Abstract
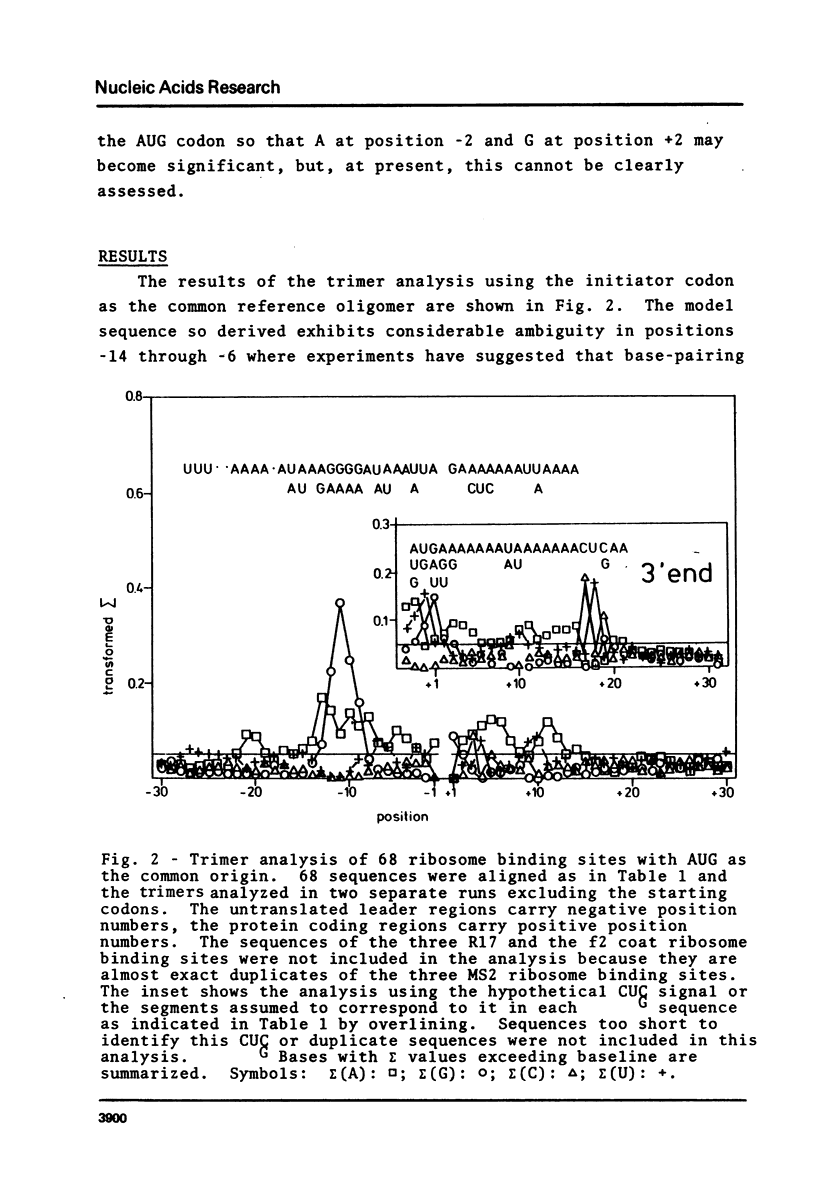
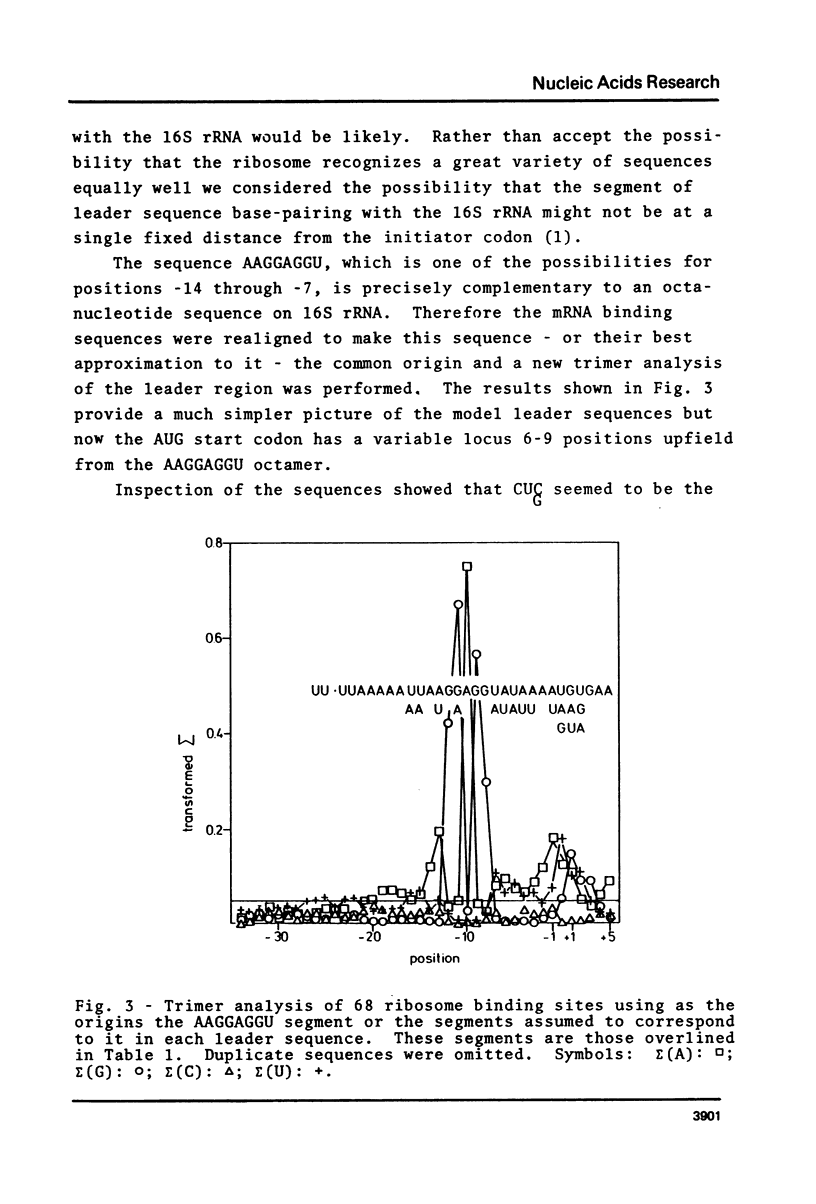
Oligonucleotide analysis, by a novel computerized procedure, was first applied to determine the sequence of an ideal E. coli promoter (Scherer et al., Nucl. Acids Res. 1978, 5:3759-3773) and has now been used to obtain the sequence of nucleotides that should be present in a messenger RNA for optimum binding to the E. coli ribosome. This sequence is: UU.UUAAAAAUUAAGGAGGUAUAUUAUGAAAAAAAUUAAAAAACUCAA AA U A AUA A CUC G. Comparison of this sequence with each of the 68 ribosome binding site sequences used to generate it shows a preference rather than an absolute requirement for a specific base in any given position. The preference for certain bases persists along the whole length of the RNA within the ribosome binding domain even though nearly half of that length includes translated codons. Thus messages without leader sequences (like lambda CI mRNA) can still have some affinity for the ribosome. Part of the model sequence is complementary to the 3'end of 16S rRNA.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Hindley J. Nucleotide sequence of a ribosome binding site on RNA synthesized in vitro from coliphage T7. Nat New Biol. 1973 Jul 4;244(131):10–13. doi: 10.1038/newbio244010a0. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Steitz J. A., Anderson C. W., Model P. Binding of mammalian ribosomes to MS2 phage RNA reveals an overlapping gene encoding a lysis function. Cell. 1979 Oct;18(2):247–256. doi: 10.1016/0092-8674(79)90044-8. [DOI] [PubMed] [Google Scholar]
- Baan R. A., Hilbers C. W., Van Charldorp R., Van Leerdam E., Van Knippenberg P. H., Bosch L. High-resolution proton magnetic resonance study of the secondary structure of the 3'-terminal 49-nucleotide fragment of 16S rRNA from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1028–1031. doi: 10.1073/pnas.74.3.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Hedgpeth J., Selzer G. B., Epstein R. H. Temperature-sensitive mutation in the initiation codon of the rIIB gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1979 Feb;76(2):700–704. doi: 10.1073/pnas.76.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. Escherichia coli lac operator mRNA affects translation initiation of beta-galactosidase mRNA. Nature. 1979 Feb 1;277(5695):407–409. doi: 10.1038/277407a0. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Blattner F. R. Sequence of the promoter-operator proximal region of the major leftward RNA of bacteriophage lambda. Nucleic Acids Res. 1975 Sep;2(9):1441–1458. doi: 10.1093/nar/2.9.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper D. E., von Hippel P. H. Nucleic acid binding properties of Escherichia coli ribosomal protein S1. II. Co-operativity and specificity of binding site II. J Mol Biol. 1978 Jul 5;122(3):339–359. doi: 10.1016/0022-2836(78)90194-8. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Buzash-Pollert E., Studier F. W. Mutations of bacteriophage T7 that affect initiation of synthesis of the gene 0.3 protein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2741–2745. doi: 10.1073/pnas.75.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegmean G., Merregaert J., Jou W. M., Raeymakers A., Volckaert G., Ysebaert M., Van de Kerckhove J. A-protein gene of bacteriophage MS2. Nature. 1975 Jul 24;256(5515):273–278. doi: 10.1038/256273a0. [DOI] [PubMed] [Google Scholar]
- Files J. G., Weber K., Miller J. H. Translational reinitiation: reinitiation of lac repressor fragments at three internal sites early in the lac i gene of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Mar;71(3):667–670. doi: 10.1073/pnas.71.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Barrell B. G., Staden R., Fiddes J. C. Nucleotide sequence of bacteriophage G4 DNA. Nature. 1978 Nov 16;276(5685):236–247. doi: 10.1038/276236a0. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Steitz J. A. Cistron specificity of 30S ribosomes heterologously reconstituted with components from Escherichia coli and Bacillus stearothermophilus. Biochemistry. 1974 May 7;13(10):2123–2129. doi: 10.1021/bi00707a020. [DOI] [PubMed] [Google Scholar]
- Grindley N. D. IS1 insertion generates duplication of a nine base pair sequence at its target site. Cell. 1978 Mar;13(3):419–426. doi: 10.1016/0092-8674(78)90316-1. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Chen J., Schaefer L., Lengyel P., Weissman S. M. Nucleotide sequence of a ribosome attachment site of bacteriophage f2 RNA. Biochem Biophys Res Commun. 1970 Jun 5;39(5):883–888. doi: 10.1016/0006-291x(70)90406-7. [DOI] [PubMed] [Google Scholar]
- Held W. A., Gette W. R., Nomura M. Role of 16S ribosomal ribonucleic acid and the 30S ribosomal protein S12 in the initiation of natural messenger ribonucleic acid translation. Biochemistry. 1974 May 7;13(10):2115–2122. doi: 10.1021/bi00707a019. [DOI] [PubMed] [Google Scholar]
- Hindley J., Staples D. H. Sequence of a ribosome binding site in bacteriophage Q-beta-RNA. Nature. 1969 Dec 6;224(5223):964–967. doi: 10.1038/224964a0. [DOI] [PubMed] [Google Scholar]
- Isono K., Isono S. Lack of ribosomal protein S1 in Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1976 Mar;73(3):767–770. doi: 10.1073/pnas.73.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Branlant C., Ebel J. P., Visentin L. P. Recognition of RNA by ribosomal protein S1: interaction of S1 with 23 S rRNA of Escherichia coli. FEBS Lett. 1977 Aug 15;80(2):255–260. doi: 10.1016/0014-5793(77)80452-3. [DOI] [PubMed] [Google Scholar]
