Abstract
The discovery of regulatory T cells (Tregs) as a crucial component of peripheral down-regulation of immunity to self and allogeneic antigens, has raised legitimate hope for the development of Treg-based clinical protocols for tolerance to allografts. The present review addresses the question of whether or not therapeutic Tregs are ready to enter the clinical transplantation arena. In the light of recent experimental observations, we will revisit some fundamentals of T cell and biology that stress the need for further studies prior to applications and provide conceptual cues for novel therapeutic approaches.
Keywords: Immune regulation, Tolerance, Cell therapy, Treg, Thymus, Deletion, Suppression
INTRODUCTION
Several reviews on the role of regulatory T cells (Tregs), the chief cell subset implicated in immune regulation, in the induction and maintenance of immune tolerance to allogeneic transplants have been published in the past few years. 1–6 It was of no surprise to find that these analyses converged on the necessity to improve Treg mediated protocols to better prevent allograft rejection and possibly induce tolerance. We should stress that although necessary, these reviews and more importantly, the field of Treg therapy, are acquainted with a monolithic perception of CD4 Tregs as “just” another subset of T lymphocytes obeying the same basic rules as those of conventional CD4 T lymphocytes (conT). Enduring notions originating from the old days of T suppressor cells (Ts), the distant cousins of Tregs, have been transposed to the new world of Tregs without convincing proof of veracity. The present review is based on the now well-documented view that although CD4 Tregs and conT lymphocytes are both made in the thymus from common precursors, they respond to different thymic developmental cues and to different peripheral signals when they reach maturation. We will review recent advances in the field that foster understanding on Treg development, antigenic specificity and suppressive functions. Alternative views on the importance of Treg-mediated regulation will also be discussed as well as potential mechanisms underlying Treg-induced tolerance to allografts.
I. DELETIONAL VERSUS REGULATORY TOLERANCE
Immune tolerance to self antigens, defined as a state of permanent unresponsiveness, is undoubtedly one of the most challenging concepts that have defied the intellect of many talented scientists for more than 150 years. Despite remarkable advances in our understanding of lymphocyte differentiation, antigen recognition, shaping of T and B lymphocyte receptors and of their signaling pathways, the conceptual foundation of immune tolerance remains elusive. At this point, it would be fair to recognize that the field has accumulated far more data showing what tolerance is not rather than what it is.
A. On the Necessity of Immune Regulation
Lymphocyte tolerance of allogeneic grafts pertains mainly to the T lymphocyte subset as B cells often require T cell help for alloreactive antibody production. 7 Part of the complexity of T cell tolerance emerges from the fascinating paradigm of thymocyte selection, which ultimately generates immunological ticking-bombs in any healthy individual. According to this principle and in agreement with compelling evidence supporting the affinity selection model for thymocytes, the T cell receptors for antigens (TCR) carried on developing thymocytes are fashioned on self peptides bound/presented on self MHC molecules (positive selection on pMHC complexes, 8–10). Following positive selection, auto-aggressive T cell clones expressing high TCR affinity for self pMHC are deleted from the selected thymocyte pool to avoid autoimmunity (negative selection; reviewed in 11, 12), a process that leaves a large repertoire of lower affinity T cells distributed in lymphoid organs. One of the implications of thymic selection is that our ability to recognize and respond to the world of “foreign” antigens rests on initial recognition of self pMHC complexes, a property that needs to be suppressed (down-regulated) in steady state conditions in order to prevent autoimmunity. Alternatively, regulation may not be required if one imagines that the set of self pMHC complexes is hidden from reactive T cells in a state of “immunological ignorance”, as suggested by R. Zinkernagel. 13
For the purpose of this review and based on results from Treg depleting studies that will be discussed later, we will argue that negative regulation is a critical component of the immune balance that keeps in check autoreactive T lymphocytes. Another implication of the selection paradigm would be that the mechanisms of T cell tolerance to syngeneic (self) and allogeneic antigens are identical as the T cell pools involved in each case is selected and regulated along similar fundamental processes. The evaluation of the respective contribution of deletional versus regulatory tolerance to allografts may, therefore, provide new insights on their role in tolerance induction and maintenance.
B. Effect of Thymic Deletion on Tolerance of Allotransplants
According to FM Burnet’s clonal selection theory, 14 anti-self reactivity is eliminated to prevent autoimmunity. The seminal work of JP Miller demonstrated that the thymus was essential in this process 15 and clonal deletion of high affinity, self-reactive thymocytes was ultimately established. 16–19 Indeed, the affinity hypothesis of the TCR for pMHC ligands correlates in large part with the outcome of positive and negative selection of thymocytes 20–22. The hypothesis depicts positive selection as resulting from productive TCR/pMHC interactions that are followed by deletion of clones expressing “too high” affinity TCR for self pMHC ligands. This is supported by numerous data, notably by those showing that a large fraction of clones with intermediate TCR affinity are rescued during thymic selection. 23 However, the anthropomorphic view of a critical TCR avidity checkpoint deciding on the fate of potentially autoreactive cells appears to be approximate and in disagreement with other important observations. For example, it has been shown that, independently of positive selection, the TCR repertoire has a propensity to interact with the MHC molecules from the same species. 24–26 During positive selection, thymocytes also have several chances to build a functional receptor via sequential rearrangements of the TCR alpha locus. 27 One would expect that the combination of these two features would increase the pool of positively selected thymocytes. In fact, the net output of positive selection is that over 90% of thymocytes died by failure of assembling “correct” TCR in so-called death by neglect. 28,29 This apparent waste of thymocytes may, nonetheless, be important for thymocyte education given that 1) antigens from dying cells can be presented by other cells (cross presentation, reviewed in 30), 2) there is a high rate of peptide exchange between medullary antigen-presenting-cells (APCs 31,32) and 3) thymic APCs ensure the rapid clearance of neglected T cells. 33
Stable hematopoietic chimeras (individuals holding a mixture of host- and donor-type bone marrow cells) are probably the best illustration of central thymic deletion leading to immune tolerance. Hematopoietic chimeras show robust tolerance to donor tissues as they indefinitely accept donor skin grafts without additional conditioning. Peripheral and thymic responses to donor antigens also vanish in hematopoietic chimeras during the entire tolerance period, a condition possibly due to deletional tolerance of host alloreactive cells on donor APCs colonizing the chimeric thymi. 34,35 Unfortunately, quantitative estimates of the extent of clonal deletion in these models are sparse due to a critical lack of techniques allowing for the tracking of polyclonal thymocytes. Using combinations of radiation chimeras able to positively select thymocytes (on pMHC complexes present on radioresistant thymic epithelial cells) but defective in negative selection on the same pMHC displayed by radiosensitive bone marrow-derived APCs, HR MacDonald and coll. suggested that roughly half of positively selected thymocytes undergo deletional selection. 28 Even so, results based on depletion of bone marrow elements via irradiation should be taken with caution as the approach often fails to completely purge the thymus of radiosensitive APCs while inflicting thymic damage that could, on their own, compromise thymocyte selection. 36 Although other evidence supports the critical role of thymic deletion in stable chimeras, it would appear that the contribution of T cell deletion is model-specific as minimal fractions of thymocytes are depleted in other models of thymus-targeted MHC expression. 33,37 Collectively, these data suggest that the substantial T cell deletion found in stable hematopoietic chimeras is inherent to unique conditions leading to chimerism. Indeed, stable chimeras result from the forced coexistence of two functional immune systems, which had to overcome their respective alloreactivity via a profound reprogramming of T cell progenitors, that included massive deletion. This would also infer that deletion of alloreactive thymocytes is not the prevalent mechanism in protocols which do not promote persistent bone marrow chimerism.
