Abstract
Neutralizing antibodies to type I interferons are of therapeutic significance i.e. are currently evaluated for the treatment of autoimmune diseases with pathogenic IFN-α production such as for systemic lupus erythematosus.
Unexpectedly, we observed that several neutralizing antibodies reportedly known to counteract IFN-α or -β activity triggered an “IFN-like” response in quiescent primary human endothelial cells leading to activation of the transcription factor ISGF3 and the expression of interferon-responsive genes. Furthermore, these antibodies were found to enhance rather than inhibit type I interferon signals, and the effect was also detectable for distinct other cell types such as PBMCs. The stimulatory capacity of anti-IFN-α/β antibodies was mediated by the constitutive autocrine production of sub-threshold IFN levels, involved the type I IFN receptor and was dependent on the Fc antibody domain, as Fab or F(ab’)2 fragments potently inhibited IFN activity.
We thus propose that a combined effect of IFN recognition by the antibody paratope and the concomitant engagement of the Fc domain may trigger an interferon signal via the respective type I IFN receptor which accounts for the observed “IFN-like” response to the neutralizing antibodies. With respect to clinical applications, the finding may be of importance for the design of recombinant antibodies versus Fab or F(ab’)2 fragments to efficiently counteract IFN activity without undesirable activating effects.
Keywords: endothelial cell, neutralizing antibody, type I interferon, Fc domain
Introduction
The inducible interferon response and the associated anti-virus, anti-tumor and immunomodulatory activities are well characterized hallmarks of the defense system. They are primarily mediated by the rapid activation of the transcription factor ISGF3 (interferon stimulated gene factor 3)4 which binds to promoter elements termed ISRE (interferon stimulated response element) and induces expression of interferon-response genes such as IFIT-1 (interferon induced protein with tetratricopeptide repeats 1) or ISG15 (interferon stimulated gene 15). In contrast, the low level, constitutive expression of type I interferons (IFN-α or -β) has now been recognized to serve distinct functions in cellular signaling and activation (1): In the absence of any known stimulus, a low basal expression level of type I interferons is maintained which results in a weak signaling event and intracellular tyrosine phosphorylation of IFNAR-1, the type I IFN receptor α-chain. The signal is considered to be “sub-threshold” i.e. does not elicit the signaling cascade that leads to transcriptional activation of interferon-response genes. However, IFNAR-1 is maintained in a “ready state” thereby promoting rapid cellular activation upon stimulation with virus, interferons or other STAT-signaling cytokines. In this context, IFNAR-1 was shown to engage in cross-talk with other receptors such as the type II IFN-γ or the IL-6 receptor – thus constituting a common “docking site” for STAT dimerization and an efficient enhancer of cellular activation (2, 3). Constitutive, low level IFN-α/β expression has been reported for mouse embryonic fibroblasts (MEFs), splenocytes, macrophages and bone marrow cells (2, 3), and was further described to promote activation of CD8+ T-cells upon T-cell receptor engagement (4) and to regulate dendritic cell differentiation (5, 6).
Considering the potency of IFN-α/β in the immune response, the application of recombinant IFNs has proven an evident and valuable therapeutic tool in the treatment of e.g. viral infections or cancer. On the other hand, type I interferons – in particular IFN-α - have been found to play a crucial role in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE), type I diabetes or autoimmune thyroid disease (7, 8). In this context, the development of a neutralizing antibody directed against multiple IFN-α subtypes has been promoted (9), and in April 2006 the first clinical trial has been launched applying a humanized IFN-α blocking mAb to SLE patients. IFN-neutralizing activities are expected to target immune effector cells such as leukocytes, but their impact further extends to other cell types such as the vessel-lining endothelial cells (ECs). In this regard, we have observed and characterized an unexpected “IFN-like” response in ECs upon exposure to neutralizing antibodies directed against type I interferons.
Materials and Methods
Cell culture
Primary ECs were isolated from human foreskin samples by dispase digest, purified via α-CD31 antibody coupled Dynabeads (Invitrogen Corp., Carlsbad, CA) and cultured in fibronectin-containing EGM2-MV growth medium (Cambrex Corp., East Rutherford, NJ) without VEGF supplementation. Purified EC cultures showed ≥ 98 % purity and viability. For separation of lymphatic and blood vessel ECs, α-podoplanin antibody coupled Dynabeads were applied. All isolates were characterized by flow cytometry for EC characteristics i.e. CD31 expression, CD34 as marker of microvessels and for E-selectin induction following stimulation with 100 ng/ml of TNFα for 4 h. Lymphatic ECs were detected by podoplanin expression. To keep the passage number low, experiments were generally repeated with different EC isolates i.e. represent biological replicates with variable inducibility. Representative results of 2-4 comparable experiments are shown (as further specified in the figure legends).
HUVECs (human umbilical vein endothelial cells) were obtained from Cambrex Corp. and grown in fibronectin-containing EGM2 medium without VEGF supplementation (Cambrex Corp., East Rutherford, NJ). 293T cells are derived from human embryonic kidney cells into which the temperature sensitive gene for SV40 T-antigen was inserted. HT-29 was isolated from a human colorectal adenocarcinoma. Both cell lines (293T, HT-29) were supplied by ATCC and cultured in Dulbeccós modified Eagle medium with 10 % FCS. Peripheral blood mononuclear cells (PBMCs) were isolated from 100 ml EDTA-treated whole blood of a healthy volunteer by standardized density gradient centrifugation using Ficoll-Paque (GE Healthcare, Chalfont St Giles, Buckinghamshire, UK) and were supplied with RPMI1640 medium containing 10 % FCS.
