Abstract
Unlike the general population, among hemodialysis patients body-mass index(BMI)is inversely related to blood pressure (BP) and mortality. To explore the reasons for this risk-factor paradox the cross-sectional association of obesity with the following factors was examined: the prevalence of hypertension, its control and echocardiographic left ventricular mass index (LVMI). Longitudinal follow-up explored the relationship of BMI with all-cause mortality. Further it explored whether poorer survival in leaner individuals was related to either high BP or greater LVMI. Among 368 hemodialysis patients both the prevalence of hypertension and its poor control were inversely related to BMI. BMI was also inversely associated with evidence of excess extracellular fluid volume but adjustment for this variable did not completely remove the inverse relationship between BP and BMI. Over 1122 patient-years of cumulative follow up (median 2.7 years) 119 (32%) patients died. In the first two years of follow up, the mortality hazard for the lowest BMI group was increased; thereafter, the survival curves were similar. Adjusting for several risk factors including BP and LVMI did not remove the inverse relationship of BMI with mortality. In conclusion, leaner patients on dialysis have a higher prevalence of hypertension, poorer control of hypertension, more LVMI, and greater evidence of extracellular fluid volume excess. However, volume only partially explains the greater prevalence or poorer control of hypertension. Leaner patients have an accelerated mortality rate in the first two years; this is not completely explained by BP, LVMI or other cardiovascular or dialysis-specific risk factors.
Keywords: Body mass index, epidemiology, hemodialysis, ambulatory blood pressure, left ventricular hypertrophy, survival
Introduction
The association of body-mass index (BMI) with mortality in the general population is well recognized 1. There are more than 400,000 patients on long-term dialysis in the United States. Among these people, many epidemiological studies indicate that unlike the general population, BMI is not inversely but directly associated with survival 2–8. The reasons for this paradoxical observation–termed risk-factor paradox or reverse epidemiology–are not completely understood.
Unlike the general population obese people on dialysis also have lower blood pressure (BP) 9, 10. The reasons underlying this paradoxical association are also unclear. One reason could be that individuals with higher BMI are better able to sequester excess extracellular fluid volume. If so, markers of extracellular fluid volume excess among these individuals would be inversely related to BMI. Also, if these markers lie in the causal pathway, correction for these markers would diminish the relationship between BP and BMI. Furthermore, if BMI is truly associated with lower BP, then it must also be associated with less target organ damage measured as left ventricular mass index(LVMI) 11. Whether this is so, is also unknown.
The survival studies relating obesity to mortality often do not have extended follow ups; some of them have been carried out only over one year 5, 6. Notably, studies in the general population have excluded deaths in the first 5 years of follow up to exclude reverse causation1. The limited duration of these studies leave open the possibility that leaner individuals have an accelerated death rate. Although this hypothesis has been proposed, so far there have been few data to support it 12. Finally, whether BP and LVMI are mediators of poorer survival among leaner individuals is also unknown.
In this study we examined the association of the prevalence and control of blood pressureas well as echocardiographic left ventricular mass index with obesity. We sought the association of echocardiographic markers of volume excess with obesity and asked the question whether excess volume is a mediator of prevalence and control of hypertension. In longitudinal follow up of up to 8 years we explored the relationship of all cause mortality and obesity and whether poorer survival in leaner individuals is related to high BP and LVMI.
Methods
Participants
Portions of this cohort have been previously described 11, 13. Patients 18 years or older who had been on chronic hemodialysis for more than 3 months, and were free of vascular, infectious or bleeding complications within one month of recruitment who were dialyzed three times a week dialysis at one of the four dialysis units in Indianapolis affiliated with Indiana University were enrolled in the study. Those who missed two hemodialysis treatments or more over one month, abused drugs, had chronic atrial fibrillation or had body mass index of 40 kg/m2 or more at screening visit were excluded. Patients who had a change in dry-weight or antihypertensive drugs within 2 weeks were also excluded. The study was approved by the Institutional Review Board of Indiana University and Research and Development Committee of the Roudebush VA Medical Center, Indianapolis and all subjects gave written informed consent.
Definition of body mass index and obesity
Body mass index was calculated as post dialysis weight (kg) divided by height (m2). Obesity was defined by BMI according to the World Health Organization 14 (the ranges used to classify are shown in Table 1).
Table 1.
