Summary
To design successful vaccines for chronic diseases, an understanding of memory CD8+ T cell responses to persistent antigen re-stimulation is critical. However, most studies comparing memory and naïve cell responses have only been performed in rapidly cleared acute infections. Herein, by comparing the responses of memory and naïve CD8+ T cells to acute and chronic lymphocytic choriomeningitis virus (LCMV) infection, we show that memory cells dominated over naïve cells and were protective when present in sufficient numbers to quickly reduce infection. In contrast, when infection was not rapidly reduced, memory cells were quickly lost, unlike naïve cells. This occurred with both transgenic and endogenous memory CD8+ T cells, and with memory cells initially generated by different vaccines. This loss of memory cells was due to a block in sustaining cell proliferation, selective regulation by the inhibitory receptor 2B4, and increased reliance on CD4+ T cell help. Thus, emphasizing the importance of designing vaccines that elicit effective CD4+ T cell help and rapidly control infection.
Introduction
Memory CD8+ T cells can provide efficient protection to re-infection due to their increased cytotoxic potential, cytokine secretion, and ability to respond to reinfection faster than naïve CD8+ T cells. Recent studies have focused on better delineating what qualities memory cells need in order to be protective and highly functional, as well as how to better design vaccines to elicit memory cells with these properties (Ahmed and Gray, 1996; Appay et al., 2008; Harty and Badovinac, 2008; Kaech et al., 2002b; Prlic et al., 2007). Because memory CD8+ T cells can provide quick and effective elimination of intracellular pathogens, vaccines designed to generate virus-specific memory CD8+ T cells represent an attractive strategy for combating persistent human viral and intracellular bacterial infections such as HIV, HCV and tuberculosis. Importantly, multiple studies have indicated that virus-specific CD8+ T cell function and proliferation are associated with decreased SIV or HIV viral loads thus, indicating that virus-specific CD8+ T cells can help control SIV and HIV infection (Ahlers and Belyakov, 2010; Bangham, 2009; Goulder and Watkins, 2008). However, studies have not been rigorously performed comparing the protective abilities and qualities of memory versus naïve CD8+ T cells during chronic infections. Because chronic antigen stimulation has been shown to be detrimental to CD8+ T cells, understanding the response of memory CD8+ T cells to persistent antigen re-stimulation is important for rational vaccine design for chronic infections.
During chronic antigen stimulation, CD8+ T cells undergo exhaustion, characterized by decreased proliferative capacity, loss of cytokine secretion, reduced cytotoxic killing abilities, and phenotypic changes, such as an increase in inhibitory molecule expression (Shin and Wherry, 2007; Wherry et al., 2003a). Upregulation of multiple inhibitory molecules has been shown to play a major role in the process of CD8+ T cell exhaustion during chronic infection. In particular, the inhibitory molecule programmed death 1 (PD-1) has been shown to play a central role in the process of CD8+ T cell exhaustion, and blocking PD-1 can partially rescue exhausted CD8+ T cells by increasing both their numbers and anti-viral function (Barber et al., 2006; Velu et al., 2009). Furthermore, other inhibitory receptors, such as lymphocyte activation gene (Lag-3) and T cell immunoglobulin and mucin domain-containing molecue-3 (Tim-3) have been shown to synergize with PD-1, and co-blockade studies have resulted in enhanced restoration of function to exhausted CD8+ T cells (Blackburn et al., 2009; Jin et al., 2010). Another inhibitory molecule upregulated by exhausted CD8+ T cells is 2B4 (CD244), however the role of this molecule in T cell exhaustion is not well understood. Most research on 2B4 has focused on its’ role on natural killer cells and recent reports have provided conflicting views as to whether 2B4 plays an inhibitory or stimulatory role on CD8+ T cells (Bengsch et al., 2010; Blackburn et al., 2009; Raziorrouh et al., 2010; Rey et al., 2006; Waggoner et al., 2010; Wang et al., 2010; Wherry et al., 2007). Our understanding of CD8+ T cell exhaustion and the roles of inhibitory receptors in this process are mainly based on studies of the primary T cell response. However, since vaccination results in a pool of pathogen-specific memory CD8+ T cells, it is important to better understand how inhibitory molecules affect the secondary response of pre-existing memory CD8+ T cells in the setting of chronic infection.
Another important aspect of T cell vaccine design is understanding the role of CD4+ T cell help in the generation of functional CD8+ T cell responses. While CD4+ T cell help has been shown to be important during CD8+ T cell primary responses to generate quality memory cells in multiple acute viral and bacterial infections (Harty and Badovinac, 2008; Northrop and Shen, 2004; Williams et al., 2006), the relative importance of CD4+ T cell help in primary and secondary CD8+ T cell responses in acute versus chronic infection has not been addressed. Therefore, understanding both the response of memory CD8+ T cells to persistent antigen re-stimulation and the role of CD4+ T cell help may be key in designing successful vaccines for chronic diseases. In the present study we addressed these questions using the lymphocytic choriomenengitis virus (LCMV) model to compare naïve and memory CD8+ T cell responses during acute and chronic infection.
Results
Memory cells are selectively lost during high antigen load or antigen persistence
To examine the effects of chronic versus acute antigen re-exposure on naïve and memory CD8+ T cells, we utilized the mouse LCMV CD8+ TCR transgenic P14 system (cells specific for LCMV Db-restricted epitope GP 33–41). Using this transgenic system allowed us to eliminate differences in TCR avidity and specificity between naïve and memory cells and allow for comparisons on a per cell basis. Furthermore, by co-transferring both the memory and naïve cells into the same mice, we were able to eliminate possible differences in environment. We altered the duration of antigen stimulation by infecting mice with either the Armstrong strain (Arm) of LCMV which results in an acute infection that is cleared by day 8–10 post-infection (p.i), or clone-13 strain (cl-13) which differs from Arm by only two amino acids and results in a highly disseminated viral infection with ~ 2 months of viremia (Ahmed et al., 1984; Wherry et al., 2003a). The memory P14 cells used in these adoptive transfer experiments were highly functional memory cells from LCMV Armstrong immune mice that produced TNF-α, IL-2 and IFN-γ upon ex-vivo re-stimulation with their cognate GP33 peptide. Furthermore, 80–90% of these memory cells had the central memory phenotype; CD44hi CD127hi CD62Lhi (Figure S1 A and B). By 7 days post-infection (p.i.), the transferred memory T cells (secondary effectors) dominated over the transferred naïve T cells (primary effectors) in the blood during acute LCMV infection, as previously described (Badovinac et al., 2003; Grayson et al., 2002; Jabbari and Harty, 2006; Masopust et al., 2006) (Figure 1A). In striking contrast, when the mice were infected with the LCMV chronic strain, cl-13, primary effectors outnumbered secondary effectors at day 7, and this difference became even more drastic as the infection progressed (Figure 1A). Importantly, similar results were obtained when the cells were transferred separately into different mice (data not shown). Moreover, transferred memory cells were also rapidly lost, unlike naïve cells, in the tissues after chronic infection (p<0.01 in spleen and p<0.005 in liver and bone marrow) (Figures 1B and 1C). These data show that secondary effectors are more detrimentally impacted by high antigen load or antigen persistence than primary effectors.
