Abstract
Purpose
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood and remains refractory to combined-modality therapy in patients with high risk disease. In skeletal myogenesis, Notch signaling prevents muscle differentiation and promotes proliferation of satellite cell progeny. Given its physiologic role in myogenesis and oncogenic role in other human cancers, we hypothesized that aberrant Notch signaling may contribute to RMS tumorigenesis and present novel therapeutic opportunities.
Experimental Design
Human RMS cell lines and tumors were evaluated by immunoblot, IHC, and RT-PCR to measure Notch ligand, receptor, and target gene expression. Manipulation of Notch signaling was accomplished using genetic and pharmacologic approaches. In vitro cell growth, proliferation, and differentiation were assessed using colorimetric MTT and BrdU assays, and biochemical/morphologic changes after incubation in differentiation-promoting media, respectively. In vivo tumorigenesis was assessed using xenograft formation in SCID/beige mice.
Results
Notch signaling is upregulated in human RMS cell lines and tumors compared to primary skeletal muscle, especially in the embryonal (eRMS) subtype. Inhibition of Notch signaling using Notch1 RNAi or γ-secretase inhibitors reduced eRMS cell proliferation in vitro. Hey1 RNAi phenocopied Notch1 loss and permitted modest myogenic differentiation, while over-expression of an activated Notch moiety, ICN1, promoted eRMS cell proliferation and rescued pharmacologic inhibition. Finally, Notch inhibition using RNAi or γ-secretase inhibitors blocked tumorigenesis in vivo.
Conclusions
Aberrant Notch-Hey1 signaling contributes to eRMS by impeding differentiation and promoting proliferation. The efficacy of Notch pathway inhibition in vivo supports the development of Notch-Hey1 axis inhibitors in the treatment of eRMS.
Keywords: Notch, Hey1, rhabdomyosarcoma, γ-secretase inhibitors, sarcoma
Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood with approximately 350 new cases diagnosed each year in the Unites States (1). There are two major histologic subtypes of RMS (embryonal, eRMS and alveolar, aRMS) with each subtype having distinct underlying genetic alterations that participate in pathogenesis. Although the precise etiology of RMS remains uncertain, it is commonly believed that tumor cells arise from muscle precursors that fail to differentiate appropriately into skeletal muscle. Current therapy for RMS includes surgery, chemotherapy, and radiation, but despite this aggressive combined-modality approach, patients with metastatic disease have an overall survival rate of less than 20% (2). Improved therapies for RMS may come from targeting signaling pathways that are aberrantly regulated and as a consequence contribute to tumor formation.
The Notch signaling pathway is highly conserved and plays a key role in a variety of important cell fate decisions, including proliferation, differentiation, and apoptosis. Notch signaling is involved in many developmental systems, including but not limited to neurogenesis, angiogenesis, hematopoiesis, and myogenesis (3). Notch signaling members include multiple transmembrane receptors and ligands that function through direct cell surface contact. Mammals possess four Notch receptors (Notch1, Notch2, Notch3, Notch4) and five Notch ligands (Jagged1, Jagged2, Dll1, Dll3, Dll4) (4). Signaling is initiated when neighboring cells come into direct contact, facilitating ligand-receptor interaction. Activation of Notch receptors by ligand binding is followed by proteolytic cleavage by ADAM10 and γ-secretase, which releases the cytoplasmic component of Notch (intracellular Notch, ICN). ICN then translocates to the nucleus where it cooperates with CSL and Mastermind to form a transcriptional activation complex that promotes expression of target genes involved in cell proliferation, differentiation, and apoptosis. Many targets of Notch signaling have been identified, with the most widely studied being members of the basic helix loop helix (bHLH) family of transcription factors including Hes (Hairy/enhancer of split) and Hey (Hairy/enhancer-of-split related with RYPW motif). Hes and Hey expression varies according to tissue type, and probably causes tissue-specific repression of target genes (5).
In skeletal myogenesis, activation of the Notch signaling pathway has been shown to inhibit the progression of muscle stem cells to committed progenitors and promote the proliferation of progenitors prior to terminal differentiation into myotubes (6). These muscle progenitor cells, called satellite cells, retain the ability to proliferate and differentiate in response to stimuli such as tissue damage and growth signals. By inhibiting muscle differentiation and promoting the proliferation of satellite cell progeny, the Notch signaling pathway functions to preserve and expand this subpopulation of muscle stem cells (7).
Aberrant Notch signaling has been implicated in a variety of pediatric cancers including T-cell leukemia and lymphoma, osteosarcoma, glioma, and medulloblastomas (8), with evidence of overexpression of multiple Notch signaling pathway ligands, receptors, and target genes in a number of malignancies (9). In contrast, the Notch signaling pathway functions as a tumor suppressor in some type of malignancies including B cell leukemia (3). The role of Notch signaling in tumorigenesis likely varies in different cell types and depends in part on tissue contexts (3, 10).
The oncogenic role of Notch in tumorigenesis can be targeted by inhibiting the proteolytic cleavage of the Notch receptors using small molecule inhibition. γ-Secretase is a large protease complex that releases the intracellular domain of the Notch receptor following ligand-receptor activation. This step in Notch signaling is pivotal in the activation of this signaling cascade, and over the past decade, small molecule inhibitors of γ-secretase have been developed and studied for their potential to inhibit Notch signaling. A phase I/II study evaluating the efficacy of Notch inhibitors in children with relapsed or refractory T-cell leukemia, lymphoma, CNS or solid tumors, recently opened (clinicaltrials.gov, NCI NCT01088763).
Given the critical role of Notch signaling in skeletal myogenesis and the oncogenic role of Notch signaling in other cancers, we hypothesized that aberrant regulation of the Notch pathway may present novel therapeutic targets in the treatment of RMS. Based on prior observations that the Notch target Hes1 was active in aRMS (11), and that the Notch pathway was important for RMS cell invasion in vitro (12), we turned our attention to investigating the role of Notch signaling in eRMS tumorigenesis, with focus on the Notch target Hey1, and the possibility that Notch pathway inhibition might have efficacy in blocking eRMS tumorigenesis in vivo.