Descriptive characteristics of the study population
| Variable | Severely Underweight | Underweight | Normal | Overweight | Obese I | Obese II | Obese III | Total | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Defining body mass index range (kg/m2) | <16 | (16, 18.5) | (18.5, 25) | (25, 30) | (30, 35) | (35, 40) | 40+ | ||
| N | 1 (0%) | 15 (4%) | 122 (33%) | 110 (30%) | 71 (19%) | 43 (12%) | 6 (2%) | 368 (100%) | |
| Body mass index (kg/m2) | 15.4 | 17.6 ± 0.7 | 22.3 ± 1.8 | 27.5 ± 1.4 | 32.5 ± 1.4 | 37.3 ± 1.6 | 40.7 ± 1.3 | 27.7 ± 5.9 | <0.0001 |
| Age (y) | 62.4 | 56.8 ± 12.4 | 55.0 ± 13.3 | 56.5 ± 12.6 | 54.3 ± 13.0 | 51.1 ± 11.8 | 49.6 ± 10.0 | 54.9 ± 12.8 | 0.3 |
| Male gender | 1 (100%) | 10 (67%) | 94 (77%) | 71 (65%) | 36 (51%) | 23 (53%) | 4 (67%) | 239 (65%) | <0.01 |
| Racial category | 0.5 | ||||||||
| Non-black | 0 (0%) | 3 (20%) | 19 (16%) | 17 (15%) | 6 (8%) | 10 (23%) | 1 (17%) | 56 (15%) | |
| Black | 1 (100%) | 12 (80%) | 103 (84%) | 93 (85%) | 65 (92%) | 33 (77%) | 5 (83%) | 312 (85%) | |
| History of smoking | <0.0001 | ||||||||
| Current | 1 (100%) | 9 (60%) | 50 (41%) | 38 (35%) | 14 (20%) | 6 (14%) | 2 (33%) | 120 (33%) | |
| Past | 0 (0%) | 4 (27%) | 41 (34%) | 32 (29%) | 27 (38%) | 15 (35%) | 1 (17%) | 120 (33%) | |
| Never | 0 (0%) | 2 (13%) | 24 (20%) | 37 (34%) | 29 (41%) | 20 (47%) | 3 (50%) | 115 (31%) | |
| History of cardiovascular disease | 0 (0%) | 3 (20%) | 38 (31%) | 39 (35%) | 26(37%) | 18 (42%) | 4 (67%) | 128 (35%) | 0.3 |
| History of diabetes mellitus | 1 (100%) | 5 (33%) | 45 (37%) | 52 (47%) | 46 (65%) | 27 (63%) | 4 (67%) | 180 (49%) | <0.0001 |
| Years on dialysis | 0.1 | ||||||||
| <1 | 0 (0%) | 3 (20%) | 40 (33%) | 38 (35%) | 28 (39%) | 20 (47%) | 5 (83%) | 134 (36%) | |
| 1–4 | 0 (0%) | 6 (40%) | 44 (36%) | 41 (37%) | 27 (38%) | 16 (37%) | 1 (17%) | 135 (37%) | |
| 4+ | 1 (100%) | 6 (40%) | 38 (31%) | 31 (28%) | 15 (21%) | 6 (14%) | 0 (0%) | 97 (26%) | |
| Pre-HD weight (kg) | 50.1 | 54.3 ± 5.1 | 68.8 ± 9.6 | 85.0 ± 10.6 | 98.1 ± 14.3 | 109.7 ± 14.2 | 132.1 ± 20.4 | 84.8 ± 20.2 | <0.0001 |
| Post-HD weight (kg) | 47.3 | 52.2 ± 4.9 | 66.3 ± 9.0 | 82.0 ± 10.2 | 95.0 ± 13.5 | 106.6 ± 13.6 | 127.4 ± 18.9 | 81.6 ± 19.5 | <0.0001 |
| Etiology of end-stage renal disease | 0.03 | ||||||||
| Diabetes mellitus | 1 (100%) | 3 (20%) | 28 (23%) | 39 (35%) | 33 (46%) | 21 (49%) | 3(50%) | 128 (35%) | |
| Hypertensive nephrosclerosis | 0 (0%) | 6 (40%) | 67 (55%) | 49 (45%) | 33 (46%) | 17 (40%) | 3 (50%) | 175 (48%) | |
| Glomerulonephritis | 0 (0%) | 3 (20%) | 8 (7%) | 6 (5%) | 1 (1%) | 1 (2%) | 0 (0%) | 19 (5%) | |
| Adult autosomal polycystic kidney disease | 0 (0%) | 0 (0%) | 1 (1%) | 4 (4%) | 0 (0%) | 1 (2%) | 0 (0%) | 6 (2%) | |
| Other | 0 (0%) | 3 (20%) | 15 (12%) | 11 (10%) | 3 (4%) | 1 (2%) | 0 (0%) | 33 (9%) | |
| Urea reduction ratio (%) | 76.3 | 80.2 ± 6.8 | 75.7 ± 8.1 | 74.2 ± 7.0 | 72.8 ± 6.1 | 71.8 ± 7.3 | 68.7 ± 5.4 | 74.3 ± 7.4 | <0.01 |
| Albumin (g/dL) | 2.5 | 3.5 ± 0.6 | 3.7 ± 0.4 | 3.7 ± 0.5 | 3.8 ± 0.4 | 3.7 ± 0.3 | 4.0 ± 0.2 | 3.7 ± 0.4 | 0.03 |
| Hemoglobin (g/dL) | 14 | 12.2 ± 1.5 | 12.2 ± 1.5 | 12.0 ± 1.6 | 12.0 ± 1.0 | 12.1 ± 1.6 | 12.5 ± 1.4 | 12.1 ± 1.5 | 0.8 |
| On antihypertensive medications | 1 (100%) | 12 (80%) | 96 (79%) | 79 (72%) | 52 (73%) | 27 (63%) | 3 (50%) | 270 (73%) | 0.4 |
| Number of antihypertensives in users | 4.0 | 1.9 ± 1.4 | 2.2 ± 1.7 | 1.8 ± 1.5 | 1.6 ± 1.3 | 1.6 ± 1.6 | 1.2 ± 1.5 | 1.8 ± 1.6 | 0.06 |
| Aspirin use | 0 (0%) | 8 (53%) | 45 (37%) | 38 (35%) | 35 (49%) | 28 (65%) | 1 (17%) | 155 (42%) | <0.01 |
| Statin use | 0 (0%) | 6(40%) | 42 (34%) | 48 (44%) | 36 (51%) | 19 (44%) | 0 (0%) | 151 (41%) | 0.1 |
| Vitamin D receptor activator use | 0 (0%) | 2 (13%) | 39 (32%) | 40 (36%) | 27 (38%) | 18 (42%) | 0 (0%) | 126 (34%) | 0.2 |
| Epoetin use | 0 (0%) | 6 (40%) | 58 (48%) | 51 (46%) | 43 (61%) | 26 (60%) | 2 (33%) | 186 (51%) | 0.2 |