In this regard, the contribution of thymic and peripheral deletion of donor-reactive T cells to allogeneic antigens has been overestimated. Most of the data on clonal deletion came from studies using non-physiological models of high affinity pMHC/TCR interactions. For example, models of bacterial or viral superantigens expressed by the donors and binding with high affinity to a large fraction of recipient TCR beta chains, have been used to evaluate the extent of donor-specific deletion (reviewed in 38). TCR transgenic animals in which high affinity TCR to donor pMHC are expressed on most of the CD4 or CD8 cells have also been used in transplantation models. 39 In either case, important deletion of “donor-reactive” T cell clones was observed but it only affected highly reactive T cells that were not representative of the polyclonal alloreactive T cell population. On the whole, there is presently no convincing evidence supporting a dominant role of central and/or peripheral T cell deletion in the induction of T lymphocyte tolerance to solid organ transplants.
If most of the clones reactive to self pMHC and cross-reactive to allogeneic complexes are not eliminated because they would make up the pool of T cells responding to pathogenic antigens, these would have to be repressed in a cell-extrinsic manner through regulatory mechanisms. Would this suppression be permanent or temporary at steady state? These points will be discussed in the following paragraphs.
C. Tregs impact the Negative Regulation of Immunity
Numerous processes of negative regulation of immune responses have been described. These include regulatory T cells (Tregs) of CD4 40–42 and CD8 43 phenotype, regulatory B cells (B-regs), 44 CD4 CD8 double negative T cells 45 and gamma/delta T cells which all interact with effector T cells and down-modulate their function. Non-lymphoid cells have likewise been implicated in regulation of alloreactivity among which plasmacytoid dendritic cells (DC) activate CD4 Tregs to lessen graft rejection 46; tolerogenic DCs tame antigen presentation to effector T cells 47; mesenchymal stem cells 48 and subsets of macrophages /monocytes 49,50 secrete immunomodulatory cytokines and soluble factors that can modulate the scope and flavor (Th1/Th17 vs Th2) of alloresponses by T, B and NKT cells. Importantly, CD4 Tregs have been considered as major players in immune regulation because of their decisive implication in fundamental pathways controlling both auto- 51–53 and allo-immunity 1,2,4; and as such they remain the focus of this review.
The notion of dominant tolerance to self antigens mediated by a dedicated lineage of T cells of thymic origin has been firmly established by the work of N. Le Douarin 54 and S. Sakaguchi and colleagues. 55 A landmark paper from the latter group established that this lineage encompassed the CD4+ CD25+ subset and had suppressive properties on immune responses including allogeneic responses. 42 The discovery of the transcription factor Foxp3, a crucial molecule required in Treg development 40,41, 56 and suppressive function, 57, 58 definitely established the basic phenotype of the previously elusive suppressor T cells. The importance of Foxp3 in Treg lineage fate was further established by showing that continuous expression of Foxp3 was necessary to maintain suppression in mature peripheral Tregs. 59 Although studies on Treg biology have been compromised by the lack of Treg clones and specific activation markers, the clear identification of the Treg subset as a primordial cell type involved in generic immune regulation was eventually established (reviewed in 60). The ultimate function of Tregs is to suppress lymphocyte responses by mechanisms which are still not well understood and that will not be discussed in this review except for the role of TCR specificity in Treg suppressive functions. 61–63
D. Treg-Dependent Negative Regulation of Allogeneic Responses
In the world of experimental transplantation, the arrival of this new regulatory cell type had diverse influences on the conceptual views of the time. The skeptical minds, forged during the days of Ts cells and the experimental fiasco that followed their discovery, 64 understandingly remained doubtful about the relevance of Tregs in immune tolerance. The more optimistic transplantation scientists saw the therapeutic potential of these newly defined regulators. Indeed, numerous transplantation models have demonstrated the importance of Tregs in harnessing alloreactivity. 1–4 However, no model has established, as of yet, clear connections between Treg TCR specificity for donor antigens, Treg-mediated suppression in vivo and prevention of graft rejection. Hence, key features of Treg biology remain to be elucidated in order to unleash their full therapeutic potential in safe and controlled clinical situations. In the absence of such knowledge, several unfounded features from Ts cells have been spuriously attributed to Tregs. Growing evidence indicate that Treg characteristics such as their TCR repertoire diversity, specificity for antigens, modality of activation and thymic development are fundamentally different from those reported for Ts and conT cells. Because these parameters have crucial bearing on the mechanism of Treg-mediated regulatory tolerance to transplants, they need to be reevaluated in the light of recent reports.
II. CELLULAR AND MOLECULAR DIVERSITY OF TREG CELLS
In terms of cellular diversity and aside from the CD4+ Foxp3+ Tregs of thymic origin, other types of CD4+ regulatory cells were derived from Foxp3− cells upon antigen stimulation in vitro in the presence of TGFβ 65 as well as in vivo. 66,67 These extra-thymic Tregs, also called induced Tregs (iTregs) as compared to the “natural” or thymic Tregs (tTregs), can also be generated with IL-2, which inhibits a potential differentiation pathway towards Th17 proinflammatory cells. 68 Other cytokines such as IL-6 interfere with the TGFp induction of iTregs to ultimately promote the TH17 lineage. 69 Finally, retinoic acid secreted by some DC subsets in the gut also allows for the conversion of Foxp3− CD4+ cells into iTregs with suppressive functions. 70–72 Together with other reports describing various hybrid phenotypes of iTreg (reviewed in 73), these observations illustrate the complex balances existing between peripheral naïve CD4 cells and environmental cues that drive lineage differentiation and specialization according to local requirements.
A. Are induced and thymic Tregs of the same kind?
Although the initial results showing that ectopic expression of Foxp3 in naïve CD4 cells conferred upon them the hallmarks of the tTreg phenotype 40,41 and function, 74 recent evidence would suggest that iTregs and tTregs differ by fundamental criteria, 75 including the fact that in vitro iTregs would present unstable Foxp3 phenotypes and suppressive functions. 76
It is commonly accepted that iTregs and tTregs share several characteristics, notably the CD4+ CD25+ phenotype as well as Foxp3 expression. The efficacy of suppression mediated by in vitro generated iTregs appears, however, to be reduced as compared to that of tTregs. 65, 77 In contrast, immune responses including alloresponses seem to be suppressed equally well by tTregs and in vivo-generated iTregs. 66, 67,72 The differences between these two cell subsets are multiple. We will briefly analyze the divergences relevant to the transplantation field. The induction phase of iTreg and tTregs relies on different molecular signals: CTLA-4 is required for the TGFβ-induction of iTregs, whereas the development of tTregs depends upon CD28. 78 This was confirmed by cross-linking of CD28 with specific mAbs that blocked the generation of Treg cells in vitro. 70 Together these studies suggest that the behavior and overall reactivity of iTregs and tTregs respond to different environmental cues which may not be equally represented in inflammatory milieus around allogeneic transplants and, therefore, these cells may not be able to act synergistically to overcome alloresponses. Another rather alarming difference emerged recently from molecular studies on the regulation of Foxp3 gene expression. Foxp3 gene transcription can be down-modulated by extrinsic factors in both the iTreg and tTreg subsets although at different levels. Given the importance of Foxp3 in Treg differentiation /function and the therapeutic potential of regulatory cells in clinical transplantation, we will closely examine recent data related to the so-called “plasticity” of the Treg phenotype.