Materials and Methods
Generation of Cell Lines and Constructs
Early passage normal human skeletal muscle myoblasts (HSMM cells, Lonza) were grown in defined media (SkGM-2 Bullet Kit). Human RMS cell lines (RD, SMS-CTR, Rh28, and Rh30) were a gift from Dr. Tim Triche of The Children’s Hospital of Los Angeles and were grown in RPMI-1640 (GIBCO) with 10% FBS. Cell line authentication was confirmed using STR analysis (Promega PowerPlex 1.2) with the assistance of the Fragment Analysis Facility at Johns Hopkins Genetic Resources Core Facility (Supplemental Table I). Using nine STR markers, we found 100% identity of our RD cell line compared to a published STR profile (13) and the ATCC database and 88.9% identity of our Rh30 cell line compared to published STR profiles (13). There are no published STR profiles for the SMS-CTR and Rh28 cell lines, however, these cell lines had unique profiles when compared to the ATCC comprehensive database. The Notch1 shRNA sequence was a gift from Dr. Chris Counter (Duke University), and annealed shRNA oligos (Supplemental Table II) were ligated into the pSUPER-retro-GFP/neo plasmids. RD and SMS-CTR cells were stably infected with Notch1 shRNA or empty vector and selected with 400 µg/ml neomycin (Gibco). Hey1 shRNA sequences (Open Biosystems, Supplemental Table II) were ligated into the pLKO.1/puro (Addgene 8453) plasmids. RD and SMS-CTR cells were stably infected with Hey1 shRNA or empty vector and selected with 1µg/ml puromycin (Sigma). ICN1 overexpression virus was a gift from Jordan Blum (Duke University). RD and SMS-CTR cells were stably infected with ICN1 or empty vector and confirmed by YFP expression.
Drug Treatments
For in vitro work, GSI-X (Calbiochem) was diluted in DMSO and added to culture media at desired concentrations. Cells were treated with GSI-X or equal volumes of DMSO for vehicle control. For in vivo work, GSI-XII (Calbiochem) was suspended in DMSO at 5mg/ml concentration. Mice were treated with GSI-XII versus DMSO control via intraperitoneal injection at a dose of 5mg/kg daily for 21 days.
Immunoblotting
Cells were lysed in Tris/RIPA buffer with standard protease inhibitors and passaged through a 21g needle to shear DNA. Protein concentration was measured by the DC assay (Bio-Rad). 60–100µg of lysate was resolved by SDS-PAGE, transferred to PVDF membrane and immunoblotted with primary monoclonal antibodies anti-NOTCH1 (Santa Cruz), anti-HEY1 (Abcam), or actin SC-8462 (Santa Cruz). Membranes were reacted with a secondary HRP-labeled goat anti-donkey (Santa Cruz), goat anti-rabbit antibody (Invitrogen-Zymed), or goat anti-mouse antibody (Invitrogen-Zymed) and developed using chemiluminescence (Amersham).
MTT assays
The MTT assay is a surrogate measure for cell number based on quantitation of the conversion of yellow methylthiazolyldiphenyl-tetrazolium bromide to purple formazan by the mitochondrial enzyme succinate dehydrogenase. In this work, the MTT assay was used to measure cell growth after genetic or pharmacologic interventions (14). For genetic interventions, cells were stably infected, selected based on antibiotic resistance or YFP positivity, the then plated in 96-well flat-bottomed plates at specific cell densities (5000 cells/well for RD cells and 7500 cells/well for SMS-CTR cells) and cultured as described above. For pharmacologic interventions, cells were plated at specific cell densities. On day 0, the media was replaced with fresh media supplemented with drug versus vehicle control. Cells were treated for a total of 48 hours with fresh drug replaced every 24 hours. At indicated time points, the culture media was supplemented with 1mg/mL MTT for three hours at 37°C, media removed, cells solubilized with DMSO, and absorbance measured at 540 nm.
BrdU Incorporation
Cells were grown at the described densities in 96-well plates for 24 hours, then proliferation measured using the Cell Proliferation ELISA BrdU kit (Roche). Cells were labeled with BrdU for three hours at 37°C and assayed according to the manufacturer’s protocol. Absorbance was measured at 370 nm.
Differentiation Assays
To assess their ability to acquire biochemical and morphologic markers of skeletal muscle differentiation, eRMS cells were cultured in DMEM-F12 supplemented with 2% horse serum (“fusion” medium) for 72 hours. Control cells were cultured in standard RPMI-1640 with 10% FBS (“growth” medium). Exposure to fusion medium, so-called because of its ability to induce individual myoblasts to fuse membranes with neighboring cells and generate elongated multinucleate myotubes indicative of terminal differentiation, is an established method used previously by our laboratory (15) to assess the ability of cells in culture to differentiate down the myogenic lineage. Biochemical and morphologic evidence of differentiation was assessed using myogenin expression by PCR, and imaging by phase contrast microscopy using a Nikon DS-Fi1 camera, respectively.
RT-PCR
Total cellular RNA was isolated using the RNA-Bee kit (TEL-TEST). Following spectrophotometric quantitation, 2µg was subject to reverse transcription using the Omniscript RT kit (QIAGEN) with Oligo-dT primers (Life Technologies Invitrogen). Standard PCR using primer sets for NOTCH1-4, DLL1, 3, and 4, JAGGED1, JAGGED2, HES1, HEY1, Myogenin, and GAPDH (Supplemental Table III) (16) was performed, with product separated on 2% agarose. GAPDH and water controls were included to verify equal RNA and specificity of cDNA input, respectively.
Tumor xenografts
Under institutional IACUC-approved protocols, and as performed (17), 10 million cells/cell line were resuspended in Matrigel (BD Biosciences) and injected subcutaneously into the flanks of SCID/beige mice in quadruplicate for genetic knockdown studies or in replicates of six for γ-secretase inhibitor studies. In drug studies, mice were treated with GSI-XII (or DMSO vehicle) via intraperitoneal injection for 21 days as previously reported, with drug suspended in DMSO to increase solubility (9). Mice were monitored biweekly, and tumor volume was estimated by external caliper measurements and calculated as ((width)2×length)/2. Mice were sacrificed at time points described in the text, at maximal tumor burden, or if demonstrating a decline in health. Following sacrifice, tumors were dissected, weighed, and portions of the tumors were snap-frozen in liquid N2 for RNA and protein evaluation or fixed in formalin for histological evaluation.
Immunohistochemistry
Human RMS tissue microarrays were provided by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute; other investigators may have received specimens from the same subjects. Staining for Notch1 (Santa Cruz) was performed with the assistance of the University of Florida Department of Pathology, Immunology, and Lab Medicine Pathology Core (Gainesville, FL). The tissue array was examined and scored by two independent observers who were blinded to the annotated histological subtype. For each tissue core, positivity for Notch1 was determined on the basis of discrete nuclear staining and a semi-quantitative score of the percentage of positive cells (0= no nuclear staining, 1= <25% staining, 2= 25–50% staining, 3= >50% staining). Paraffin-embedded tumor xenografts were sectioned and stained with H&E to assess tumor morphology, Ki67 (Thermo Scientific) to assess for proliferation, and Hey1 (Abcam) to assess inhibition of Notch signaling. A pathologist with experience in the evaluation of pediatric sarcomas (R.C.B.) evaluated slides.
Statistical analysis
Data are presented as means and standard errors of the mean. Comparisons between groups were performed using t-tests and two-way ANOVA. Differences were considered statistically significant at the p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) levels.