| Mean ambulatory systolic BP (mmHg) | 149.7 | 137.1 ± 27.6 | 139.2 ± 21.6 | 134.3 ± 20.8 | 133.0 ± 19.2 | 130.2 ± 19.4 | 125.2 ± 7.0 | 135.2 ± 20.9 | 0.1 |
| IVC diameter at expiration (mm) | 7.3 | 16.75 ± 10.45 | 15.22 ± 5.11 | 15.26 ± 5.76 | 15.92 ± 5.28 | 13.36 ± 4.16 | 17.25 ± 5.98 | 15.25 ± 5.67 | 0.3 |
| Left atrial diameter (cm) | 3.73 | 3.84 ± 0.84 | 4.09 ± 0.77 | 4.08 ± 0.76 | 4.18 ± 0.75 | 4.27 ± 0.66 | 4.89 ± 0.58 | 4.12 ± 0.76 | 0.1 |
| IVC diameter index at expiration (mm/m2) | 4.65 | 10.26 ± 5.76 | 8.56 ± 3.02 | 7.87 ± 2.94 | 7.80 ± 2.86 | 6.26 ± 2.05 | 7.26 ± 1.83 | 8.04 ± 3.16 | <0.01 |
| Left atrial diameter index (cm/m2) | 2.38 | 2.40 ± 0.55 | 2.29 ± 0.44 | 2.10 ± 0.42 | 2.04 ± 0.43 | 2.02 ± 0.41 | 2.09 ± 0.24 | 2.16 ± 0.45 | <0.01 |
| Left ventricular mass index (g/m2) | 130 | 146.5 ± 60.3 | 151.5 ± 50.1 | 141.7 ± 40.0 | 123.9 ± 35.1 | 139.9 ± 41.2 | 156.4 ± 15.1 | 141.8 ± 44.5 | 0.01 |
| Left ventricular hypertrophy | 1 (100%) | 9 (60%) | 83 (68%) | 76 (69%) | 37 (52%) | 26 (60%) | 5 (83%) | 237 (64%) | 0.04 |
Measurements
Ambulatory BP Monitoring and definitions of hypertension
Ambulatory BP monitoring was performed either after the first or mid-week hemodialysis session for 44 hours. Ambulatory BP was recorded every 20 minutes during the day (6 AM to 10 PM) and every 30 minutes during the night (10 PM to 6 AM) using a Space lab 90207 ambulatory blood pressure monitor (Space Labs Medical Inc, Redmond, WA, USA) in the non-access arm, as reported previously 15. In this study, patients who had less than 8 hours of ambulatory BP recordings were noted to have inadequate measurement and were excluded.
Hourly average of ambulatory BP was first computed. These averages were then averaged over the 44 hours of recording to yield the overall ambulatory BP. Ambulatory blood pressure values greater than or equal to 135/85 mmHg were considered hypertensive 16. Also, any patient on antihypertensives was considered to be hypertensive. If the ambulatory blood pressure was 135/85 mmHg or more then the patient was considered to be poorly controlled.
Echocardiograms
Two-dimensional guided M-mode echocardiograms were performed by research echocardiographic technicians, 30–60 minutes following dialysis, in the dialysis unit with a digital cardiac ultrasound machine (Cypress Acuson, Siemens Medical)within 10 days of ambulatory BP recording as previously reported 11.
The left atrial diameter indexed for body surface area and the IVC diameter in expiration also indexed for body surface area were imaged as previously described 17. They have previously been shown to be markers of volume and therefore chosen as ECF volume markers for this analysis 17.
Data Analysis
Descriptive statistics for demographic and clinical variables related to the categories of BMI were provided. Race was combined into two categories, black and non-black. Dialysis vintage was categorized into three groups, dialysis less than a year, dialysis one to four years and dialysis more than four years. The number of antihypertensives was capped at four, as generally few patients were on more than 4 medications.
Odds ratio for prevalence was calculated by logistic regression. BMI categories were used as determinants with the highest category used as reference. Given the small number of patients, normal, underweight and severely underweight BMI categories were merged as were grade 2 or 3 obesity categories. A multivariable model was then used to adjust for the following covariates: gender, smoking, urea reduction ratio, aspirin use, diabetes and serum albumin. These covariates were selected based on their association with BMI. Stepwise forward-selection logistic regression was performed with factors added at the .15 level of significance. Similar models were fitted for poor control of hypertension.
