Abstract
The target of rapamycin (TOR) is a critical intracellular regulator of the immune system. Recent studies have suggested that immunosuppression by TOR inhibition may be mediated by modulating differentiation of both effector and regulatory CD4 T cell subsets. However, it was paradoxically shown that inhibiting TOR signaling has immunostimulatory effects on the generation of long-lived memory CD8 T cells. Beneficial effects of TOR inhibition have also been observed with dendritic cells and hematopoietic stem cells. This immune modulation may contribute to lifespan extension seen in mice with mTOR inhibition. Here, we review recent findings on TOR modulation of innate and adaptive immune responses, and discuss potential applications of regulating TOR to provide longer and healthier immunity.
Introduction
The target of rapamycin (TOR) is evolutionally conserved from yeast to human and is an important kinase that regulates cell growth and metabolism in response to environmental signals [1,2]. It has become increasingly apparent that mammalian TOR (mTOR) activity is implicated in many of the physiological abnormalities associated with cancer, various metabolic syndromes, and aging [1,2]. mTOR exerts its effects through two different complexes, mTOR complex1 (mTORC1) and mTORC2, that have distinct roles in cellular actions (Fig. 1). mTORC1 is known to regulate many key cellular processes including autophagy, translation, ribosome biogenesis, and transcription. On the other hand, mTORC2 mediates organization of the actin cytoskeleton, and also controls cell survival (Fig. 1). Rapamycin, an inhibitor of mTOR, has been extensively used in numerous experimental settings to understand the role of the mTOR pathway. In clinical settings, rapamycin is given to transplant recipients as an immunosuppressant, and its primary effect of immunosuppression has long been considered to be due to inhibition of T cell proliferation. However, recent studies using rapamycin as well as genetic manipulation of the different components of the mTOR signaling pathway have revealed more complex mechanisms for the immunosuppression [3-5]. Furthermore, paradoxical immunostimulatory effects of rapamycin have also been reported by several groups [3-5]. In this review, we summarize and discuss recent findings regarding how mTOR signaling regulates various components of the immune system.
Fig. 1. The mTOR signaling pathway.
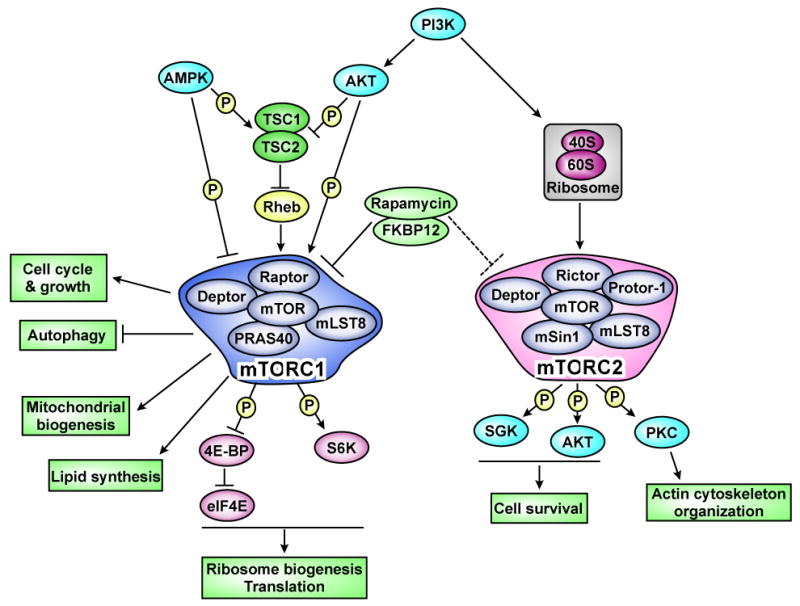
mTOR regulates many cellular activities through two distinct complexes; mTORC1 and mTORC2. mTORC1 is inhibited by the rapamycin-FKBP12 complex. mTORC2 is usually insensitive to rapamycin, but prolonged treatment decreases the mTORC2 activity in some cells. The ribosome is recently found to regulate activation of mTORC2 [68]. Arrows and bars represent activation and inhibition, respectively.