B. Stability of Treg Phenotypes
Several studies have now reported loss of Foxp3 expression as well as suppression in murine but also human enriched Tregs from the iTreg and tTreg type. 73, 76 Although the phenotypic plasticity of Tregs is of prime interest to Treg cell biologists, the termination of Foxp3 expression in functional Tregs is an obvious concern for transplantation immunologists. More worries were raised from reports on loss of Treg function coinciding with the conversion of ex-Tregs into pathogenic autoreactive T cells. 79 Recent mechanistic studies of Treg instability have, however, suggested alternative means to curtail Treg conversion in murine and human Tregs. 80
The group of J. Demengeot showed that injections of purified Foxp3+ peripheral tTregs into lymphopenic RAG knock-out hosts led to the extinction of Foxp3 expression in roughly half of the injected Tregs, a process associated with a concomitant loss of suppression tested in vitro. 81 This alarmist rate of conversion should, however, be reevaluated in view of other results. First, lymphopenic environments represent non-physiological conditions in which lymphocyte-made cytokines and other factors cannot contribute to Treg maintenance in vivo. Concurring with this, CD4 lymphopenia observed in patients transplanted with allogeneic hematopoietic stem cells altered Treg homeostasis. 82 In murine models, conversion towards Foxp3 negative cells was increased in the absence of TGFβ 83,84 but limited to a minor fraction of cells (below 10%) in lymphorepleted hosts. 79, 85 Second, the lack of iTreg- and tTreg-specific markers complicate data interpretation as their respective contribution in Treg phenotype instability cannot be determined. Emerging evidence from molecular genetic analyses suggest that the induction and maintenance of high levels of Foxp3 production are two distinct phases which are individually controlled by intrinsic and extrinsic factors in iTregs and tTregs. 76, 86 Thus, it would be untimely to extend the interpretation of data obtained on in vitro-generated iTregs to the entire Treg population, the cellular and functional heterogeneity of which remains to be better defined.
C. Molecular Parameters of Treg Heterogeneity
In order to channel the discussion toward parameters relevant to the use of iTregs or tTregs in tolerance therapy, we will briefly examine the molecular status of Foxp3 expression in these two regulator sets. Some intronic and highly conserved CpG islands from the Foxp3 gene are fully demethylated in CD4 T cells expressing high levels of Foxp3; i.e., tTregs. 83 Conversely, CpG regions are partially methylated in TGFβ-induced murine and human iTregs (known to be unstable), suggesting that the Foxp3high phenotype is not firmly “clamped” in iTregs. 87,88 Confirmations of the role of CpG methylation in the instability of Foxp3 expression were provided by several sources: 1) treatment of naïve CD4 T cells with inhibitors of DNA methylation promoted Foxp3 expression 89; 2) deletion of CpG regions differentially affected the development of tTregs and iTregs, suggesting that each region may have a different role; and 3) recent elegant studies from the group of AY Rudensky identified 3 key non-coding regions (CNS1–3) that control the fate of Foxp3 expression and, thereby, Treg development. 76,90
In summary, a novel view of Treg heterogeneity would be that epigenetic mechanisms enroll different CNS cis-elements to control the induction phase and commitment/clamping phase of Foxp3 expression. 90 More specifically, demethylation of CNS3, previously called Treg-specific demethylation region or TSDR, correlates with higher frequencies of iTregs and tTregs; as anticipated, mice deficient in the CNS1 element, which contains a TGFβ-responsive sequence, have normal tTregs but lack iTregs. CNS2, on the other hand, is not required for initial Foxp3 expression, but binds the Foxp3 protein following demethylation to promote prolonged Foxp3 expression in Treg progeny (i.e. stability). Of high relevance to the transplantation field, concordant evidence indicate that the CNS2 region from purified iTregs does not bind Foxp3, 90 likely because of incomplete demethylation. 79 These results strongly suggest that the cellular heterogeneity of Tregs, which includes Foxp3high tTregs and Foxp3+ iTreg cells exhibiting helper/Treg hybrid phenotypes, may correspond to a mixture of cell subsets, each responding to different environmental cues and each arrested at various stages along the same differentiation line. 73,90 In this scheme, Foxp3high tTregs with high suppressive activity would represent the ultimate stages of the Treg genetic program with essentially no option for phenotypic reversal toward helper functions. 76 More importantly, these findings indicate that the regulatory/suppressive properties of in-vitro expanded iTregs would be unstable due to a lack of appropriate signals ensuring sustained high Foxp3 expression.
III. BASIC TREG FUNCTIONS ASSOCIATED WITH REGULATORY TOLERANCE
Three primary issues will be examined in the context of Treg-mediated regulatory tolerance to allogeneic transplants: 1) Treg thymic differentiation, which may provide clues on their mode of selection and thereby on their suppressive functions; 2) the TCR specificity of Tregs; and, 3) the role of Treg TCR specificity in the mechanism of suppression.
A. Treg Differentiation: Everything Occurs in the Thymus
It is well established that the TCR plays a key role in lymphocyte lineage commitment in the thymus through transduction of intracellular signals of certain strength and duration (reviewed in 91). Because the initial differentiation of thymic Treg progenitors also proceeds through positive selection, it was of no surprise to see that TCR engagement to pMHCII was crucial to Treg development. 92, 93 Like their non-Treg CD4+ counterparts, Treg precursors emerge in the thymus 94–96 following a first step of positive selection on pMHCII complexes displayed on thymic epithelium. 96–99 The consensus view on Treg development indeed stops at this point as opinions and data interpretations diverge on the timing of Foxp3 expression, nature of selecting pMHCII, role of TCR affinity and process of Treg maturation. We would like to propose a model of Treg differentiation that, at present, accounts for recent observations on Treg commitment and reconciles some experimental divergences. This model is based on the following observations:
TCR transgenic mice that have no alternative other than to use the imposed TCR (animals defective in the recombination activating gene (RAG)), have no Foxp3+ Tregs. 76, 99,100 From this, we deduced that a second step of positive selection on cognate pMHCII was required to ensure specific Treg development. In other words, transgenesis with rearranged TCR genes would bypass the positive selection step of conT and Treg cells but would not license Treg “maturation” because of the absence of cognate pMHCII for the transgenic TCR. In agreement with this view, mice expressing a monoclonal TCR cloned from non-Treg cells of the same MHC background failed to use this TCR on Tregs. 99,101 Likewise, co-expression of the TCR and cognate model antigens by double transgenic animals led to the deletion of transgenic conT cells and the emergence of Foxp3+ cells bearing the monoclonal TCR. 97, 102,103
As referred to above, Treg development requires Foxp3 expression that is contingent on TCR signaling, thereby suggesting that TCR sensing of certain pMHCII complexes in the thymus is critical to Treg commitment.
Subset of DCs, notably the plasmacytoid DC and CD8lo, SIRPα+ DC, migrating from the blood and bone marrow, are particularly poised to facilitate Treg differentiation. 46,103
Human Tregs mature in the medulla of the thymus around the Hassall’s corpuscles, a diffuse structure in murine thymi. The mechanisms that favor DC to become tolerogenic, i.e. furthering Treg maturation in the thymic medulla, are still unknown although local expression of IL-7 and thymic stromal lymphopoietin (TSLP) seems to be involved. 104,105 In agreement with these findings, antigen-specific tTregs were produced following targeted expression of a model antigen to medullary thymic epithelial cells (mTEC) via the Aire promoter control. 31 At first glance, these results appear contradictory to reports suggesting that expression of pMHCII in the thymic medulla is dispensable for Treg development. 96,98 However, T. Laufer’s group as well as ours have shown that Tregs from the mutant K14 mouse used in the cited experiments are not functionally mature, 96 especially in their lack of MHC restriction in suppression (Germana, S. personal communication).
Although significant numbers of Foxp3+ cells are not detected by flowcytometry prior to the CD4-8 double positive stage, 4,76 basal levels of Foxp3 transcripts were observed in double negative (DN) thymocytes, which have not yet displayed cell surface TCR. 106 Low levels of Foxp3 transcription in DN cells were increased following cross-linking with anti-CD28 mAb, suggesting that early Foxp3 expressers are already susceptible to extrinsic signaling via CD28; a crucial signal controlling thymic Treg development. 78,107
Sequence analyses indicate that Treg and naïve CD4 T cells show minimal overlap of their TCR repertoires. There is a large repertoire overlap between Tregs and self-reactive T cells. 108–110 In contrast, Tregs and naïve CD4 cells have limited sharing in TCR gene usage. 111 Thus, the TCR specificities of Tregs are essentially different from those carried by the TCR of effector CD4 cells that they ultimately control.
