Abstract
Many eukaryotic channels, transporters and receptors are activated by phosphatidyl inositol bisphosphate (PIP2) in the membrane, and every member of the eukaryotic inward rectifier potassium (Kir) channel family requires membrane PIP2 for activity. In contrast, a bacterial homolog (KirBac1.1) is specifically inhibited by PIP2. We speculate that a key evolutionary adaptation in eukaryotic channels is the insertion of additional linkers between trans-membrane and cytoplasmic domains, revealed by new crystal structures, that convert PIP2 inhibition to activation. Such an adaptation may reflect a novel evolutionary drive to protein structure,; one that was necessary to permit channel function within the highly negatively charged membranes that evolved in the eukaryotic lineage.
Keywords: inward rectifier, gating, structure, K ATP, PIP2, KCNJ, KirBac
Perhaps the most universally recognized regulators of ion channel gating, after membrane voltage, are the phosphorylated phosphatidyl inositols, the archetype being PI(4,5)P2 (PIP2). In many eukaryotic channels, transporters and receptors, including voltage-gated K channels,1–4 epithelial Na channels,5 the transient receptor potential (TRP) family of channels,6 the Na+-Ca2+ exchanger,7 and P2X receptor channels,8 increased PIP2 in the membrane stimulates activity. Likewise, every member of the eukaryotic inward rectifier potassium channel (Kir or KCNJ) family requires membrane PIP2 for activity.9,10 How, and—teleologically—why, PIP2 activates these channels and transporters has been difficult to assess at the biochemical level, partly because of the complexity of cell-based systems typically used to study them. Over the past five years, the ability to express and purify active bacterial homologs of inward rectifier channels (KirBacs) has allowed us to study regulation of pure channel protein in lipid bilayers of defined composition, and has led to the surprising realization that, in contrast to the above, these cousins of eukaryotic Kir channels are specifically inhibited by PIP2.11,12
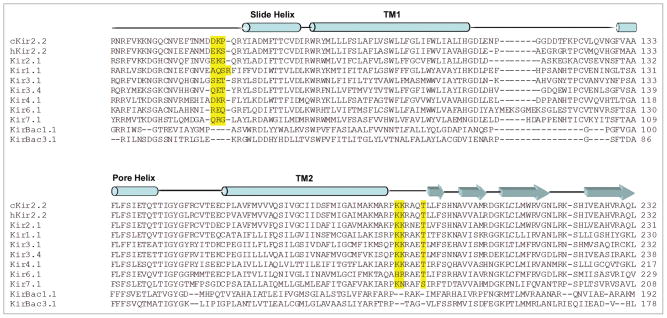
We have suggested that this paradoxical behavior might be the result of missing key residues in the KirBac structure that are crucially involved in PIP2 binding and transduction in eukaryotic Kir channels.11 These key residues are located in two short linker regions that connect the large cytoplasmic domain to the transmembrane (TM) pore-forming region of the channel. Alignments of KirBac and eukaryotic Kir sequences (Fig. 1) reveal that each of these linkers is longer by three residues in the eukaryotic Kirs. Additionally, the second linker, between TM2 and the cytoplasmic domain, contains two charged residues which, when mutated, invariably causes activation loss of PIP213,14 and loss of PIP2 binding.15 These three residue insertions are predicted to displace the cytoplasmic domain away from the membrane surface, and as the new structure of the chicken Kir2.2 channel reveals,16 this is indeed the case (Fig. 2).
Figure 1.
Sequence Alignment of Eukaryotic and Bacterial Inward Rectifier K+ Channels. Eukaryotic Kirs have a prominent 3 residue insertion (highlighted in yellow) in both the N- and C-terminal linkers that link the cytoplasmic domain to the transmembrane domains. These insertions, which include key residues for PIP2 activation of eukaryotic Kir channels, are predicted to displace the cytoplasmic domain away from the membrane surface.
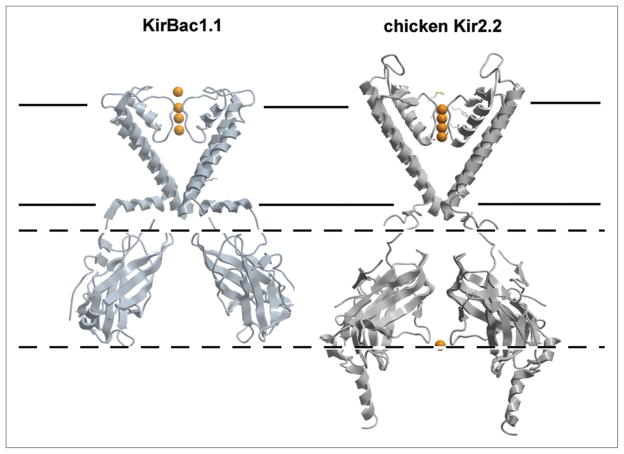
Figure 2.