The role of TOR in adaptive immunity
Regulation of CD8 T cell responses
CD8 T cells play an important role in controlling viral infections and intracellular bacterial and parasitic infections by directly killing infected cells as well as by producing pro-inflammatory cytokines [6-8]. It is becoming increasingly clear that CD8 T cells are also involved in immunity against tumors and there is a growing interest in developing anti-tumor vaccines that stimulate CD8 T cell responses [7,9]. During the past few years, considerable progress has been made in understanding the role of the mTOR pathway in CD8 T cell responses. One example is that mTOR activity regulates T cell trafficking by altering expression of cell surface receptors important for migration into lymphoid organs. Naïve T cells express the lymph node-homing receptors CD62L and CCR7. Activated T cells are known to downregulate these receptors, and this downregulation in part facilitates their migration to the periphery toward sites of infection [10]. Inhibiting mTOR in activated CD8 T cells with rapamycin enhances expression of both CD62L and CCR7, and these CD8 T cells improve their ability to home to secondary lymphoid tissues [11]. This redirection of activated CD8 T cells into secondary lymphoid tissues might promote allograft survival in transplant recipients by relocating allogeneic effector T cells from transplanted organs.
In addition to T cell trafficking, several reports have recently shown that mTOR plays an important role in memory CD8 T cell differentiation [12-18]. After an acute viral infection, activated CD8 T cells clonally expand and differentiate into effector cells that clear virus-infected cells. This expansion phase is followed by a contraction phase during which 90-95% of the effector T cells die and the surviving 5-10% of the antigen-specific T cells become memory cells [6]. Thus, the surviving effector cells are considered memory precursor cells and can be distinguished from terminal effector cells by their surface expression of IL7R and KLRG1[19-22]. Our group has identified the surprisingly immunostimulatory effect of rapamycin on memory CD8 T cell differentiation [17]. Rapamycin treatment during the T cell expansion phase increased the quantity of memory CD8 T cells by increasing the number of memory precursor effector cells (Fig. 2) [17]. Thus, the treatment resulted in a similar number of antigen specific effector CD8 T cells at the peak of the clonal expansion compared to untreated mice, but reduced apoptotic cell death during the contraction phase (Fig. 2) [17]. On the other hand, inhibiting mTOR with rapamycin during the contraction phase accelerated the effector cells to memory CD8 T cell differentiation and improved the protective capacity and longevity of memory cells (Fig. 2) [17]. Consequently, rapamycin treatment during both the expansion and contraction phase enhanced not only the magnitude but also the quality of memory CD8 T cells (Fig. 2)[17]. Experiments using RNA interference demonstrated that rapamycin effects were intrinsic to antigen specific CD8 T cells. Knocking down raptor, a component of mTORC1 (Fig. 1), in antigen specific CD8 T cells resulted in similar effects to those observed with rapamycin treatment [17], suggesting that the mTORC1 pathway regulates memory CD8 T cell differentiation. In addition, Choi et al. have independently demonstrated an enhancement of CD8 T cell responses in rapamycin treated mice [18]. These studies show that targeting the mTOR pathway in CD8 T cells has adjuvant effects on generation of memory CD8 T cell immunity. However, it should be noted that administration of a very high dose of rapamycin prevented CD8 T cell responses [17]. These data collectively indicate that mTOR signaling is required for CD8 T cell responses and also suggest that the level of mTOR activity plays an essential role in regulating memory T cell generation (Fig. 2).
Fig. 2. mTORC1 regulates memory CD8 T cell differentiation.
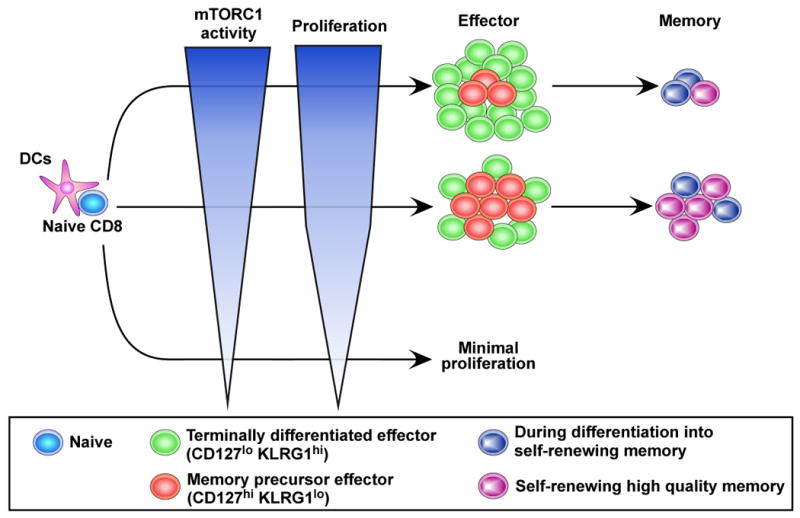
mTORC1 signaling is required for initial T cell proliferation as well as generation of effector T cells. Reducing mTORC1 signaling with rapamycin enhances induction of memory precursor effector cells, resulting in increased number of memory CD8 T cells. It also accelerates memory CD8 T cell differentiation, and improves memory T cell quality than normally found.