Next logistic regression models were constructed with each of the two markers of volume (IVC diameter and left atrial diameter) used separately to predict the prevalence of (Model 3 and 4 in table 2) or the lack of control of hypertension(Model 4 and 5 in table S1). Sensitivity analyses were performed using multiple imputation for missing data for all logistic models using the “mi” set of commands in Stata.
Table 2.
Odds ratios for prevalence of hypertension by ambulatory blood pressure monitoring
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Independent variable | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Body mass index | ||||||||
| Normal or underweight | 2.85 (1.28, 6.34) | 0.01 | 2.83 (1.08, 7.42) | 0.03 | 2.09 (0.76, 5.72) | 0.2 | 3.07 (1.08, 8.76) | 0.04 |
| Overweight | 1.70 (0.78, 3.70) | 0.2 | 1.30 (0.53, 3.19) | 0.6 | 1.43 (0.53, 3.82) | 0.5 | 1.81 (0.66, 4.96) | 0.3 |
| Obese I | 1.78 (0.75, 4.23) | 0.2 | 1.27 (0.47, 3.43) | 0.6 | 0.93 (0.33, 2.59) | 0.9 | 1.58 (0.52, 4.76) | 0.4 |
| Obese II/III | 1.00 (ref cat) | 1.00 (ref cat) | 1.00 (ref cat) | 1.00 (ref cat) | ||||
| p value for trend | 0.02 | 0.02 | 0.07 | 0.03 | ||||
| Diabetes mellitus | 1.78 (0.95, 3.31) | 0.07 | 1.92 (0.97, 3.78) | 0.06 | 1.78 (0.90, 3.52) | 0.1 | ||
| Serum albumin (g/dL) | 2.03 (1.06, 3.90) | 0.03 | 2.05 (1.01, 4.17) | 0.05 | 1.83 (0.90, 3.72) | 0.1 | ||
| Left atrial diameter index (cm/m2) | 2.12 (0.98, 4.56) | 0.06 | ||||||
| IVC diameter at expiration index (mm/m2) | 0.99 (0.89, 1.10) | 0.8 | ||||||
| Continuous body mass index | 0.95 (0.91, 1.00) | 0.04 | 0.95 (0.90, 0.99) | 0.03 | 0.96 (0.90, 1.01) | 0.1 | 0.94 (0.89, 1.00) | 0.06 |
A linear regression model was used to predict echocardiographic left ventricular mass index (Model 1, table 3). This model was further adjusted for the following covariates: gender, smoking, urea reduction ratio, aspirin use, diabetes and serum albumin using stepwise multivariable regression with covariates added at the 0.15 level of significance (Model 2, table 3). This model was further adjusted for interdialytic ambulatory systolic BP (Model 3, table 3). Sensitivity analyses were performed using multiple imputationas above.
Table 3.
Association of body mass index with left ventricular mass index
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Independent variable | Mean (95% CI) | P | Mean (95% CI) | P | Mean (95% CI) | P |
| Body mass index | −1.13 (−1.96, −0.30) | <0.01 | −0.78 (−1.73, 0.17) | 0.1 | −0.21 (−1.12, 0.71) | 0.7 |
| Male gender | 6.22 (−5.10, 17.54) | 0.3 | 9.67 (−1.15, 20.50) | 0.08 | ||
| Diabetes mellitus | −16.97 (−27.20, −6.74) | <0.01 | −24.26 (−34.21, −14.30) | <0.0001 | ||
| Serum albumin (g/dL) | −20.95 (−32.23, −9.68) | <0.001 | −15.98 (−26.82, −5.14) | <0.01 | ||
| Urea reduction ratio (%) | −0.70 (−1.46, 0.06) | 0.07 | −0.56 (−1.29, 0.16) | 0.1 | ||
| Ambulatory systolic BP (mmHg) | 0.70 (0.46, 0.95) | <0.0001 | ||||
Survival was analyzed by Kaplan-Meier methods (Figure 3) and Cox proportional hazards regression (Table 4). BMI was used as a continuous variable in the Cox model (Model 1, table 4). The proportionality assumption was violated, therefore all subsequent models contained the BMI x time interaction term. Introducing this interaction term showed no violation of the proportionality assumption for other covariates (as tested by the Schoenfeld residuals). The model was further adjusted for covariates shown in Table 4.
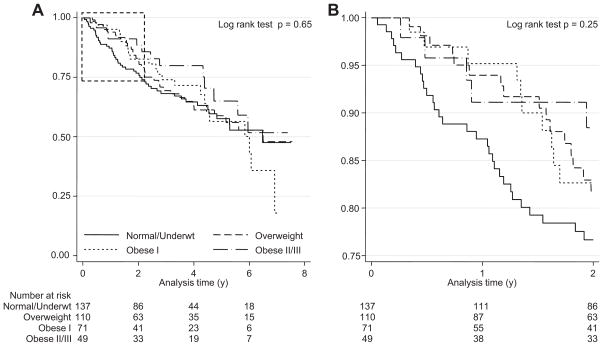
Figure 3.