Structural Comparison of Bacterial and Eukaryotic Kir channels. Closed-state structures of KirBac1.1 (PDB entry: 1P7B) and chicken Kir2.2 (PDB entry: 3YJC). For clarity, chain A and C TM domains, and chain B and D cytoplasmic domains are shown. Notably the tetrameric assembly of the chicken Kir2.2 soluble domain is rotated ~60° compared to the KirBac1.1 structure, and is displaced away from the cell membrane resulting in minimal interaction between the slide helix and the soluble domain in this structure.
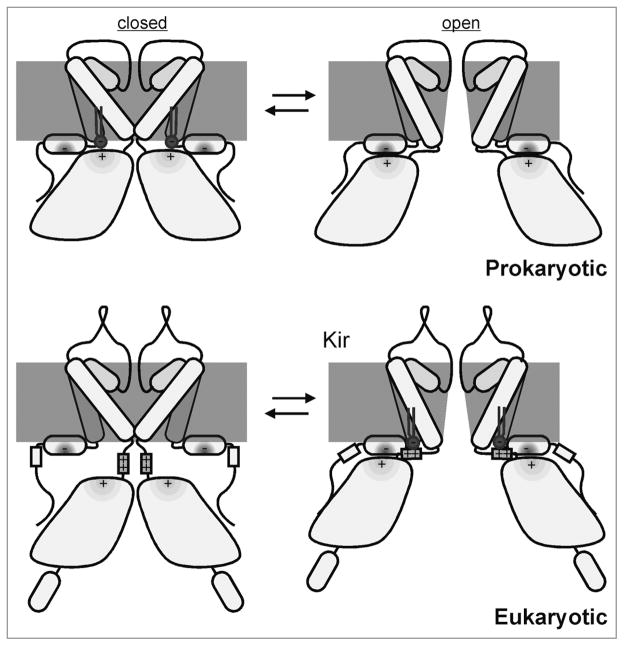
How does this displacement convert inhibition of KirBacs into activation PIP2 of eukaryotic Kir channels? Interactions between the slide helix and the cytoplasmic domain of Kir channels have been suggested to play a key role in channel gating. Mutations which disrupt this interaction can destabilize the open state and favor channel closure, although the ability of these proteins to bind PIP2 remains intact.17 Thus, we can speculate that the shorter linkers in KirBacs energetically favor interactions between the slide helix and the cytoplasmic domain, leading to opening of the channel in the absence of PIP2 (Fig. 3) Binding of PIP2 to KirBacs may act to destabilize this interaction, separating the cytoplasmic domain from the slide helix, leading to channel closure (Fig. 3). In eukaryotic Kirs, the longer linker would minimize the interaction between the slide helix and the cytoplasmic domain, and thereby keep the channel closed in membranes that lack PIP2. However, the PIP2 head-group can extend up to 17 Å from the surface of the bilayer,18,19 and PIP2 binding may pull the cytoplasmic domain back towards the membrane, restoring its interaction with the slide helix to drive channel opening (Fig. 3).
Figure 3.
Mechanism of PI(4,5)P2 gating in Kir channels. In prokaryotic KirBac channels, short TM-cytoplasmic domain linkers may permit energetically favorable interactions between the slide helix and cytoplasmic domain to open the channel in the absence of PIP2 (top right). The addition of PIP2 to the membrane may act to destabilize this interaction, separating the cytoplasmic domain from the slide helix, leading to channel closure (top left). The longer linker in eukaryotic Kirs minimizes the interaction between the slide helix and cytoplasmic domain in the absence of PIP2, keeping the channel in the closed state (bottom left). Binding of PIP2 may recoil the cytoplasmic domain towards the membrane allowing for restored interaction with the slide helix to drive channel opening (bottom right).