The important question of how mTOR signaling controls memory CD8 T cell differentiation is yet to be fully addressed. Many cellular activities such as cell growth, proliferation, autophagy, and translation are regulated by mTOR downstream signaling. Choi and co-workers suggested that modulating mTOR dependent fatty acid metabolism is essential for enhancing CD8 T cell memory [18]. Another study by Shrikant and colleagues proposed that mTOR signaling determines memory CD8 T cell fate by regulating expression of the transcription factors T-bet and Eomesodermin [16]. However, the mechanistic details of how mTOR activity controls fatty acid metabolism and the expression of the transcription factors remain unknown. Thus, investigating the direct downstream signaling of the mTOR pathway will be an essential step for achieving a better understanding of the role of mTOR in memory CD8 T cell differentiation.
Regulating differentiation into CD4 T helper cell subsets
CD4 T helper cells represent an important arm of the adaptive immune system as they orchestrate humoral as well as cellular immune responses. There are multiple lineages of CD4 T cells that include helper subsets (Th1, Th2, Th17, and follicular helper T or Tfh) and a regulatory subset (Treg) [23-25]. Emerging evidence suggests that mTOR activity regulates development of these CD4 T cell subsets [3,4,26-31]. Powell and colleagues have shown that differentiation into Th1, Th2, and Th17 is inhibited in CD4 T cells with a conditional deletion of mTOR (Fig. 3)[31]. This differentiation failure was associated with decreased phosphorylation of STAT as well as insufficient induction of lineage specific transcription factors [31]. Recently, the same group has further extended these studies by genetically deleting mTORC1 or mTORC2 signaling components in T cells [30]. Abrogating mTORC1 activity by deletion of Rheb, an upstream activator of mTORC1 (Fig. 1), resulted in CD4 T cells that failed to develop to either Th1 or Th17 cells, while differentiation into Th2 remained intact upon in vitro stimulation [30]. On the other hand, rictor-deficient T cells, in which mTORC2 signaling (Fig. 1) was blocked, differentiated into Th1 and Th17 cells but lost their ability to differentiate into Th2 cells [30]. Thus, these studies indicated that mTORC1 and mTORC2 differentially regulate the generation of CD4 T helper subsets. However, it should be noted that there is another report showing that not only Th2 but also Th1 differentiation was inhibited in CD4 T cells lacking rictor [29]. As Powell and colleagues discuss in their paper, this discrepancy in the effect of mTORC2 on Th1 lineage between these two independent studies may be due to the system used for rictor deletion. One study used Lck-Cre expression to remove loxp-flanked rictor alleles [29], while CD4-Cre was used in the other [30]. Because Lck is expressed at a much earlier stage of T cell development compared to the CD4 protein, deletion of rictor in a different stage of T cell development may influence subsequent Th1 differentiation by changing naïve T cell properties. Despite the discrepancy regarding Th1 induction, both studies show that mTORC2 signaling is required for Th2 differentiation and is dispensable for Th17 differentiation. Collectively, these data indicate that the two distinct mTOR pathways play a critical role in regulating development of Th1, Th2, and Th17 subsets (Fig. 3). However, there are still many unresolved questions concerning the relationship between helper T cell responses and mTOR activity. For example, whether or not mTOR has any impact on generation of Tfh cells is unknown (Fig. 3). Because Tfh cells provide necessary help for optimal production of antibodies by B cells [23,32], the answer to this question may be essential in understanding the fundamental role of mTOR in protective immunity. Another important question is how the level of mTOR activation affects helper T cell differentiation. Genetic deletion of mTOR pathway-related genes results in a complete or strong block of the mTOR signaling (Fig. 3). As discussed above, in vivo memory CD8 T cell formation is dependent on the level of mTOR activity (Fig. 2) [17]. Thus, it will be interesting to examine if partial inhibition of mTOR activity in vivo still prevents development of CD4 T helper cell subsets, or causes different outcomes compared to genetic deletion (Fig. 3). Furthermore, in addition to these effector helper T cell subsets, it is also important to investigate how memory CD4 T cell formation might be modulated by mTOR signaling (Fig. 3).
