouse ventricular myocytes by constitutively active PKC-betaII. Am J Physiol Cell Physiol. 2002;282:C768–C774. doi: 10.1152/ajpcell.00494.2001. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Lai M, Maeno-Hikichi Y, Zhang JF. Essential role of the LIM domain in the formation of the PKCepsilon-ENH-N-type Ca2+ channel complex. Cell Signal. 2006;18:215–224. doi: 10.1016/j.cellsig.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, et al. Association of CaV1.3 L-type calcium channels with Shank. J Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.