From an evolutionary perspective, the differential PIP2 regulation of prokaryotic and eukaryotic Kir channels may provide a fascinating illustration of the interplay of ligands and the evolution of protein structure. It is noteworthy that bacterial membranes typically do not contain PIP2 or other phosphoinositide lipids. Instead, the dominant lipids are phosphatidylethanolamines (PE), and phosphatidylglycerols (PG),20 in which KirBac channels are active.11,21 As eukaryotic organisms evolved, PIP2 and other acidic lipids became increasingly concentrated in plasmalemmal membranes. The unwonted inhibitory effect of PIP2 on KirBac1.1 activity is such that at the predicted PIP2 concentrations in mammalian membranes (~1% of phospholipids),22,23 KirBac-based channel activity would be completely suppressed.11 By contrast, the requirement for PIP2 for activity would render eukaryotic Kir channels inactive in bacterial membranes and in intracellular membranes of the ER and Golgi, which also lack PIP2. It is tempting to speculate that the 3 residue insertions in the cytoplasmic domain-TM domain linkers evolved to allow eukaryotic Kir channels to (i) be functionally active in membranes that evolved to contain PIP2 for other critical cellular functions and/or (ii) take advantage of differences in membrane composition of the various cellular compartments, thereby protecting cells from undesirable channel activity during the trafficking process. Given the breadth of eukaryotic membrane proteins that are sensitive to PIP2, this may be a more generally observable evolutionary mechanism. As more genomes are sequenced and advanced lipidomics are employed to resolve the compositions of specific membranes, this hypothesis can be rigorously examined.
References
- 1.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–20. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, et al. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–75. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 3.Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, et al. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–70. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- 4.Bian J, Cui J, McDonald TV. HERG K(+) channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ Res. 2001;89:1168–76. doi: 10.1161/hh2401.101375. [DOI] [PubMed] [Google Scholar]
- 5.Yue G, Malik B, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–9. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 6.Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch. 2007;455:157–68. doi: 10.1007/s00424-007-0275-6. [DOI] [PubMed] [Google Scholar]
- 7.Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–9. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Yang M, Ting AT, Logothetis DE. PIP(2) regulates the ionic current of P2X receptors and P2X(7) receptor-mediated cell death. Channels (Austin) 2007;1:46–55. [PubMed] [Google Scholar]
- 9.Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–95. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 10.Logothetis DE, Jin T, Lupyan D, Rosenhouse-Dantsker A. Phosphoinositide-mediated gating of inwardly rectifying K(+) channels. Pflugers Arch. 2007;455:83–95. doi: 10.1007/s00424-007-0276-5. [DOI] [PubMed] [Google Scholar]
- 11.Enkvetchakul D, Jeliazkova I, Nichols CG. Direct modulation of Kir channel gating by membrane phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2005;280:35785–8. doi: 10.1074/jbc.C500355200. [DOI] [PubMed] [Google Scholar]
- 12.Cheng WW, Enkvetchakul D, Nichols CG. KirBac1. 1: it’s an inward rectifying potassium channel. J Gen Physiol. 2009;133:295–305. doi: 10.1085/jgp.200810125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyng SL, Cukras CA, Harwood J, Nichols CG. Structural determinants of PIP(2) regulation of inward rectifier K(ATP) channels. J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, et al. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–44. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- 15.Soom M, Schonherr R, Kubo Y, Kirsch C, Klinger R, et al. Multiple PIP2 binding sites in Kir2. 1 inwardly rectifying potassium channels. FEBS Lett. 2001;490:49–53. doi: 10.1016/s0014-5793(01)02136-6. [DOI] [PubMed] [Google Scholar]
- 16.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science. 2009;326:1668–74. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decher N, Renigunta V, Zuzarte M, Soom M, Heinemann SH, et al. Impaired interaction between the slide helix and the C-terminus of Kir2. 1: a novel mechanism of Andersen syndrome. Cardiovasc Res. 2007;75:748–57. doi: 10.1016/j.cardiores.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Haider S, Tarasov AI, Craig TJ, Sansom MS, Ashcroft FM. Identification of the PIP2-binding site on Kir6. 2 by molecular modelling and functional analysis. EMBO J. 2007;26:3749–59. doi: 10.1038/sj.emboj.7601809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sansom MS, Bond PJ, Deol SS, Grottesi A, Haider S, et al. Molecular simulations and lipid-protein interactions: potassium channels and other membrane proteins. Biochem Soc Trans. 2005;33:916–20. doi: 10.1042/BST20050916. [DOI] [PubMed] [Google Scholar]
- 20.Phung LV, Tran TB, Hotta H, Yabuuchi E, Yano I. Cellular lipid and fatty acid compositions of Burkholderia pseudomallei strains isolated from human and environment in Viet Nam. Microbiol Immunol. 1995;39:105–16. doi: 10.1111/j.1348-0421.1995.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Gan JH, Paynter JJ, Tucker SJ. Cloning and functional characterization of a superfamily of microbial inwardly rectifying potassium channels. Physiol Genomics. 2006;26:1–7. doi: 10.1152/physiolgenomics.00026.2006. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Watras J, Loew LM. Kinetic analysis of receptor-activated phosphoinositide turnover. J Cell Biol. 2003;161:779–91. doi: 10.1083/jcb.200301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–75. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]