Figure 1. Naïve and memory CD8 T cell responses during acute versus chronic infection.
1×103 of each memory (Thy1.1+1.2+) P14 and naïve P14 T cells (Thy1.1+1.2−) were co-transferred into naïve B6 mice. On the following day the mice were infected with either LCMV Arm (Acute) or LCMV cl-13 (chronic). Blood was taken at days 7, 14, 21 & 32 post-infection. (A) Percent of transferred memory and naïve P14 cells in the blood after infection. (B and C) Cells were co-transferred as above and mice were infected with either an acute (2×102 pfu) or chronic (2×106 pfu) dose of LCMV cl-13 (B) Representative dot plots and (C) numbers of transferred cells in the tissues day 14 p.i. Results representative of two to six independent experiments with 4–6 mice per group. Statistical comparisons were performed with the unpaired Students t test *p<0.05, **p< 0.01. Error bars represent SEM.
We wanted to eliminate the possibility that the loss of secondary effector T cells during chronic infection was due to differences in tropism between the Arm and cl-13 strains of LCMV. Although these two strains differ by only two amino acids, the increased receptor binding affinity of the cl-13 strain alters the viral tropism, allowing it to infect cell types that the Arm strain does not (Durbeej et al., 1998; Mueller et al., 2007; Sevilla et al., 2000). Therefore, we transferred naïve and memory P14 T cells into mice and then infected them with either a low or high dose of LCMV cl-13. Mice infected with a low dose of cl-13 (2×102 pfu) had no detectible viremia at day 4 p.i. and the infection was rapidly cleared as an acute infection, unlike the viral persistence seen in mice infected with the high dose (2×106 pfu) of cl-13 (data not shown). Infection with the low dose of cl-13 resulted in the secondary effector P14’s outnumbering the primary effectors (Figures 1B and C), similar to that in acute Arm infection. Furthermore, by altering the dose and route of Armstrong infection, we changed the length of antigen presence by a few days, but this also resulted in differential survival or loss of transferred memory CD8+ T cells. With the typical acute dose of Arm (2×105 pfu) given i.p. or iv., secondary effector cells predominated, as shown previously. However, when the higher dose of Arm (2×106 pfu) was given i.v. the situation was changed, and the primary effector cells dominated over the secondary effector cells (Figure S1C). It should be noted that this effect was less drastic than that seen after chronic cl-13 infection, where antigen amounts were much higher and the duration of viral persistence was longer. Taken together, these data show that high antigen load or persistence rather than viral tropism affect memory cell expansion and/or survival.
Next, we asked whether this observation was applicable to non-transgenic memory CD8+ T cells. We sorted and transferred either LCMV-specific DbGP33 tetramer+ memory CD8+ T cells (specific for the same epitope as P14 T cells) or memory P14 T cells along with an equal number of naïve P14 T cells. We observed the same phenomenon of selective loss of the memory cells with both the non-transgenic memory T cells and the transgenic memory P14 cells during chronic infection, while both persisted during acute infection (Figure S1D, data not shown).
Central and effector memory cells generated by multiple vaccines are lost during antigen persistence
Because different vaccines or viruses can result in memory cells of varying phenotypes and function, we asked whether the initial vaccine or virus used to create the memory cells impacted the ability of memory cells to persist during chronic antigen exposure. To address this, we generated memory P14 cells using multiple vaccines or viruses: LCMV, an adenovirus vector expressing the LCMV glycoprotein (GP), or vaccinia virus expressing the LCMV GP33 epitope. It is important to note that memory CD8+ T cells can be divided into two main subsets, central and effector memory T cells, that have different proliferative abilities and effector properties upon re-stimulation, at differing anatomical locations (Masopust et al., 2001; Sallusto et al., 1999; Wherry and Ahmed, 2004; Wherry et al., 2003b). To control for differences due to altered ratios of central to effector memory CD8+ T cells generated by different viruses or vaccines, we sorted the memory P14 cells into CD62Lhi central (Tcm) or CD62Llo effector (Tem) memory cell populations and co-transferred each of them individually with naïve P14 T cells (Figure 2A). Because central memory cells (Tcm) have more proliferative capacity than effector memory cells (Tem)(Wherry et al., 2003b), we found, as expected, that only Tcm cells dominated the naïve P14 cells during acute infection (Figures 2B–D). However, after chronic infection, both Tcm and Tem cells were preferentially lost in the blood and tissues no matter which vaccine or virus was used to initially create them (Figures 2B–D). Thus, even purified highly proliferative central memory CD8+ T cells were unable to persist during chronic antigen stimulation. Furthermore, this is applicable to memory cells generated by an adenovirus vector, vaccinia virus or LCMV Arm.
Figure 2. Memory cells are preferentially lost during chronic antigen exposure, regardless of the vaccine or virus used to generate the memory cells.
Memory P14’s (Thy1.1+1.2−) were generated by either Arm, Ad-5 GP or VV-33 infection. At day 46 p.i. memory cells were sorted based on CD62L expression. Tcm (CD62L+) and Tem (CD62L−) cells were each co-transferred with naive P14’s (Thy1.1+1.2+) and the mice were infected the following day with an acute (2×102 pfu) or chronic (2×106 pfu) dose of LCMV cl-13. (A) Experimental set-up. (B) Representative flow plots of Tcm memory and naïve P14 cells in the blood at day 14 p.i. and (C) numbers in the tissues at day 26 p.i. (D) Representative flow plots of Tem memory and naïve P14 cells in the blood at day 14 p.i. and (E) numbers in the tissues at day 26 p.i. Results representative of 6 mice per group. *p<0.05, **p< 0.01, ***p<0.005. Error bars represent SEM.
