Abstract
Severe hypoxia caused by a lack of vascular supply and an inability to retrieve encapsulated islets transplanted in the peritoneal cavity for biopsy and subsequent evaluation are obstacles to clinical application of encapsulation strategies for islet transplantation. We recently proposed an omentum pouch model as an alternative site of encapsulated islet transplantation and have also described a multi-layer microcapsule system suitable for coencapsulation of islets with angiogenic protein in which the latter could be encapsulated in an external layer to induce vascularization of the encapsulated islet graft. The purpose of the present study was to determine the angiogenic efficacy of fibroblast growth factor (FGF-1) released from the external layer of the new capsule system in the omentum pouch graft. We prepared 2 groups of alginate microspheres, each measuring ~600 μm in diameter with a semipermeable poly-L-ornithine (PLO) membrane separating 2 alginate layers. While one group of microcapsules contained no protein (control), FGF-1 (1.794 μg/100 microcapsules) was encapsulated in the external layer of the other (test) group. From each of the 2 groups, 100 microcapsules were transplanted separately in an omentum pouch created in each normal Lewis rat and were retrieved after 14 days for analysis of vessel density using the technique of serial sample sections stained for CD31 with quantitative three-dimensional imaging. We found that FGF-1 released from the external layer of the test microcapsules induced a mean ± SD vessel density (mm2) of 198.8 ± 59.2 compared with a density of 128.9 ± 10.9 in pouches measured in control capsule implants (P = .03; n = 5 animals/group). We concluded that the external layer of our new alginate microcapsule system is an effective drug delivery device for enhancement of graft neovascularization in a retrievable omentum pouch.
The Edmonton protocol provided the stimulus for a renewed surge of interest in islet transplantation.1 However, like pancreas transplantation and other organ transplantation procedures, routine use of islet transplantation in type 1 diabetic patients is hampered by 2 major obstacles, the severe shortage of human organs and the need to use immunosuppressive drugs to prevent transplant rejection.2 Islet immunoisolation by microencapsulation prior to transplantation was proposed as a potential solution to overcome these 2 barriers more than 3 decades ago.3 In turn, clinical application of the microencapsulation technology has been impeded by various factors including severe hypoxia caused by a lack of vascular supply and an inability to retrieve encapsulated islets transplanted in the peritoneal cavity for biopsy and subsequent evaluation.2 Some investigators have suggested an omentum pouch as an alternative site of islet transplantation,4,5 from where encapsulated islet transplants are easily retrievable.2 To enhance vascular supply and retrievability of encapsulated islet grafts, we recently described a redesigned alginate-poly-L-ornithine-alginate (APA) microcapsule to serve the dual purpose of islet immunoisolation and angiogenic protein delivery to induce graft neovascularization in the omentum pouch.2 Our proposed new encapsulation scheme consists of an inner alginate core layer for immunoisolating islets, separated by a poly-L-ornithine (PLO)-selective membrane from a thinner external alginate layer for controlled release of angiogenic protein.2,6,7 The goal of the present study was to determine the efficacy of fibroblast growth factor (FGF-1) released from the external layer of the microcapsule in the induction of angiogenesis in the omentum pouch graft.
METHODS
Chemicals
Sodium chloride, calcium chloride, and PLO hydrochloride (molecular weight 15,000–30,000) were purchased from Sigma-Aldrich (St. Louis, Mo, United States). Low viscosity (20–200 mPa s) ultra-pure sodium alginate with high mannuronic acid content (LVM) and high guluronic acid content (LVG) were purchased from Nova-Matrix (Oslo, Norway). LVM and LVG were reported by the manufacturer to have molecular weights of 75–200 kd and a guluronic/mannuronic ratio of ≤1 and ≥1.5, respectively. Human fibroblast growth factor (FGF-1) was purchased from Peprotech (Rocky Hill, NJ, United States) and heparin sodium was purchased from McKesson (Durham, NC, United States).
FGF-1 Preparation
FGF-1 was reconstituted with 37 μL of 5 mmol/L sodium phosphate and 0.1% bovine serum albumin to a final concentration of 270 μg/μL. The stock concentration of FGF-1 and heparin sodium were added to 1.5% LVG alginate to a final concentration of 5 U heparin + 3 μg FGF-1 per μL 1.25% LVG.