Results
The Notch-Hey1 axis pathway is upregulated in human eRMS cell lines and tumor samples
To determine if the Notch signaling pathway is upregulated in human RMS, we first analyzed four human RMS cell lines and examined a previously published cohort of human RMS tumors for expression of Notch pathway receptors, ligands, and target genes. RT-PCR analysis of RNA extracted from two human eRMS (RD and SMS-CTR) and two human aRMS cell lines (Rh28 and Rh30) compared to non-transformed primary human skeletal muscle myoblasts (HSMMs) as well as in silico analysis of microarray expression data of primary human RMS tumors from a publicly available database (18) demonstrated upregulation of multiple Notch signaling members (Supplemental Figure 1). While the upregulation of many pathway members suggested that Notch signaling is likely important for both RMS subtypes, most intriguing was that the Notch target HEY1, a transcriptional repressor downstream of total cellular Notch signaling and a critical inhibitor of myogenesis, was upregulated in eRMS compared to aRMS cells. Thus in this work, we focus on the eRMS RMS subypte, with emphasis on the Notch-Hey1 axis. First, we verified by semi-quantitative RT-PCR and immunoblot that Hey1 mRNA and protein are upregulated in human eRMS cell lines compared to non-transformed HSMMs and aRMS cell lines (Fig.1A). Second, in silico analysis of microarray expression data of primary human RMS tumors from a publicly available database (18) showed that relative Hey1 expression is low in fusion-positive aRMS, but high in eRMS (Fig.1B). Finally, an RMS tissue microarray containing human eRMS, aRMS, and normal skeletal muscle cores was probed for activated Notch1 protein expression, using standard immunohistochemistry, as a surrogate for Hey1 protein expression. As expected in tissues of skeletal muscle origin (19), there was diffuse membrane and cytoplasmic staining of Notch1 in all samples. However, nuclear Notch1 staining, which represents the transcriptionally active form of Notch1, while absent in skeletal muscle and moderately expressed in aRMS, was highly expressed in eRMS (Fig.1C). Taken together, these data suggest that the Notch pathway is preferentially upregulated in human eRMS cell lines and tumor tissue, and that the Notch effector Hey1 may be playing a vital role in eRMS tumorigenesis.
Figure 1. Notch signaling is upregulated in eRMS cell lines and human tumors.
A, Semi-quantitative PCR (top) and immunoblot (bottom) analysis of the Notch target gene Hey1 in human skeletal muscle myoblasts (HSMMs) in two eRMS cell lines and two aRMS cell lines, compared to GAPDH and Actin controls, respectively. B, Relative Hey1 mRNA expression in human RMS samples as mined in silico from (18). C, Immunohistochemical analysis of nuclear Notch1 staining of a human RMS tumor tissue microarray which included 14 human skeletal muscle controls, 42 eRMS tumors, and 93 aRMS tumors. Also shown are representative sections of Notch1 staining from each of the skeletal muscle, eRMS, and aRMS subgroups, magnification 40x. See Methods for explanation of statistical analyses.
Genetic suppression of Notch signaling blocks eRMS cell growth in vitro
Given the critical role for Notch signaling in skeletal myogenesis, and the prevalence of the Notch target Hey1 in eRMS cell lines and tissue, we next investigated whether genetic suppression of this pathway would block eRMS tumorigenesis. To this end, we stably expressed an shRNA to Notch1 in RD and SMS-CTR eRMS cells. Using immunoblot and semi-quantitative RT-PCR, we confirmed knockdown of Notch1 and also concomitant knockdown of Hey1 (Fig.2A). Then, using a standard MTT assay, we showed that compared to empty vector controls, suppression of Notch1 in both cell lines inhibited cell growth over time (Fig.2B). Since Hey1 is downstream of Notch1, we predicted that its knockdown would phenocopy Notch1 loss. To this end, we generated an shRNA to Hey1 and demonstrated knockdown of Hey1 at both the protein and mRNA levels in RD and SMS-CTR eRMS cells (Fig.2C). Following this, we measured cell growth over time and noted an inhibition of eRMS cell growth in cells expressing the Hey1 shRNA (Fig.2D and Supplemental Fig.2).
Figure 2. Genetic suppression of Notch signaling decreases eRMS cell growth in vitro.
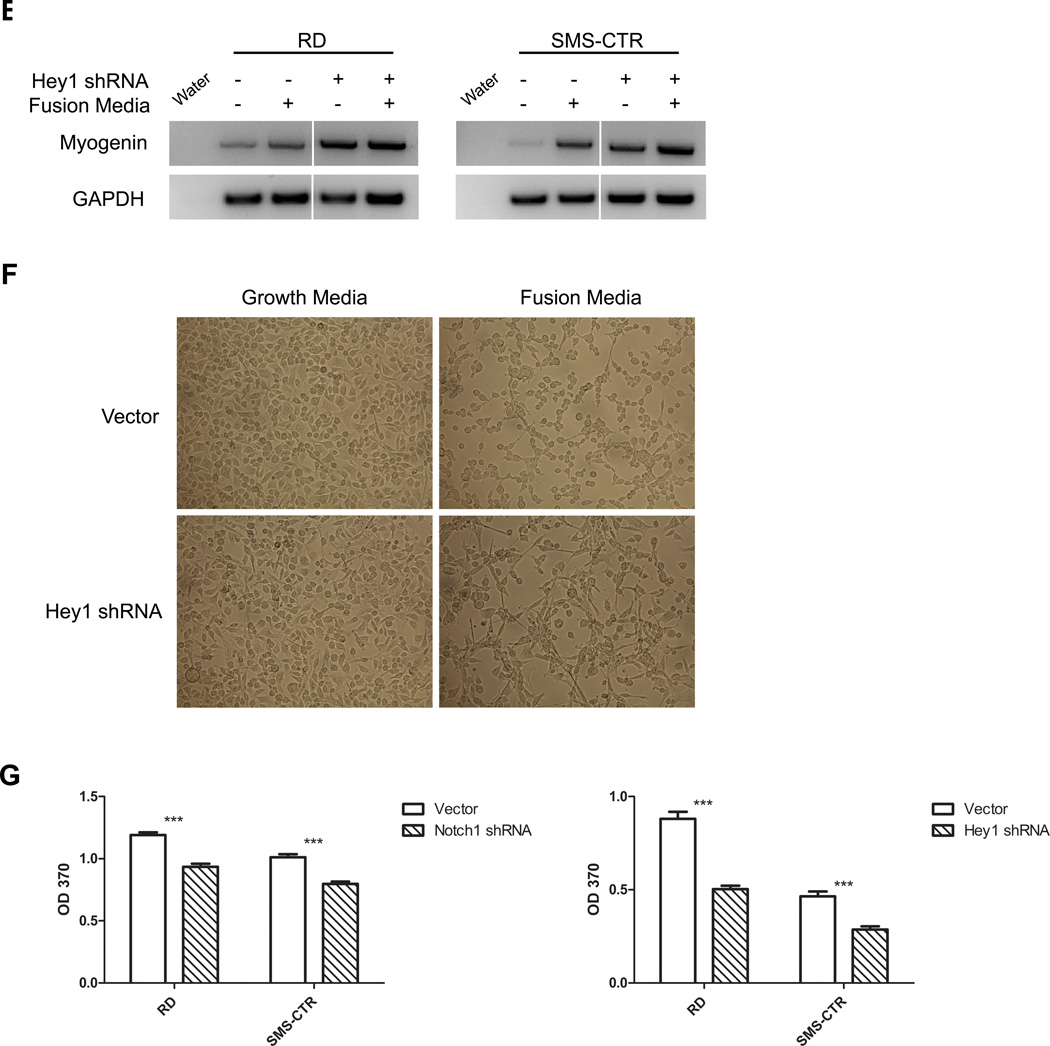
A, Immunoblot (left) and semi-quantitative PCR (right) of Notch1 and Hey1, and B, MTT growth assay of RD and SMS-CTR eRMS cells stably expressing either vector or Notch1 shRNA. C, Immunoblot (left) and semi-quantitative PCR (right) of Hey1 and D, MTT growth assay of RD and SMS-CTR cells stably expressing either vector or Hey1 shRNA. Actin and GAPDH used as loading controls. E, Myogenin expression by RT-PCR in RD and SMS-CTR cells stably expressing Hey1 shRNA. F, Morphology under light microscopy of RD cells stably expressing Hey1 shRNA and cultured in growth-promoting versus differentiation-promoting media. 10x magnification. G, Proliferation analysis of RD and SMS-CTR cells stably expressing vector or Notch1 shRNA as measured by BrdU incorporation.