Lastly, to verify that the loss of secondary effectors is related to antigen persistence and not something specific to the LCMV system, we co-transferred naïve and memory P14 cells and immunized the mice with a persistent adenovirus 5 vector expressing the LCMV glycoprotein. Intramuscular injection of E1-deleted replication deficient adenoviral vectors expressing a transgene into mice results in detectable amounts of the transgene for 5–6 weeks post-injection, indicating that these vectors result in persistence of a low amount of protein antigen (Tatsis et al., 2007). As seen after chronic LCMV infection, memory cells were selectively lost during persistent adenovirus infection (Figure S2), thus, verifying that this phenomenon is not specific to the LCMV system, but is related to antigen chronicity.
Secondary effectors have a block in sustaining cell proliferation
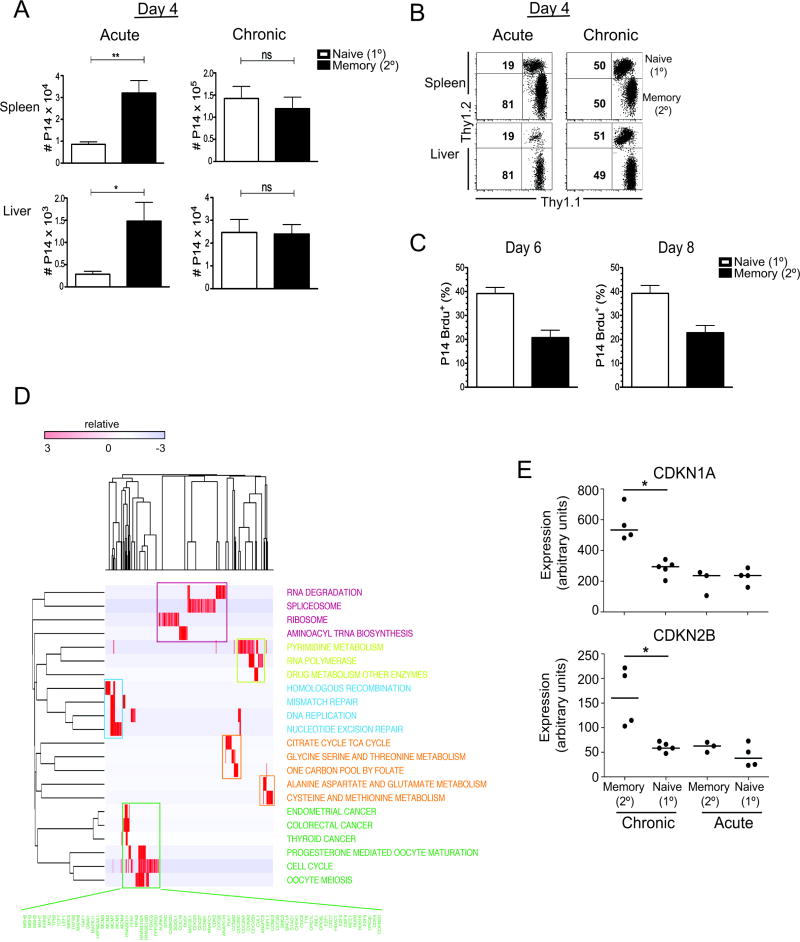
We wanted to determine whether the relative decrease in numbers of secondary effectors to primary effectors was due to a difference in initial recruitment of the cells, increased cell death, and/or decreased proliferation of the secondary effector P14 T cells. By day 4 during acute LCMV infection, there were already significantly more secondary effectors than primary effectors in the tissues (p<0.01 in spleen, p<0.05 in liver) (Figures 3A and 3B). In contrast, at day 4 during chronic LCMV infection there were equal numbers of transferred memory and naïve cells in the spleens and livers (Figures 3A and 3B). This indicates that although secondary effectors are selectively lost during antigen persistence, their initial recruitment is similar to primary effectors. To understand the possible role that cell proliferation and death play in the loss of secondary effectors, mice were given BrdU i.p. for 6 hours at days 6 and 8 post-infection with chronic LCMV and cell proliferation was assessed by BrdU incorporation. By day 6 p.i., there was a reduction in the proliferation of the secondary effectors, with only ~21% of the secondary effectors incorporating BrdU compared to ~39% of primary effectors (Figure 3C); an effect that was maintained at day 8 p.i. (~20% of secondary and ~43% of the primary effectors were BrdU+) (Figure 3C). Interestingly cell death of the secondary effectors was either similar, at day 6 p.i. (~14% of the secondary and ~18% of the primary effectors were 7AAD+), or reduced, at day 8 p.i. (~12% of the secondary and ~25% of the primary effectors were 7AAD+), compared to primary effectors (data not shown). Thus, indicating that the decreased number of secondary effectors is due to decreased proliferative abilities compared to primary effectors in the context of antigen persistence.
Figure 3. Initial recruitment of naïve and memory cells is similar during chronic infection, however memory cells have a block in sustaining cell proliferation.
(A and B) 5×103 of each memory (Thy1.1+1.2−) and naïve (Thy1.1+1.2+) P14 cells were transferred into B6 mice and the mice were infected the following day with either an acute (2×102 pfu) or chronic (5×106 pfu) dose of LCMV cl-13. (A) Numbers and (B) representative flow plots of naïve and memory P14’s in the spleens and livers at day 4 p.i. (C) 1×103 of each memory (Thy1.1+1.2−) and naïve (Thy1.1+1.2+) P14 cells were transferred into B6 mice and the mice were infected the following day with 2×106 cl-13. Percent of P14 cells that are Brdu+ 6 hours after Brdu i.p. injection at days 6 & 8 p.i. (D) Modular view of genes upregulated in primary vs secondary effectors at day 8 post-chronic infection. Genes upregulated in primary effectors were tested for enrichment with the KEGG collection of annotated gene-sets corresponding to major biological processes using Gene set enrichment analysis (GSEA). The expression of genes contained in the leading edges of gene-sets that were significantly enriched are displayed as a heatmap matrix, and clustered by gene (column) and row (gene-set). A cluster of gene sets related to RNA processing are colored purple; nucleotide synthesis, yellow; DNA replication, blue; metabolism, orange; and proliferation green. Genes contained in the proliferation module are listed in green. (E) Relative gene expression values of CDKNA1 (p21/Cip) (P=0.002) and CDKNB2 (p15) (P=0.002). Results representative of two or three independent experiments with 4–6 mice per group (A–C). *p<0.05, **p< 0.01, ns=p>0.05 (A–C). Error bars represent SEM.