A: Survival by BMI categories. Figure 3B Enlarged version of the dotted area of Figure 3A.
During the first two years of follow up, the mortality hazard for the lowest BMI group was increased; thereafter, the survival curves were similar.
Table 4.
Effects of body mass index on survival
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Independent variable | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Body mass index (kg/m2) | 0.98 (0.95, 1.01) | 0.1 | 0.92 (0.87, 0.97) | <0.01 | 0.91 (0.85, 0.97) | <0.01 | 0.90 (0.85, 0.96) | <0.01 |
| Age (y) | 1.03 (1.02, 1.05) | <0.001 | 1.03 (1.01, 1.05) | <0.01 | ||||
| Black | 0.57 (0.31, 1.05) | 0.07 | 0.69 (0.36, 1.34) | 0.3 | ||||
| Male gender | 1.27 (0.82, 1.96) | 0.3 | 1.39 (0.88, 2.20) | 0.2 | ||||
| Diabetes mellitus | 1.57 (0.99, 2.47) | 0.05 | 1.38 (0.86, 2.23) | 0.2 | ||||
| Cardiovascular disease | 1.55 (1.03, 2.32) | 0.03 | 1.67 (1.11, 2.52) | 0.01 | ||||
| Serum albumin (g/dL) | 0.78 (0.49, 1.26) | 0.3 | 0.83 (0.52, 1.33) | 0.4 | ||||
| Ambulatory systolic BP (mmHg) | 1.01 (1.00, 1.02) | 0.2 | 1.01 (1.00, 1.02) | 0.2 | ||||
| Left ventricular mass index (g/m2) | 1.00 (0.99, 1.00) | 0.4 | 1.00 (0.99, 1.00) | 0.4 | ||||
| Dialysis vintage (y) | 1.09 (1.03, 1.16) | <0.01 | 1.10(1.04, 1.16) | <0.001 | ||||
| Serum creatinine (mg/dL) | 0.93 (0.85, 1.02) | 0.1 | ||||||
| Body mass index * time | 1.002 (1.000, 1.003) | <0.01 | 1.002 (1.000, 1.004) | 0.02 | 1.002 (1.001, 1.004) | <0.01 | ||
All analyses were conducted using Stata 11.0 (Stata Corp, College Station, TX). The P values reported are two-sided and taken to be significant at <0.05.
Results
Of the 441 patients who consented, 1 was missing BMI, 7 had inadequate ambulatory BP recordings and 65 had none. These 368 patients who had measurements of both BMI and interdialytic ambulatory BP formed the study cohort: 316 (86%) of these also had echocardiographic data (please see http://hyper.ahajournals.org Figure S1).
Table 1 shows the characteristics of the patients by body mass index categories. Overall, we studied 368 patients with a mean BMI of 27.7, age 55 years, two thirds were men, one third were active smokers and 85% were Black. The etiology of ESRD was diabetes mellitus in 35% and hypertensive nephrosclerosis in nearly half. BMI was associated with gender (more obesity among women), smoking (smokers were leaner), diabetes, urea reduction ratio, serum albumin and aspirin use (more aspirin use among obese).There was more evidence of volume expansion by echocardiographic criteria among leaner patients.
Table 2 shows the odds ratio for the prevalence of hypertension by interdialytic ambulatory BP monitoring. The unadjusted model (Model 1) showed a significant inverse relationship between the odds of being hypertensive and BMI categories. In a separate model, BMI used as a continuous variable also showed a similar inverse relationship(OR 0.95, p=0.04). Model 2 shows odds ratios for only the significant determinants in which BMI categories were adjusted in a stepwise forward logistic regression for the following variables: gender, smoking, urea reduction ratio, aspirin use, diabetes and serum albumin. Diabetes mellitus and serum albumin emerged as significant predictors of prevalence of hypertension. However, BMI categories still remained significantly and inversely associated with prevalent hypertension. As a continuous variable, BMI adjusted for diabetes and serum albumin remained significantly and inversely associated with hypertension (OR 0.95, p=0.03). Further adjustment of Model 2 for left atrial diameter (Model 3) reduced the strength of the statistical association of BMI categories (p=0.07) and BMI (OR 0.96, p=0.1) with hypertension. However, upon imputing for missing data showed that the statistical association of BMI categories (p=0.04) and BMI (OR 0.95, p=0.07) with hypertension were both improved. Adjustment of Model 2 for IVC diameter in expiration (Model 4) removed the strength of the statistical association of BMI (OR 0.94, p=0.06) but not BMI categories (p=0.03) with hypertension. Thus BMI categories were significantly and inversely related to hypertension even after accounting for markers of volume.