Treatment with neutralizing antibodies and rIFN
Two days prior to stimulation, ECs were seeded in growth medium at 7×105 cells per 30 mm dish (293T and HT-29 cells at 2×106 cells) to yield a confluent cell layer within 24 hours. Culture medium was then exchanged to EGM2-MV containing 5 % FCS but no additional growth factor supplements and cells were allowed to adopt a quiescent state over the next 24 h. Antibodies were added on day 3 at various concentrations and for the time periods indicated. 7×105 PBMCs in 500 μl RPMI1640 medium were stimulated immediately after isolation without prior culture period.
Recombinant interferon and blocking mAbs targeting either IFN-α (clones #2 and #13: MMHA-2 and MMHA-13, respectively) or IFN-β (clones #3 and #12: MMHB-3 and MMHB-12, respectively) as well as the IFNAR chain 2 (MMHAR-2) were all obtained from PBL Biomedical Laboratories (Piscataway, NJ). ELISA assays for detection of human IFN-α or -β were also manufactured by PBL and carried out essentially as described (10). Neutralizing antibodies to IFN-γ (NIB42) or the IFNGR α-chain (GIR-208) were supplied by BD Biosciences (San Jose, CA); for mouse IgG1 isotype control the MOPC-21 clone was applied (Sigma-Aldrich Corp., St. Louis, MO). TNFα was kindly provided by H. R. Alexander (NCI, NIH, Bethesda, MD), whereas LPS was obtained from Sigma-Aldrich Corp.
Real-time RT-PCR
Total RNA was isolated from cell cultures with Qiagen RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany), 500 ng RNA were reverse transcribed with oligo(dT) primers using the Superscript III First Strand Synthesis System (Invitrogen Corp., Carlsbad, CA) and the generated cDNA was diluted 1:25 prior to PCR analysis. Real-time PCR was performed with SYBR Green PCR Core Reagents (Applied Biosystems, Foster City, CA) and the following primer sets for IFIT-1 (900 nM forward primer 5′-GCA GAA CGG CTG CCT AAT TT-3′, 900 nM reverse primer 5′-TCA GGC ATT TCA TCG TCA TC-3′), ISG-15 (300 nM forward primer 5′-GAG AGG CAG CGA ACT CAT CT-3′, 300 nM reverse primer 5′-AGC TCT GAC ACC GAC ATG G-3′), IFN-β (50 nM forward primer 5′-AGC ACT GGC TGG AAT GAG AC-3′, 300 nM reverse primer 5′-TCC TTG GCC TTC AGG TAA TG-3′), IFN-α1 (300 nM forward primer 5′-GCC TCG CCC TTT GCT TTA CT-3′, 300 nM reverse primer 5′-CTG TGG GTC TCA GGG AGA TCA-3′, PrimerBank ID 13128950a1), IFN-α2 (300 nM forward primer 5′-GCT TGG GAT GAG ACC CTC CTA-3′, 300 nM reverse primer 5′-CCC ACC CCC TGT ATC ACA C-3′, PrimerBank ID 11067751a1), BCoR (300 nM forward primer 5′-AGA CGA CAT GCT CTC AGC AA-3′, 50 nM reverse primer 5′-GAT CCT ATG GGC CGT GCT 3′), housekeeping genes β2-microglobulin (50 nM forward primer 5′-GAT GAG TAT GCC TGC CGT GTG-3′, 50 nM reverse primer 5′-CAA TCC AAA TGC GGC ATC T-3′) and β-actin (900 nM forward primer 5′-CTG GAA CGG TGA AGG TGA CA-3′, 300 nM reverse primer 5′-AAG GGA CTT CCT GTA ACA ATG CA-3′). With the exception of IFNs which do not contain introns, all primer sets span at least one exon/intron gene boundary. Primer sets for IFN-α1 and IFN-α2 were retrieved from the PrimerBank (11). Each sample was assayed in triplicate with the GeneAmp 5700 Sequence Detection System (Applied Biosystems) for 45 cycles of 15 sec at 95 °C followed by 1 min at 60 °C and a final dissociation protocol to screen for false amplification products. mRNA levels for IFIT-1, ISG15, BCoR or IFNs were deduced from the on-plate dilution series of a standard cDNA and were normalized to housekeeping gene values as previously described (12). Real-time PCR data are given as mean and standard deviation of triplicate samples. The value obtained for the untreated control sample was generally set to 1 and changes in mRNA expression upon stimulation are given in relation to the untreated control.
Immunoblotting
Endothelial whole cell extracts were generated essentially as described (13, 14) i.e. in lysis buffer containing 1 % NP-40, 0.5 % deoxycholic acid and the Complete Mini-Protease Inhibitor Cocktail (Roche, Indianapolis, IN). 30 μg of total protein were separated on PAGE mini gels and subjected to wet blotting. Immunodetection was performed with polyclonal rabbit antiserum against IFIT-1 (generous gift by Ganes Sen, Cleveland, OH) at a 1:2000 dilution or ISG-15 (Rockland Immunochemicals, Inc., Gilbertsville, PA) at a 1:200 dilution.
EC transfection with siRNA
Three distinct Stealth siRNA duplex oligonucleotides for IFN-β gene silencing (HSS10523-2, −3, −4) as well as the respective negative control siRNAs with matched GC-content were obtained from Invitrogen (Carlsbad, CA). 2×106 proliferating ECs were harvested in 400 μl RPMI1640 with 10 % FCS and siRNA was added. Cells were then subjected to electroporation at 200 V, 960 μF essentially as described (15). Stimulation of ECs with anti-IFN mAbs or rIFN was performed 24 to 48 h post transfection. A mix of the three IFN-β siRNAs (f.c. 1 μM each) proved to be more effective than single application of the IFN-β siRNA variants (data not shown).