To gain insight into the mechanism of eRMS cell growth inhibition in response to Notch pathway suppression, we investigated two cellular processes that are predicted to be supported by Notch signaling and also contribute to tumorigenesis: prevention of differentiation and promotion of proliferation. For the former, we cultured eRMS cells under conditions (“fusion” media; see Materials & Methods) that would stimulate differentiation down the myogenic lineage, then assessed for biochemical and morphologic evidence of differentiation. Given the Notch pathway’s role in impeding skeletal muscle differentiation, we predicted that Hey1 knockdown would at minimum induce expression of pro-myogenic skeletal muscle transcription factors such as myogenin, and at most induce multinucleate myotube formation indicative of terminal differentiation. We found that compared to cells with an empty vector, eRMS cells with an shRNA to Hey1 indeed showed increased myogenin, which was enhanced in fusion media (Fig.2E). Hey1 knockdown also caused a change in cellular morphology from round to spindle-shaped, with intercellular protrusions reminiscent of myotubes, also most obvious in fusion media (Fig.2F). However, Hey1 knockdown did not result in a homogenous population of multinucleate myotubes, indicating that under these conditions, Hey1 loss was not sufficient to induce terminal differentiation.
To investigate the effect of Notch pathway inhibition on proliferation, we evaluated the impact of Notch1 and Hey1 knockdown on RD and SMS-CTR BrdU uptake in vitro. While cells expressing empty vector showed no change, those with an shRNA to Notch1 or Hey1 showed decreased BrdU incorporation (Fig.2G), indicating an inhibition of proliferation. There was no evidence of increased apoptosis in these experiments as measured by caspase cleavage in vitro and TUNEL staining in vivo (data not shown). In summary, genetic suppression of Notch signaling inhibited the growth of human eRMS cell lines, with some contribution from stimulation of differentiation, but more profoundly due to inhibition of cell proliferation.
Pharmacologic inhibition of Notch signaling blocks eRMS cell growth in vitro
To complement the genetic inhibition studies above, and reflecting the reality of how patients with RMS would be treated, we next evaluated pharmacologic blockade of Notch signaling in the same eRMS cell lines using GSI-X, a commercially available γ-secretase inhibitor (20). To characterize the effect of GSI-X on Notch activity in eRMS cells in vitro, we first determined the concentration of GSI-X required to functionally inhibit Notch signaling. Using expression of ICN1 and Hey1 as readouts for Notch pathway inhibition, we found that 10µM GSI-X unequivocally inhibited the Notch pathway (Fig.3A). We also observed that the same dose exerted a phenotypic effect on eRMS cell lines in vitro (Fig.3B). Next, GSI-X treatment was compared to DMSO vehicle and found to inhibit the growth of both eRMS cell lines in vitro (Fig.3C). We found that eRMS cells were more sensitive to gamma-secretase inhibition than aRMS cells, with significantly lower IC50 values (Fig.3D), which may reflect the greater expression of the Notch pathway in eRMS. Finally, to complement our genetic data showing that suppression of Notch1 largely blocked eRMS cell growth by inhibition of proliferation, we evaluated the ability of GSI-X to inhibit BrdU incorporation. In both RD and SMS-CTR cell lines, BrdU incorporation was significantly decreased in the presence of GSI-X (Fig.3E).
Figure 3. Pharmacologic inhibition of Notch signaling blocks eRMS cell growth in vitro.
A, Immunoblot of ICN1 and Hey1 in RD cells treated with increasing doses of GSI-X. B, Dose responsive inhibition of RD and SMS-CTR cell growth treated with increasing GSI-X concentrations, as measured by MTT assay. C, Cell growth analysis as measured by MTT assay in RD and SMS-CTR cell lines treated with GSI-X versus DMSO vehicle. D, Sensitivity to GSI-X inhibition in two eRMS cell lines and two aRMS cell as measured by IC50. E, Proliferation analysis of RD and SMS-CTR cells treated with GSI-X versus DMSO vehicle as measured by BrdU incorporation.
Since similar to other drugs GSI-X could have off-target effects, even at low micromolar dosing, we sought to prove that GSI-X-mediated inhibition of cell growth was specific and could be rescued by amplified Notch signaling. To this end, we stably expressed a constitutively active ICN1 (21) in RD and SMS-CTR eRMS cells, confirmed by immunoblot (Fig.4A.) Functionality of this constitutively active ICN1 was demonstrated both by its ability to upregulate Hey1 (Fig.4A) and to stimulate cell growth in vitro (Fig.4B). Cells expressing ICN1 were rescued from the increasing doses of GSI-X compared to cells expressing an empty vector, since the IC50 of cells expressing ICN1 increased (Fig.4C and Fig.4D). Finally, cells expressing ICN1 had increased BrdU incorporation compared to cells expressing an empty vector (Fig.4E), again implicating proliferation as the dominant effect of Notch signaling in eRMS. In summary, pharmacologic blockade of Notch signaling using the γ-secretase inhibitor GSI-X inhibited RMS cell growth in a manner parallel to genetic suppression.
Figure 4. Constitutively active ICN1 increases cell growth and confers resistance to GSI in eRMS cells.