Fig. 3. The role of mTOR in differentiation of helper and regulatory T cell subsets.
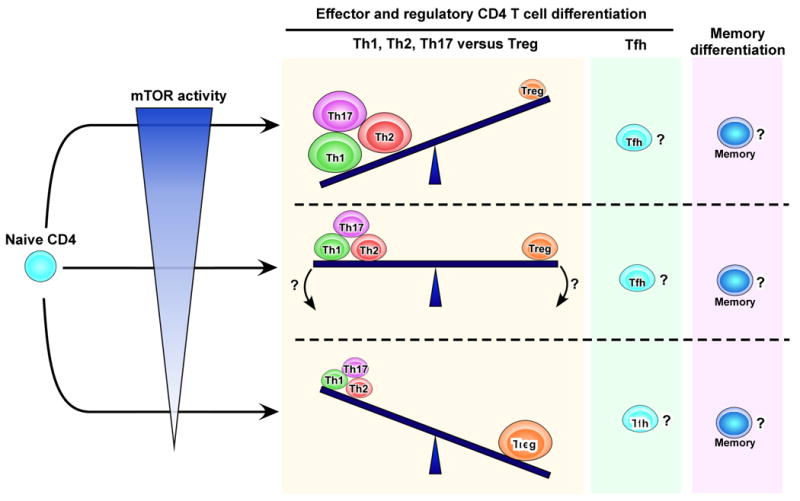
Sufficient mTOR activity induces effector CD4 T helper subsets. On the other hand, a complete or strong block of the signaling prevents the generation of these effector cells, instead promotes Treg differentiation. The effects of partial inhibition of mTOR activity in vivo on effector versus regulatory T cell differentiation are not fully understood. The role of mTOR pathway in development of Tfh as well as memory CD4 T cells is yet to be defined.
Regulation of B cell immunity
B cells represent the adaptive immune arm responsible for producing protective anti-pathogen antibodies. Several groups have shown that the mTOR pathway is required for development and maturation of B cells [33-35]. Genetic deletion of Sin1, an essential component of mTORC2 (Fig. 1), prevents B cell development by inhibiting mTORC2 dependent Akt activity[34]. Similarly, decreased mTOR activity mediated by disruption of mTOR transcription interfers with normal B cell development, leading to decreased number of peripheral B cells with those cells showing altered phenotypes and impaired function [33]. Interestingly, hyperactivation of mTOR signaling also impairs B-cell maturation [35]. Conditional knockout of TSC1, an upstream inhibitor of mTORC1 (Fig. 1), in B cells using CD19-cre resulted in accumulation of immature B cells and loss of marginal zone B cells [35]. These recent findings indicate that the activation level of mTOR signaling may be critical for B cell development and function.
In contrast to B cell development, less is known about the biological role of the mTOR pathway in homeostasis and activation of mature B cells. Work done by Rajewsky and colleagues suggest that B cell receptor-dependent PI3 kinase signals are necessary for mature B cell survival [36]. Because mTOR is one of the major downstream targets for PI3 kinase, it will be interesting to examine if the mTOR pathway influences mature B cell survival. Furthermore, it will be important to investigate the role of mTOR signaling in antigen-induced activation of mature B cells and their consequential differentiation into germinal center B cells, memory B cells, and plasma cells.
The role of TOR in innate immunity
In addition to adaptive immunity, mTOR can also modulate innate immunity. Through their ability to secrete pro-inflammatory cytokines and to present foreign antigens to CD4 T cells, dendritic cells (DCs) represent a key link between innate and adaptive immune systems. mTOR inhibition with rapamycin prevents Flt3L-driven development of conventional and plasmacytoid DCs (cDCs and pDCs) [37,38]. In contrast, hyperactivation of the PI3K-AKT-mTOR pathway by deletion of PTEN, an inhibitor of this signaling pathway, promoted DC development, suggesting that PI3K-AKT-mTOR pathway downstream of Flt3L signaling regulates DC development [37]. Furthermore, inhibiting either PI3K or mTOR decreased the generation of human myeloid DCs (mDCs) [39]. Recent reports have identified functional impairment of rapamycin treated DCs as well [40,41]. Bone-marrow derived DCs generated in the presence of rapamycin show poor ability to induce allogeneic T cell responses, and rather enhanced induction of Tregs [40,41]. Taken together, these data suggest that activation of mTOR signaling is required for the generation of functional DCs.