We next sought to characterize the major differences between naive and memory cells at a molecular level. We compared the gene expression profiles of naive (primary effector) CD8+ T cells with memory (secondary effector) CD8+ T cells at day 8 post-infection in the setting of chronic infection, and tested for enrichment of classes of genes corresponding to major biological processes in each cell type. We found that there was highly significant upregulation of sets of genes related to cell cycle and proliferation in primary effectors compared to secondary effectors in chronic infection (Fig 3E, Table S3). In addition, genes related to RNA processing, amino acid synthesis, mitochondrial metabolism and DNA repair were highly upregulated in primary vs. secondary effector T cells in chronic infection. In contrast, there was significant upregulation of cell cycle dependent kinase inhibitors (CDKi’s) CDKN1A (p21/Cip) (P = 0.002, FDR = 0.18) and CDKN2B (p15) (P = 0.002, FDR = 0.18) in secondary effectors compared to primary effectors in chronic infection. Some evidence of increased expression of proliferation-associated genes was also evident in the primary vs secondary effector comparison in acute infection, however, CDKi’s were not upregulated in secondary effectors in acute infection. Thus in chronic infection, the global expression pattern of primary effectors was consistent with more robust proliferation and metabolic activity than secondary effector CD8+ T cells.
2B4 selectively regulates memory cells during chronic infection
We then asked whether the preferential loss of memory cells during chronic infection could be contributed to increased CD8+ T cell exhaustion of the memory cells compared to naïve cells. From day 5 to day 14 post-chronic infection, both the primary and secondary P14 T cells acquired a decreasing potential to make TNF-α, IFN-γ, and IL-2 after ex-vivo peptide re-stimulation with cognate GP33 peptide, indicating that both are undergoing the typical CD8+ T cell exhaustion seen in chronic infection (Figure S4). To evaluate the development of the exhausted state at a global level, we analyzed gene expression profiles from primary and secondary CD8+ T cells at day 8 post-acute or chronic infection. We compared these profiles with published microarray data of exhausted CD8+ T cells at ~D21 during chronic LCMV infection (Wherry et al., 2007). Gene-set enrichment analysis showed highly significant upregulation of the exhausted CD8+ T cell gene signature during chronic infection in both the primary and secondary effectors (Figures 4A and 4B). The most enriched genes from the exhausted signature in both primary and secondary effectors were highly similar (Figure 4C) and, with few exceptions, these genes were expressed at the same magnitude amongst the primary and secondary effector samples (Table S4). These findings suggest that both memory and naïve cells develop the molecular and functional properties of T cell exhaustion to a similar degree and argue against a more extreme exhausted phenotype in secondary effectors.
Figure 4. The role of 2B4 in regulating virus-specific memory CD8 T cells during chronic infection.
(A and B) Gene set enrichment analysis (GSEA) of a signature of genes associated with exhausted CD8 T cells in the rank ordered list of genes differentially expressed in (A) primary effectors at day 8 in chronic vs. acute infection and (B) secondary effectors at day 8 in chronic vs. acute infection. (C) Genes at the leading edge of enrichment in primary effector GSEA (green set) and secondary effector GSEA (orange set) are largely overlapping. (D) Genes differentially expressed at day 8 in secondary effectors in chronic infection compared to secondary effectors in acute infection and primary effectors in either infection. Each column represents an individual sample, each row a gene and cells colored to indicate relative expression. Top 200 genes upregulated or downregulated are shown, ranked by the signal-to-noise metric, 2B4 (Cd244) is indicated. (E) Relative expression values of Cd244, Tim-3, Lag-3 and PD-1 (F and G) 2×103 Wild-type (2B4 sufficient) or 2B4-deficient (Cd244−/−) naïve and memory P14’s (all Thy1.1+1.2+) were individually transferred into mice and on the following day the mice were infected with 2×106 pfu cl-13 i.v. (F) Representative flow plots of P14’s in the blood at day 7 p.i. and (G) number of P14 T cells per 106 PBMC at days 7,14 &23 post-infection. Results representative of 12 mice per group (F and G). *p<0.05, **p< 0.01, ***p<0.005 (F and G).
In order to identify candidate mechanisms involved in the selective loss of secondary effectors in chronic infection, we identified genes selectively upregulated in that population (compared to naïve cells after chronic infection, and naïve cells and memory cells after acute infection). Among the most differentially upregulated genes in secondary effectors in chronic infection, we found several inhibitory receptors (Figures 4D and 4E). Whereas the genes encoding PD-1 and Lag-3 were expressed at equivalent levels in primary and secondary effectors during chronic infection (p>0.05), 2B4 and Tim-3 were among the most highly upregulated in secondary effectors (4.4 fold increase in secondary vs. primary effectors in chronic infection, P=0.002 and 2.2 fold increase, P=0.002, respectively) (Figure 4E). It is important to note that all of these inhibitory receptors were also highly expressed on primary effectors after chronic infection, as compared to acute infection. However, expression of these receptors was highest on secondary effectors after chronic infection (Figure 4E).
To understand the role of inhibitory receptors in the loss of memory cells during antigen persistence, we performed in vivo functional studies. Blocking PD-1 or Lag-3 signaling by administering PD-L1 or Lag-3 blocking antibodies to mice infected with chronic LCMV after adoptive co-transfer of memory and naïve P14 cells did not lead to the increased survival of memory cells over naïve cells (data not shown). Therefore, while the inhibitory receptors PD-1 and Lag-3 play a role in the functional exhaustion of both the secondary and primary virus-specific effector CD8+ T cells during persistent antigen stimulation, these receptors do not inhibit memory cells more than naïve cells. These results were consistent with the microarray data, since neither PD-1, nor Lag-3 was differentially expressed between primary and secondary effector CD8+ T cells during chronic infection. Thus, PD-1 and Lag-3 do not appear to play a role in the selective loss of secondary effectors during chronic antigen stimulation.
