SUMMARY
Hookworm infection is associated with anaemia and malnutrition in many resource-limited countries. Ancylostoma hookworms have previously been shown to modulate host cellular immune responses through multiple mechanisms, including reduced mitogen-mediated lymphocyte proliferation, impaired antigen presentation/processing, and relative reductions in CD4+ T cells in the spleen and mesenteric lymph nodes. Syrian hamsters were depleted of CD4+ for up to 9 days following intraperitoneal injection (200 μg) of a murine anti-mouse CD4 monoclonal IgG (clone GK1·5). CD4+ T-cell-depleted hamsters infected with the hookworm Ancylostoma ceylanicum exhibited a threefold higher mean intestinal worm burden and more severe anaemia than animals that received isotype control IgG. In addition, depletion of CD4+ T cells was associated with impaired cellular and humoral (serum and mucosal) immune responses to hookworm antigens. These data demonstrate an effector role for CD4+ T cells in hookworm immunity and disease pathogenesis. Ultimately, these studies may yield important insights into the relationship between intestinal nematode infections and diseases that are associated with CD4+ T-cell depletion, including HIV.
Keywords: hookworm, Ancylostoma ceylanicum, CD4+ T cells, nematode, anemia
INTRODUCTION
Hookworms are blood-feeding nematodes that infect nearly 800 million people worldwide and are a leading cause of anaemia and malnutrition. Hookworm infection is also associated with alterations in host cellular immune responses (1,2) which could impact host-responses to administered vaccines and co-infecting pathogens, including HIV, malaria and tuberculosis (3–5). In animal models, Ancylostoma hookworm infection is associated with impaired lymphocyte proliferation, functional defects in antigen presentation/processing and increased secretion of nitric oxide (6). Similar effects have been observed in other helminthiases, and suggest varying degrees of cellular immune suppression mediated by infection with intestinal and tissue worms (7).
Alteration of lymphocyte homeostasis, as manifested by depletion of CD4+ T-cell populations, has been suggested as a possible mechanism through which hookworms modulate host immune responses to facilitate parasite survival (2,6,8). Studies in murine models have demonstrated a role for T cells in mediating protective immunity to a variety of nematodes (9–11). More recently, the role of CD4+CD25+ regulatory T cells (Tregs) (12), in regulating the host inflammatory response to helminth infections has been explored (13–15), although the role this lymphocyte subset plays in the immune response to infection remains controversial.
We report here data from studies aimed at defining the role of CD4+ T cells in mediating host immune responses to hookworm infection. Hamsters depleted of CD4+ cells using a monoclonal antibody demonstrated significant impairment of humoral, cellular and mucosal immune responses following infection with Ancylostoma ceylanicum. CD4+ T cells were also shown to play a role in controlling hookworm infection in vivo, as infected animals treated with the α-CD4 IgG exhibited more severe anaemia and a nearly threefold higher intestinal worm burden than isotype IgG-treated controls. These studies support prior evidence suggesting that hookworm-associated depletion of CD4+ lymphocyte populations represents a specific parasite survival strategy. Moreover, the data add to our understanding of how co-infection with hookworm may influence immune responses to other pathogens in human populations.
MATERIALS AND METHODS
Hookworm life cycle
The A. ceylanicum life cycle was maintained in Syrian hamsters (16,17). Experiments were approved by the Yale Animal Care and Use Committee. For infection studies, animals received 75 third stage (L3) A. ceylanicum larvae by oral gavage. Weight and blood haemoglobin levels were measured as described (16), and at the time of sacrifice, adult hookworms were manually recovered from the intestines.
Immunodepletion of CD4+ T cells
The rat anti-mouse CD4 monoclonal antibody L3T4 (clone GK1·5) and isotype control IgG (rat IgG2b) were obtained from eBioscience (San Diego, CA, USA). The Anti-CD4 antibody cross-reacts with hamster CD4 on T lymphocytes (6), but not monocytes or macrophages (18,19). To assess the dose of antibody necessary for effective depletion, groups of hamsters (n = 3) were injected intraperitoneally (IP) with increasing amounts (100, 150 and 200 μg) of anti-CD4 IgG, or 200 μg of isotype control antibody. Animals were sacrificed 2 days following injection and the percentages of CD4+ T cells in the spleens were analysed by fluorescence-activated cell sorter (FACS) (6). In a subsequent experiment, nine hamsters were injected with 200 μg of anti-CD4 IgG or isotype control and groups of three hamsters were sacrificed 3, 6 and 9 days later.
To define the effect of CD4 cell depletion on hookworm pathogenesis, eight hamsters were each injected IP with 200 μg of anti-α-CD4 antibody, while equal numbers received 200 μg of control IgG. Two days later, five hamsters in each group were infected with 75 A. ceylanicum larvae (L3), and three uninfected animals in each group served as controls. Animals received additional injections with anti-CD4 or isotype control IgG on days 8 and 18 post-infection (PI). The animals were sacrificed at day 30 PI, and blood, intestine, spleen and mesenteric lymph nodes (MLNs) were collected for analyses.
Fluorescence-activated cell sorter assay
Single cell preparations (106 cells) of splenocytes or MLN cells were incubated with fluorescein isothiocyanate (FITC)-labelled goat anti-Syrian hamster IgG and phycoerythrin (PE)-labelled α-mouse CD4 (L3T4) for 30 min on ice. Cell surface determinant data were acquired on 100 000 cells/sample using the FACSCalibur cytometer and FlowJo software (Treestar, Ashland, OR, USA).