In addition, rapamycin exerts immunosuppressive effects on mature DCs. pDCs provide a first line of defense against viral infections by producing type I IFN. Pulendran and co-workers have shown that treating pDCs with rapamycin impairs their type I IFN production through suppression of IRF7 activity [42]. Another report has also demonstrated immunosuppression with rapamycin on monocyte-derived DCs (moDCs) [43]. Rapamycin treatment results in decreased production of both pro-inflammatory (IL-12, IL-6, TNF-a) and anti-inflammatory (IL-10) cytokines in LPS-stimulated moDCs [43]. Rapamycin-induced functional impairment of moDCs was also indicated by the decreased expression of costimulatory molecules, which affected their capacity to stimulate allogeneic T cells [43]. In contrast, several reports have recently demonstrated that rapamycin potentiates the ability of mature mDCs to promote T cell responses [43-45]. Rapamycin treatment enhances production of IL-12 from mDCs upon stimulation with Toll-like receptor (TLR) ligands [43-45]. The drug also decreases production of the immunosuppressive cytokine IL-10 [43-45]. Consistent with these observations, mDCs stimulated in the presence of rapamycin promote greater T cell responses [43]. Thus, the mTOR pathway in DCs has both immuno-stimulatory and inhibitory effects that seem to be DC-type specific.
Another important effect of mTOR on DCs is the modulation of their capacity to perform antigen presentation. Autophagy, a lysosomal degradation pathway, is known to be important for antigen presentation on MHC class II molecules. A recent in vivo study demonstrated that the autophagic activity in DCs is required for optimal antigen presentation via MHC class II to stimulate CD4 T cells [46]. Because the mTOR pathway negatively regulates autophagic activity, this study suggested that rapamycin could improve the ability of antigen presentation in DCs by enhancing autophagy. Indeed, Jagannath and colleagues observed an improvement of in vitro antigen presentation by DCs via rapamycin-induced autophagy [47]. Similarly, in experiments where live attenuated mycobacteria-infected, rapamycin-treated DCs were used as a vaccine, they showed enhanced antigen presentation, which resulted in higher Th1 responses and superior protection upon challenge with virulent Mycobacterium tuberculosis [47]. Interestingly, HIV seems to impair antigen presentation in DCs by inhibiting autophagy; thereby the virus may evade early immune responses [48]. DCs at mucosal sites are one of the primary targets for HIV, and it was shown that a rapid shutdown of autophagy-mediated HIV antigen processing and presentation by HIV infection occurs via activating the mTOR pathway and results in decreased activation of HIV specific CD4 T cells [48].
TOR and Foxp3+ regulatory T cell (Treg) differentiation
In vitro stimulation of naïve CD4 T cells in the presence of high concentrations of the cytokine TGF-β results in their differentiation into an inhibitory Foxp3+ regulatory T cell subset, with Foxp3 being the master transcription factor that mediates the differentiation process [4]. The involvement of mTOR in the differentiation of Tregs was indicated by the in vitro and in vivo observations that rapamycin can selectively promote their expansion [49,50]. These results lead to the premise that the tolerogenic effects of rapamycin are, at least partially, mediated through its ability to preferentially promote Tregs differentiation. Powell and colleagues confirmed such observations by demonstrating the preferential differentiation of mTOR-deficient naïve CD4 T cells into Foxp3+ cells (Fig. 3) [31]. Several reports provided further evidence for the regulatory role of mTOR signaling in differentiation of Tregs [28,51-54]. First, studies showed that constitutively active signaling molecules that are upstream of mTOR, such as PI3K and Akt, inhibit Foxp3 expression in a rapamycin-sensitive manner [51,52]. Second, studies by Francisco et al. demonstrated that PD1/PD-L1 interaction can, through inhibiting the Akt-mTOR axis, promote the generation and maintenance of Tregs [54]. Third, studies by Liu et al. showed that sphingosine 1-phoshate (S1P)-mediated activation of the Akt-mTOR axis inhibits the development and functionality of Foxp3+ Tregs [53]. Recently, Liu and colleagues used CD4 T cells that express the S1P receptor, S1P1, or cells in which the receptor for TGF-β (TGFβRII) was genetically deleted to show that neither cell type is able to upregulate Foxp3 in response to TGF-β stimulation, suggesting that the S1P-mediated effect on Tregs may be mediated by interfering with TGF-β receptor signaling [28]. The authors further dissected the interaction between S1P1 and TGF-β receptor signaling showing that S1P1 signaling attenuated the activity of Smad3, the transcription factor that mediates TGF-β induction of Tregs, [28].