ISGF3 Reporter Gene Assay
Electroporation of 2×106 ECs at 200 V, 960 μF was performed with a total of 30 μg DNA. A combination of 29 μg pISRE-Luc plasmid with 5 ISRE sites directing expression of the luciferase reporter gene (Stratagene, La Jolla, CA) and 1 μg of the constitutive β-galactosidase expression plasmid pCMVβ for normalization (Clontech, Mountain View, CA) was applied. Cells were then seeded in 30 mm wells at 1×106 to yield a confluent cell layer within 24 hours. Stimulation with anti-IFN-α mAb #2 (6 μg/ml) or rIFN-α (100 pg/ml) was carried out for 4 and 24 hours. Cells were then harvested in 40 μl lysis buffer and samples (à 10 μl) were assayed in triplicate for luciferase as well as β-galactosidase activity applying the Tropix Dual-Light System according to manufacturer’s instructions (Applied Biosystems, Foster City, CA) for chemiluminescent detection with a Wallac Victor3 multilabel counter (Perkin Elmer Life Sciences, Boston, MA). Luciferase activity as measured in relative light units (RLU) was normalized to the corresponding β-galactosidase value.
Generation of Fab and F(ab’)2 fragments
The anti-IFN-α mAb #2 or mIgG1 isotype control antibody were subjected to ficin digest at 37 °C in the presence of 10 mM cysteine for Fab versus 1 mM cysteine for F(ab’)2 fragment generation according to the instructions of the “ImmunoPure IgG1 Fab and F(ab’)2 Preparation Kit” (Pierce Biotechnology, Rockford, IL). For control, an antibody fraction was treated comparably in ImmunoPure Digestion Buffer but without addition of ficin protease. The resulting antibody fragments were evaluated under reducing as well as non-reducing conditions by Western blotting with ImmunoPure peroxidase-conjugated goat anti-mouse IgG (H+L) antiserum (Pierce) and digest efficiency was found to be ≥ 90 %. Control human Fab/kappa fragments were obtained from Bethyl Laboratories, Inc. (Montgomery, TX).
Results
Primary endothelial cells as isolated from human dermal microvessels (HDMECs) were subjected to standard in vitro culture. When cells were exposed to increasing doses of neutralizing antibodies directed against IFN-α or -β (in the absence of any exogenously added type I interferon) an “interferon-like” response was observed (Fig. 1): The dose-dependent induction of IFN-responsive genes such as IFIT-1 and ISG15 was detected at the mRNA level by real-time RT-PCR as well as at the protein level by immunoblotting. The effect was verified for four different, commercially available blocking mAbs targeting either IFN-α (clones #2, 13) or IFN-β (clones #3, 12) as illustrated in figures 1 and 4. Furthermore, the stimulatory capacity of anti-IFN-α and -β mAbs was additive (Fig. 2 A) and IFIT-1 mRNA induction was observed at all time-points (2, 4, 6, 8 h) investigated (data not shown). All mAbs tested were of the mouse IgG1 isotype with κ light chains. An appropriate isotype control did not induce IFIT-1 or ISG15 expression in HDMECs.
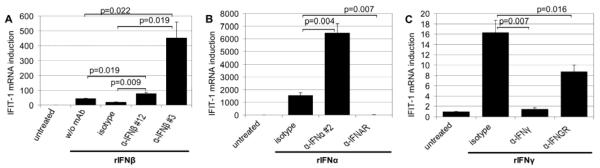
FIGURE 1.
Dose-dependent induction of interferon-responsive genes by neutralizing antibodies to type I IFN. Endothelial cell cultures were treated for 4 h with 0.8, 3 or 12 μg/ml of anti-IFN-α mAb clone #2 (A, n=7), clone #13 (B, n=2) or anti-IFN-β mAb clone #3 (C, n=3). The non-specific mIgG1 isotype control was applied at 12 μg/ml. Real-time RT-PCR analysis of endothelial RNA was performed to evaluate mRNA levels of IFIT-1 and ISG15. Each sample was assayed in triplicate and the number of comparable experiments performed is given for each figure (n). IFIT-1 induction by antibody treatment was statistically significant (p≤0.03) for all mAbs and concentrations. Comparably, p-values ≤0.01 were recorded for ISG15 induction with the exception of anti-IFN-α mAb clone #13 at the lowest concentration (p=n.s.). Protein expression (D, n=2) of IFIT-1 and ISG15 was investigated by immunoblotting of whole cell extracts following 5 h of stimulation with anti-IFN-α mAb clone #2 at 12 μg/ml (*non-specific, cross-reactive protein).
FIGURE 4.
Enhanced response to rIFN-α or -β in the presence of neutralizing antibodies to type I IFN. (A, n=3) HDMECs were stimulated with rIFN-β (10 pg/ml) for 4 h in the absence or presence of anti-IFN-β mAb clone #3 or #12, or mIgG1 isotype control at 12 μg/ml each. (B, n=2) EC treatment for 6 h with rIFN-α (10 pg/ml) was combined with 12 μg/ml of mIgG1 isotype control, anti-IFN-α mAb clone #2 or anti-IFNAR antibody. (C, n=3) Comparably, ECs were exposed for 6 h to 10000 U/ml of rIFN-γ and 12 μg/ml of mIgG1 isotype control, anti-IFN-γ or anti-IFNGR antibody. Endothelial RNA was analyzed for IFIT-1 mRNA expression by real-time RT-PCR.