Lymphocyte proliferation assay
Spleens and MLNs were harvested from naïve and infected hamsters. For histological evaluation, 5–8 μm sections of paraffin embedded MLNs were stained with haematoxylin and eosin (H&E) for examination by light microscopy (6). For proliferation studies, splenocytes were plated in triplicate (105 per well) and stimulated with Concanavalin A (ConA; 1 μg/mL; Sigma, St Louis, MO, USA) or hookworm extracts (HEX, 25 μg/mL) made from soluble adult worm homogenates (17) for 24 h at 37°C, 5% CO2. Proliferation of cells was estimated by 5-bromo 2′-deoxyuridine incorporation using a colorimetric kit (Roche Diagnostics, Penzberg, Germany) (6). The stimulation index (SI) was calculated as a ratio of mean OD at 450 nm of stimulated cultures to unstimulated cultures.
Antibody responses by ELISA and Immunoblotting
Serum (IgG) and mucosal (IgA) antibody responses to pooled adult hookworm excretory/secretory (ES) proteins were measured by ELISA using previously described methods (20,21). IgG ELISA was performed using pooled serum collected from anti-CD4-treated animals and control animals at day 30 PI. Mucosal IgA responses to hookworm antigens were measured in concentrated intestinal flush (20) collected from CD4-depleted animals and control animals at day 30 PI. Bound antibodies were detected using HRP-labelled secondary IgG and ABTS substrate.
Parasite-specific IgG production was also assessed by immunoblot (20). Two micrograms of ES and 200 ng of recombinant ES protein rAceES-2 (21) were subjected to SDS–PAGE and proteins were blotted onto nitrocellulose membranes. Blocked membranes were probed with pooled hamster serum (day 30 PI) diluted 1 : 1000 in 5% milk/phosphate buffer saline-tween (PBS-T). Washed membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-hamster IgG, with detection using chemiluminescence.
Statistical analysis of data
Data are presented as mean ± SE. Unpaired t tests were performed to assess statistical significance between data from CD4-depleted and isotype control groups. For multiple group comparisons, one-way ANOVA was performed followed by Tukey-Kramer Multiple Comparison test. P-values <0·05 were considered significant.
RESULTS
Intraperitoneal injection of anti-CD4 IgG induces a sustained depletion of hamster CD4+ T lymphocytes
As measured by FACS, IP injection of anti-CD4 IgG resulted in depletion of the CD4+ target cell population. At day 3 PI, FACS analysis determined that 0·36 ± 0·03% of spleen lymphocytes in anti-CD4 IgG-treated animals were CD4+, compared to15·9 ± 0·7% in the isotype control-treated animals (P < 0·0001; Figure 1). At 6 days following injection, the mean percentage of CD4+ T cells in anti-CD4 IgG-treated hamsters was 0·42 ± 0·04%, compared to 22·1 ± 1·3% in isotype controls (P < 0·0001). At day 9, these percentages were 0·5 ± 0·02% vs. 25·5 ± 1·1% respectively (P < 0·0001).
Figure 1.
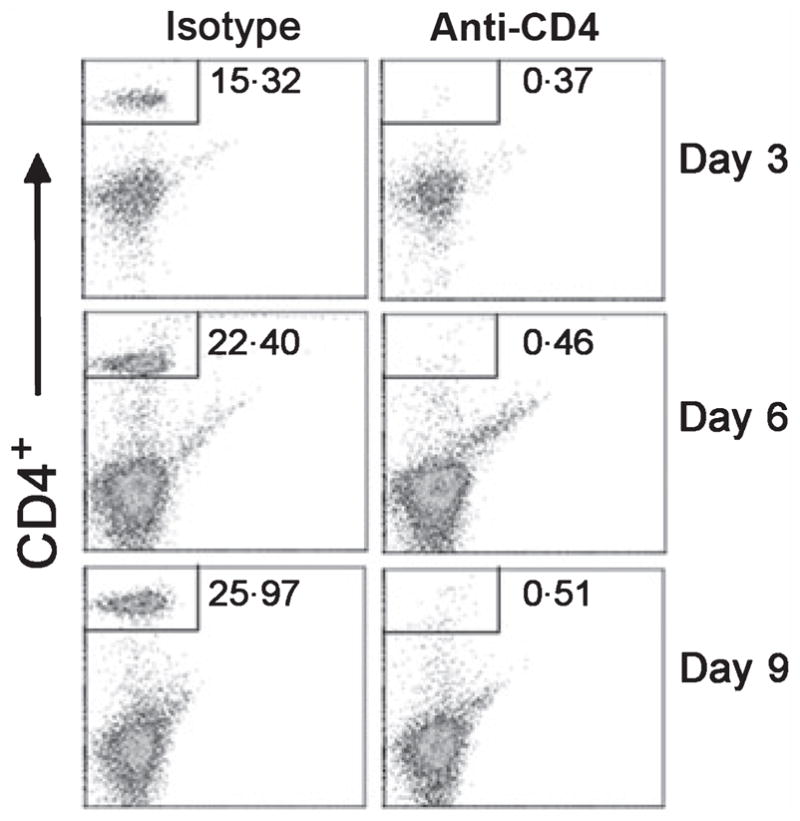
Fluorescence-activated cell sorter (FACS) analysis of splenocytes from hamsters injected with anti-CD4 antibody. Hamsters were injected intraperitoneally (IP) with 200 μg of rat anti-mouse CD4 monoclonal antibody (clone GK1·5) or an isotype control IgG. The percentage of CD4+ cells (upper left quadrant box) in the spleens of isotype control (left panels) and CD4-depleted hamsters (right panels) were assessed by FACS at days 3, 6 and 9 post-depletion. Data are representative FACS profiles of CD4+ T-cell-depleted animals and isotype IgG-treated controls.