- Lee N., Carbon J. Nucleotide sequence of the 5' end of araBAD operon messenger RNA in Escherichia coli B/r. Proc Natl Acad Sci U S A. 1977 Jan;74(1):49–53. doi: 10.1073/pnas.74.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N. E. coli lactose operon ribosome binding site. Nature. 1974 Jun 14;249(458):647–649. doi: 10.1038/249647b0. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Musso R. E., de Crombrugghe B., Pastan I., Sklar J., Yot P., Weissman S. The 5'-terminal nucleotide sequence of galactose messenger ribonucleic acid of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4940–4944. doi: 10.1073/pnas.71.12.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A., Abelson J. The regulatory region of the biotin operon in Escherichia coli. Nature. 1978 Dec 14;276(5689):689–694. doi: 10.1038/276689a0. [DOI] [PubMed] [Google Scholar]
- Pieczenik G., Model P., Robertson H. D. Sequence and symmetry in ribosome binding sites of bacteriophage f1 RNA. J Mol Biol. 1974 Dec 5;90(2):191–124. doi: 10.1016/0022-2836(74)90368-4. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. Operators and promoters in the OR region of phage 434. Nucleic Acids Res. 1979 Apr;6(4):1495–1508. doi: 10.1093/nar/6.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtle R. M., Pirtle I. L., Inouye M. Homologous nucleotide sequences between prokaryotic and eukaryotic mRNAs: the 5'-end sequence of the mRNA of the lipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1978 May;75(5):2190–2194. doi: 10.1073/pnas.75.5.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Yanofsky C. An intercistronic region and ribosome-binding site in bacterial messenger RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2399–2403. doi: 10.1073/pnas.72.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Arfsten A. E., Reusser F., Nomura M. DNA sequences of promoter regions for the str and spc ribosomal protein operons in E. coli. Cell. 1978 Sep;15(1):215–229. doi: 10.1016/0092-8674(78)90096-x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Roberts T. M., Kacich R., Ptashne M. A general method for maximizing the expression of a cloned gene. Proc Natl Acad Sci U S A. 1979 Feb;76(2):760–764. doi: 10.1073/pnas.76.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Scherer G. E., Walkinshaw M. D., Arnott S. A computer aided oligonucleotide analysis provides a model sequence for RNA polymerase-promoter recognition in E.coli. Nucleic Acids Res. 1978 Oct;5(10):3759–3773. doi: 10.1093/nar/5.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Scherer G., Hobom G., Kössel H. Nucleotide sequence of cro, cII and part of the O gene in phage lambda DNA. Nature. 1978 Mar 30;272(5652):410–414. doi: 10.1038/272410a0. [DOI] [PubMed] [Google Scholar]
- Selker E., Yanofsky C. Nucleotide sequence of the trpC-trpB intercistronic region from Salmonella typhimurium. J Mol Biol. 1979 May 15;130(2):135–143. doi: 10.1016/0022-2836(79)90422-4. [DOI] [PubMed] [Google Scholar]