Table S1 (please see http://hyper.ahajournals.org) shows the odds ratio for the control of hypertension. The unadjusted model (Model 1) showed a significant inverse relationship between the odds of being poorly controlled hypertensive and BMI categories. BMI used as a continuous variable also showed a similar inverse relationship (OR 0.96, p=0.01). Model 2 shows odds ratios for only the significant determinants in which BMI categories were adjusted in a stepwise forward logistic regression for the variables noted in methods. Only serum albumin emerged as weak direct determinant of control of hypertension. However, BMI categories remained significantly and inversely associated with lack of control of hypertension. BMI adjusted for serum albumin remained significantly and inversely associated with hypertension (OR 0.96, p=0.02). Further adjustment of Model 2 for number of antihypertensive medications (Model 3) reduced the statistical association of BMI categories (p=0.09) and BMI (OR 0.97, p=0.1) with lack of control of hypertension. Imputation for missing data did not alter the results. Adjustment of Model 3 for left atrial diameter indexed for body surface area (Model 4) increased the statistical association of BMI (OR 0.96, p=0.05) and BMI categories (p=0.04) with the lack of control of hypertension. Adjustment of Model 3 for IVC diameter in expiration (Model 5) removed the statistical association of BMI (OR 0.96, p=0.1) but not BMI categories (p=0.05) with the lack of control of hypertension.
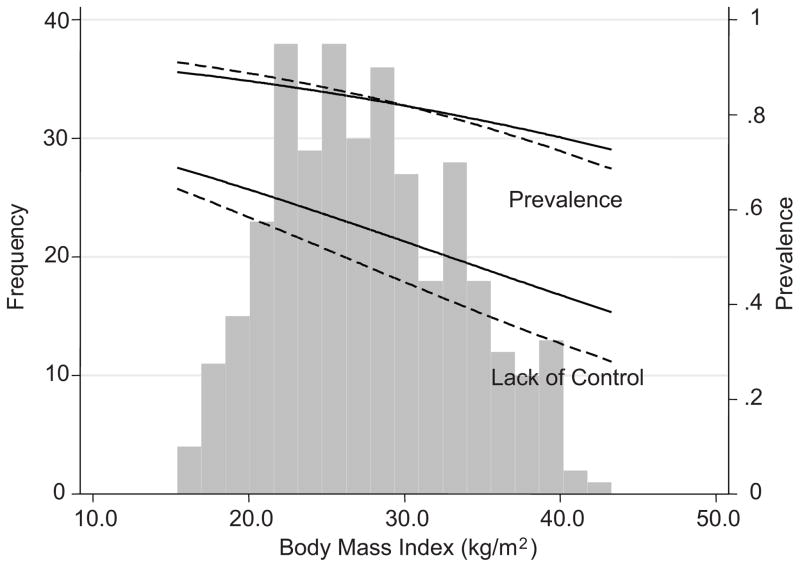
The distribution of BMI and the prevalence and lack of control of hypertension is shown in Figure 1. The solid lines represent the unadjusted estimates. The dotted lines are either prevalence adjusted for diabetes and serum albumin or lack of control adjusted for serum albumin.
Figure 1. Distribution of BMI, prevalence of hypertension and lack of control of hypertension.
The solid lines represent the unadjusted estimates. The dotted lines are either prevalence adjusted for diabetes and serum albumin or lack of control adjusted for serum albumin. Prevalence of hypertension or lack of control are shown on the right Y-axis.
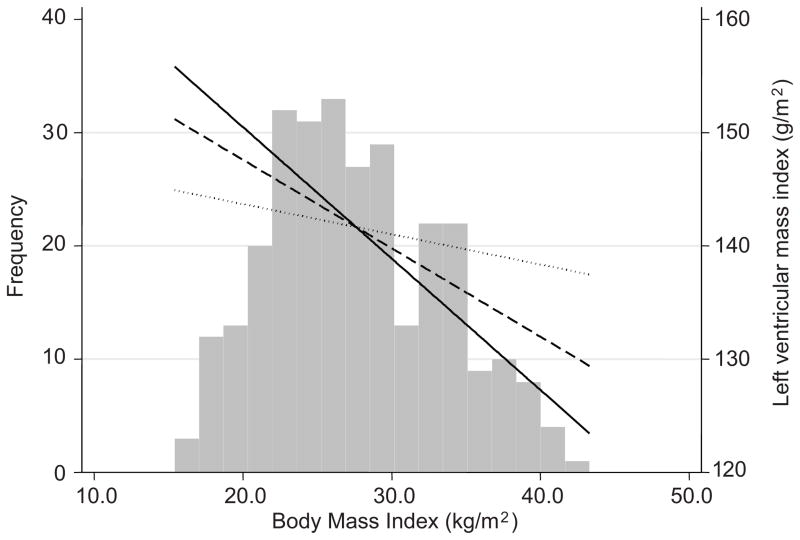
We next evaluated the association of BMI with target organ damage (left ventricular mass index (LVMI)). Table 3 shows a significant and inverse relationship of BMI with unadjusted LVMI(Model 1). The model intercept was 172.8 g/m2. Stepwise forward multivariable linear regression for the following variables: gender, smoking, urea reduction ratio, aspirin use, diabetes and serum albumin removed the statistical association of BMI with LVMI. The significant determinants of LVMI are shown in Model 2. Further adjustment of Model 2 for interdialytic ambulatory systolic BP removed the association of BMI with LVMI nearly completely (model 3). Figure 2 shows the association of unadjusted LVMI with BMI (solid line), multivariate adjusted for variables shown in Model 2 (dashed line) and further adjusted for interdialytic ambulatory systolic BP (dotted line).
Figure 2. Association of unadjusted LVMI with BMI.