Next, because Cd244 (2B4) was the inhibitory receptor gene most upregulated on memory cells compared to naïve cells, we sought to determine whether the increased expression of 2B4 played a functional role in the loss of secondary effectors during chronic infection. Because the available antibodies used to block 2B4 in vitro are cell depleting in vivo, we bred 2B4-deficient mice (Cd244−/−) with LCMV-specific P14 TCR transgenic mice to obtain 2B4-deficient P14 TCR transgenic mice (Cd244−/− P14). We transferred memory and naïve wild-type or Cd244−/− P14 T cells into naïve mice and then subsequently infected the mice with chronic LCMV. The phenotype and function of the naïve Cd244−/− P14 cells versus wild-type naïve P14 cells as well as the memory Cd244−/− P14 cells versus wild-type memory P14 cells were identical pre-transfer (data not shown). Interestingly, the transferred 2B4 deficient-memory cells were able to persist during chronic infection, unlike wild-type (2B4 sufficient) cells (p<0.01 at days 7 &14, and p<0.005 at day 23), and there was no observable difference between the wild-type and 2B4-deficient naïve cells (p>0.05) (Figure 4F and 4G). These data document a role for 2B4 in the preferential loss of secondary effectors during antigen persistence
Memory cells are more dependent on CD4 T cell help than naïve cells during antigen persistence
We have established that inhibitory receptors play an enhanced role in regulating memory cells during chronic infection. Therefore, we next wanted to find a way to overcome the increased regulation of these cells and enhance memory cell persistence during chronic infection. Because CD4+ T cell help has been shown to be important for CD8+ T cell function during viral infections (Harty and Badovinac, 2008; Northrop and Shen, 2004; Wherry, 2011; Williams et al., 2006) we investigated whether increased LCMV-specific CD4+ T cell help could rescue the transferred memory cells during chronic infection. To answer this question, we co-transferred LCMV GP61 epitope-specific CD4+ T cells (Smarta) at the time of naïve and memory CD8+ T cell co-transfer into normal CD4+ intact B6 mice. After infection with chronic LCMV, secondary effectors in the mice receiving Smarta cells outnumbered the primary effectors in multiple tissues and the blood (p < 0.005) (Figures 5A–C). Overall, this resulted in greater than a 3 fold increase of memory cells in the spleens of mice receiving Smarta cells compared to those without, while the number of naïve cells remained similar between the two groups during chronic infection (p<0.005) (Figure 5B). These data indicate selective rescue of the transferred memory cells. The increase in secondary effectors was only seen during chronic infection, as neither the primary nor secondary effector CD8+ T cell numbers changed with Smarta cell co-transfer during acute infection (Figures 5A–C). Furthermore, the rescue of the CD8+ T cells by co-transfer of Smarta cells during chronic infection was not due to reduced viral loads at this early time point after CD4+ T cell transfer, as both the chronically infected mice receiving Smarta cells and those without Smarta cells had serum viral titers between 105 and 106 log10 pfu/ml at day 6.5 p.i. (data not shown). Thus, our data indicate that during prolonged high antigen stimulation the memory CD8+ T cells are more reliant on CD4+ T cell help than naïve cells.
Figure 5. The role of CD4 T cell help in primary and secondary responses during acute versus chronic infection.
1×103 of each memory (Thy1.1+1.2+) and naïve (Thy1.1+1.2−) P14’s were co-transferred with or without 1×106 LCMV-GP61 specific CD4 T cells (Smartas) into naïve mice. The next day mice were infected with either an acute (2×102) or chronic (2×106) dose of LCMV cl-13.(A) Representative dot plot of naïve and memory P14 T cells in the spleen, liver and lung at day 6.5 p.i. (B) Numbers of naïve and memory P14 T cells in the spleen at day 6.5 p.i. (C) Percent of naïve and memory P14 T cells in blood at day 6.5 p.i. (D) Mice were set-up as in A–D and were treated with MR1 antibody or PBS on day −1,0,3 and 6 p.i. Numbers of naïve and memory P14 T cells in spleen at day 6.5 p.i. In the figures and legends, +CD4 help indicates mice receiving Smarta cells, − CD4 help indicates mice that do not receive Smarta cells. Results are representative of two (D) or three (A–C) independent experiments with 4–6 mice per group. ***p<0.005. Error bars represent SEM.
To understand the mechanism in which CD4+ T cell help recues memory CD8+ T cells during chronic infection, we first asked whether this rescue was linked to the increased immunoregulation of memory cells by 2B4. To address this, we assessed 2B4 expression on memory CD8+ P14 T cells in chronically infected mice after transfer of Smarta cells. 2B4 was expressed at equivalent levels on memory CD8+ T cells at day 6.5 post-infection whether or not they were rescued by added CD4+ T cell help (Figure S5A), thus, suggesting that 2B4 does not play a role in the rescue of memory cells by CD4+ T cell help during chronic infection, and instead these are two independent mechanisms.
Secondly, because CD40 and CD40L interactions have been shown to be important for CD4+ T cell help of CD8+ T cells (Bourgeois et al., 2002; Grewal and Flavell, 1998), we asked the question of whether CD40:CD40L interactions were playing a role in the rescue of memory CD8+ T cells by CD4 T cell help. We co-transferred memory and naïve CD8+ P14 T cells along with Smarta cells and blocked this pathway by the use of an antibody that blocks CD40L (MR1). We administered MR1 one day before infection with chronic LCMV (D−1), on the day of infection (D0) and every three days thereafter, and sacrificed the mice at day 6.5 post-infection. The blockade of CD40:CD40L interactions in mice that did not receive Smarta cells resulted in a slight reduction of both memory and naïve P14 T cells, as it inhibits endogenous CD4+ T cell help (Figure 5D). However, the number of naïve P14 T cells did not differ between the mice treated with MR1 and those treated with MR1 that received Smarta cells. The blockade of CD40:CD40L interactions resulted in a significant reduction in the expansion of memory P14 T cell numbers seen after Smarta cell transfer (p<0.001), showing that a mechanism in which CD4+ T cell help rescue. Furthermore, blockade of CD40L did not significantly affect Smarta cell expansion at this early time point (Figure S5B). Thus, our data indicates that during prolonged high antigen stimulation memory CD8+ T cells are more reliant on CD4+ T cell help than naïve cells and CD40:CD40L interactions may be involved.
The ability of naïve and memory CD8+ T cells to control acute versus chronic infection
In order to better understand how the increased regulation of memory cells during chronic infection impacts protective immunity, we assessed the ability of memory and naïve cells to control LCMV infection. To address this, we performed two sets of experiments. In one experiment, we transferred a constant number of naïve or memory P14 T cells and varied the dose of LCMV infection, while in the second, we varied the number of naïve and memory P14 T cells transferred and infected the mice with the chronic dose of LCMV cl-13. To begin, we transferred 3×104 naïve or memory P14 T cells (~ 3×103 of each cells after accounting for an ~10% take of the cells post-injection) into mice that were subsequently infected with increasing doses of LCMV cl-13 and assessed viral titers in the spleens at days 2, 3 and 5 post-infection. By day 5 p.i. memory P14 T cells significantly decreased viral load in mice infected with 2×102 and 2×104 pfu of cl-13. However, in contrast, these low numbers of memory and naïve P14 T cells were incapable of reducing virus levels in mice infected with higher and more persistent doses of cl-13 (2×105 and 2×106) (Figure 6A). These data indicate that on a per cell basis, memory cells are better than naïve cells at controlling acute or low dose infections, however small numbers of memory or naïve cells are unable to control high dose and/or persistent infections.