CD4+ T-cell depletion is associated with altered lymphocyte homeostasis in the setting of A. ceylanicum infection
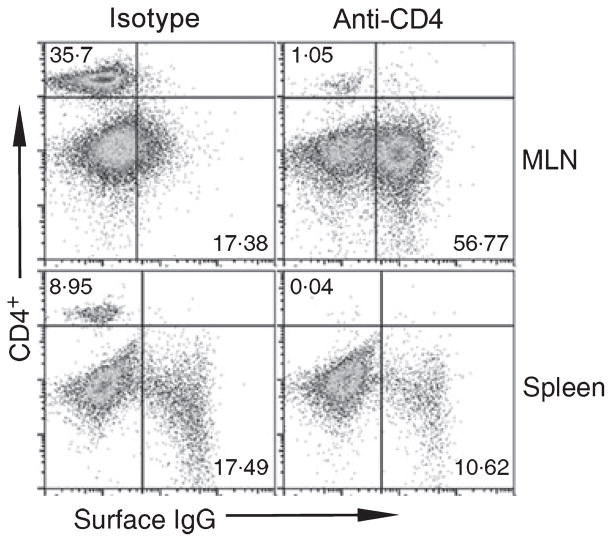
To define the role of CD4+ T cells in mediating susceptibility to hookworm, hamsters were injected with anti-CD4 IgG (or isotype control IgG) on days 2, 8 and 18 PI, followed by sacrifice at day 30 PI. Figure 2 shows representative CD4+ and IgG+ FACS profiles from anti-CD4 IgG and isotype control-treated animals. The percentage of CD4+ T cells in mesenteric lymph node cells from anti-CD4 IgG-treated animals (1·1 ± 0·07%) was reduced compared with isotype controls (36·3 ± 1·5%; P = 0·0007), indicating that the effect of immunodepletion was sustained throughout the course of infection. The percentage of CD4+ T cells in splenocyte preparations was also greatly reduced in anti-CD4 IgG-treated animals (0·04 ± 0·01%) compared to isotype IgG-treated hamsters (9·1 ± 0·7%; P = 0·002). Depletion of CD4+ T cells also impacted the profile of surface IgG+ B cells during the course of infection. As shown in Figure 2, the isotype control-treated group showed a single homogeneous population of mostly surface IgG− cells, consistent with past observations on the effect of A. ceylanicum infection on lymphocyte homeostasis (6). In contrast, infected animals that received anti-CD4 IgG showed two distinct populations of B cells (surface IgG+ and surface IgG−) in MLN preparations, similar to the pattern seen in uninfected animals (6). In splenocytes, immunodepletion of CD4+ T cells was associated with a reduction in the percentage of surface IgG+ B cells (10·7 ± 0·2%) compared with that measured in isotype control IgG-treated animals(17·4 ± 0·5%), a difference that was statistically significant (P = 0·03).
Figure 2.
FACS staining for CD4 and surface IgG in lymphocytes from CD4+ T-cell-depleted and isotype control-treated hamsters infected with A. ceylanicum. At day 30 PI, infected hamsters that received anti-CD4 IgG demonstrate persistent reduction in the proportion of CD4+ T cells (upper left quadrant box) in the mesenteric lymph node (MLN) cells (top panels) and spleen (bottom panels). Infected animals treated with the isotype control IgG showed expected reduction in the percentage of CD4+ T cells in the setting of A. ceylanicum infection, compared with uninfected animals [(6) and Figure 1]. Staining for surface IgG shows preservation of IgG+ B cells in the MLN cells of infected animals that received anti-CD4 IgG. Data are representative FACS profiles of CD4-depleted animals and isotype IgG-treated controls.
CD4+ T-cell depletion is associated with greater intestinal worm burdens and reduced blood haemoglobin levels following hookworm infection
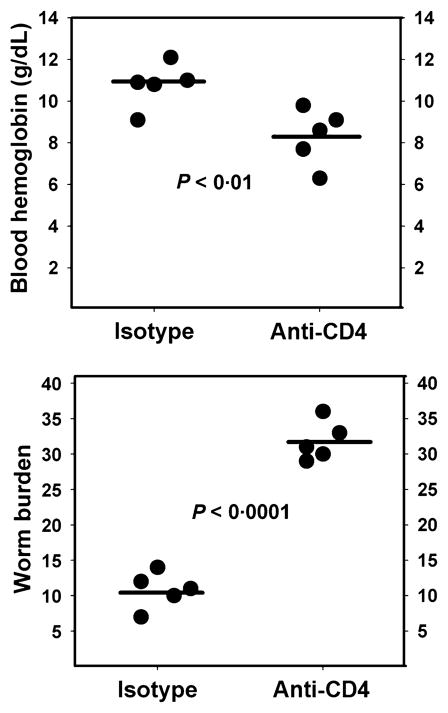
As shown in Figure 3, at day 30 PI, the mean number of intestinal worms recovered from the anti-CD4 IgG-treated hamsters was approximately threefold higher than that from infected isotype controls (32 ± 1 vs. 11 ± 1; P < 0·0001). Of note, the worm burden in Isotype IgG-treated animals is consistent with our prior observations at day 30 PI following infection with 75 A. ceylanicum L3 (6). A 23% reduction in blood haemoglobin levels was also noted in isotype control-treated hamsters, compared to CD4+ T-cell-depleted animals (8·3 ± 0·6 g/dL vs. 10·8 ± 0·5 g/dL; P = 0·01). Both infected groups had lower mean blood haemoglobin levels than uninfected controls (isotype control: 15·4 ± 1·0 g/dL; CD4-depleted: 16·5 ± 0·8 g/dL).