Unadjusted estimate is shown by the solid line, multivariate adjusted (for variables shown in Model 2, Table 3) by the dashed line and further adjusted for interdialytic ambulatory systolic BP by the dotted line. The relationship between BMI and LVMI was not significant after adjusted for interdialytic ambulatory systolic BP.
Cumulative follow up for 1122 patient-years of 368 patients with a median follow-up of 2.7 years culminated in 119 (32%) deaths. Out of 138 normal or underweight patients, 49 (36%) died. Out of 110 overweight patients, 34 (31%) died. Out of 71 patients mildly obese (BMI < 35), 23 (32%) died. Out of 49 moderately or severely obese patients (BMI >= 35), 13 (27%) died.
Figure 3A shows the survival according to BMI categories. In the first two years of follow up, the mortality hazard for the lowest BMI group was increased(Figure 3B which shows the enlarged version of the dotted area of Figure 3A); thereafter, the survival curves were similar. These graphs indicate violation of the proportionality assumption therefore Table 4 shows the hazard ratio for the unadjusted model without accounting for the proportionality assumption (Model 1) and after interacting the predictor with time (Model 2). We next added the following variables to the model: age, sex, race, serum albumin, history of diabetes, history of cardiovascular disease, dialysis vintage, left ventricular mass index, and systolic ambulatory blood pressure(Model 3). Even adjusting for these variables neither did mitigate the strength of the association between BMI and mortality nor remove the statistical significance of this inverse association. Serum creatinine in dialysis patients is a proxy for muscle mass. We added this term to the model to explore further the relationship between BMI and mortality further (Model 4). Adding serum creatinine also did not explain the increased mortality associated with a lower BMI. However, adjusting for serum creatinine in those with low or normal body mass index removed the association of mortality with BMI (data not shown).
Discussion
An inverse relationship between both the prevalence of hypertension and its poor control was associated with BMI. Those who were the leanest had the greatest prevalence of hypertension and also the poorest control. Similarly, an inverse relationship between left ventricular mass index and BMI was found. Thus, those who were leaner had a greater left ventricular mass index. The excess prevalence of hypertension and poor control of hypertension among lean hemodialysis patients may be due to the following reasons. First, obese patients may sequester excess fluid volume in the extracellular space more effectively than lean people and therefore not get hypertensive. Although echocardiographic evidence of volume excess among leaner patients (increased left atrial diameter or inferior vena cava diameter both indexed for body surface area)was found, these markers were by themselves insufficient to obliterate the inverse association between hypertension and BMI. Second, increased muscle mass may be associated with increased renalase expression in those with higher BMI 18. Renalase, a catecholamine-metabolizing enzyme is expressed in skeletal muscle and can reduce the circulating catecholamine levels 18, 19. This in turn would be associated with less prevalence of hypertension and better control. Neither muscle mass nor plasma renalase concentration was measured in our patients so we are unable to confirm or refute this possibility.
The association of increased BMI with lower mortality was first reported in the Diaphane collaborative study in younger French dialysis patients treated with long-term dialysis during the 1970’s 2. With one notable exception 20, these observations have now been confirmed in several cohorts 3–8. This study not only confirms the inverse association of BMI with mortality it provides some mechanistic insights. For example, adjustment for numerous explanatory variables for mortality–including ambulatory systolic BP and left ventricular mass index–did not remove the association of excess mortality and lower BMI. More importantly, the relationship of BMI and mortality was not constant over time. Leaner individuals showed accelerated mortality in the short term. However, after the first two years, the mortality curves were similar. Also notable was the lack of graded relationship between BMI and mortality. Thus, having a BMI of <25 (normal or underweight) was associated with increased mortality; however, being overweight, or progressively increasing levels of obesity was not associated with increasing mortality. Thus, obesity by itself does not appear to confer a survival advantage; in contrast, being underweight or normal weight on hemodialysis confers an increased risk for mortality. These patients may have a greater burden of illness. In this cohort they were more often smokers and also had a lower serum albumin. The results support the hypothesis proposed by Beddhu and associates 12. These investigators proposed that lean (low muscle mass) people have an accelerated rate of death. People who are obese have a high mortality rate but one that does not reach that of the leaner individuals. Our data provide direct support for this hypothesis. Notably, in a cohort of patients followed for 5 years, the effect of BMI on survival was also found to be time dependent; as noted in our study, the highest risk of death attributable to under nutrition or low BMI was noted to be in the first two years 3.
There is an additional potential mechanism to explain these observations. The risk of increased mortality in this group of normal or underweight individuals could be due to misclassification of obesity. We have previously reported that the negative predictive value of BMI to detect obesity in CKD is only 45% 21. Thus, a normal BMI does not rule out obesity. Although body composition was not measured in this cohort, it is unlikely that hemodialysis patients in are normal or underweight have low body fat. In fact, it is this group of patients that is more likely to have sarcopenia (and therefore body fat proportion that may be comparable to those with overt obesity). As an example, among ESRD patients in Sweden, protein-energy wasting was measured by the subjective global assessment of nutrition 22. This condition was equally prevalent in patients with low, normal and high BMIl ending support to the condition of “obese sarcopenia”. In this cohort, BMI per se did not predict mortality. However, for each BMI group, protein-energy malnutrition was associated with increased death risk. Serum creatinine is a surrogate for muscle mass in hemodialysis patients and has been inversely and independently associated with mortality 8. Adjusting for serum creatinine in those with low or normal body mass index removed the association of mortality with BMI. This suggests that low muscle mass (sarcopenia) may confer excess mortality risk among hemodialysis patients. This is further supported by subjective global assessment (SGA) of nutrition among hemodialysis patients; malnutrition as assessed by SGA is associated with a remarkably increased early mortality 23.