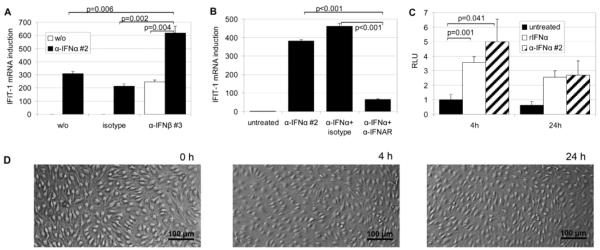
FIGURE 2.
Characterization of the mechanisms involved in the induction of interferon-responsive genes by neutralizing antibodies to type I IFN. (A, n=3) HDMECs were challenged for 4 h by single or combined treatment with 3 μg/ml of anti-IFN-α mAb clone #2 and anti-IFN-β mAb clone #3 (or mIgG1 isotype control). Endothelial RNA and corresponding cDNA were then analyzed for IFIT-1 mRNA expression by real-time PCR which demonstrated an additive effect of anti-IFN-α and -β antibodies. (B, n=3) IFIT-1 induction by 3 μg/ml of anti-IFN-α mAb clone #2 was blocked by the addition of anti-IFNAR antibody at 1 μg/ml versus mIgG2a isotype control. (C, n=2) Activation of the interferon-responsive transcription factor ISGF3 was evaluated by reporter gene assay measuring luciferase activity in relative light units (RLU) after EC stimulation for 4 h or 24 h with 6 μg/ml of anti-IFN-α mAb clone #2 or rIFN-α (100 pg/ml) for comparison. (D) Endothelial cultures after 0, 4 or 24 h of stimulation with 6 μg/ml of anti-IFN-α mAb clone #2 were investigated by phase contrast microscopy to document that no apparent changes in EC morphology were triggered by antibody treatment.
A first indication on the potential mechanism underlying the extraordinary “intrinsic” ability of the type I interferon antibodies to induce IFN-responsive genes came from the observation that IFIT-1 induction by anti-IFN-α or -β mAbs was abolished in the presence of IFNAR-blocking antibodies (Fig. 2 B). Thus, the type I interferon receptor seemed to be involved even in the absence of exogenously added IFN. Furthermore, antibody treatment resulted in the activation of the ISGF3 transcription factor as monitored by reporter gene assay (Fig. 2 C) indicating an interferon-like signal resulting in the transcriptional regulation of IFIT-1 and ISG15. Exposure of endothelial cells to the IFN neutralizing antibodies was well tolerated and did not result in any apparent changes in morphology (Fig. 2 D) or signs of apoptosis (data not shown) over prolonged time periods. Concomitant pro-inflammatory activation of ECs by LPS or TNFα led to a partial reduction but could not prevent the endothelial “interferon-like” response to the antibodies (Fig. 3 A).
FIGURE 3.
“Interferon-like” response to neutralizing antibodies by different EC variants or other cell types. (A, n=2) HDMECs stimulated with pro-inflammatory mediators such as LPS at 1 μg/ml or TNFα at 100ng/ml were concomitantly treated with 12 μg/ml of anti-IFN-α mAb clone #2 for 4 h. (B, n=2) EC populations isolated from blood (BECs) or lymphatic (LECs) skin vessels or from human umbilical veins (HUVECs) were exposed to 12 μg/ml of anti-IFN-α mAb clone #2 or mIgG1 isotype control for 4 hours. (C, n=2) Comparably, freshly isolated PBMCs (peripheral blood mononuclear cells) or in vitro cultures of 293T and HT-29 cells were exposed to 12 μg/ml of anti-IFN-α mAb clone #2 for 4 hours. Total RNA and corresponding cDNA were analyzed for IFIT-1 mRNA expression by real-time PCR.
The tested cell isolates mostly consisted of a mixture of vascular and lymphatic ECs originating from human skin microvessels. When analyzed in separate cultures, comparable responses were elicited in both cell populations (Fig. 3 B). In addition, endothelial cultures derived from larger vessels (human umbilical vein endothelial cells, HUVECs) were highly responsive to the type I interferon antibodies. When investigating other human cell types, we found that the phenomenon was not restricted to ECs but could also be observed in freshly isolated PBMCs when treated with the respective IFN blocking mAbs (Fig. 3 C). In contrast, the effect was essentially absent in antibody-treated 293T or HT-29 cell cultures representing a human embryonic kidney and colon carcinoma cell line, respectively.
Since all the antibodies tested on HDMECs were established neutralizing monoclonals, we proceeded to test their blocking abilities in EC combination treatment with rIFN and mAb (Fig. 4). Four hours of incubation with rIFN-β (10 pg/ml) induced IFIT-1 mRNA levels by about 40-fold. Interestingly, addition of blocking mAbs (at 12 μg/ml) targeting IFN-β resulted in a further increase of IFIT-1 expression by 2 to 10-fold depending on the antibody applied (clone #12 and #3, respectively). A similar phenomenon was observed when combining rIFN-α with anti-IFN-α blocking mAbs. In contrast, a neutralizing antibody directed against the type I interferon receptor IFNAR completely abrogated the endothelial response to rIFN-α. Furthermore, when IFIT-1 expression was triggered by recombinant type II interferon (10000 U/ml rIFN-γ), the induction was efficiently blocked by the addition of neutralizing antibody targeting either IFN-γ or the corresponding type II receptor.