Several pieces of evidence indicate that, as for conT cells, TCR affinity for self pMHCII may be the main driving force of Treg maturation. But conversely to conT, Treg clones with high affinity for self pMHCII would be the only ones kept in the mature Treg pool. CD28-deficient mice that cannot produce TCR of high affinity for self are deficient in Tregs but not in conT cells. 78,106 Treg failed to develop in TCR transgenic animals that express low affinity TCR. 112 Other studies argue to the fact that high affinity TCR for self is likely a stabilizing factor rather than a means for Foxp3+ clones to escape deletion during negative selection. Importantly, CD4+ T cells from Foxp3-deficient mice that expressed high affinity TCRs for self pMHCII were not deleted and became auto-reactive, whereas these TCRs were “preferentially” found on Tregs from Foxp3-sufficient animals. 108,113 This strongly suggests that the TCR specificities for self of Tregs and naïve CD4 cells are different and that the stable mature tTreg phenotype results from the combined effects of Foxp3 expression and high TCR reactivity to self pMHCII.
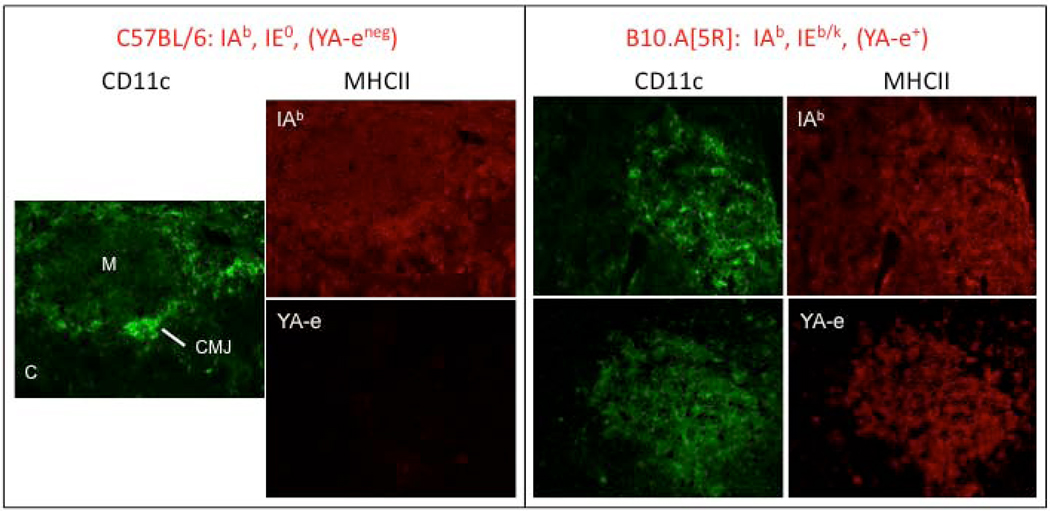
The nature and/or density of thymic peptides presented by pMHC are different between the thymus cortex and medulla. The thymic medulla, the birthplace of Tregs (point 4), preferentially expresses in mTEC a set of genes for potentially tissue-specific antigens under the control of the transcription factor Aire. 114,115 Nonetheless, Aire-defective mice showed similar Treg numbers, percentages and functions as their wild-type counterparts suggesting that Aire is not the only genetic system controlling the diversity of the medullary peptides presented 116. MHCII proteins themselves represent another source of medullary peptides. For example, a single self MHCII peptide derived from the IEα chain (positions 52–68) is presented by the IAb allele on 10% of MHCII molecules exposed on B cells. 117 The IEα amino-acid sequence is conserved across mammals and is highly expressed on bone marrow-derived CD11+ DC in the thymic medulla (Figure 1; 117, 118). Recent experiments from our group have shown that the provision of the correct pMHCII (IEα52–68 peptide on IAb) in the medulla of transgenic mice expressing the cognate TCR, promoted the development of transgenic Foxp3+ Tregs (Germana, S. et al., unpublished). Hence, we surmised that certain self peptides are overrepresented in confined areas of the medulla to select high affinity TCR and clinch high Foxp3 expression in Tregs.
FIGURE 1. Immunohistologic studies of thymus sections from C57BL/6 and B10.A[5R] mice.
Sections were stained with an anti-CD11c mAb (Green), MHCII-IAb (IAb, red) or [IAb + IEp]-specific mAb (YA-e). The medulla (M), cortex (C) and cortical medullary junction (CMJ) are indicated. All sections were examined at 200X magnification. Figure shows that thymi from B6 mice, which do not express the IEα 52–68 peptide, are IAb+ in both cortex and medulla but YA-e negative (left panel). In contrast, B10.A (5R) mice thymi showed expression of IAb (on cortex APC and medullary DC), whereas the IEα peptide was presented almost exclusively on medullary DCs (right panels).
B. Thymic Treg Lineage Commitment
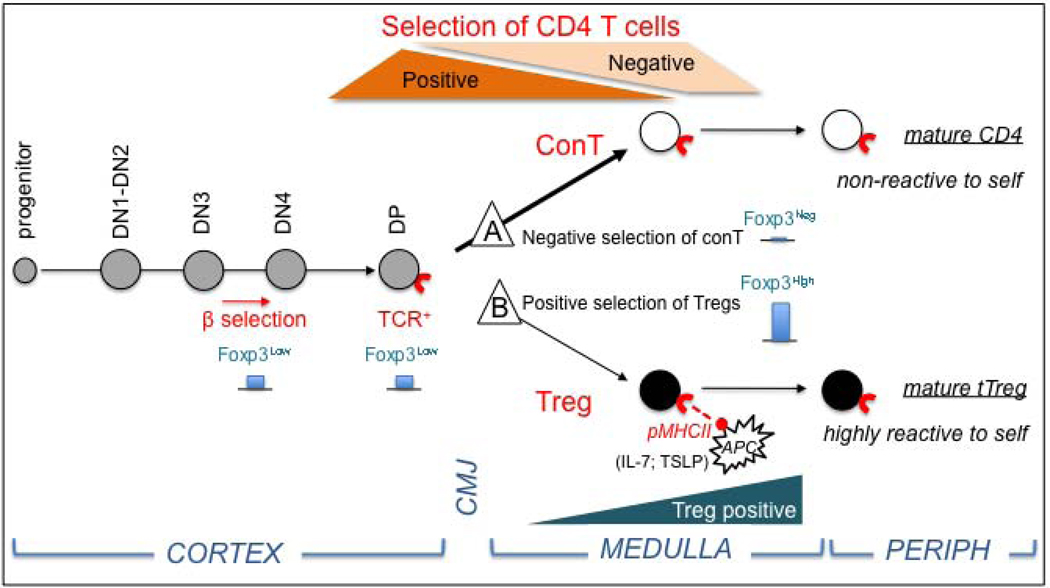
The Treg differentiation model we propose, called the “double selection model” extends on previous studies from C Janeway’s group suggesting that different sets of pMHCII may contribute to positive and negative selection of conT cells. 117 The possibility of a second step of selection for Tregs was inspired by the studies of YJ Liu and colleagues who projected a model of Treg selection on high affinity to self antigens in the medulla. 105,119 As shown in Figure 2, our model predicts that thymocytes at the CD4 and CD8 double negative stage 3 (DN3) that express low levels of Foxp3 mRNA, are common precursors of conT and Treg cells that would appear after positive selection at the double positive stage (DP). Further selection processes involve the negative selection of conT cells on bone marrow derived APCs displaying pMHCII essentially at the cortical medullary junction. This would lead to deletion of clones with high affinity TCR for self pMHCII and turn-off Foxp3 expression in the bulk of medium affinity clones that would further mature into CD4 helper T cells (Figure 2, path A). Tregs would develop from a small subset of DP high affinity thymocytes engaging dominant pMHCII complexes displayed in the thymic medulla. The overwhelming expression of a limited set of medullary peptides (such as the IEα peptide) in confined milieus secreting Treg-prone factors (such as TSLP and IL-7 in the vicinity of Hassall’s corpuscles in humans), will allow pre-Tregs to escape deletion and to clinch high Foxp3 expression (Figure 2, path B).