Figure 3.
Effect of CD4+ T-cell depletion on hookworm infection intensity and anaemia. (a) Hamsters that received an IP injection of 200 μg of anti-CD4 IgG or the isotype control IgG at days 2, 8 and 18 post-infection (PI) exhibited an approximately threefold higher intestinal worm burden on day 30 PI. (b) CD4+ T-cell-depleted animals also exhibited lower blood haemoglobin levels (8·3 ± 0·6 g/dL vs. 10·8 ± 0·5 g/dL; P = 0·01), consistent with greater intensity of infection. Both infected groups had lower mean blood haemoglobin levels than uninfected controls (isotype control: 15·4 ± 1·0 g/dL; CD4-depleted: 16·5 ± 0·8 g/dL).
Depletion of CD4+ T cells is associated with impaired cellular immune responses to mitogen and hookworm antigens
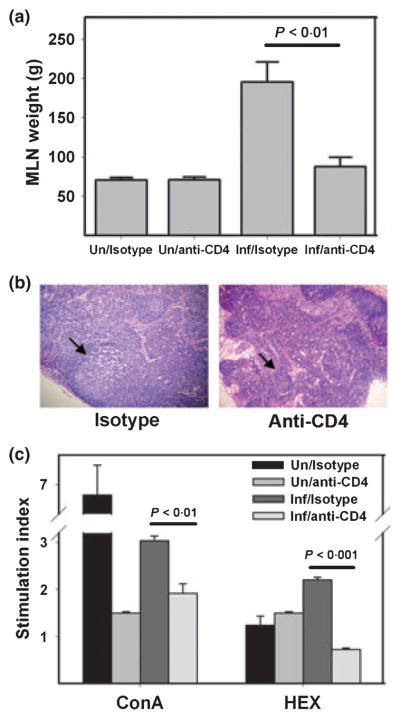
Hamsters infected with A. ceylanicum exhibit enlarged MLNs, with histological evidence of germinal centre activation compared to uninfected controls (6). To assess the effect of CD4+ T-cell depletion on lymph node responses, we measured the weights of MLNs from each of the experimental groups. As in Figure 4(a), the mean MLN weights were equivalent in the uninfected isotype control and anti-CD4 IgG-treated hamsters at day 30 PI (71 ± 3 mg and 71 ± 4 mg respectively). However, in the infected groups, the mean MLN weight of isotype control-treated hamsters (196 ± 20 mg) was more than twice that of CD4-depleted animals (88 ± 12 mg; P < 0·01). Consistent with this observation, H&E stained sections of MLNs from hookworm-infected isotype control hamsters were notable for enlarged and activated germinal centres, compared to sections from controls (Figure 4).
Figure 4.
CD4+ T-cell depletion is associated with impaired activation of mesenteric lymph nodes and reduced lymphocyte proliferative capacity. (a) Mean weights of MLNs removed from infected hamsters and uninfected controls at day 30 PI. All values are mean ± SE. (b) Representative MLN sections stained with H&E at day 30 PI shows large, activated germinal centre (arrow) in an infected isotype control-treated animal (left panel), compared to similar section from an L3T4-treated hamster (right panel). Both images are at 10× magnification. (c) Stimulation indices (SI) of MLN cells of isotype control and CD4+ T-cell-depleted hamsters stimulated with ConA or soluble hookworm extracts (HEX) at day 30 PI. All values are mean ± SE, and corresponding P-values are shown above brackets.
To further define the effector functions of CD4+ T cells in A. ceylanicum infection, MLN cells harvested at day 30 PI were stimulated in vitro with ConA or soluble HEX (6). Shown in Figure 4(c), the SI of MLN cells exposed to ConA was higher in the group of infected hamsters treated with isotype control IgG than in anti-CD4 IgG-treated animals (3·0 ± 0·1 vs. 1·9 ± 0·2; P < 0·001). Also, MLN cells from infected isotype control-treated hamsters stimulated with HEX demonstrated a threefold greater proliferative response than those from anti-CD4 IgG-treated animals (SI: 2·2 ± 0·1 vs. 0·7 ± 0·03; P < 0·001). When stimulated with ConA, the SI of MLN lymphocytes from naïve isotype control-treated animals was 6·9 ± 0·2, while that from anti-CD4 IgG-treated hamsters was 1·5 ± 0·03 (P < 0·001), a difference potentially accounted for by the absence of CD4+ T cells in the anti-CD4 IgG-treated animals. Splenocytes harvested from CD4+ T-cell-depleted hamsters at day 30 PI and stimulated in vitro (ConA, HEX) exhibited a reduction in SI that was similar to MLN cells.