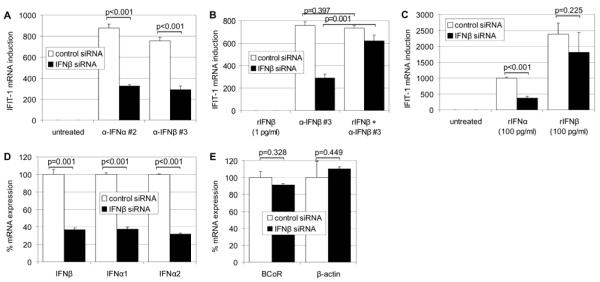
As the ability of the type I interferon antibodies to induce IFN-responsive genes was not dependent on but could be enhanced by exogenously added IFN, we hypothesized that endothelial cells might constitutively express low levels of autocrine type I interferon contributing to the effects observed. To test our hypothesis, silencing of IFN-β gene expression was achieved by transiently transfecting HDMEC cultures with double-stranded siRNAs. When HDMECs were challenged with IFN-β siRNA or non-specific (control) siRNA, no induction of IFIT-1 was observed i.e. there was no endothelial response to the uptake of chemically modified dsRNA oligonucleotides (Fig. 5 A). 24 hours post-transfection, ECs were exposed to anti-IFN-α or -β mAbs at 12 μg/ml. Induction of IFIT-1 mRNA was markedly inhibited by IFN-β gene silencing as opposed to control siRNA treatment (Fig. 5 A): IFIT-1 expression levels in response to type I interferon antibodies were reduced to about 40 %. The IFN silencing efficiency of these samples was evaluated by real-time RT-PCR analysis and equaled the effect seen for IFIT-1. However, not only IFN-β mRNA levels but also IFN-α1 and IFN-α2 transcripts were decreased by about 60 % in the presence of IFN-β siRNA (Fig. 5 D). In contrast, the detectable mRNA expression of non-related EC genes such as the transcriptional regulator BCoR or the housekeeping gene β-actin were not affected by IFN-β versus control siRNA (Fig. 5 E). To reverse the effect of IFN-β gene silencing, ECs were pretreated with “sub-threshold” concentrations of IFN-β at 1 pg/ml (corresponding to 0.1 U/ml) for 2 h and were then challenged with anti-IFN-β blocking mAb (Fig. 5 B). As expected, rIFN-β at 1 pg/ml was below the signal threshold i.e. was insufficient per se to induce IFIT-1 expression. However, the low-dose pretreatment restored EC responsiveness to anti-IFN-β neutralizing mAb to 84 %. Comparably, EC activation by high-dose (100 pg/ml) IFN-α was inhibited to 38 % due to IFN-β gene silencing, whereas high-dose IFN-β could partially overcome the block and achieve expression levels of 76 % (Fig. 5 C).
FIGURE 5.
IFN-β gene silencing inhibits the “IFN-like” response of ECs to IFN-neutralizing mAbs. Following EC transfection with IFN-β siRNA or non-specific control siRNA, cells were challenged with 12 μg/ml of anti-IFN-α mAb clone #2 or anti-IFN-β mAb clone #3 for 2 h (A, n=3). To reverse the effect of IFN-β gene silencing, EC cultures were pretreated with “sub-threshold” concentrations of rIFN-β (1 pg/ml) for 2 h with or without subsequent addition of 12 μg/ml of IFN-β mAb clone #3 for another 2 h (B, n=2). Treatment of siRNA transfected ECs with high-dose (100 pg/ml) rIFN-α or -β for 4 h (C, n=4) was followed by RNA isolation and real-time RT-PCR detection of IFIT-1 expression. (D, n=3) Silencing efficiencies for IFN-β as well as IFN-α1 and IFN-α2 were determined by real-time RT-PCR and are shown for EC samples stimulated for 2 h with 12 μg/ml anti-IFN-β mAb clone #3. Comparably, the effect of IFN-β siRNA versus non-specific control siRNA was tested on transcript levels of non-related EC genes such as BCoR and β-actin for the same samples (E, n=3).
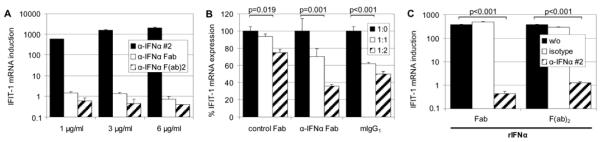
Having established that endogenously expressed type I interferon mediates the unexpected EC responsiveness to IFN-neutralizing mAbs, we then questioned whether antibody binding to Fcγ receptors on the cell surface might contribute to the effects observed. Despite the fact that all our in vitro experiments were conducted in the presence of 5 % FCS in culture medium thus supplying an excess of bovine IgG, binding of the mouse monoclonal anti-IFN antibodies to human endothelial Fcγ receptors could not be excluded. We therefore added increasing concentrations of mIgG1 isotype antibody to our reactions (Fig. 6 B): A dose-dependent decline in EC responsiveness i.e. in IFIT-1 mRNA induction by interferon neutralizing mAbs was observed. This prompted us to further investigate the requirement for the Fc domain i.e. we generated Fab as well as F(ab’)2 fragments from anti-IFN-α blocking mAb #2 by ficin digest. When comparing the Fab and F(ab’)2 fragments to the intact antibody (subjected to a mock treatment without addition of ficin protease, for control) the intact molecule retained its dose-dependent stimulatory capacity, whereas the corresponding Fab or F(ab’)2 fragments could not induce IFIT-1 mRNA expression in HDMECs (Fig. 6 A). However, the generated fragments exhibited strong neutralizing capacity for recombinant IFN-α which was not observed when Fab or F(ab’)2 fragments of the control mIgG1 isotype antibody were applied (Fig. 6 C): IFIT-1 mRNA induction in response to EC treatment with 10 pg/ml of rIFN-α was entirely abolished by 1 μg/ml of Fab or F(ab’)2 fragments from anti-IFN-α blocking mAb #2 which documents the actual interferon neutralizing potency attributed to the orginal antibody by the manufacturer. In comparison, the fragments where less potent in competing i.e. inhibiting the activity of the intact Ig molecule: Increasing the amount of Fab fragment while maintaining the concentration of intact anti-IFN-α mAb #2 at 1 μg/ml led to a 64 % drop in IFIT-1 transcript levels at a ratio of 2:1. In contrast, a comparable amount of unrelated control Fab fragment led to a non-specific quenching of IFIT-1 induction in the range of 25 % (Fig. 6 B).