FIGURE 2. The double selection model for Treg differentiation.
Starting at the CD4, 8 double negative stage 3 (DN3), thymocytes show low Foxp3 gene transcription (blue bars). Positive selection of common precursors of conventional (conT) and Treg cells occurs at the double positive stage (DP). Further selection processes involve negative selection of conT cells (A) by deletion of clones with high affinity TCR for self pMHCII on APCs in the cortical medullary junction (CMJ), leaving the majority of clones with medium affinity (thick arrow) to mature into functional Foxp3 negative, CD4+ helper T cells. Treg lineage differentiation (B) includes a second selection step (Treg positive) in which few CD4+Foxp3lo thymocytes (thin arrow) engage in high affinity interactions with overly expressed medullary pMHCII to secure Foxp3 high expression in Treg permissive micro environments (IL-7 and TSLP).
No model is perfect; the double selection model, nonetheless, accounts for the poor overlap between Treg and conT cell TCR repertoires since the high reactivity against self is eliminated in conT cells but kept in Tregs due to the compartmentalization of thymocyte selection. Failure of detecting transgenic TCR Tregs in mice expressing transgenic TCR against model (i.e. non-self) antigens also finds explanation in this scenario. In further support of the model, recent studies have shown that many autoreactive thymocytes persist in confined niches of the medullary microenvironment. 120 Of relevance to tolerance protocols involving Tregs, this model predicts that beside their disconcerting instability, donor-specific iTregs from graft recipients would not reach the regulatory efficacy of professional tTregs as their TCR would come from non-Treg cells and would at best cross-react to allogeneic pMHCII. Further studies are evidently required to fully understand the dichotomy of tTreg and iTreg TCR specificity in order to optimize their use in the clinic. According to this model, it is of no surprise that there is, so far, no data documenting a better efficacy of iTregs over that of tTregs in clinical trials (see below).
C. Specificity of Treg Activation and Suppression
The apparent difference between the TCR specificities of tTreg and conT cells raises the possibility that tTregs would not be “donor-specific” as often stated in the transplantation literature. During thymic selection the pMHCII complexes, which engage the TCR of Treg precursors are, in essence, the selecting antigens guiding Treg TCR specificity. 92,93 Therefore, thymic commitment and peripheral activation of Tregs are antigen-specific but not necessarily donor-specific. The perspective of activated conT cells being suppressed only by Tregs of similar TCR specificity is a remnant of the Ts ages. This view rested on the development of two similar TCR specificity repertoires that would be equally distributed on suppressor and effector cells, a condition which has never been demonstrated. 64 Although, donor-specific iTregs with effective suppressive functions have been described in several models, 121–123 these data could not conclude on the real TCR specificities of tTregs because of potential cross-reactivity to allogeneic pMHCII. Such iTregs suppressed in a donor-specific fashion although the mechanism could be “artifactual” since there was potential competition between conT and iTreg cells - using the same TCRs - for the same determinants (reviewed in 4).
Conversely, the mechanism of tTreg suppression has been shown to be antigen-nonspecific (i.e. unrelated to the specificity of the suppressed conT cells) and dependent on initial cell-cell contacts. 124, 125 In vitro studies showed mandatory contacts between Tregs and APCs 62, 126 or Tregs and ConT cells, 127, 128 prior to suppression. The mechanisms of tTreg suppression are still not well-understood. These include the secretion by activated Tregs of immuno-modulatory cytokines (IL-10, IL-35, TGFβ…), the Treg-mediated down-modulation of antigen presentation on APCs and the direct killing or inactivation by Tregs of conT cells following T-T cell contacts (reviewed in 4, 61–63). The cell-cell contact requirement for Tregs of specificity A suppressing in vitro and in vivo conT cell stimulation by unrelated antigen B 63,129, 130 suggests that the activator determinant of tTregs should be different from and in the vicinity of the activator determinant for the effector conT cells. Figure 3 depicts two possible situations in which such proximity may occur. The 3 cell model brings the two determinants on the same APC (Figure 3A), whereas the 2 cell model sequentially presents the regulatory determinant on the activated conT cells. We have recently tested these models using allogeneic APC as stimulators of conT cells. Results supported the 2 cell model by demonstrating that Treg-mediated suppression was MHCII-restricted (i.e. Tregs could suppress conT activation only if the latter cells were from the same MHCII background). Additional data suggest that pMHCII captured from APCs and transferred on the surface of responding conT cells may serve as docking signals for Tregs (Germana, S. et al. unpublished results). These observations would argue for a Treg suppressive mechanism that only targets activated effectors exposing pMHC flags to be recognized by neighboring Tregs. Anti-pathogen responses will be granted because of local inhibition of Treg functions by massive TLR activation generated by many pathogens. 131 Conversely, in conditions favoring regulatory tolerance to allografts - such as with MHCII-matched transplants - unwanted rejection responses would be repressed locally by Tregs activated by proper pMHCII complexes that are common between donor and recipient. 132,133
FIGURE 3. Suppression of Conventional T cells by Tregs of different specificity.
(A). In the 3 cell model, CD4+Foxp3+ Tregs and CD4+Foxp3neg conventional T cells (conT) recognize different determinants from the same antigen (red triangle) presented by promiscuous pMHC complexes that are displayed on antigen presenting cells (APC). The dashed line indicates the T-T contacts required for suppression (Supp.) of conT cell activation by stimulated Tregs. (B). In the 2 cell-model, Treg and conT cells interact independently of the TCR engagement of the conT cell.
D. Influence of Cell Trafficking on Treg-Mediated Tolerance to Transplants
Following transplantation of vascularized allografts donor dendritic cells, primary residents of normal tissues, 134 migrate to local lymph nodes where they participate in the presentation of donor antigens to both conventional CD4 and CD8 T cells (reviewed in 135). Like alloreactive lymphocytes, peripheral tTregs are recruited in lymph nodes following activation and expression of homing receptors. 136 However, efficient suppression of anti-donor immune responses in vivo is contingent on tTreg migration from the lymph nodes to inflammatory sites of the transplant as shown in tagging experiments performed in a skin transplantation model 137 or in vascularized allotransplants. 46,138 Similar migratory properties were seen for Tregs infiltrating infected tissues, 139 for those able to control GVHD 140 or autoimmunity. 141 Hence, when stimulated by pMHCII complexes exposed on APCs from draining lymph nodes – including those with donor peptides - host Tregs traffic to the inflamed sites to exert local suppression of effector T cells according to mechanisms evoked earlier. As it appears that Treg suppression in vivo is confined to local inflamed sites where they have been recruited, 138,142 there is no need to invoke systemic suppression to explain regulatory tolerance to self or allogeneic tissues.