Figure 6. The ability of naïve and memory cells to control LCMV infection.
(A and B) 3×104 of memory or naïve P14 T cells were transferred into naïve B6 mice. The next day the mice were infected with 2×102, 2×104, 2×105 or 2×106 pfu of LCMV cl-13 iv. (A) Viral titer in the spleen on days 2, 3 and 5 p.i., as assayed by plaque assay on Vero E6 cells. (B–D) 1×103, 1×104, 3×104, 1×105 or 2.5×105 naïve or memory Thy1.1+ P14 T cells were transferred into naïve mice. The next day mice were infected with 2×106 pfu of LCMV cl-13 iv. (B) Viral titer in the serum on days 4, and 8 p.i. (C) Representative dot plots of naïve and memory P14 T cells in the PBMC at day 8 p.i. (D) Viral titer in the serum in mice receiving either no cells, or 1×103 memory or naïve P14 cells on day 14 p.i. Results are representative of 3–6 mice per group per time point. *p<0.05, ***p<.005, ns=p>0.05.
Next, to determine the ability of naïve and memory cells to control virus in the case of high viral load, we titrated the number of memory and naïve P14 CD8+ T cells that we transferred into mice that were subsequently infected with a chronic dose (2×106 pfu) of LCMV cl-13, and assessed viral titers in the serum. No reduction in viremia was seen in any group at day 4 post-infection. However, by day 8 p.i., the transfer of greater numbers of P14 T cells, 1×105 or 2.5×105, resulted in a larger expansion of memory cells in the PBMC and increased reduction of viral titers, in contrast to naïve cell transfers (Figures 6B and C). These data indicate that in cases where there are sufficient numbers of functional memory cells to rapidly reduce or clear the virus, memory CD8+ T cells are more efficient than naïve cells. In contrast, in instances with lower numbers of naïve or memory cells present, where virus was not quickly reduced, the naïve cells outnumbered memory cells at day 8 post-infection, but there was little effect on viral load by either naïve or memory cells (Figures 6B and C). By day 14 p.i. there was a small, but significant, decrease in the viral titers of the mice receiving 1×103 naïve P14 T cells compared to memory cells (p=0.0327), (Figure 6D), indicating that in some situations of high viral load where virus is not rapidly eliminated, naïve cells can be more effective at slightly reducing viral loads than memory cells. Taken together, these data show that memory cells provide effective control of low dose infection, and can provide control of high dose infections in situations where they are present in large enough numbers to rapidly control viral loads, thwarting the virus from persisting. However, in instances where memory cells are unable to quickly clear infection, the cells undergo regulatory mechanisms and are rapidly lost.
Discussion
In this study, we reveal the unexpected limitations of memory CD8+ T cells in the context of high viral load or persistent antigen. Memory cells provide quick and effective responses in the case of short-lived antigen stimulation. However, in stark contrast, our data show that memory cell function and survival is tightly regulated during high antigen load or antigen persistence. Unlike naïve CD8+ T cells, memory cells have more TCR signaling molecules clustered at the TCR, increased loci accessibility of genes involved in effector functions, and amplified immediate cytokine production, creating a hyperresponsive state (DiSpirito and Shen, 2010). Thus, increased regulation of memory cells compared to naïve cells may be an evolved mechanism to prevent excessive immunopathology in the host. Two recent reports by Hinrichs et al. show that adoptive transfer of effector cells derived from naïve rather than memory CD8+ T cells mediate superior anti-tumor immunity in a mouse model, and secondly that after in vitro stimulation effector cells derived from naive human CD8+ T cells have an increased proliferative capacity and are less terminally differentiated than effectors derived from memory human CD8+ T cells, and, thus, primary effectors may be a superior cell population to use for adoptive immunotherapy (Hinrichs et al., 2009; Hinrichs et al., 2011). Our data support the concept that secondary effectors are more terminally differentiated than primary CD8+ effector T cells during chronic antigen stimulation. An increased expression of NK cell receptors are found on terminally differentiated or senescent human T cells (Koch et al., 2007), and in parallel, we found that NK cell receptors such as KLRG1 were up-regulated on secondary effectors during chronic infection.
Another property associated with senescence or terminal differentiation is reduced proliferative capacity. In this study, we demonstrate that virus-specific memory and naïve CD8+ T cells are initially recruited similarly during situations of high antigen load. However, since in low dose acute infection, the memory cells already outnumber the naïve cells at this time point, this may suggest that the memory cells are already undergoing regulatory mechanisms before, or as early as, day 4 post-infection in situations of high dose infection. Moreover, we show both experimentally and by gene profiling, that after this equal initial recruitment of naïve and memory cells, memory cells have less proliferative capacity than naïve cells early in chronic infection. Thus, overall, constant antigen stimulation, like that seen in a high dose infection or antigen persistence, may quickly drive memory cells to a more terminally differentiated state.