A, Immunoblot analysis of ICN1 and Hey1 in RD and SMS-CTR cells infected with either empty control or ICN1 overexpression plasmid. B, Cell growth analysis as measured by MTT assay in RD and SMS-CTR cells stably expressing either vector or ICN1. C, Dose response curves of RD and SMS-CTR cells stably expressing either empty vector or ICN1 treated with increasing doses of GSI-X. D, Sensitivity to GSI-X in RD and SMS-CTR cells infected with empty vector versus ICN1 as measured by IC50s. E, In vitro proliferation analysis as measured by BrdU assay in RD and SMS-CTR cells infected with empty vector versus ICN1.
Both genetic and pharmacologic inhibition of Notch signaling blocks eRMS tumorigenesis in vivo
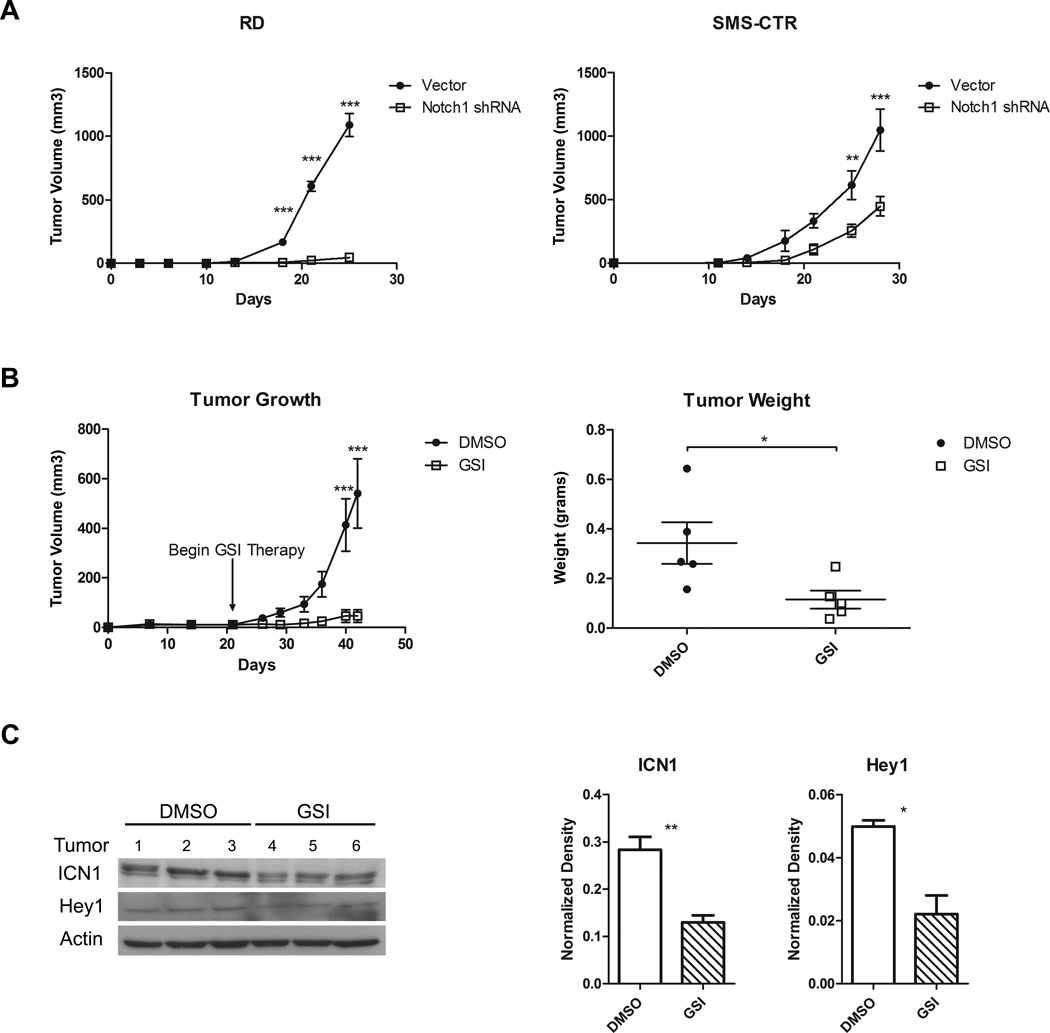
Although the in vitro data above suggested that eRMS cell growth could be blocked by Notch inhibition, whether through genetic silencing of single Notch family members such as Notch1 or Hey1, or through Notch receptor pharmacologic blockade, the gold standard for assessing the importance of a target in tumorigenesis is through in vivo assays. To this end, the eRMS cell lines expressing Notch1 shRNA versus empty vectors were evaluated as subcutaneous xenografts in SCID/beige mice (Fig.5A). Suppression of Notch1 in both RD and SMS-CTR cells blocked tumor growth compared to empty vector, with a more dramatic effect in the RD cells (Fig.5A, left panel).
Figure 5. Inhibition of Notch signaling decreases RMS tumorigenesis in vivo.
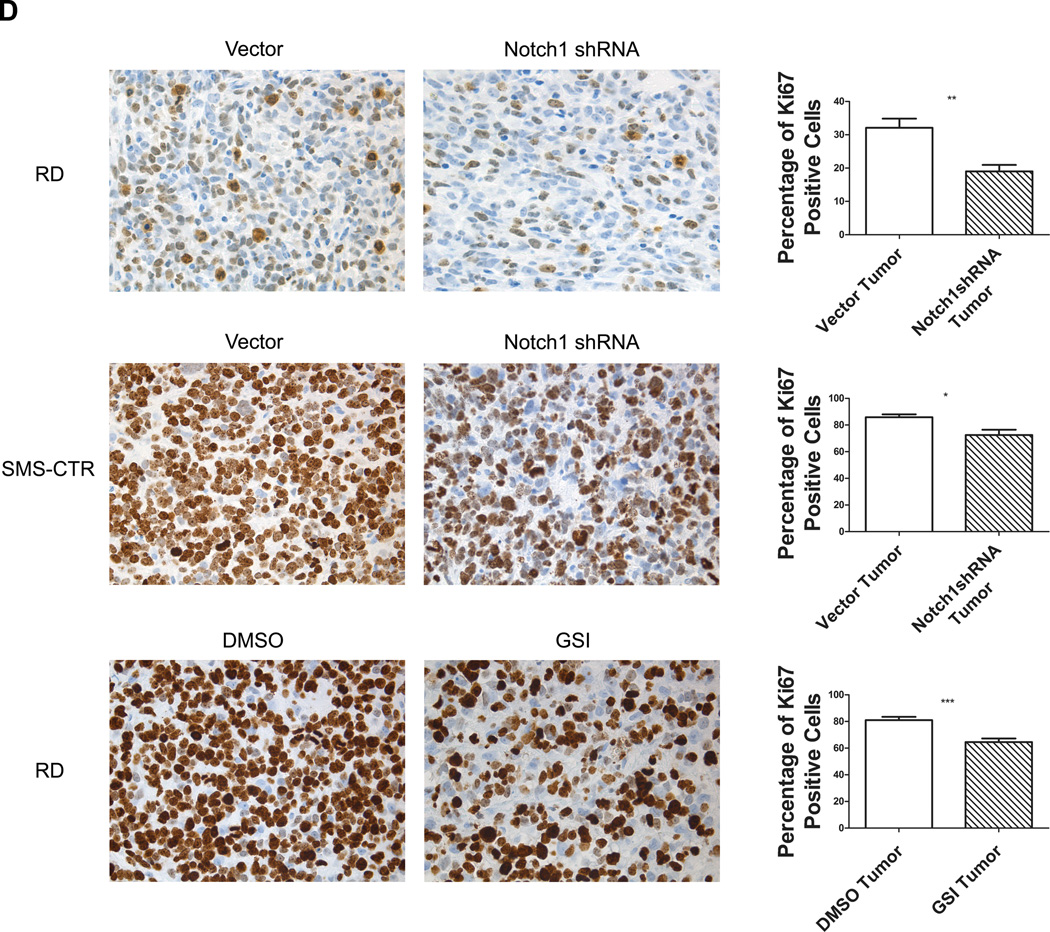
A, Tumor growth of RD and SMS-CTR eRMS cells stably expressing either vector or Notch1 shRNA assessed in vivo as subcutaneous xenografts in SCID/beige mice. B, Tumor growth over time (left panel) and harvested tumor weights (right panel) of RD cells injected as subcutaneous xenografts in SCID/beige mice and treated with either DMSO vehicle control or GSI-X. C, Immunoblot analysis of ICN1 and Hey1 in protein lysates from xenograft tumors treated with either DMSO vehicle control or GSI-X. These blots were quantitated using densitometry. D, In vivo cell proliferation as measured by Ki67 IHC staining in RD and SMS-CTR tumors with inhibition of the Notch signaling pathway using Notch1 shRNA or GSI-X.