As far as the transplantation field is concerned, considering Tregs as active “guardians for life” of tolerance, to quote a commentary from J. Hill and colleagues, 143 may be an overstatement as permanent suppression of allograft rejection by infiltrating Tregs awaits demonstration. Two sets of studies have substantiated the notion of constantly active Treg patrollers. In the first set, the ablation of peripheral Tregs via anti-CD25 mAb treatments and reinjection of non-Treg cells in thymectomized newborn nude mice, correlated with the onset of autoimmune syndromes, suggesting a crucial role for CD25+ Tregs in maintaining self-reactivity at bay. 42 However, the data should be interpreted with caution in view of the fact that immunodeficient animals often show weak immunity and altered lymphocyte homeostasis that could, on their own, account for the effects observed. In line with this concern, similar CD25 depletion protocols rarely cause autoimmunity in adult recipients. 144 More recent experiments from AY Rudensky’s group used the Foxp3DTR transgenic mouse in which treatments with diphtheria toxin kill Foxp3+ Tregs. Such studies examined the effects of profound in vivo depletion of Foxp3+ cells on newborn and adult mouse immunity to self antigens. 51 They convincingly established that Treg depletion done immediately after birth reproduced the phenotype observed in thymectomized as well as anti-CD25 treated animals. Furthermore, Foxp3+ cell ablation in adults induced autoimmune disorders associated with massive myeloid cell infiltrations in multiple organs. The authors suggested that an increase in self antigen presentation by infiltrating DCs was the likely cause of immune deregulation. Although, these results would agree with a continuous suppression of self-reactive T cells by Tregs, the generalization of this principle to the control of allograft rejection may be premature or even incorrect. Indeed, depletion of adult Tregs requires prolonged treatments with high doses of toxin (50 times more toxin than for other cell types 145,146), leading to potential tissue toxicity and inflammation unrelated to the obliteration of Tregs. In agreement with this, autoimmune disorders observed in “natural” Foxp3 deficient scurfy mice affected many fewer organs that in the Treg-depleted Foxp3DTR animals. 147
Convincing results supporting a permanent negative control of allograft rejection by host Tregs would have to show that tolerant recipients of fully healed transplants, which at that point should be considered as auto-transplants with minimal mononuclear cell infiltrates, will reject their grafts and not their own tissues upon Foxp3+ cell depletion.
IV. TREG CELL THERAPY: ARE WE READY YET?
A. Taking from Murine Transplantation Models
There have been numerous attempts at inducing transplantation tolerance via donor-specific iTregs that were expanded/selected ex-vivo and re-injected around the time of transplantation. Unfortunately, most of these approaches were unsuccessful on their own. They required adjunct therapies including irradiation and donor bone marrow, 148 donor specific transfusion and antibody treatments 123 or costimulatory blockade. 149 Beneficial effects of Treg infusions on allograft survival were observed in bone marrow transplantation models in which donor Tregs reduced the incidence of GVHD 150 and promoted tolerance to semi-allogeneic bone marrow cells in irradiated recipients. 121 Interestingly, tolerance was equally effective following the injections of donor-specific or non-specific Tregs. Such findings were interpreted as a “paradoxical behavior of Tregs” according to the donor-specific suppression principle, 122 but are indeed compatible with the rationale of this review which considers Treg and conT activations as two unrelated events.
The experiments from H Waldmann’s laboratory on infectious tolerance, this ability of Tregs from tolerant mice to transfer tolerance to heart allografts in naïve recipients by recruiting conT cells to become adjunct iTregs, 5,151 would be another example where iTreg may control allograft rejection. However, other studies in autoimmunity and infection models have failed to demonstrate systemic CD4+ Foxp3− → CD4+ Foxp3+ conversion in vivo. In spontaneous autoimmune encephalomyelitis (EAE), thymic Tregs conferred protection towards EAE but did not co-opt recipient CD4 cells to mitigate autoimmunity. 152 Similarly, little if any peripheral conversion was seen in the BDC2.5 model of diabetes in which comparison of TCRα usage between tTreg, iTreg and conT cells revealed no peripheral CD4 conversion in the context of inflammation and cognate antigen presentation. 130 Conversion of non-Treg into Treg cells also failed in animals infected with either Listeria monocytogenes 74 or Leishmania. 153 Thus, it seems that there is no clear evidence as yet in favor of a significant contribution of iTregs in the peripheral regulation of immune responses to self or allogeneic antigens. We should however take these conclusions with moderation as they came from laboratory mice that have limited exposure to pathogens and commensal flora. As suggested by Curotto de Lafaille and colleagues, it is possible that iTregs constitute a specialized subset of regulators responding to local micro-environmental cues present in the guts, skin or lungs with the prime mission of preventing chronic inflammation and associated immunity to pathogens and food allergens. 75 The question as to whether iTregs are significantly contributing to harnessing rejection of allografts remains open in part because of a lack of lineage-specific markers to assess the respective role of iTregs and tTregs.
In contrast to iTregs amplified ex-vivo, encouraging results were gathered in approaches promoting Treg activation/recruitment in vivo. Induction of transplantation tolerance was reported in graft recipients pre-conditioned with donor tolerogenic DCs generated ex vivo, 46,154–156 although the differentiation conditions and tolerogenic phenotype of the DCs were not always well defined. 134 Others studies have confirmed that Tregs activated in vivo did preferentially react to the indirect antigen presentation pathways, 148 strongly suggesting that they engage self pMHCII complexes. Experiments from our laboratory demonstrated that MHCII peptides presented on MHCII were crucial complexes for Treg stimulation in vivo and tolerance induction. We showed that pre-transplantation transgenesis with donor-MHCII genes, generated MHCII peptides in host APCs, and induced tolerance to fully allogeneic allografts without additional therapy. 133,157 Tolerance was achieved in two animal models, spread to immune responses against all antigenic disparities, was donor MHCII-specific and transferrable to immunocompetent hosts only via tolerant Tregs. This approach is presently the only model of a defined Treg TCR specificity that has led to full allogeneic tolerance without additional treatment. Other promising approaches using low doses of soluble IL-2 to extend Treg survival 158 or of IL-2 and anti-IL-2 mAb complexes to improve Treg activation 159 have shown that Treg function can be preferentially targeted in vivo in a non-antigen specific fashion.
B. Translational Treg therapy
Although murine and human Tregs share a large number of features such as developmental and functional characteristics, human Tregs have their peculiarities. 60 Contrary to mouse, human CD4+ CD25+ effector T cells transiently express Foxp3 upon activation. Tregs and activated CD4 effectors can, however, be identified with the CD127 marker. 160,161 To this day, human Tregs are less understood than their murine counterparts. Hence, gathering further information on their biology is crucial to devising effective Treg protocols for transplantation tolerance. Until recently, clinical trials involving human Tregs have been pre-clinical tests on the feasibility and safety of ex-vivo Treg expansions/infusions in patient recipients of hematopoietic stem cells (HSC). 162 Two recent trials were proven safe and did not demonstrate infusional toxicity. The first trial was based on infusions of non-expanded, donor Tregs in post-HSCT patients, whereas the second involved donor-independent expansion of Tregs isolated from umbilical cord blood. Preliminary data on the outcome of Treg therapy in the second trial suggest that the Treg effects on allogeneic GVHD are modest but significant. A reduced incidence of grade II-IV GVHD (from 61% to 43%; p = 0.05) was observed between the trial and control groups with no deleterious effect on risks of infection. 163 It is evidently too early to conclude on the efficacy of Treg therapy in these models. It would also be important to gather information in models of vascularized transplants.
The risks associated with the ex vivo iTreg expansion against potentially undesired allogeneic pMHC and the phenotypic instability of donor-specific iTregs, which can revert to alloreactive CD4 effectors, have to be weighed in regard to the anticipated benefits of iTreg infusions. Treg therapy has undoubtedly a bright future, which in large part is contingent on the elucidation of the “natural” TCR specificity of tTregs. Defining the natural pMHCII activators of Treg suppression will provide cues to avoid partial activation and/or phenotypic reversion that are presently the most troublesome risks associated with Treg cellular therapy.
CONCLUSIONS
Compelling experimental evidence indicate that professional thymic Tregs (tTregs) are not conventional CD4 T lymphocytes with additional suppressive capabilities. Such reagents -called iTregs - are man made and pale ersatz Tregs which do not acquire the full properties of professional Tregs. The differentiation pathway, TCR specificity and mode of action of iTregs and tTregs are fundamentally different. Because most of iTregs derive from mature conventional CD4 T cells bearing a TCR repertoire that poorly overlap with that of tTregs, the two types of regulators respond to different micro-environmental cues. Notably, the adaptive immune responses of CD4 lymphocytes (and iTregs) seem to have evolved towards the use of polymorphic receptors to discern the changing diversity of pathogenic antigens. Conversely, immune regulation by a dedicated subset of professional regulatory cells (tTregs) would operate upon recognition of non-immunogenic “innate signals” likely made from a limited set of self pMHCII complexes. These points have to be factored in when devising Treg-based protocols adapted to clinical transplantation. In a recent update on the clinical value of Treg therapy, L Turka and colleagues concluded that “attempts to translate Tregs towards clinical utility have met with unanticipated difficulties”. 6 We would disagree with this statement by reiterating that infusions of unstable, non-professional, donor-specific iTregs that include, at best, a small fraction of suppressors cross-reacting to allogeneic pMHCII, have a poor chance to succeed since these cells will not encounter optimal conditions for activation and ensuing suppression. The concepts and data discussed here emphasize the relevance of utilizing naturally occurring Tregs in clinical protocols; they are de facto more stable and better regulators of T cell responses than their precarious iTreg substitutes. Further investigations on the nature of natural activators of tTregs must be conducted with the objective to harness the real therapeutic potential of this cell subset in clinical transplantation.