CD4+ T-cell depletion is associated with impaired serum and mucosal antibody responses to hookworm antigens
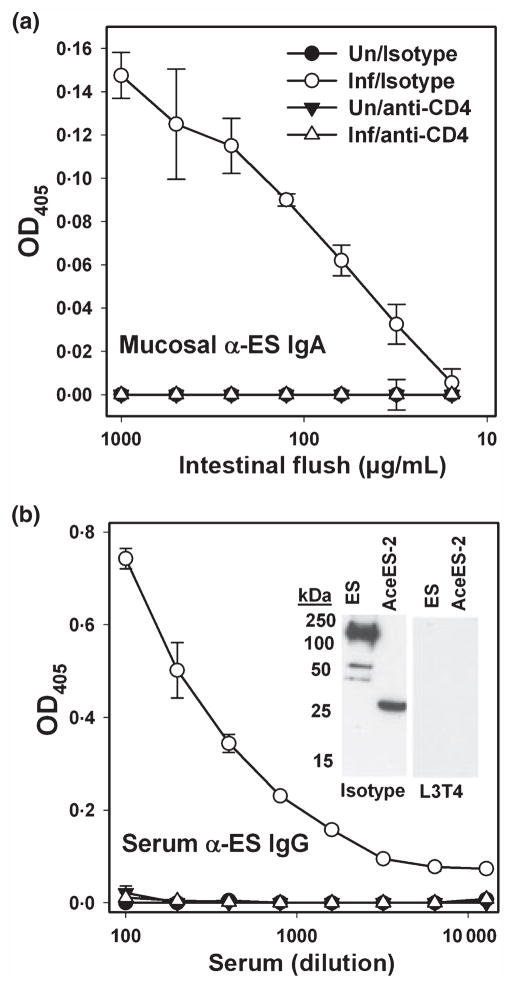
We have demonstrated that hamsters infected with A. ceylanicum generate robust serum IgG and mucosal IgA responses directed at hookworm ES proteins (17,20,22). To assess the potential effect of CD4+ T-cell depletion on parasite-specific humoral responses, we measured mucosal IgA and serum IgG directed at A. ceylanicum ES in each of the experimental groups (20). As shown in Figure 5(a), ES-specific IgA was detected at day 30 PI in the intestinal flush of isotype control-treated hamsters, but not in the anti-CD4 IgG-treated group. Likewise, CD4+ T-cell-depleted animals generated no serum IgG response to ES (Figure 5b). The serum IgG ELISA result was confirmed by immunoblot (Figure 5b, inset), which demonstrates recognition of multiple ES proteins, including the recombinant hookworm ES protein rAceES-2 (21), in isotype control, but not in anti-CD4 IgG-treated animals. These results demonstrate an essential role for CD4+ T cells in mediating humoral (IgA and IgG) immune responses to hookworm infection.
Figure 5.
CD4+ T-cell depletion impairs hookworm specific mucosal IgA and serum IgG responses. (a) At day 30 PI, intestinal flush samples collected from naïve and infected hamsters were assessed for the presence of IgA directed against hookworm excretory/secretory (ES) proteins. (b) Serum samples were also analysed for the presence of IgG against hookworm ES by ELISA. Inset shows recognition by immunoblot of ES and the recombinant secretory protein AceES-2 by isotype IgG-treated animals (left panel), but not those that received anti-CD4 IgG (right panel).
DISCUSSION
The data presented here affirm a role for CD4+ T cells in mediating a protective immune response to A. ceylanicum infection, similar to that reported in models of other parasitic helminths (7,9–11,23,24). The mechanism(s) through which depletion of hamster CD4+ T cells results in increased hookworm disease pathogenesis and greater intensity of infection remain(s) unknown, although the effector functions of this class of lymphocytes have been carefully studied in various murine models of helminth infection. For instance, expulsion of Trichuris muris is inhibited in CD4+ T-cell-depleted, but not in anti-CD8+ antibody-treated mice (10). In SCID mice, Betts et al. demonstrated a central role for CD4+ T cells in mediating protective immunity to T. muris (25). It has also been shown that adoptive transfer of CD4+ T cells from Trichinella spiralis-infected mice induces a protective phenotype in naïve recipients (26–28), demonstrating a central role in the control of trichinellosis. In vivo depletion of CD4+ T cells has also been reported to impair resistance to infection with Schistosoma mansoni (29), and similar results have been obtained in models of infection with the nematodes Heligmosomoides polygyrus, Nippostrongylus brasiliensis and Brugia pahangi (9,30,31).
To our knowledge, this is the first demonstration that hookworm pathogenesis can be modulated by targeting a specific component of the cellular immune response, specifically CD4+ T cells, using a monoclonal antibody. A major reason for limited understanding of host immune responses to hookworm is the lack of a suitable murine host model that accurately reproduces the pathogenic mechanisms and clinical features of disease in humans infected with Ancylostoma spp. and Necator americanus. Although few reagents are available for characterization of immune responses in M. auratus, including monoclonal antibodies to cell surface markers and immunoglobulin subtypes, the hamster is a most efficient and highly reproducible model for the study of hookworm pathogenesis (17,32,33). Moreover, because A. ceylanicum is a recognized parasite of humans (34–37), immune evasion mechanisms defined in the hamster host may be more reflective of strategies utilized by species that more commonly infect people, e.g. A. duodenale and N. americanus.