To assess the impact of Notch pharmacologic inhibition by GSI-XII, RD cells were injected as xenografts into SCID/beige mice, as above. When palpable tumors developed, the mice were randomly divided into two groups and treated with either GSI-XII or DMSO vehicle control daily for 21 days. As predicted by the genetic suppression, mice treated with GSI-XII exhibited decreased tumor growth by volume and tumor weight (Fig.5B). To confirm that Notch signaling was being inhibited in this xenograft model, tumor lysates were probed for ICN1 and Hey1 protein expression (Fig. 5C). ICN1 downregulation in the GSI treated samples was evident by immunoblot, and quantitated as a greater than 50% decrease compared to DMSO control. Changes in Hey1 were not as demonstrable due to faint bands and limited tumor tissue, although densitometry suggested downregulation in the actual tumors. Finally, given the results of our in vitro work demonstrating that inhibition of eRMS cell growth was due in part to reduced cell proliferation, we examined sections of tumor tissue from the xenografts resulting from either the genetic suppression or pharmacologic inhibition experiments. In both methods of Notch signaling inhibition, the percentage of Ki67 positive cells was significantly decreased (Fig.5D), further implicating proliferation as a mechanism of Notch-mediated tumorigenesis in eRMS.
In summary, inhibition of Notch signaling, whether through genetic knockdown of one Notch receptor, or pharmacologic inhibition with a γ-secretase inhibitor, blocked eRMS tumorigenesis in vivo, suggesting that the Notch pathway may be a useful therapeutic target in patients with eRMS.
Discussion
In this work, we demonstrate using in vitro and in vivo, genetic and pharmacologic approaches, that the Notch signaling pathway is upregulated in the embryonal variant of RMS (eRMS), and that inhibition of the Notch pathway blocks eRMS tumorigenesis. Emphasis is placed on the role of the Notch target Hey1, known to be important in skeletal myogenesis. Previously published work demonstrated a role for Notch signaling in aRMS tumorigenesis, as the Notch target Hes1 was found to allow evasion of differentiation and promote proliferation in the RhJT aRMS cell line (11, 22), and Notch inhibition reduced both eRMS and aRMS invasiveness in vitro (12). Thus our study focuses on the role of Notch signaling in eRMS tumorigenesis, establishes a role for Hey1 in eRMS, and demonstrates the efficacy of Notch inhibition in an in vivo pre-clinical model.
An oncogenic role for Notch was first identified in human T-cell ALL during the 1980s. Since then, aberrant Notch signaling has been implicated in a variety of pediatric and adult malignancies including breast, pancreatic, bone, and brain (3, 8). Whether Hes or Hey predominates as the ultimate Notch effector depends upon cellular context, as for example Hes1 is important in osteosarcoma (23, 24), while Hey1 is key in glioblastoma (5, 25). The oncogenic role of Notch signaling appears related to its role in normal tissue maintenance, where it functions in many cell types to prevent terminal differentiation and maintain populations of undifferentiated progenitor cells including pancreatic, breast, and neural stem cells (26–28). In select types of cancer, these same properties of the Notch signaling pathway function to support a population of cancer stem cells, as described in both brain (29) and pancreatic cancer (26).
In the case of skeletal muscle, the Notch-Hey1 axis is critical for the proliferation and preservation of muscle stem cells, termed satellite cells. This appears to occur through both prevention of differentiation and promotion of proliferation of satellite cells and their progeny. For example, constitutive expression of Hey1 prevents myoblast differentiation by repressing pro-myogenic transcription factors such as Mef2C and myogenin (30, 31), while inhibiting Notch signaling recruits satellite cells to undergo differentiation and induces myotube hypertrophy, thus reducing satellite cell number (7). Notch-Hey1 signaling also promotes proliferation of satellite cells, allowing expansion and maintenance of a reserve population (6, 32). Although not addressed in the current study, it is possible that dysregulated Notch signaling is similarly preventing differentiation and promoting the proliferation of RMS tumor-propagating cells.
Our findings suggest that Notch signaling supports RMS tumorigenesis predominantly through promotion of proliferation. We found that inhibition of Notch signaling via genetic and pharmacologic techniques decreased eRMS proliferation in vitro and in vivo, while overexpression of the Notch pathway using a constitutively active Notch1 receptor resulted in increased eRMS proliferation in vitro. Most importantly, pharmacologic inhibition of the Notch pathway using a γ-secretase inhibitor decreased proliferation and tumor growth in vivo. A lesser, but still apparent role for Notch signaling in eRMS tumorigenesis is through the prevention of myogenic differentiation. When Hey1 expression was knocked down using shRNA, myogenin expression increased, reflecting more commitment to the myogenic lineage. This is consistent with the known role of Hey1 in binding the myogenin promoter and blocking its transcription (31), and also consistent with previous published data showing that both dnHes1 and GSI treatment increase myosin heavy chain mRNA, another marker of commitment to the myogenic lineage, in the presence of fusion medium (11). Since we did not see overt evidence of terminal differentiation in our in vivo xenograft studies, it appears that Notch pathway inhibition is insufficient to induce terminal differentiation in eRMS, a finding consistent with our in vitro Hey1 knockdown data, previous analyses of Notch inhibition in skeletal muscle (30, 33), and the known difficulty in driving RMS cells in vitro to terminal differentiation (15, 17). However, even in the absence of terminal differentiation, this modest effect may have therapeutic consequences, particularly if Notch inhibition in eRMS promotes a transition from self-renewal to differentiation fates.