ACKNOWLEGMENTS
The author is grateful to Ms. Sharon Germana and Dr. Christene Huang for their critical review of the manuscript. I am equally thankful to Drs. Gilles Benichou and Emmanuel Zorn for fruitful discussions on numerous concepts detailed in this review. This work was supported by NIH grants # AI063408 and AI064344 to C. LG.
Glossary
Abbreviations
- APC
Antigen-presenting cell
- ConT
conventional CD4 T lymphocytes
- MHC
major histocompatibility complex
- MHCI
MHC class I
- MHCII
MHC class II
- pMHC
MHC+ peptide complex
- TCR
T cell receptor for antigen
- Tregs
regulatory T cells
- Ts
T suppressor cells
REFERENCES
- 1.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nature reviews. 2003 Mar;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 2.Long E, Wood KJ. Regulatory T cells in transplantation: transferring mouse studies to the clinic. Transplantation. 2009 Nov 15;88(9):1050–1056. doi: 10.1097/TP.0b013e3181bb7913. [DOI] [PubMed] [Google Scholar]
- 3.Wieckiewicz J, Goto R, Wood KJ. T regulatory cells and the control of alloimmunity: from characterisation to clinical application. Curr Opin Immunol. 2010 Oct;22(5):662–668. doi: 10.1016/j.coi.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008 May 30;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann H, Chen TC, Graca L, Adams E, Daley S, Cobbold S, Fairchild PJ. Regulatory T cells in transplantation. Semin Immunol. 2006 Apr;18(2):111–119. doi: 10.1016/j.smim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010 Oct;6(10):577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 7.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 8.Matzinger P. Why positive selection? Immunol Rev. 1993;135:81–117. doi: 10.1111/j.1600-065x.1993.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 9.Berg LJ, Pullen AM, Fazekas de St.Groth B, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 10.Ashton-Rickardt P, van Kaer L, Schumacher TNM, Ploegh HL, Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+ T cells in the thymus. Cell. 1993;73:1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 11.Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 12.Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002 Jul;185(1):126–135. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 13.Zinkernagel R. On observing and analyzing disease versus signals. Nature immunology. 2007 Jan;8(1):8–10. doi: 10.1038/ni0107-8. [DOI] [PubMed] [Google Scholar]
- 14.Burnet F. The Clonal Selection Theory of Acquired Immunity. Nashville, TN: Vanderbilt University Press; 1958. [Google Scholar]
- 15.Miller JF. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 16.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 17.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 18.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 19.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 20.Berg LJ, Frank GD, Davis MM. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell. 1990 Mar 23;60(6):1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- 21.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994 Feb 25;76(4):651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 22.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996 Jun 13;381(6583):616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 23.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000 Dec;13(6):829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 24.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005 Jul 29;122(2):247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Blackman M, Yague J, Kubo R, Gay D, Coleclough C, Palmer E, Kappler J, Marrack P. The T cell repertoire may be biased in favor of MHC recognition. Cell. 1986 Nov 7;47(3):349–357. doi: 10.1016/0092-8674(86)90591-x. [DOI] [PubMed] [Google Scholar]
- 26.Merkenschlager M, Graf D, Lovatt M, Bommhardt U, Zamoyska R, Fisher AG. How many thymocytes audition for selection? The Journal of experimental medicine. 1997 Oct 6;186(7):1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrie HT, Livak F, Schatz DG, Strasser A, Crispe IN, Shortman K. Multiple rearrangements in T cell receptor alpha chain genes maximize the production of useful thymocytes. The Journal of experimental medicine. 1993 Aug 1;178(2):615–622. doi: 10.1084/jem.178.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Meerwijk JP, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. The Journal of experimental medicine. 1997 Feb 3;185(3):377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991 Aug 9;66(3):533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 30.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 31.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature immunology. 2007 Apr;8(4):351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 32.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. The Journal of experimental medicine. 2009 Jul 6;206(7):1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994 Nov 3;372(6501):100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 34.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001 Apr;14(4):417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 35.Sykes M. Mechanisms of tolerance induced via mixed chimerism. Front Biosci. 2007;12:2922–2934. doi: 10.2741/2282. [DOI] [PubMed] [Google Scholar]
- 36.Ramsdell F, Fowlkes BJ. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990 Jun 15;248(4961):1342–1348. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 37.Laufer TM, Dekoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on the thymus cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 38.Robey E, Fowlkles BJ. Selective events in T cell development. AnnRevImmunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 39.Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nature reviews. 2003 Feb;3(2):147–158. doi: 10.1038/nri1002. [DOI] [PubMed] [Google Scholar]
- 40.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003 Apr;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 41.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. 2003 Feb 14;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 43.Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nature immunology. 2004 May;5(5):469–471. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- 44.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010 Jan 29;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Thomson CW, Lee BP, Zhang L. Double-negative regulatory T cells: non-conventional regulators. Immunol Res. 2006;35(1–2):163–178. doi: 10.1385/IR:35:1:163. [DOI] [PubMed] [Google Scholar]
- 46.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nature immunology. 2006 Jun;7(6):652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 47.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nature reviews. 2007 Aug;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005 Feb 15;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 49.Brem-Exner BG, Sattler C, Hutchinson JA, Koehl GE, Kronenberg K, Farkas S, Inoue S, Blank C, Knechtle SJ, Schlitt HJ, Fandrich F, Geissler EK. Macrophages driven to a novel state of activation have anti-inflammatory properties in mice. J Immunol. 2008 Jan 1;180(1):335–349. doi: 10.4049/jimmunol.180.1.335. [DOI] [PubMed] [Google Scholar]
- 50.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002 Jan 15;168(2):689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 51.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007 Feb;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 52.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nature reviews. 2010 Dec;10(12):849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature immunology. 2005 Apr;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 54.Le Douarin N, Corbel C, Bandeira A, Thomas-Vaslin V, Modigliani Y, Coutinho A, Salaun J. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol Rev. 1996;149:35–53. doi: 10.1111/j.1600-065x.1996.tb00898.x. 35–53. [DOI] [PubMed] [Google Scholar]
- 55.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice II Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. The Journal of experimental medicine. 1982 Dec 1;156(6):1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003 Apr;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 57.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nature immunology. 2002 Jan;3(1):33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 58.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008 Oct 10;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 59.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature immunology. 2007 Jan 14; doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nature reviews. 2010 Jul;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 61.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews. 2008 Jul;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009 Oct;21(10):1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 63.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009 May;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Bloom BR, Salgame P, Diamond B. Revisiting and revising suppressor T cells. Immunol. Today. 1992 Apr;13(4):131–136. doi: 10.1016/0167-5699(92)90110-S. [DOI] [PubMed] [Google Scholar]
- 65.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003 Dec 15;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. The Journal of experimental medicine. 2004 May 17;199(10):1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature immunology. 2005 Dec;6(12):1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 68.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007 Mar;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006 Feb;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. The Journal of experimental medicine. 2007 Aug 6;204(8):1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta} and retinoic acid dependent mechanism. The Journal of experimental medicine. 2007 Aug 6;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007 Jul 13;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 73.Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol. 2010 Oct;22(5):575–582. doi: 10.1016/j.coi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005 Mar;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 75.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009 May;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 76.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009 May;30(5):616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007 Nov;27(5):786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 78.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nature immunology. 2005 Feb;6(2):152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 79.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nature immunology. 2009 Sep;10(9):1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009 Apr;39(4):948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 82.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, Cutler C, Ho VT, Alyea EP, Antin JH, Soiffer RJ, Ritz J. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. The Journal of clinical investigation. 2010 May 3;120(5):1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007 Feb;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007 Jun 1;178(11):6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 85.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. The Journal of experimental medicine. 2010 Jul 5;207(7):1347–1350. doi: 10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. The Journal of experimental medicine. 2007 Jul 9;204(7):1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Turbachova I, Hamann A, Olek S, Huehn J. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007 Sep;37(9):2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 89.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008 Jun;38(6):1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 90.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010 Feb 11;463(7282):808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nature reviews. 2008 Oct;8(10):788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van de Keere F, Tonegawa S. CD4 T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J ExpMed. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olivares-Villagomez D, Wand Y, Lafaille JJ. Regulatory CD4+ T Cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J ExpMed. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162(9):5317–5326. [PubMed] [Google Scholar]