The work reported here demonstrates that cross-reacting murine monoclonal antibodies can be used effectively to characterize immune responses in the hamster. We confirmed that IP administration of anti-mouse CD4 antibody (L3T4; GK1·5 clone) (9,10,38,39) to hamsters induced a 98% depletion in vivo, with the effect of a single injection lasting at least 9 days (Figure 1). The anti-CD4 IgG was then used to characterize the role of CD4+ T lymphocytes in mediating disease pathogenesis and immune responses to the hookworm A. ceylanicum. It is worth noting that the same antibody was used to deplete and detect CD4+ T cells, which could potentially obscure detection of positive cells by subsequent FACS. Thus, while we cannot rule out the possibility that the FACS data underestimate the actual numbers of circulating CD4+ T cells, the clear differences in clinical and parasitological outcomes between depleted and control animals strongly suggest that antibody treatment achieved the intended effect.
Depletion of CD4+ cells in hamsters was associated with a threefold increase in intestinal worm burden, as well as a 23% decrease in blood haemoglobin levels following primary infection (Figure 3). In addition, anti-CD4 IgG-treated hamsters did not exhibit enlargement of MLNs in the context of hookworm infection, compared to controls (Figure 4a), consistent with histological analysis showing smaller germinal centres in CD4+ T-cell-depleted hamsters (Figure 4b). Treatment with anti-CD4 IgG also was associated with reduced lymphocyte proliferation to ConA or hookworm antigens (HEX), unlike MLN cells harvested from isotype control-treated animals (Figure 4c). These data demonstrate that CD4+ T cells constitute the major cell subpopulation capable of responding to mitogen and hookworm-specific protein stimulation. Previous studies investigating the proliferation of hamster cells to hookworm proteins or mitogen did not identify specific effector lymphocyte populations (40). However, based on the data reported here, the lymphocytes responding to stimulation with hookworm antigens are most likely CD4+ T cells.
Infected CD4+ T-cell-depleted animals failed to mount a measurable secretory IgA response to A. ceylanicum ES antigens, compared to isotype antibody-treated controls (Figure 5a). This observation is of interest in light of our previous demonstration that oral immunization with soluble hookworm protein extracts confers partial protection against anaemia and growth delay following challenge infection, consistent with generation of a protective mucosal immune response (21). More recently, we have demonstrated that resistance to infection in the hamster model is associated with the production of specific IgA directed against hookworm ES (20). This lack of antibody production in CD4+ cell-depleted animals (Figure 5) is consistent with findings of reduced MLN size following infection, compared with isotype-treated controls (Figure 4a). Histological evaluation also demonstrated that MLNs from depleted animals failed to produce germinal centres (Figure 4b), which represent sites of antibody production in the draining lymph nodes (41). These data support a role for CD4+ T cells in humoral immunity to hookworm, presumably via stimulation of B lymphocytes to mature, proliferate and produce antigen-specific IgA and/or IgG antibodies.
Animals treated with anti-CD4 IgG also failed to mount a measurable serum IgG response to adult hookworm ES (Figure 5b). In contrast, isotype control-treated animals produced serum IgG directed against both pooled ES, as well as the recombinant AceES-2, a highly immunoreactive secretory protein produced early in the course of infection with A. ceylanicum (21,42). As has been shown, passive transfer of serum from twice-infected animals to naïve hamsters is associated with reduced clinical sequelae (anaemia, growth delay) following challenge infection, suggesting that antibodies to hookworm proteins play a measurable, although not fully protective role in mediating resistance to infection (17). It has also been demonstrated that systemic immunization with pooled ES, and in some instances single recombinant hookworm secretory proteins, will confer partial protection against A. ceylanicum, further evidence for a contributory role of antigen-specific IgG (21,43,44). The observation that CD4+ T cell depletion abrogates anti-ES IgG and IgA responses suggests an important potential mechanism through which these cells mediate acquired immunity to A. ceylanicum in this model. In the absence of CD4+ T cells, hamsters are unlikely to mount a protective humoral (IgG and IgA) immune response, which typically develops in association with attrition of adult worms between days 20 and 30 PI (6,20).
We have previously reported that hookworm infection is associated with depletion of CD4+ T cells in the hamster (6). The demonstration that CD4+ T cells are important in the control of infection and disease pathogenesis raises the possibility that depletion of this cell type represents a potential survival strategy of the parasite, designed to enhance its ability to remain attached to the intestinal mucosa by blunting the host immune response. The observation that hookworm infection is exacerbated in the absence of CD4+ T cells is particularly significant in light of the high rates of co-infection with HIV among people living in areas endemic for hookworm and other soil transmitted nematodes, Ascaris lumbricoides and T. trichiura (45,46). Although the relationship between HIV and hookworm infection is less clear, it has nonetheless been suggested that de-worming of HIV-infected individuals may improve certain clinical parameters, e.g. viral load and CD4+ T-cell numbers (3,47). Interestingly, data from a recent retrospective study has shown that introduction of highly active antiretroviral therapy in an HIV-infected population at risk for co-infection with intestinal nematodes was associated with a significant reduction in prevalence of A. lumbricoides, T. trichiura and hookworm (48), suggesting that the relationship between helminth and HIV co-infection is bi-directional.
In summary, CD4+ T cells mediate components of the host immune response to the hookworm A. ceylanicum, and may play a role in controlling intensity of infection. Taking together data from previous investigations, we propose a potential mechanism, specifically depletion of CD4+ T cells, through which hookworm could alter the clinical course of other infections, further supporting the implementation of strategies aimed at integrating control of intestinal nematodes with programmes targeting co-endemic infectious diseases. And finally, given that humans are fully permissive hosts for A. ceylanicum, the data presented here further suggest that the hamster model recapitulates features of hookworm disease seen in endemic areas.