Multi-Layer Microbead Synthesis and Encapsulation of FGF-1 in the Outer Layer of the APA Microcapsule
Microcapsules were prepared aseptically in a biosafety cell culture hood. Then, 1.5% LVM was extruded through an 8-channel microfluidic device at a flow rate of 2 mL/min with an air jacket pressure of 18 psi to form 500–600-μm diameter capsules. The capsules were extruded into a 100 mmol/L CaCl2 solution, and allowed to crosslink for 15 minutes. Capsules were then strained through a 100-μm cell strainer and rinsed with 25 mL of a mixture of 22 mmol/L CaCl2 and 0.9% NaCl. The capsules were subsequently placed in a 0.1% PLO solution and rocked for 20 minutes. Capsules were then rinsed with 25 mL 0.9% saline and mixed with either 1.25% LVG + ultrapure water (control) or 1.25% LVG with FGF-1 and heparin for 45 minutes. The capsules were finally rinsed with 0.9% saline to remove unbound alginate and dried prior to implantation.
Microbead Implantation in the Omentum Pouch
The animal surgical procedure was approved by Wake Forest University Institutional Animal Care and Use Committee. Under general anesthesia, the 2 groups (5/group) of normal Lewis rats underwent a mini-laparotomy and the greater omentum was mobilized and placed onto moist gauze. A 4-0 uncoated vicryl suture in a running fashion was placed along the edge of the omentum. Frequent irrigation with saline was ensured through the entire procedure to prevent the omentum from drying up. For each animal, 100 empty (control) or FGF-1–loaded microcapsules were placed onto the exposed omentum, the suture was pulled up and tied, creating a pouch for the microcapsules. After hemostasis was ensured, the surgical incision was closed using a standard surgical technique. After 2 weeks of implantation, the animals were humanely killed and immediately after the animal terminal procedure was performed, the recipients underwent a full-laparotomy, the omentum pouch containing the microcapsules was harvested, fixed in formalin, and paraffin embedded. Specimens were serially sectioned (5-μm thickness) for immunohistochemical analysis.
Immunohistochemistry and Image Analyses
Serial sections were stained for CD31, a sensitive marker of endothelial cells (ECs).8 Deparaffinized and rehydrated sections underwent steam antigen retrieval using Dako target retrieval solution (Dako, Carpinteria, Calif, United States) prior to immunohistological staining. Specimens were stained following an indirect procedure using rabbit anti-human CD31 (Santa Cruz Biotechnology, Santa Cruz, Calif, United States) or rabbit anti-alpha smooth muscle actin (Abcam, Cambridge, Mass) and a biotinylated anti-rabbit secondary antibody using the Vectastain Elite ABC kit (Vector Labs, Burlingame, Calif, United States). Sections were digitally imaged (20x objective; 0.017 μm/pixel) using an Axiovert 200 inverted microscope (Carl Zeiss, Oberkochen, Germany). Areas stained positive for CD31 were manually selected using the Axiovision AC. The blood vessels per tissue view area were then counted based on CD31 staining, and the vessel density was calculated as previously described.8
Statistical Evaluation of Data
The mean ± SD vessel density measured in animals implanted with FGF-1–loaded microcapsules was compared with that measured in control animals using Student t test and a value of P < .05 was accepted as significant.
RESULTS
Samples of CD31-positive stains are shown in Figure 1a for control animals that received empty microcapsule implants and in Figure 1b for animals that were implanted with FGF-1–loaded microcapsules. Quantitative analysis of these CD31-positive stains showed that FGF-1 released from the external layer of the test microcapsules induced a mean ± SD vessel density (mm2) of 198.8 ± 59.2 compared with a density of 128.9 ± 10.9 in pouches with control capsules (P = .03; n = 5 animals/group).
Fig 1.
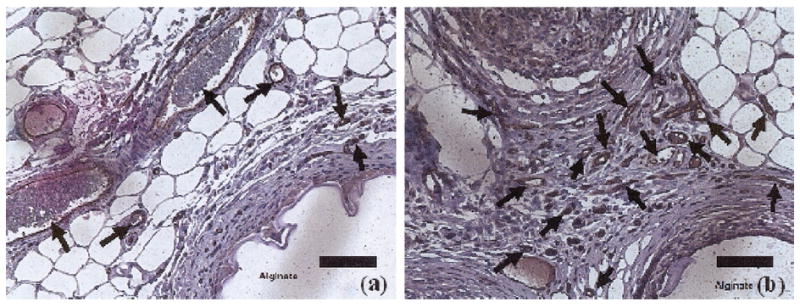
CD31-positive stains as a measure of new blood vessel formation: (a) control = empty microcapsule implants; and (b) test samples of FGF-1–loaded microcapsule implants. Brown areas are positive for CD31.