- 95.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature immunology. 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 96.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. The Journal of experimental medicine. 2001 Aug 20;194(4):427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature immunology. 2002 Aug;3(8):756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 98.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nature immunology. 2009 Jun;10(6):610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007 Aug 6;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DiPaolo RJ, Shevach EM. CD4+ T-cell development in a mouse expressing a transgenic TCR derived from a Treg. Eur J Immunol. 2009 Jan;39(1):234–240. doi: 10.1002/eji.200838772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002 May 1;168(9):4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 103.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008 Oct 15;112(8):3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005 Aug 25;436(7054):1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 106.Nazarov-Stoica C, Surls J, Bona C, Casares S, Brumeanu TD. CD28 signaling in T regulatory precursors requires p56lck and rafts integrity to stabilize the Foxp3 message. J Immunol. 2009 Jan 1;182(1):102–110. doi: 10.4049/jimmunol.182.1.102. [DOI] [PubMed] [Google Scholar]
- 107.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000 Apr;12(4):431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 108.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature immunology. 2006 Apr;7(4):401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 109.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006 Aug;25(2):249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 110.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007 Jun 1;178(11):7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 111.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004 Aug;21(2):267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 112.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. The Journal of experimental medicine. 2004 Nov 15;200(10):1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Z, Benoist C, Mathis D. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc Natl Acad Sci USA. 2005 Oct 11;102(41):14735–14740. doi: 10.1073/pnas.0507014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kyewski B, Derbinski J, Gotter J, Klein L. Promiscuous gene expression and central T-cell tolerance: more than meets the eye. Trends Immunol. 2002 Jul;23(7):364–371. doi: 10.1016/s1471-4906(02)02248-2. [DOI] [PubMed] [Google Scholar]
- 115.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002 Nov 15;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 116.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005 Aug;23(2):227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 117.Murphy DB, Lo D, Rath S, Brinster RL, Flavell RA, Slanetz A, Janeway CA., Jr A novel MHC class II epitope expressed in thymic medulla but not cortex. Nature. 1989 Apr 27;338(6218):765–768. doi: 10.1038/338765a0. [DOI] [PubMed] [Google Scholar]
- 118.Rudensky AY, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353(6345):660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 119.Liu YJ. A unified theory of central tolerance in the thymus. Trends Immunol. 2006 May;27(5):215–221. doi: 10.1016/j.it.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 120.Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK, Robey EA. The impact of negative selection on thymocyte migration in the medulla. Nature immunology. 2009 Aug;10(8):823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joffre O, Gorsse N, Romagnoli P, Hudrisier D, van Meerwijk JP. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood. 2004 Jun 1;103(11):4216–4221. doi: 10.1182/blood-2004-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Graca L, Le Moine A, Lin CY, Fairchild PJ, Cobbold SP, Waldmann H. Donor-specific transplantation tolerance: The paradoxical behavior of CD4+CD25+ T cells. Proc Natl Acad Sci USA. 2004 Jun;24:101, 10122–10126. doi: 10.1073/pnas.0400084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002 Feb 1;168(3):1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998 Dec;10(12):1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 125.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. The Journal of experimental medicine. 1998 Jul 20;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008 Jul 22;105(29):10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shevach EM, Suri-Payer E, Amar AZ, Thornton AM. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 128.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007 Nov 22;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 129.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164(1):183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 130.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. The Journal of experimental medicine. 2007 Sep 3;204(9):2039–2045. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003 Feb 14;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 132.White-Scharf ME, Banerjee P, Sachs DH, LeGuern C. Applications of gene therapy in transplantation. Gene Ther Mol Biol. 1997;1:333–344. [Google Scholar]
- 133.LeGuern C. Regulation of T Lymphocyte Functions by MHC Class II Self-Presentation. Trends Immunol. 2003 Dec;24(12):633–638. doi: 10.1016/j.it.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 134.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007 Nov;37 Suppl 1:S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 135.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010 Mar;234(1):5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 136.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. The Journal of experimental medicine. 2005 Sep 19;202(6):771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y, Waldmann H, Hori S, Cyster JG, Watanabe T, Miyachi Y, Kanagawa O, Kabashima K. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. The Journal of clinical investigation. 2010 Mar 1;120(3):883–993. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. The Journal of experimental medicine. 2002 Jun 17;195(12):1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002 Dec 5;420(6915):502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 140.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005 Nov 1;106(9):3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. The Journal of experimental medicine. 2005 Nov 21;202(10):1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009 Feb;30(2):277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hill JA, Benoist C, Mathis D. Treg cells: guardians for life. Nature immunology. 2007 Feb;8(2):124–125. doi: 10.1038/ni0207-124. [DOI] [PubMed] [Google Scholar]
- 144.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002 Jun 15;168(12):5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 145.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002 Aug;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lahl K, Sparwasser T. In Vivo Depletion of FoxP3(+) Tregs Using the DEREG Mouse Model. Methods in molecular biology (Clifton, NJ) 2011;707:157–172. doi: 10.1007/978-1-61737-979-6_10. [DOI] [PubMed] [Google Scholar]
- 147.Chen GY, Chen C, Wang L, Chang X, Zheng P, Liu Y. Cutting edge: Broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol. 2008 Apr 15;180(8):5163–5166. doi: 10.4049/jimmunol.180.8.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008 Jan;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. The Journal of experimental medicine. 2001 Jun 4;193(11):1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002 May 15;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 151.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. Infectious transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 152.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci USA. 2002;99(12):8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. The Journal of experimental medicine. 2006 Mar 20;203(3):777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Connell PJ, Li W, Wang Z, Specht SM, Logar AJ, Thomson AW. Immature and mature CD8alpha+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002 Jan 1;168(1):143–154. doi: 10.4049/jimmunol.168.1.143. [DOI] [PubMed] [Google Scholar]
- 155.Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, Wang Z, Thomson AW. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010 Jan 15;184(2):624–636. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thomson AW, Turnquist HR, Zahorchak AF, Raimondi G. Tolerogenic dendritic cell-regulatory T-cell interaction and the promotion of transplant tolerance. Transplantation. 2009 May 15;87(9 Suppl):S86–S90. doi: 10.1097/TP.0b013e3181a2dcec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.LeGuern C, Akiyama Y, Tanaka K, Germana S, Iwamoto Y, Fernandez L, Houser S, Benichou G. Intracellular MHC class II controls regulatory tolerance to allogeneic transplants. J Immunol. 2010 Mar 1;184(5):2394–2400. doi: 10.4049/jimmunol.0803664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008 May;28(5):687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009 Mar 30;206(4):751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. The Journal of experimental medicine. 2006 Jul 10;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine. 2006 Jul 10;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009 May;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011 Jan 20;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]