Acknowledgments
This work was supported by NIH grant AI58980 and a Hellman Family Fellowship from the Office of the President of Yale University. The authors are grateful to Diane McMahon-Pratt, George Porter and Keke Fairfax for technical assistance and thoughtful suggestions.
Footnotes
Disclosures: The authors do not have a commercial or other association that might pose a conflict of interest (e.g. pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents).
References
- 1.Geiger SM, Massara CL, Bethony J, Soboslay PT, Correa-Oliveira R. Cellular responses and cytokine production in post-treatment hookworm patients from an endemic area in Brazil. Clin Exp Immunol. 2004;136:334–340. doi: 10.1111/j.1365-2249.2004.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onyemelukwe GC, Musa BO. T-lymphocyte subsets in patients with hookworm infection in Zaria, Nigeria. Afr J Med Med Sci. 2001;30:255–259. [PubMed] [Google Scholar]
- 3.Borkow G, Teicher C, Bentwich Z. Helminth-HIV coinfection: should we deworm? PLoS Negl Trop Dis. 2007;1:e160. doi: 10.1371/journal.pntd.0000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walson JL. Treatment of helminth co-infection in individuals with HIV-1: a systematic review of the literature. PLoS Negl Trop Dis. 2007;1:e102. doi: 10.1371/journal.pntd.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper PJ, Chico M, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondji B, Bungiro RD, Harrison LM, et al. Role for nitric oxide in hookworm-associated immune suppression. Infect Immun. 2008;76:2560–2567. doi: 10.1128/IAI.00094-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenson JS, O’Connor R, Osborne J, Devaney E. Infection with Brugia microfilariae induces apoptosis of CD4(+) T lymphocytes: a mechanism of immune unresponsiveness in filariasis. Eur J Immunol. 2002;32:858–867. doi: 10.1002/1521-4141(200203)32:3<858::AID-IMMU858>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Geiger SM, Caldas IR, Mc Glone BE, et al. Stage-specific immune responses in human Necator americanus infection. Parasite Immunol. 2007;29:347–358. doi: 10.1111/j.1365-3024.2007.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bancroft AJ, Grencis RK, Else KJ, Devaney E. The role of CD4 cells in protective immunity to Brugia pahangi. Parasite Immunol. 1994;16:385–387. doi: 10.1111/j.1365-3024.1994.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 10.Koyama K, Tamauchi H, Ito Y. The role of CD4+ and CD8+ T cells in protective immunity to the murine nematode parasite Trichuris muris. Parasite Immunol. 1995;17:161–165. doi: 10.1111/j.1365-3024.1995.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 11.Vickery AC, Vincent AL, Sodeman WA., Jr Effect of immune reconstitution on resistance to Brugia pahangi in congenitally athymic nude mice. J Parasitol. 1983;69:478–485. [PubMed] [Google Scholar]
- 12.Doetze A, Satoguina J, Burchard G, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 13.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 14.Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 15.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 16.Held MR, Bungiro RD, Harrison LM, Hamza I, Cappello M. Dietary iron content mediates hookworm pathogenesis in vivo. Infect Immun. 2006;74:289–295. doi: 10.1128/IAI.74.1.289-295.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bungiro RD, Jr, Greene J, Kruglov E, Cappello M. Mitigation of hookworm disease by immunization with soluble extracts of Ancylostoma ceylanicum. J Infect Dis. 2001;183:1380–1387. doi: 10.1086/319867. [DOI] [PubMed] [Google Scholar]
- 18.DuChateau BK, Jensen JR, England DM, Callister SM, Lovrich SD, Schell RF. Macrophages and enriched populations of T lymphocytes interact synergistically for the induction of severe, destructive Lyme arthritis. Infect Immun. 1997;65:2829–2836. doi: 10.1128/iai.65.7.2829-2836.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuChateau BK, Munson EL, England DM, et al. Macrophages interact with enriched populations of distinct T lymphocyte subsets for the induction of severe destructive Lyme arthritis. J Leukoc Biol. 1999;65:162–170. doi: 10.1002/jlb.65.2.162. [DOI] [PubMed] [Google Scholar]
- 20.Bungiro RD, Jr, Sun T, Harrison LM, Shoemaker CB, Cappello M. Mucosal antibody responses in experimental hookworm infection. Parasite Immunol. 2008;30:293–303. doi: 10.1111/j.1365-3024.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 21.Bungiro RD, Jr, Solis CV, Harrison LM, Cappello M. Purification and molecular cloning of and immunization with Ancylostoma ceylanicum excretory-secretory protein 2, an immunoreactive protein produced by adult hookworms. Infect Immun. 2004;72:2203–2213. doi: 10.1128/IAI.72.4.2203-2213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bungiro RD, Harrison LM, Cappello M. Ancylostoma ceylanicum excretory/secretory protein 1: purification and molecular cloning of a major secretory protein from adult hookworms. Mol Biochem Parasitol. 2002;119:147–151. doi: 10.1016/s0166-6851(01)00408-x. [DOI] [PubMed] [Google Scholar]