Our study has the following limitations: we did not measure body composition or 24 hour urine creatinine excretion. Assessed by 24 hour urine creatinine excretion, Beddhu et al 24 have previously demonstrated that normal or increased muscle mass confers a survival advantage of high BMI among CKD patients on long-term dialysis. We also did not measure change in BMI over time so we are unable to assess the impact of change in BMI on mortality. However others have previously shown that a drop in BMI over six months 4 or post dialysis weight loss is associated with an increased risk for mortality 8. Other metrics of increased visceral fat such as waist circumference were not measured. However, it has been noted previously that a higher waist circumference even after adjusting for BMI is associated with a higher all-cause and cardiovascular mortality 25. Strengths of our study include the measurement of interdialytic ambulatory BP and echocardiographic LVMI as mediators of the inverse relationship between BMI and mortality.
Perspective
This study shows that leaner patients on dialysis have a higher prevalence of hypertension, poorer control of hypertension, and greater evidence of extracellular fluid volume excess. However, the latter only partially explains the greater prevalence or poorer control of hypertension. Leaner patients also have evidence of more left ventricular mass index mostly because of higher interdialytic ambulatory blood pressure. Leaner patients have an accelerated mortality rate in the first two years. Subsequently, the mortality rate among these patients matches ones with higher BMI. The accelerated mortality rate is not completely explained by hypertension, left ventricular hypertrophy or other cardiovascular or dialysis-specific risk factors. Further research to explain the mechanistic relationship between low BMI and increased mortality is needed.
Supplementary Material
Acknowledgments
Sources of Funding
Supported by grant number 2RO1-NIDDK062030-07 from NIH-NIDDK.
Footnotes
Disclosure
none
Reference List
- 1.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degoulet P, Legrain M, Reach I, Aime F, Devries C, Rojas P, Jacobs C. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31:103–110. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 3.Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31:997–1006. doi: 10.1053/ajkd.1998.v31.pm9631845. [DOI] [PubMed] [Google Scholar]
- 4.Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, Young EW. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 5.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 7.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64:1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salahudeen AK, Fleischmann EH, Bower JD, Hall JE. Underweight rather than overweight is associated with higher prevalence of hypertension: BP vs BMI in haemodialysis population. Nephrol Dial Transplant. 2004;19:427–432. doi: 10.1093/ndt/gfg523. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 12.Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial. 2004;17:229–232. doi: 10.1111/j.0894-0959.2004.17311.x. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Andersen MJ, Bishu K, Saha C. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006;69:900–906. doi: 10.1038/sj.ki.5000145. [DOI] [PubMed] [Google Scholar]
- 14.Physical status :the use and interpretation of anthropometry : report of a WHO Expert Committee. Geneva: World Health Organization; 1995. WHO Expert Committee on Physical Status: the Use and Interpretation of Anthropometry. [PubMed] [Google Scholar]
- 15.Agarwal R. Supervised atenolol therapy in the management of hemodialysis hypertension. Kidney Int. 1999;55:1528–1535. doi: 10.1046/j.1523-1755.1999.00359.x. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R, Bouldin JM, Light RP, Garg A. Inferior vena cava diameter and left atrial diameter measure volume but not dry weight. Clin J Am Soc Nephrol. 2011;6:1066–1072. doi: 10.2215/CJN.09321010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–1280. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desir GV. Regulation of blood pressure and cardiovascular function by renalase. Kidney Int. 2009;76:366–370. doi: 10.1038/ki.2009.169. [DOI] [PubMed] [Google Scholar]
- 20.de Mutsert R, Snijder MB, van der Sman-de Beer, Seidell JC, Boeschoten EW, Krediet RT, Dekker JM, Vandenbroucke JP, Dekker FW. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol. 2007;18:967–974. doi: 10.1681/ASN.2006091050. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Bills JE, Light RP. Diagnosing obesity by body mass index in chronic kidney disease: an explanation for the “obesity paradox?”. Hypertension. 2010;56:893–900. doi: 10.1161/HYPERTENSIONAHA.110.160747. [DOI] [PubMed] [Google Scholar]
- 22.Honda H, Qureshi AR, Axelsson J, Heimburger O, Suliman ME, Barany P, Stenvinkel P, Lindholm B. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86:633–638. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- 23.de Mutsert R, Grootendorst DC, Boeschoten EW, Brandts H, van Manen JG, Krediet RT, Dekker FW. Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. Am J Clin Nutr. 2009;89:787–793. doi: 10.3945/ajcn.2008.26970. [DOI] [PubMed] [Google Scholar]
- 24.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 25.Postorino M, Marino C, Tripepi G, Zoccali C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53:1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.