DISCUSSION
The 2 major attractions of the microencapsulated islet technology are the possibility of performing islet transplantation without the use of risky immunosuppressive drugs and the potential to increase the islet donor source. Routinely, encapsulated islet transplantations have been performed in the peritoneal cavity.2,9-15 However, one of the limitations of intraperitoneal transplantation of microencapsulated islets is that the cells are subjected to a prolonged period of hypoxia extending from the time of isolation, purification, and microencapsulation through the immediate posttransplantation period, which necessitates the requirement for large quantities of islets to achieve normoglycemia in studies performed in large diabetic animals and human subjects. Islets are very highly metabolically active tissues that require a lot of oxygen. They constitute about 1% of the pancreatic mass, but receive approximately 10% of the blood flow to the pancreas, thus indicating a disproportionate supply of oxygen to meet metabolic demands.16 We had previously suggested that, using our restructured APA microcapsule, the external alginate layer could be used for controlled release of angiogenic protein to induce neovascularization of encapsulated islet grafts in an omentum pouch.2,6,7 In the present study, we have shown that FGF-1 encapsulated in this external layer can indeed enhance angiogenesis in the omentum pouch, thus setting the stage for further experiments on islets coencapsulated with angiogenic protein in this new model of the microencapsulation technology. It is hoped that the enhanced vascularization of the microencapsulated islet graft would result in sustained graft function and the use of lower numbers of encapsulated islets to achieve long-term normoglycemia. Current studies are investigating the rate and stability of vessel formation and whether this improved vascularization increases islet graft function. In conclusion, our data showed that the external layer of our new alginate microcapsule system is an effective drug delivery device for enhancement of encapsulated islet neovascularization in a retrievable omentum pouch.
Acknowledgments
Supported by the National Institutes of Health (grant no. RO1DK080897), the Vila Rosenfeld Estate, Greenville, NC, for research in Dr Opara’s laboratory, and the National Science Foundation (grant no. 0852048) and Department of Veterans Affairs for research support in Dr Brey’s laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Opara EC, Mirmalek-Sani S-H, Khanna O, et al. Design of a bioartificial pancreas. J Investig Med. 2010;58:831. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim F, Sun A. Microencapsulated islets as bioartificial pancreas. Science. 1980;210:908. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 4.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3:281. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 5.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59:1285. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna O, Moya ML, Opara EC, et al. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95:632. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna O, Moya ML, Greisler HP, et al. Multilayered alginate microcapsules for sustained release of angiogenic proteins from encapsulated cells. Am J Surg. 2010;200:655. doi: 10.1016/j.amjsurg.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moya ML, Garfinkel MR, Liu X, et al. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J Surg Res. 2010;160:208. doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soon-Shiong P, Feldman E, Nelson R, et al. Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation. 1992;54:769. doi: 10.1097/00007890-199211000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Soon-Shiong P, Heintz RE, Merideth N, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:343. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Ma X, Zhou D, et al. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98:1417. doi: 10.1172/JCI118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calafiore R, Calabrese G, Basta G, et al. Microencapsulated pancreatic islet allograft into non- immunosuppressed patients with type 1 diabetes. Diabetes Care. 2006;29:137. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 13.Dufrane D, Goebbels RM, Saliez, et al. Six month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation. 2006;81:1345. doi: 10.1097/01.tp.0000208610.75997.20. [DOI] [PubMed] [Google Scholar]
- 14.Elliott RB, Escobar L, Tan PLJ, et al. Live encapsulated porcine islets from type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Adcock J, Kuhtreiber W, et al. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation. 2008;85:331. doi: 10.1097/TP.0b013e3181629c25. [DOI] [PubMed] [Google Scholar]
- 16.Jansson L, Hellerstrom C. Stimulation by glucose of the blood flow to the pancreatic islets of the rat. Diabetologia. 1983;25:45. doi: 10.1007/BF00251896. [DOI] [PubMed] [Google Scholar]