- Senear A. W., Steitz J. A. Site-specific interaction of Qbeta host factor and ribosomal protein S1 with Qbeta and R17 bacteriophage RNAs. J Biol Chem. 1976 Apr 10;251(7):1902–1912. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman W. G., Reichardt L. F., Yaniv M., Heinemann S. F., Kaiser A. D., Eisen H. Bidirectional transcription and the regulation of Phage lambda repressor synthesis. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3156–3160. doi: 10.1073/pnas.69.11.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague K. U., Steitz J. A., Grenley R. M., Stocking C. E. 3' terminal sequences of 16S rRNA do not explain translational specificity differences between E. coli and B. stearothermophilus ribosomes. Nature. 1977 Jun 2;267(5610):462–465. doi: 10.1038/267462a0. [DOI] [PubMed] [Google Scholar]
- Squires C., Lee F., Bertrand K., Squires C. L., Bronson M. J., Yanofsky C. Nucleotide sequence of the 5' end of tryptophan messenger RNA of Escherichia coli. J Mol Biol. 1976 May 15;103(2):351–381. doi: 10.1016/0022-2836(76)90317-x. [DOI] [PubMed] [Google Scholar]
- Staples D. H., Hindley J., Billeter M. A., Weissmann C. Localization of Q-beta maturation cistron ribosome binding site. Nat New Biol. 1971 Sep 15;234(50):202–204. doi: 10.1038/newbio234202a0. [DOI] [PubMed] [Google Scholar]
- Staples D. H., Hindley J. Ribosome binding site of Q-beta RNA polymerase cistron. Nat New Biol. 1971 Sep 15;234(50):211–212. doi: 10.1038/newbio234211a0. [DOI] [PubMed] [Google Scholar]
- Steege D. A. 5'-Terminal nucleotide sequence of Escherichia coli lactose repressor mRNA: features of translational initiation and reinitiation sites. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4163–4167. doi: 10.1073/pnas.74.10.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steege D. A. A ribosome binding site from the pR RNA of bacteriophage lambda. J Mol Biol. 1977 Aug 25;114(4):559–568. doi: 10.1016/0022-2836(77)90178-4. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Bryan R. A. Two ribosome binding sites from the gene 0-3 messenger RNA of bacteriophages T7. J Mol Biol. 1977 Aug 25;114(4):527–543. doi: 10.1016/0022-2836(77)90176-0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Oligonucleotide sequence of replicase initiation site in Q RNA. Nat New Biol. 1972 Mar 22;236(64):71–75. doi: 10.1038/newbio236071a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Specific recognition of non-initiator regions in RNA bacteriophage messengers by ribosomes of Bacillus stearothermophilus. J Mol Biol. 1973 Jan;73(1):1–16. doi: 10.1016/0022-2836(73)90155-1. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Steege D. A. Characterization of two mRNA-rRNA complexes implicated in the initiation of protein biosynthesis. J Mol Biol. 1977 Aug 25;114(4):545–558. doi: 10.1016/0022-2836(77)90177-2. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Billeter M. A., Kahane S., Weissmann C., Hindley J., Porter A. Molecular basis for repressor activity of Q replicase. Nat New Biol. 1972 Jun 7;237(75):166–170. doi: 10.1038/newbio237166a0. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]