FIGURE 6.
Antibody domains involved in IFIT-1 induction by IFN-neutralizing antibodies. (A, n=4) HDMECs were exposed for 4 h to 1, 3 or 6 μg/ml of intact (control-treated) anti-IFN-α mAb clone #2 or a generated Fab or F(ab’)2 fragment of clone #2. Incubation for 4 h was followed by real-time RT-PCR analysis of endothelial RNA for IFIT-1 mRNA expression. In comparison to the full-length antibody, the fragments were incapable of eliciting IFIT-1 mRNA expression (p≤0.029 for all concentrations of intact mAb versus fragments applied). (B, n=4) The full-length anti-IFN-α mAb clone #2 (1 μg/ml) was mixed at a ratio of 1:1 or 1:2 with the corresponding generated Fab fragment, a non-specific pool of unrelated Fab fragments or with an intact mIgG1 isotype control. (C, n=3) HDMEC stimulation with recombinant IFN-α at 10 pg/ml for 4 h was challenged with concomitant administration of 1 μg/ml Fab or F(ab’)2 fragments generated from anti-IFN-α mAb clone #2 or from a mIgG1 isotype antibody.
Discussion
In the work presented, we have investigated the unexpected induction of IFN-regulated genes IFIT-1 and ISG15 in primary endothelial cells which were exposed to antibodies known to neutralize type I interferon. Despite the absence of exogenously added IFN, an “IFN-like” signal was observed involving the type I interferon receptor and leading to the activation of the transcription factor ISGF3. The potency of eliciting this response varied among the antibodies applied – apparently relating to differences in binding affinities rather than the specificity for IFN-α or -β. Depending on the clone, antibody concentrations in the range of 1-10 μg/ml were sufficient to induce IFIT-1 mRNA levels comparably achieved by EC stimulation with 10 pg/ml of rIFN-β (40-fold). It is of interest to note that plasma levels of neutralizing antibodies reached in clinical applications are well within or even higher than the concentration range tested in our in vitro setting (16, 17).
Since all antibodies applied in our analyses were reported to neutralize interferon bioactivity - as confirmed by the manufacturer in cytopathic effect inhibition assays and as subsequently verified for the Fab and F(ab’)2 fragments in our experiments - we investigated the combined application of rIFN and mAb on HDMECs. The full-length type I IFN blocking antibodies were found to enhance rather than inhibit the endothelial response to IFN-α or IFN-β. In contrast, neutralizing antibodies targeting IFN-γ or the interferon receptors IFNAR and IFNGR potently repressed IFIT-1 induction by recombinant interferon. We have thus provided evidence that the ability of neutralizing antibodies to elicit an “interferon-like” response or to further enhance the effects of type I interferon, is only observed for mAbs targeting IFN-α or -β in this cellular context.
Since the response was inhibited by concomitant administration of IFNAR blocking antibodies even in the absence of exogenously added rIFN, we hypothesized that endothelial cells might constitutively express low levels of type I interferons – as previously reported for other cell types (2, 3) – which might contribute to the effects observed. When testing EC culture supernatants, interferon was not detectable at ELISA sensitivities of 4 U/ml (IFN-α) or 10 U/ml (IFN-β). However, considering that basal “sub-threshold” IFN concentrations were reported to range around 0.1 U/ml (2, 3) ELISA sensitivity may have been limiting (data not shown). We therefore proceeded to block the potential constitutive production of type I interferon by siRNA application. The approach was limited to IFN-β, since silencing of IFN-α genes is difficult to accomplish due to the variety of IFN-α subtypes that may be expressed. Furthermore, basal expression of IFN-α in fibroblasts was shown to be dependent on the constitutive IFN-β production – arguing for a predominant role of IFN-β in the low level, basal expression of type I interferons (18). Comparably, we observed that the application of IFN-β siRNA led to the concurrent down-regulation of IFN-β and IFN-α subtypes generally expressed in endothelial cells (19). Silencing of IFN-β expression in HDMECs was found to greatly reduce the “interferon-like” response to IFN blocking mAbs: IFIT-1 expression levels were reduced to about 40 % irrespective of the antibody specificity to IFN-α or -β, thus reflecting the impact of IFN-β siRNA on the overall type I interferon expression. We therefore propose that ECs maintain a basal level of IFN expression and IFNAR phosphorylation which allows for their “interferon-like” response to IFN-blocking mAbs. Since the constitutive, weak IFN signal is also known to be a prerequisite for the efficient cellular response to a high-level IFN challenge in e.g. MEFs (3), we conducted a control experiment with 100 pg/ml of rIFN-α or -β. IFIT-1 induction by high-dose interferon was similarly impaired by IFN-β silencing. These results further support our argument for an essential, basal IFN-β expression in primary endothelial cells which promotes their capacity for efficient and rapid cellular activation.