- 23.Cox FE, Liew EY. Centrefold: T-cell subsets and cytokines in parasitic infections. Parasitol Today. 1992;8:371–374. doi: 10.1016/0169-4758(92)90173-y. [DOI] [PubMed] [Google Scholar]
- 24.Scott P, Kaufmann SH. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12:346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 25.Urban JF, Jr, Schopf L, Morris SC, et al. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 26.Grencis RK, Lee TD, Wakelin D. Adoptive transfer of immunity to Trichinella spiralis in mice: generation of effective cells by different life cycle stages. Int J Parasitol. 1985;15:195–202. doi: 10.1016/0020-7519(85)90087-6. [DOI] [PubMed] [Google Scholar]
- 27.Grencis RK, Riedlinger J, Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology. 1985;56:213–218. [PMC free article] [PubMed] [Google Scholar]
- 28.Riedlinger J, Grencis RK, Wakelin D. Antigen-specific T-cell lines transfer protective immunity against Trichinella spiralis in vivo. Immunology. 1986;58:57–61. [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips SM, Lin JJ, Galal N, Tung AS, Linette GP, Perrin PJ. Resistance in murine schistosomiasis is contingent on activated IL-2 receptor-bearing L3T4+ lymphocytes, negatively regulated by Lyt-2+ cells, and uninfluenced by the presence of IL-4. J Immunol. 1991;146:1335–1340. [PubMed] [Google Scholar]
- 30.Katona IM, Urban JF, Jr, Finkelman FD. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988;140:3206–3211. [PubMed] [Google Scholar]
- 31.Katona IM, Urban JF, Jr, Kang SS, Paul WE, Finkelman FD. IL-4 requirements for the generation of secondary in vivo IgE responses. J Immunol. 1991;146:4215–21. [PubMed] [Google Scholar]
- 32.Garside P, Behnke JM. Ancylostoma ceylanicum in the hamster: observations on the host-parasite relationship during primary infection. Parasitology. 1989;98 (Pt 2):283–289. doi: 10.1017/s003118200006220x. [DOI] [PubMed] [Google Scholar]
- 33.Garside P, Behnke JM, Rose RA. The immune response of male DSN hamsters to a primary infection with Ancylostoma ceylanicum. J Helminthol. 1989;63:251–260. doi: 10.1017/s0022149x00009068. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury AB, Schad GA. Ancylostoma ceylanicum: a parasite of man in Calcutta and environs. Am J Trop Med Hyg. 1972;21:300–301. doi: 10.4269/ajtmh.1972.21.300. [DOI] [PubMed] [Google Scholar]
- 35.Areekul S, Radomyos P, Viravan C. Experimental infection of Ancylostoma ceylanicum in man. J Med Assoc Thai. 1970;53:190–194. [PubMed] [Google Scholar]
- 36.Velasquez CC, Cabrera BC. Ancylostoma ceylanicum (Looss, 1911) in a Filipino woman. J Parasitol. 1968;54:430–431. [PubMed] [Google Scholar]
- 37.Yoshida Y, Okamoto K, Chiu JK. Ancylostoma ceylanicum infection in dogs, cats, and man in Taiwan. Am J Trop Med Hyg. 1968;17:378–381. doi: 10.4269/ajtmh.1968.17.378. [DOI] [PubMed] [Google Scholar]
- 38.Suss G, Eichmann K, Kury E, Linke A, Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 1988;56:3081–3088. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim LC, England DM, Glowacki NJ, DuChateau BK, Schell RF. Involvement of CD4+ T lymphocytes in induction of severe destructive Lyme arthritis in inbred LSH hamsters. Infect Immun. 1995;63:4818–4825. doi: 10.1128/iai.63.12.4818-4825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendez S, Valenzuela JG, Wu W, Hotez PJ. Host cytokine production, lymphoproliferation, and antibody responses during the course of Ancylostoma ceylanicum infection in the Golden Syrian hamster. Infect Immun. 2005;73:3402–3407. doi: 10.1128/IAI.73.6.3402-3407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekkens MJ, Liu Z, Liu Q, et al. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 42.Bungiro RD, Jr, Cappello M. Detection of excretory/secretory coproantigens in experimental hookworm infection. Am J Trop Med Hyg. 2005;73:915–920. [PubMed] [Google Scholar]
- 43.Chu D, Bungiro RD, Ibanez M, et al. Molecular characterization of Ancylostoma ceylanicum Kunitz-type serine protease inhibitor: evidence for a role in hookworm-associated growth delay. Infect Immun. 2004;72:2214–2221. doi: 10.1128/IAI.72.4.2214-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendez S, Zhan B, Goud G, et al. Effect of combining the larval antigens Ancylostoma secreted protein 2 (ASP-2) and metalloprotease 1 (MTP-1) in protecting hamsters against hookworm infection and disease caused by Ancylostoma ceylanicum. Vaccine. 2005;23:3123–3130. doi: 10.1016/j.vaccine.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 46.Bundy D, Sher A, Michael E. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol Today. 2000;16:273–274. doi: 10.1016/s0169-4758(00)01689-6. [DOI] [PubMed] [Google Scholar]
- 47.Walson JL, Otieno PA, Mbuchi M, et al. Albendazole treatment of HIV-1 and helminth co-infection: a randomized, double-blind, placebo-controlled trial. AIDS. 2008;22:1601–1609. doi: 10.1097/QAD.0b013e32830a502e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachur TP, Vale JM, Coelho IC, Queiroz TR, Chaves Cde S. Enteric parasitic infections in HIV/AIDS patients before and after the highly active antiretroviral therapy. Braz J Infect Dis. 2008;12:115–122. doi: 10.1590/s1413-86702008000200004. [DOI] [PubMed] [Google Scholar]