The mechanisms behind the observed preferential expansion of Foxp3+ CD4 cells in the absence of mTOR signaling are yet to be fully defined. A growing body of evidence suggests that T cell differentiation is tightly coupled to the cell's metabolic state and this is how mTOR, a key sensor and integrator of the cellular nutrient and energy status, may be a major player in determining T cell fate [4,55]. Along these same lines, it has been postulated that Tregs are metabolically less active than effector CD4 T cell subsets and therefore less functionally dependent on mTOR signaling [4]. Indeed Michalek et al. recently demonstrated that at least in vitro the levels of the glucose transporter (Glut1) and that of glycolysis are higher in effector CD4 T cells in comparison to Tregs [27]. However, the question of how the intracellular metabolic state of Tregs vs. effector CD4 T cell subsets causes the differential responsiveness of Tregs to mTOR signaling inhibition remains unanswered. Matarese and colleagues have demonstrated, using in vitro and in vivo experimental systems, that there is an inverse correlation between the proliferative capacity of Tregs and the adipocyte-derived anorexigenic hormone leptin and leptin-mediated signaling [56]. Recently, the same group demonstrated that the levels of leptin control mTOR activation in Tregs, and that leptin secretion and expression of its receptor on Tregs are significantly reduced by transient rapamycin treatment [57]. In the latter study, the authors observed a modest increase in mTOR signaling, as indicated by increased S6 phosphorylation, in Tregs isolated from leptin receptor deficient mice upon CD3/CD28 stimulation. Taking these results together, Procaccini and colleagues suggested that the leptin-mTOR signaling axis may act as an integrator for various metabolic signals in Tregs, controling their responsiveness to stimulation [57].
Longevity and healthier immunity with TOR modulation
It is becoming increasingly apparent that reduced TOR signaling extends lifespan in many model organisms [58-61]. Evidence that TOR signaling regulates mammalian lifespan was obtained from experiments in which the administration of rapamycin in elderly mice increased their survival [62]. Similarly, deletion of S6K1, a downstream effector of mTORC1 (Fig. 1), also prolonged lifespan [63]. Furthermore, a recent study demonstrated that rapamycin reverses the phenotype of cells obtained from patients with Hutchinson-Gilford progeria syndrome, a lethal genetic disorder that mimics rapid aging [64]. Because mTOR signaling is a major nutrient-sensing pathway, the effects on aging by inhibiting this signaling may be similar to what is seen in dietary restriction that is known to extend lifespan of diverse organisms [65]. The mechanistic details of how downregulation of mTOR exerts anti-aging effects are not fully understood, but several downstream actions of mTOR such as metabolism, translation, and autophagy are suggested to be involved in the anti-aging effects [58-61]. Here we will discuss the contribution of immunity to lifespan extension by inhibiting mTOR signaling.
Several studies have recently shown that mTOR activity is associated with immune senescence [17,66,67]. As mentioned in a previous section, rapamycin treatment minimizes generation of terminally differentiated effector CD8 T cells that undergo apoptosis during the contraction phase [17]. Instead, the drug increases the number of long-lived memory CD8 T cells by enhancing self-renewing ability [17]. This result interestingly indicates that rapamycin works on a cellular level to extend lifespan. Furthermore, memory CD8 T cells generated in the presence of rapamycin display improved proliferative and protective capacity against infections [17]. Therefore, enhanced memory CD8 T cell immunity with rapamycin treatment might help extend lifespan by protecting animals against infectious diseases. In addition to memory T cells, the activity of mTOR seems to play a critical role in maintaining a functional naïve T cell population. Deletion of TSC1, an upstream inhibitor of mTORC1, in T cells results in loss of naïve T cells from peripheral tissues, due to enhanced apoptosis [67]. These mice show impaired T cell responses to bacterial infection, and rapamycin treatment partially improves survival of TSC1-/- T cells [67]. Because mTORC1 hyperactivation with TSC1 knockout triggers the feedback loop that inhibits PI3 kinase and AKT activity, it will be important to further examine if these results are directly caused by the active mTORC1 signal, or are indirectly mediated by inhibition of PI3 kinase and/or Akt.