The further investigations focused on the potential involvement of Fc domains in the endothelial activation by IFN-neutralizing antibodies. As the mAbs tested were of the mIgG1 isotype, a cross-reaction with human Fc gamma receptors seemed feasible. When comparing the full-length antibody with Fab or F(ab’)2 fragments of an IFN-α blocking mAb, the fragments did not elicit an “interferon-like” response in endothelial cells thus pointing to the importance of the antibody Fc domain. Furthermore, the fragments potently inhibited IFIT-1 induction by recombinant interferon i.e. they exhibited the expected IFN neutralizing capacity and they could compete for the activity of the corresponding full-length antibody. The latter was, however, not as effective as the inhibition of rIFN. This might potentially relate to a better accessibility of intact antibody to autocrine interferon if the antibody was membrane-associated i.e. bound to Fc receptors. The observation that the EC response to intact anti-IFN mAb was also reduced in the presence of a mIgG1 isotype antibody (containing an Fc-portion), further suggested involvement of Fc receptors. Endothelial cells are known to express Fcγ receptors with an apparent heterogeneity depending on the vessel type. Various isoforms of FcγRII as well as FcγRI and FcRn have been detected on ECs with CD32 being the most prominent on HDMECs (20, 21). Yet, we could not demonstrate CD32 expression on our endothelial isolates nor block the effect of the anti-IFN antibodies by concomitant treatment with a neutralizing antibody directed against CD32 (data not shown). However, these results do not exclude the potential involvement of an endothelial Fc receptor other than CD32.
Interestingly, Fc receptors have been localized to EC caveolar membrane sections which sets them in close proximity to IFNAR molecules (3, 22). With respect to their interrelation, two settings may be envisioned. The mere local proximity of IFNAR and Fc receptors might serve to sequester autocrine interferon at the cell surface: IFN-neutralizing antibodies on FcRs might thus increase the local type I IFN concentration beyond the signal threshold provided that IFN bound to the blocking mAbs can be released i.e. passed onto IFNAR. Alternatively, a direct receptor interaction between FcR and IFNAR could occur, initiated by the anti-IFN-α/β antibodies and resulting in IFNAR activation beyond the basal “ready state”. Both players, IFNAR and Fcγ receptors have been reported to engage in diverse receptor cross-talk (2, 3, 23). Thus, whether Fc receptors are indeed involved in the “IFN-like” response to the neutralizing mAbs or whether the antibody Fc domain mediates engagement of another, as yet unidentified cellular factor is of prime interest for further investigations.
The activating potential of neutralizing IFN antibodies was observed for all types of endothelial cells tested and was not abolished by concomitant pro-inflammatory activation of ECs. In addition, the fact that IFN-α/β neutralizing antibodies did not only trigger an “IFN-like” response on quiescent cells but could also enhance the effect of a high-dose interferon challenge, emphasizes the potentially adverse systemic implications. The induction level of interferon-responsive genes varied to some extent with primary endothelial cell isolates. When other cell types were investigated, a heterogeneous response was observed which may relate to Fc repertoire or differences in the potency of the signaling cascade. Interestingly, the “IFN-like” response to monoclonal neutralizing antibodies directed against human type I interferon was also recorded for human PBMCs. While this observation extends the potential clinical impact these antibody effects might have, it seems intriguing why these effects have not been noted previously by other groups in comparable experiments on leukocytes. In preliminary investigations we have gathered an indication pointing to the importance of the antibody type: While all the antibodies presented herein were monoclonals of the mIgG1 isotype, we did not find an IFN-like response to rabbit polyclonal anti-IFN antiserum in our experimental setting (data not shown). Thus, the nature and isotype of the antibody may be a crucial determinant.
The clinical application of recombinant monoclonal antibodies targeted at IFN-α is at the current therapeutic focus of autoimmune diseases. In light of the recent launch of the first clinical trial testing an IFN-α neutralizing mAb for the treatment of SLE, a possibly pleiotropic antibody effect would seem of particular concern. Our results would suggest that enhanced neutralizing efficiency might be achievable by testing Fab or F(ab’)2 fragments versus full-length immunoglobulins in systemic settings.
Acknowledgments
We would like to thank R. Oehler and P. Petzelbauer for their helpful suggestions and thorough evaluation of this manuscript.
Footnotes
Abbreviations used in this paper: EC, endothelial cell; HDMEC, human dermal microvessel endothelial cell; HUVEC, human umbilical vein endothelial cell; IFIT-1, interferon induced protein with tetratricopeptide repeats 1; IFNAR, interferon alpha receptor; IFNGR, interferon gamma receptor; ISG15, interferon stimulated gene 15; ISGF3, interferon stimulated gene factor 3; ISRE, interferon stimulated response element; MEF, mouse embryonic fibroblast; SLE, systemic lupus erythematosus
This work was supported by the Austrian CLEXO grant no. 7 and the Austrian Science Fund FWF grant no. P20074-B13.