The data presented here show that both primary and secondary effector CD8+ T cells are prone to CD8+ T cell exhaustion during chronic infection, as indicated by the microarray data and the decreased ability of the cells to make cytokines as infection progressed. Although the inhibitory receptors PD-1 and Lag 3 do not appear to play a role in the selective loss of secondary effectors, our data implicate 2B4 as an immunomodulatory molecule that regulates memory cell expansion or survival during persistent antigen stimulation. Consistent with 2B4 mRNA and protein being up-regulated on exhausted CD8+ T cells during chronic LCMV, increased surface expression on virus-specific CD8+ T cells is found during human chronic diseases, such as HCV, HBV, HIV, HTLV-1 and melanomas (Bengsch et al., 2010; Casado et al., 2005; Enose-Akahata et al., 2009; Raziorrouh et al., 2010; Tarazona et al., 2002). The role of 2B4 on CD8+ T cell responses has not been well studied. In addition, the dual stimulatory and inhibitory role that 2B4 is known to play on NK cells further complicates our understanding of this molecule (Chlewicki et al., 2008). 2B4 has been reported to be positively correlated with good human CTL function (Rey et al., 2006). Yet, in contrast, other studies indicate an inhibitory role for 2B4 on CD8+ T cells in both human and mouse chronic infections ((Bengsch et al., 2010; Blackburn et al., 2009; Raziorrouh et al., 2010). Furthermore, work performed with natural killer cells has shown that inhibitory function of 2B4 is related to increased 2B4 expression, increased receptor crosslinking, and lower expression of signaling lymphocyte activation molecule-associated protein (SAP) (Chlewicki et al., 2008). Therefore, the fact that our gene profiling of primary and secondary effector CD8+ T cells at day 8 post-acute and chronic LCMV infection showed that 2B4 is most highly up-regulated on the secondary effectors during chronic infection, and yet SAP expression was similar among all cell classes (primary and secondary effectors in both acute and chronic infection), is consistent with a negative regulatory role for 2B4 on memory cells during antigen persistence. In conclusion, we have found an important role for 2B4 in selectively affecting the survival and/or expansion of secondary CD8+ T cell effectors during chronic infection, implying that a better understanding of the role of 2B4 on CD8+ T cells is needed and may be helpful for vaccine design.
While it is well established that CD4+ T cell help is most important during primary responses to create productive memory cells, our work now highlights the selective increased dependence of memory recall responses on CD4+ T cell help in the context of antigen persistence. It is well known that CD8+ T cells require CD4+ T cell help during some mouse and human infections, including chronic infections (Harty and Badovinac, 2008; Matloubian et al., 1994; Northrop and Shen, 2004; Wherry, 2011; Williams et al., 2006). However, mainly these studies have addressed the role of CD4+ T cell help during the generation of primary CD8+ T cell responses and how this loss of CD4+ T cell help during initial priming affects the recall responses of these cells, rather than focusing on whether CD4+ T cell help is important during memory recall responses. Surprisingly, our data indicate that memory virus-specific CD8+ T cell responses are more reliant on CD4+ T cell help than naive virus-specific CD8+ T cell responses during antigen persistence. These data indicate that CD4+ T cell help may be an important component of vaccine strategy for chronic infections. Thus, there is a need to better understand the mechanism and requirements for CD4+ T cell help of secondary CD8+ T cell responses during chronic infection to aid in optimal vaccine design for these infections.
These data presented in this paper have important implications towards the design of T cell vaccines against chronic infections and tumors. While memory CD8+ T cells are much more effective than naïve cells at controlling various acute infections, our data show that they are tightly regulated in situations of high viral load or antigen persistence. When left with low numbers of antigen and/or pathogen-specific memory CD8+ T cells post-vaccination, and subsequent infection resulting in high doses of antigen or persistent antigen that is not rapidly controlled, our data indicate that these memory cells are unable to persist due to increased regulatory mechanisms. Furthermore, these memory cells are more reliant on CD4+ help, implying that an important component of vaccine design in situations of high antigen chronicity may be the ability to elicit and maintain large numbers of antigen-specific CD4+ T cells. However, our data also indicate that designing vaccines which result in large pools of highly functional memory CD8+ T cells that rapidly lower antigen loads can provide viral control of high dose or persistent infections, an idea supported by previous experiments in SIV and LCMV (Haase, 2010; Li et al., 2009). Moreover, two recent human HIV vaccine trials, the Merck STEP trail and Thai RV144 trail, show little efficacy in reducing the set-point of infection during chronic infection, however in the RV144 Thai trial some efficacy is seen in the form of decreased acquisition of infection (Buchbinder et al., 2008; Rerks-Ngarm et al., 2009). Lastly, a recent vaccine study in the SIV model shows efficacy when virus was rapidly controlled early after acquisition (Hansen et al., 2011). These studies emphasize the importance of designing vaccines that quickly control or prevent chronic infections. In cases where infection is rapidly controlled, memory cells are unlikely to become subject to this tight regulatory control. Thus, the number and quality of memory CD8+ T cells elicited by a vaccine may greatly impact the success of vaccines for high dose or persistent infection. Furthermore, designing vaccines that elicit large pools of CD4+ T cell help and/or increasing strategies to overcome immunoregulation by inhibitory receptors, may be necessary for a successful vaccine outcome.
Experimental Procedures
Mice and Infections
Six-week old female C57BL/6 mice were purchased from the Jackson Laboratory. P14 TCR transgenic mice and Smarta TCR transgenic mice were bred in house. 2B4 deficient P14 TCR transgenic mice were made by breeding P14 TCR transgenic mice with 2B4 knockout mice (2B4−/−). 2B4−/− mice were generated using C57BL/6 ES cells (Bruce 4) by replacement of exon 2 and 3 by a LoxP site and were kept on the C57BL/6 background (S.C., unpublished data). All 2B4−/− P14 mice were analyzed for P14 TCR expression and genotyped before use to make sure that both alleles of 2B4 were interrupted. For acute infections mice were infected with either 2×105 pfu Arm or 2×102 pfu cl-13, unless otherwise noted. For chronic infections mice were infected with 2×106 pfu cl-13. For persistent Ad-5 GP infection, mice were infected with 1010 vp Ad-5 GP. Ad-5 GP was kindly provided by G.A. Spies and M.J. McElrath (Fred Hutchinson Cancer Research Center). Virus levels were assayed by plaque assays as previously described (Wherry et al., 2003a). All mice were used in accordance with the Emory University Institutional Animal Care and Use Committee Guidelines.
Cell transfers
We created memory cells using the LCMV-specific TCR transgenic system as described (Kaech et al., 2002a). Briefly, we transferred a small number of P14’s (~1×105), unless otherwise noted, and then infected the mice with 2×105 pfu Arm ip. For the comparison of multiple vaccines/viruses, 1×104 P14 were transferred in to mice that were then infected with 2×105 pfu Arm LCMV or 1×106 pfu VV-33 or 109 vp of AD-5 GP. Memory cells were isolated at >45 days p.i. using CD8 T cell isolation kit (Miltenyi biotech) or alternatively by sorting on CD8+ Thy1.2− CD62L+ or CD62L− cells on a BD FACS Vantage or BD Aria. 1×103 (or 5×103 for analysis at day 4 p.i.) of each memory and naïve P14 T cells were transferred 1 day pre-infection into mice for all co-transfer experiments. 1×106 Smarta CD4 T cells were transferred after isolation from spleens of naïve Smarta TCR transgenic mice with a CD4+ T cell isolation kit (Miltenyi biotech).