An interesting question raised by our study is the reason for the redundancy of Notch pathway members in RMS. A likely explanation is the importance of this signaling pathway in skeletal myogenesis and the necessity for multiple receptors and ligands to allow for amplification of signal and autocrine/paracrine signaling. However, it is also possible that each ligand/receptor has independent, disparate effects in addition to the canonical Notch signaling pathway, and the pattern of upregulation may contribute in other ways to the oncogenic effect in RMS. Finally, although not part of the current investigation, there may be upstream influences promoting such strong Notch upregulation in eRMS. Possible drivers of Notch signaling may include oncogenic Ras, which has been reported to activate Notch signaling (34), and mutations of which have been described in eRMS tumors and cell lines (35).
Another interesting finding of this study is the differential expression and activation of the Notch pathway in eRMS versus aRMS. Previous work by Roma et al demonstrated the broad upregulation of Notch signaling in RMS tumors, and while they did not report a dramatic upregulation in eRMS subtype, their data suggest a trend to increased Notch target gene expression in eRMS versus aRMS (12). In the current work, we specifically show that the eRMS subtype is more sensitive to inhibition by γ-secretase inhibitors than aRMS. The reason for this difference in Notch expression and sensitivity between the two subtypes is unclear, however at least two possibilities exist. First, it is possible that upregulation of the Notch signaling pathway is driven by an eRMS-specific mechanism. As mentioned above, several studies have shown that dysregulation or mutational activation of Ras signaling may contribute to eRMS pathogenesis (35–37), and the Notch signaling pathway is a putative downstream effector of oncogenic Ras (34). Alternatively, dissimilar cells of origin of eRMS and aRMS may be responsible for the difference in Notch signaling. It has previously been suggested that eRMS might arise from satellite cells (36, 38, 39), and these cells have upregulated Notch signaling when compared to other muscle precursor cells (32). Therefore, eRMS tumors may arise from a cell of origin which already has upregulated Notch signaling.
Despite the clear impact of Notch pathway inhibition in these pre-clinical experiments, the feasibility of Notch inhibition in humans with cancer may be challenging. γ-secretase inhibitors, while currently in phase I and II testing, are known to cause gastrointestinal toxicity resulting from goblet cell differentiation and hyperplasia (40, 41). Thus approaches to abrogate this toxicity, including the use of steroids and intermittent schedules of γ-secretase inhibitors, or additional pharmacologic approaches to inhibit the Notch pathway may be necessary (42–44).
In summary, new therapies are needed in the treatment of RMS. However, further improvements in cure rates may not be possible with the addition of new cytotoxic chemotherapy regimens alone. Instead, new treatment strategies that target aberrantly regulated signaling pathways in RMS may be necessary. Decreasing the regenerative potential of RMS cells by permitting differentiation and inhibiting proliferation may represent an alternative approach to treating RMS. The Notch-Hey1 signaling pathway is known to prevent differentiation, promote proliferation, and preserve stem cells in normal skeletal muscle. Our study demonstrates that the Notch-Hey1 axis plays a similar role in eRMS tumorigenesis and with the in vivo pre-clinical data firmly supports supports the development and evaluation of Notch-Hey1 axis inhibitors in the treatment of eRMS.
Translational Relevance.
Outcomes for children and adolescents with high risk rhabdomyosarcoma (RMS) remain dismal despite the evaluation of new chemotherapy regimens in controlled clinical trials. To improve survival, the underlying mechanisms of sarcomagenesis must be identified and targeted therapies developed to supplement current cytotoxic regimens. The Notch signaling pathway is critical for skeletal muscle self-renewal and contributes to tumor maintenance in many types of cancer. Our work demonstrates that the Notch-Hey1 axis signaling pathway is aberrantly upregulated in embryonal RMS (eRMS) and that inhibition of this pathway blocks eRMS tumorigenesis through permission of early differentiation and suppression of proliferation. Decreasing the regenerative potential of eRMS cells by inhibition of Notch-Hey1 signaling may offer a novel therapeutic strategy in the treatment of this cancer.
Supplementary Material
Acknowledgements
We thank Darrell Yamashiro (Columbia University, NYC), Oren Becher, David Kirsch, and Dan Wechsler (Duke University) for helpful discussions. We thank Melinda Hollingshead (Biological Testing Branch, NCI) for advice on the in vivo drug studies.
Financial support
This research was supported by NIH grants 5K12 HD043494, R01 CA122706, and the CureSearch Scott Carter Foundation (to C.M.L.), and a Pfizer Oncology Clinical Fellowship Award (to B.C.B.).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Huh WW, Skapek SX. Childhood rhabdomyosarcoma: new insight on biology and treatment. Curr Oncol Rep. 2010 Nov;12(6):402–410. doi: 10.1007/s11912-010-0130-3. [DOI] [PubMed] [Google Scholar]
- 2.Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4(1):34–44. [PubMed] [Google Scholar]
- 3.Zweidler-McKay PA. Notch signaling in pediatric malignancies. Curr Oncol Rep. 2008 Nov;10(6):459–468. doi: 10.1007/s11912-008-0071-2. [DOI] [PubMed] [Google Scholar]
- 4.Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikhina M, et al. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 2008 Jun 15;68(12):4727–4735. doi: 10.1158/0008-5472.CAN-07-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulleman E, Quarto M, Vernell R, Masserdotti G, Colli E, Kros JM, et al. A role for the transcription factor HEY1 in glioblastoma. J Cell Mol Med. 2009 Jan;13(1):136–146. doi: 10.1111/j.1582-4934.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buas MF, Kadesch T. Regulation of skeletal myogenesis by Notch. Exp Cell Res. 2010 Nov 1;316(18):3028–3033. doi: 10.1016/j.yexcr.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzmann M, Bonnieu A, Duret C, Vernus B, Barro M, Laoudj-Chenivesse D, et al. Inhibition of Notch signaling induces myotube hypertrophy by recruiting a subpopulation of reserve cells. J Cell Physiol. 2006 Sep;208(3):538–548. doi: 10.1002/jcp.20688. [DOI] [PubMed] [Google Scholar]
- 8.Shih I-M, Wang T-L. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007 Mar 1;67(5):1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 9.Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008 Feb 15;111(4):2220–2229. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 10.Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, et al. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum Mol Genet. 2009 Apr 15;18(8):1464–1470. doi: 10.1093/hmg/ddp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008 Aug 22;321(5892):1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roma J, Masia A, Reventos J, Sanchez de Toledo J, Gallego S. Notch pathway inhibition significantly reduces rhabdomyosarcoma invasiveness and mobility in vitro. Clin Cancer Res. 2011 Feb 1;17(3):505–513. doi: 10.1158/1078-0432.CCR-10-0166. [DOI] [PubMed] [Google Scholar]