Furthermore, it has been reported that age-related changes in hematopoietic stem cells (HSCs) are linked to mTOR activity [66]. The function of HSCs deteriorates with age, and such defects of HSCs are believed to contribute to the weakened adaptive immune responses often seen in the elderly. Zheng and colleagues have shown that mTOR signaling is activated in HSCs derived from old mice and is associated with their functional defects [66]. In these mice, rapamycin treatment restored the ability for the self-renewal and hematopoiesis of HSCs, which resulted in an improved protective immune responses against a lethal influenza virus challenge [66]. Collectively, these reports suggest that inhibiting mTOR induces long-lasting healthier immunity and this may partially provide extended lifespan in rapamycin treated animals [17,66,67].
Conclusions
It has become clear that inhibiting mTOR signaling has a variety of effects on the immune system. In particular, the paradoxical immunostimulatory effects of rapamycin may present a novel approach to enhance immunogenicity of vaccines. Such beneficial consequences may be contributing to the observed rapamycin-mediated lifespan extension. On the other hand, inhibiting mTOR suppresses certain types of immune cells, which in turn seems to prevent allograft rejection in transplant settings. The underlying mechanisms of such contradictory effects of mTOR inhibition on different immune cells and clinical/experimental settings are not fully understood, but the levels of mTOR signaling may partly determine stimulatory or inhibitory effects on immunity as discussed in this review. It will be critical to further investigate the relationship between the levels of mTOR activity and immune responses for a better understanding of the role mTOR in immunity. Furthermore, defining the important mTOR downstream pathways in the different immune cells will be essential for the full exploitation of such pathways in improving anti-pathogen immune responses.
Highlights.
mTOR regulates memory CD8 T cell differentiation.
Differentiation into both effector and regulatory CD4 T cells is modulated by mTOR.
mTOR has both stimulatory and inhibitory effects on DCs.
Inhibiting mTOR has anti-aging effects and extends life span.
Rapamycin rejuvenates functional ability of HSCs from aged mice.
Acknowledgments
R.A. was supported in part by the National Institutes of Health (Grant AI30048 and Grant AI088575).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Koichi Araki, Email: karaki@emory.edu.
Ali H. Ellebedy, Email: ali.ellebedy@emory.edu.
Rafi Ahmed, Email: rahmed@emory.edu.
References
- 1.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Salmond RJ, Zamoyska R. The influence of mTOR on T helper cell differentiation and dendritic cell function. Eur J Immunol. 2011;41:2137–2141. doi: 10.1002/eji.201141523. [DOI] [PubMed] [Google Scholar]
- 4.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev. 2010;235:234–243. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaech SM, Wherry EJ. Heterogeneity and Cell-Fate Decisions in Effector and Memory CD8(+) T Cell Differentiation during Viral Infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 9.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 10.Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–157. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Rao RR, Araki K, Pollizzi K, Odunsi K, Powell JD, Shrikant PA. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity. 2011;34:541–553. doi: 10.1016/j.immuni.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, Ford ML, Ahmed R, Kirk AD, Larsen CP. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant. 2011;11:613–618. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104:643–652. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. Using an in vitro system, this paper suggests that effector and memory CD8 T cell differentiation can be modified by mTOR activity-dependent T-bet/Eomesodermin expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. The authors demonstrate that mTOR is a major regulator of memory CD8 T cell differentiation and that rapamycin treatment enhances both quntity and quality of memory CD8 T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. Using TRAF6 deficient mice and microarray analysis, this paper identified that fatty acid metabolism is involved in memory CD8 T cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 23.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1a-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. The authors demonstrate that S1P1-induced inhibition of Tregs development is mediated by its signaling through the Akt-mTOR pathway and antagonizing the function of TGF-β by interfering with sustained activity of the signal transducer Smad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. This paper shows that mTORC2 is required for both Th1 and Th2 differentiation, and is despensable for Th17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. Using conditional knockout mice, the authors show that mTORC1 and mTORC2 have distinct roles in differentiation of helper T cell subsets; mTORC1 is required Th1 and Th17 differentiation, while mTORC2 is necessary for Th2 differentiation. Inhibiting mTORC2 did not inhibit Th1 differentiation; this is different from #29 paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. The authors demonstrate that differentiation into Th1, Th2, and Th17 is inhibited in CD4 T cells with a conditional deletion of mTOR, while Treg differentiation is enhanced in these cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Readinger JA, DuBois W, Janka-Junttila M, Robinson R, Pruitt M, Bliskovsky V, Wu JZ, Sakakibara K, Patel J, et al. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood. 2011;117:1228–1238. doi: 10.1182/blood-2010-05-287821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazorchak AS, Liu D, Facchinetti V, Di Lorenzo A, Sessa WC, Schatz DG, Su B. Sin1-mTORC2 suppresses rag and il7r gene expression through Akt2 in B cells. Mol Cell. 2010;39:433–443. doi: 10.1016/j.molcel.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benhamron S, Tirosh B. Direct activation of mTOR in B lymphocytes confers impairment in B-cell maturation andloss of marginal zone B cells. Eur J Immunol. 2011;41:2390–2396. doi: 10.1002/eji.201041336. [DOI] [PubMed] [Google Scholar]
- 36*.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. Using genetic approach, this paper demonstrates that the PI3 kinase-FOXO1 pathway activated by BCR signals plays a critical role in mature B cell survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sathaliyawala T, O'Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 39.van de Laar L, Buitenhuis M, Wensveen FM, Janssen HL, Coffer PJ, Woltman AM. Human CD34-derived myeloid dendritic cell development requires intact phosphatidylinositol 3-kinase-protein kinase B-mammalian target of rapamycin signaling. J Immunol. 2010;184:6600–6611. doi: 10.4049/jimmunol.0903089. [DOI] [PubMed] [Google Scholar]
- 40.Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, Metes D, Thomson AW. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 42.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Horl WH, Saemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 44*.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. This paper shows that DCs stimulated with LPS in the presence of rapamycin produce higher levels of IL-12 and less IL-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. Similar to #44 paper, the authors show that rapamycin alters IL-12 and IL-10 production from DCs as well as monocytes. Also, an in vivo study demonstrates that rapamycin modulates innate immunity and protects mice from lethal listeria monocytogenes challenge. [DOI] [PubMed] [Google Scholar]
- 46.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 48.Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R, Garcia E, Dinkins C, Leuba F, Wu L, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 50.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 51.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. Using genetic deletion and overexpression approaches, this paper was the first to provide evidence that S1P1-mediated activation of Akt-mTOR signaling interferes with developments and function of Tregs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol. 2010;22:655–661. doi: 10.1016/j.coi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol Med. 2010;16:247–256. doi: 10.1016/j.molmed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 57**.Procaccini C, De Rosa V, Galgani M, Abanni L, Cali G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. The authors demonstrate that rapamycin reduces the expression of leptin and its receptor in Tregs and that Tregs from Leptin receptor deficient mice show reduced mTOR activation, indicating that the leptin-mTOR signaling axis may act as an integrator for energy and metabolic cues that control Tregs development and homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–465. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- 59.Kaeberlein M, Kennedy BK. Hot topics in aging research: protein translation and TOR signaling, 2010. Aging Cell. 2011;10:185–190. doi: 10.1111/j.1474-9726.2010.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selman C, Withers DJ. Mammalian models of extended healthy lifespan. Philos Trans R Soc Lond B Biol Sci. 2011;366:99–107. doi: 10.1098/rstb.2010.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans DS, Kapahi P, Hsueh WC, Kockel L. TOR signaling never gets old: aging, longevity and TORC1 activity. Ageing Res Rev. 2011;10:225–237. doi: 10.1016/j.arr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. This paper was the first to provide evidence that rapamycin extends lifespan of mammals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in hutchinson-gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 65.Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 66**.Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. This paper demonstrates that in vivo administration of rapamycin rejuvenates function of HSCs from aged mice. Also, similar to paper #62, continuous rapamycin treatment extends lifespan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011 doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]