References
- 1.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell. Microbiol. 2006;8:907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 2.Mitani Y, Takaoka A, Kim SH, Kato Y, Yokochi T, Tanaka N, Taniguchi T. Cross talk of the interferon-alpha/beta signalling complex with gp130 for effective interleukin-6 signalling. Genes Cells. 2001;6:631–640. doi: 10.1046/j.1365-2443.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- 3.Takaoka A, Mitani Y, Suemori H, Sato M, Yokochi T, Noguchi S, Tanaka N, Taniguchi T. Cross talk between interferon-gamma and - alpha/beta signaling components in caveolar membrane domains. Science. 2000;288:2357–2360. doi: 10.1126/science.288.5475.2357. [DOI] [PubMed] [Google Scholar]
- 4.Ogasawara K, Hida S, Weng Y, Saiura A, Sato K, Takayanagi H, Sakaguchi S, Yokochi T, Kodama T, Naitoh M, De Martino JA, Taniguchi T. Requirement of the IFN-alpha/beta-induced CXCR3 chemokine signalling for CD8+ T cell activation. Genes Cells. 2002;7:309–320. doi: 10.1046/j.1365-2443.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- 5.Honda K, Mizutani T, Taniguchi T. Negative regulation of IFN-alpha/beta signaling by IFN regulatory factor 2 for homeostatic development of dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2416–2421. doi: 10.1073/pnas.0307336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichikawa E, Hida S, Omatsu Y, Shimoyama S, Takahara K, Miyagawa S, Inaba K, Taki S. Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3909–3914. doi: 10.1073/pnas.0400610101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selmi C, Lleo A, Zuin M, Podda M, Rossaro L, Gershwin ME. Interferon alpha and its contribution to autoimmunity. Curr. Opin. Investig. Drugs. 2006;7:451–456. [PubMed] [Google Scholar]
- 8.Stewart TA. Neutralizing interferon alpha as a therapeutic approach to autoimmune diseases. Cytokine Growth Factor Rev. 2003;14:139–154. doi: 10.1016/s1359-6101(02)00088-6. [DOI] [PubMed] [Google Scholar]
- 9.Chuntharapai A, Lai J, Huang X, Gibbs V, Kim KJ, Presta LG, Stewart TA. Characterization and humanization of a monoclonal antibody that neutralizes human leukocyte interferon: a candidate therapeutic for IDDM and SLE. Cytokine. 2001;15:250–260. doi: 10.1006/cyto.2001.0934. [DOI] [PubMed] [Google Scholar]
- 10.Brostjan C, Bayer A, Zommer A, Gornikiewicz A, Roka S, Benko T, Yaghubian R, Jakesz R, Steger G, Gnant M, Friedl J, Stift A. Monitoring of circulating angiogenic factors in dendritic cell-based cancer immunotherapy. Cancer. 2003;98:2291–2301. doi: 10.1002/cncr.11776. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brostjan C, Bellon T, Sobanov Y, Lopez Botet M, Hofer E. Differential expression of inhibitory and activating CD94/NKG2 receptors on NK cell clones. J. Immunol. Methods. 2002;264:109–119. doi: 10.1016/s0022-1759(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 13.Brostjan C, Anrather J, Csizmadia V, Natarajan G, Winkler H. Glucocorticoids inhibit E-selectin expression by targeting NF-κB and not ATF/c-Jun. J. Immunol. 1997;158:3836–3844. [PubMed] [Google Scholar]
- 14.Brostjan C, Anrather J, Csizmadia V, Stroka D, Soares M, Bach FH, Winkler H. Glucocorticoid-mediated repression of NF-κB activity in endothelial cells does not involve induction of IκBα synthesis. J. Biol. Chem. 1996;271:19612–19616. doi: 10.1074/jbc.271.32.19612. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez JL, Coll T, Ciudad CJ. A highly efficient electroporation method for the transfection of endothelial cells. Angiogenesis. 2004;7:235–241. doi: 10.1007/s10456-004-4180-8. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb AB, Masud S, Ramamurthi R, Abdulghani A, Romano P, Chaudhari U, Dooley LT, Fasanmade AA, Wagner CL. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. J. Am. Acad. Dermatol. 2003;48:68–75. doi: 10.1067/mjd.2003.10. [DOI] [PubMed] [Google Scholar]
- 17.Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J. Clin. Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 18.Erlandsson L, Blumenthal R, Eloranta ML, Engel H, Alm G, Weiss S, Leanderson T. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr. Biol. 1998;8:223–226. doi: 10.1016/s0960-9822(98)70086-7. [DOI] [PubMed] [Google Scholar]
- 19.Lagos D, Trotter MW, Vart RJ, Wang HW, Matthews NC, Hansen A, Flore O, Gotch F, Boshoff C. Kaposi sarcoma herpesvirus-encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood. 2007;109:1550–1558. doi: 10.1182/blood-2006-05-024034. [DOI] [PubMed] [Google Scholar]
- 20.Devaraj S, Davis B, Simon SI, Jialal I. CRP promotes monocyte-endothelial cell adhesion via Fcgamma receptors in human aortic endothelial cells under static and shear flow conditions. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1170–1176. doi: 10.1152/ajpheart.00150.2006. [DOI] [PubMed] [Google Scholar]
- 21.Groger M, Sarmay G, Fiebiger E, Wolff K, Petzelbauer P. Dermal microvascular endothelial cells express CD32 receptors in vivo and in vitro. J. Immunol. 1996;156:1549–1556. [PubMed] [Google Scholar]
- 22.Mineo C, Shaul PW. Circulating cardiovascular disease risk factors and signaling in endothelial cell caveolae. Cardiovasc. Res. 2006;70:31–41. doi: 10.1016/j.cardiores.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz Stern A, Rosales C. Cross-talk between Fc receptors and integrins. Immunol. Lett. 2003;90:137–143. doi: 10.1016/j.imlet.2003.08.004. [DOI] [PubMed] [Google Scholar]