- 13.Kang MH, Smith MA, Morton CL, Keshelava N, Houghton PJ, Reynolds CP. National Cancer Institute pediatric preclinical testing program: model description for in vitro cytotoxicity testing. Pediatric Blood & Cancer. 2011 Feb;56(2):239–249. doi: 10.1002/pbc.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ancrile B, Lim K-H, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes & Development. 2007 Jul 15;21(14):1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linardic CM, Naini S, Herndon JE, 2nd, Kesserwan C, Qualman SJ, Counter CM. The PAX3-FKHR fusion gene of rhabdomyosarcoma cooperates with loss of p16INK4A to promote bypass of cellular senescence. Cancer Res. 2007 Jul 15;67(14):6691–6699. doi: 10.1158/0008-5472.CAN-06-3210. [DOI] [PubMed] [Google Scholar]
- 16.Patel NS, Li J-L, Generali D, Poulsom R, Cranston DW, Harris AL. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res. 2005 Oct 1;65(19):8690–8697. doi: 10.1158/0008-5472.CAN-05-1208. [DOI] [PubMed] [Google Scholar]
- 17.Naini S, Etheridge KT, Adam SJ, Qualman SJ, Bentley RC, Counter CM, et al. Defining the cooperative genetic changes that temporally drive alveolar rhabdomyosarcoma. Cancer Res. 2008 Dec 1;68(23):9583–9588. doi: 10.1158/0008-5472.CAN-07-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davicioni E, Anderson JR, Buckley JD, Meyer WH, Triche TJ. Gene expression profiling for survival prediction in pediatric rhabdomyosarcomas: a report from the children's oncology group. Journal of Clinical Oncology. 2010 MaR 1;28(7):1240–1246. doi: 10.1200/JCO.2008.21.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldi A, De Falco M, De Luca L, Cottone G, Paggi MG, Nickoloff BJ, et al. Characterization of tissue specific expression of Notch-1 in human tissues. Biology of the Cell. 2004 May;96(4):303–311. doi: 10.1016/j.biolcel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, et al. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. BR J Cancer. 2009 Jun 16;100(12):1957–1965. doi: 10.1038/sj.bjc.6605060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamata S, Du C, Li K, Lavau C. Notch1 perturbation of hemopoiesis involves non-cell-autonomous modifications. Journal of Immunology. 2002 Feb 15;168(4):1738–1745. doi: 10.4049/jimmunol.168.4.1738. [DOI] [PubMed] [Google Scholar]
- 22.Sang L, Roberts JM, Coller HA. Hijacking HES1: how tumors co-opt the anti-differentiation strategies of quiescent cells. Trends in Molecular Medicine. 2010 Jan;16(1):17–26. doi: 10.1016/j.molmed.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Yang Y, Nolo R, Zweidler-McKay PA, Hughes DPM. Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene. 2010 May 20;29(20):2916–2926. doi: 10.1038/onc.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Yang Y, Zweidler-McKay PA, Hughes DPM. Critical role of notch signaling in osteosarcoma invasion and metastasis. Clinical Cancer Research. 2008 May 15;14(10):2962–2969. doi: 10.1158/1078-0432.CCR-07-1992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Gaetani P, Hulleman E, Levi D, Quarto M, Scorsetti M, Helins K, et al. Expression of the transcription factoRHEY1 in glioblastoma: a preliminary clinical study. Tumori. 2010 Jan–Feb;96(1):97–102. doi: 10.1177/030089161009600116. [DOI] [PubMed] [Google Scholar]
- 26.Mullendore ME, Koorstra J-B, Li Y-M, Offerhaus GJ, Fan X, Henderson CM, et al. Ligand-dependent Notch signaling is involved in tumor initiation and tumor maintenance in pancreatic cancer. Clinical Cancer Research. 2009 ApR 1;15(7):2291–2301. doi: 10.1158/1078-0432.CCR-08-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006 Mar;5(3):483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 28.Woo S-M, Kim J, Han H-W, Chae J-I, Son M-Y, Cho S, et al. Notch signaling is required for maintaining stem-cell features of neuroprogenitor cells derived from human embryonic stem cells. BMC Neurosci. 2009;10:97. doi: 10.1186/1471-2202-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li Y-M, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006 Aug 1;66(15):7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 30.Buas MF, Kabak S, Kadesch T. Inhibition of myogenesis by Notch: evidence for multiple pathways. J Cell Physiol. 2009 Jan;218(1):84–93. doi: 10.1002/jcp.21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buas MF, Kabak S, Kadesch T. The Notch effector Hey1 associates with myogenic target genes to repress myogenesis. J Biol Chem. 2010 Jan 8;285(2):1249–1258. doi: 10.1074/jbc.M109.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. 2005 Aug–Oct;16(4–5):612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008 Jan 10;2(1):50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002 Sep;8(9):979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 35.Martinelli S, McDowell HP, Vigne SD, Kokai G, Uccini S, Tartaglia M, et al. RAS signaling dysregulation in human embryonal Rhabdomyosarcoma. Genes Chromosomes Cancer. 2009 Nov;48(11):975–982. doi: 10.1002/gcc.20702. [DOI] [PubMed] [Google Scholar]
- 36.Langenau DM, Keefe MD, Storer NY, Guyon JR, Kutok JL, Le X, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes & Development. 2007 Jun 1;21(11):1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Takita J, Hiwatari M, Igarashi T, Hanada R, Kikuchi A, et al. Mutations of the PTPN11 and RAS genes in rhabdomyosarcoma and pediatric hematological malignancies. Genes Chromosomes Cancer. 2006 Jun;45(6):583–591. doi: 10.1002/gcc.20322. [DOI] [PubMed] [Google Scholar]
- 38.Linardic CM, Downie DL, Qualman S, Bentley RC, Counter CM. Genetic modeling of human rhabdomyosarcoma. Cancer Res. 2005 Jun 1;65(11):4490–4495. doi: 10.1158/0008-5472.CAN-04-3194. [DOI] [PubMed] [Google Scholar]
- 39.Tiffin N, Williams RD, Shipley J, Pritchard-Jones K. PAX7 expression in embryonal rhabdomyosarcoma suggests an origin in muscle satellite cells. BRJ Cancer. 2003 Jul 21;89(2):327–332. doi: 10.1038/sj.bjc.6601040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004 Nov;82(1):341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 41.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005 Jun 16;435(7044):959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 42.Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009 Jan;15(1):50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Real PJ, Ferrando AA. NOTCH inhibition and glucocorticoid resistance in T cell acute lymphoblastic leukemia. Leukemia. 2009 Aug;23(8):1374–1377. doi: 10.1038/leu.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teachey DT, Seif AE, Brown VI, Bruno M, Bunte RM, Chang YJ, et al. Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood. 2008 Jan 15;111(2):705–714. doi: 10.1182/blood-2007-05-087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.