Lymphocyte isolation and flow cytometry
Lymphocytes were isolated from the spleen, liver, lung, lymph nodes, IEL, bone marrow and blood as previously described (Barber et al., 2006; Masopust et al., 2001). All antibodies were purchased from BD Biosciences (San Diego, CA) except CD44, Thy1.1 and Thy1.2 (Biolegend) and anti-PD-1, anti-Tim-3 (ebioscience). MHC Class I tetramers were prepared and used as previously described (Wherry et al., 2003a). Intracellular cytokine staining was performed as previously described (Wherry et al., 2003a). Cells were analyzed on a Canto or LSR II flow cytometer (BD Immunocytometry Systems). Dead cells were excluded by gating on Live/Dead NEAR IR (Invitrogen).
In Vivo Blockade and other in vivo Treatments
For blockade of the PD-1 pathway, 200μg of rat anti-mouse PD-L1 antibody (10F.9G2 prepared in house) or rat IgG2b isotype control was administered intraperitoneally on day 0, 3 and 6. For blockade of the Lag-3 pathway, 200μg of rat anti-mouse Lag-3 (C9B7W from Biolegend) antibody was administered intraperitoneally on day 0, 3 and 6. For blockade of CD40L (CD154), 500ug of hamster anti-mouse CD154 (MR1 from BioXcell) antibody was administered intraperitoneally on day −1, 0, 3 and 6.
Proliferation by in vivo BrdU incorporation
For assessment of proliferation by Brdu, mice were were given 1mg of Brdu ip at day 6 and 8 p.i. and sacrificed 6 hours later. Brdu staining was carried out using the APC or FITC Brdu Kit (BD Biosciences) according to manufacturers instructions.
Microarray and analysis
Day 8 primary and secondary effectors from acute and chronic LCMV infected mice were FACS sorted based on CD8 and the congenic markers Thy1.1 and Thy1.2. RNA was isolated using a RNeasy kit (Qiagen) according to manufacturer’s protocol. RNA was amplified, biotinylated and hybridized on mouse 430.2 Affymetrix microarray chips at the Functional Genomics Shared Resource (Vanderbilt University, TN, USA). Prior to analysis, microarray data were pre-processed and normalized using robust multi-chip averaging, as previously described (Haining et al., 2008). Genes that are differentially expressed between two classes were ranked using the GenePattern software package (Gould et al., 2006). The statistical significance of differentially expressed genes and hierarchical clustering was performed using GenePattern (Gould et al., 2006). Gene set enrichment analysis (GSEA) was performed as described previously (Subramanian et al., 2005).
Statistical Analysis
All data were analyzed using Prism 5.0 (GraphPad).
Supplementary Material
Acknowledgments
We thank R. Karaffa and S. Durham for FACS sorting at the Emory University School of Medicine Flow Cytometry Core Facility, and we thank the members of the Ahmed Lab for helpful discussions. This work was supported by the National Institutes of Health (NIH) grant P01 AI030048 (to R.A.) and the American Cancer Society (ACS) postdoctoral fellowship PF-09-134-01-MPC (to B.Y.).
Footnotes
Accession numbers: The microarray data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.hih.gov/gds) under the accession number GSE30962
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115:1678–1689. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- Bangham CR. CTL quality and the control of human retroviral infections. Eur J Immunol. 2009;39:1700–1712. doi: 10.1002/eji.200939451. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado JG, Soto R, DelaRosa O, Peralbo E, del Carmen Munoz-Villanueva M, Rioja L, Pena J, Solana R, Tarazona R. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–1171. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- DiSpirito JR, Shen H. Quick to remember, slow to forget: rapid recall responses of memory CD8+ T cells. Cell Res. 2010;20:13–23. doi: 10.1038/cr.2009.140. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- Enose-Akahata Y, Matsuura E, Oh U, Jacobson S. High expression of CD244 and SAP regulated CD8 T cell responses of patients with HTLV-I associated neurologic disease. PLoS Pathog. 2009;5:e1000682. doi: 10.1371/journal.ppat.1000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J, Getz G, Monti S, Reich M, Mesirov JP. Comparative Gene Marker Selection suite. Bioinformatics. 2006 doi: 10.1093/bioinformatics/btl196. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- Haining WN, Ebert BL, Subrmanian A, Wherry EJ, Eichbaum Q, Evans JW, Mak R, Rivoli S, Pretz J, Angelosanto J, et al. Identification of an evolutionarily conserved transcriptional signature of CD8 memory differentiation that is shared by T and B cells. Journal of Immunology. 2008;181:1859–1868. doi: 10.4049/jimmunol.181.3.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002a;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002b;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Koch S, Larbi A, Ozcelik D, Solana R, Gouttefangeas C, Attig S, Wikby A, Strindhall J, Franceschi C, Pawelec G. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O’Mara L, Southern PJ, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop JK, Shen H. CD8+ T-cell memory: only the good ones last. Curr Opin Immunol. 2004;16:451–455. doi: 10.1016/j.coi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Raziorrouh B, Schraut W, Gerlach T, Nowack D, Gruner NH, Ulsenheimer A, Zachoval R, Wachtler M, Spannagl M, Haas J, et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934–1947. doi: 10.1002/hep.23936. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Rey J, Giustiniani J, Mallet F, Schiavon V, Boumsell L, Bensussan A, Olive D, Costello RT. The co-expression of 2B4 (CD244) and CD160 delineates a subpopulation of human CD8+ T cells with a potent CD160-mediated cytolytic effector function. Eur J Immunol. 2006;36:2359–2366. doi: 10.1002/eji.200635935. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazona R, DelaRosa O, Casado JG, Torre-Cisneros J, Villanueva JL, Galiani MD, Pena J, Solana R. NK-associated receptors on CD8 T cells from treatment-naive HIV-infected individuals: defective expression of CD56. AIDS. 2002;16:197–200. doi: 10.1097/00002030-200201250-00008. [DOI] [PubMed] [Google Scholar]
- Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Taniguchi RT, Mathew PA, Kumar V, Welsh RM. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J Clin Invest. 2010;120:1925–1938. doi: 10.1172/JCI41264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Calpe S, Westcott J, Castro W, Ma C, Engel P, Schatzle JD, Terhorst C. Cutting edge: The adapters EAT-2A and -2B are positive regulators of CD244- and CD84-dependent NK cell functions in the C57BL/6 mouse. J Immunol. 2010;185:5683–5687. doi: 10.4049/jimmunol.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003a;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003b;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.