Abstract
Background
Increased access to combination antiretroviral therapy in areas co-endemic for tuberculosis (TB) and HIV-1 infection is associated with an increased incidence of immune reconstitution inflammatory syndrome (TB-IRIS) whose cause is poorly understood.
Methods
A case-control analysis of pro- and anti-inflammatory cytokines in TB-IRIS patients sampled at clinical presentation, and similar control patients with HIV-TB prescribed cART who did not develop TB-IRIS. Peripheral blood mononuclear cells were cultured in the presence or absence of heat killed M. tuberculosis (MTB) for 6 and 24 hours.
Results
Stimulation with MTB increased the abundance of many cytokine transcripts with IL-1 Beta, IL-5, IL-6, IL-10, IL-13, IL-17A, IFN-gamma, GM-CSF and TNF being greater in stimulated TB-IRIS cultures. Analysis of the corresponding proteins in culture supernatants, revealed increased IL-1Beta, IL-2, IL-6, IL-8, IL-10, IL-12p40, IFN gamma, GM-CSF and TNF in TB-IRIS cultures. In serum, higher concentrations of TNF, IL-6, and IFN-gamma were observed in TB-IRIS patients̃ Serum IL-6 and TNF decreased during prednisone therapy in TB-IRIS patients.
Conclusions
These data suggest that cytokine release contributes to pathology in TB-IRIS. IL-6 and TNF were consistently elevated and decreased in serum during corticosteroid therapy. Specific blockade of these cytokines may be rational approach to immunomodulation in TB-IRIS.
Keywords: HIV-1, Immune reconstitution inflammatory syndrome, Immunology, Tuberculosis
Introduction
The dual epidemic of HIV-1 infection and tuberculosis (TB) is one of the most formidable health challenges in sub-Saharan Africa[1]. The annual risk of tuberculosis amongst HIV-1 infected persons in high incidence settings can exceed 20% and an estimated 1.37 million new cases of HIV-1 associated TB occurred globally in 2007[2, 3]. Combined antiretroviral therapy (cART) reduces the risk of tuberculosis in HIV-1 infected persons by 59-80% [2, 4, 5] and integrated (as opposed to sequential) anti-TB and cART therapies are associated with a mortality reduction of greater than 50%[6]. Earlier initiation of cART will be liable to further increase observation of the HIV-TB immune reconstitution inflammatory syndrome (TB-IRIS). Initially recognised in the context of non-tuberculous mycobacterial infection [7]; the risk factors of TB-IRIS include a high antigen load, low nadir CD4 count and short interval between commencement of anti-TB and cART therapies[8-13]. TB-IRIS presents temporally as two forms. Paradoxical TB-IRIS occurs in patients who are diagnosed with active TB prior to cART, are improving on TB treatment and then during early cART develop an immune-mediated paradoxical reaction with new or recurrent clinical and/or radiologic manifestations of TB. Less well defined is unmasking TB-IRIS which occurs in a subset of patients who are diagnosed with active TB while on cART (cART-associated TB), presenting with unusually accelerated or inflammatory features of TB during the first 3 months of cART[14].
Paradoxical TB-IRIS has been reported in 8-43% of patients starting cART while on TB treatment [8, 9, 11-13, 15-18]. Common features are recurrence of TB symptoms, fever, lymphadenitis, enlarging serous effusions, new or recurrent infiltrates on chest radiograph, and subcutaneous or deep tissue abscesses [19, 20]. Multiple organ systems may be involved. Frequently patients experience high fevers, persistent tachycardia and weight loss indicative of significant systemic involvement [20]. The duration of paradoxical TB-IRIS is significant: typically 2-3 months [13, 14, 21, 22], but cases lasting over one year are reported [13, 14, 21]. Mortality is difficult to estimate as most series are reported from centres experienced in supportive management: but can be as high as 10-13% [12, 23]. A consensus clinical case definition for paradoxical TB-IRIS has been published [14] that has been independently validated by two research groups [19, 24].
Given that the incidence of TB-IRIS is likely to increase, its pathogenesis is important to understand in order to inform specific therapies. Studies have tended to be either anecdotal or small, which is an important limitation considering the highly heterogeneous nature of the condition. An early feature associated with TB-IRIS was conversion of a negative TST to strongly positive after cART [18]. Hengel et al. [25] noted expansion of terminally differentiated tuberculin PPD specific CD4 T cells by flow cytometric analysis in TST positive patients during cART in the absence of TB-IRIS. Subsequent work has documented highly dynamic M. tuberculosis antigen specific Th1 T cell expansions are clearly associated with cART mediated immune restoration in TB co-infected persons [26-29], although their absence from some patients with TB-IRIS and their presence in similar patients without TB-IRIS brings into question whether the association is causal [27, 29]. One possibility is that such Th1 expansions are associated with defective restoration of regulatory T cell function. However TB-IRIS had no difference in the numbers of CD4+FoxP3+ positive cells (assumed regulatory) when compared to similar patients who did not develop the syndrome[27], observations subsequently replicated by others [30, 31]. TB-IRIS has also been associated with Killer Immunoglobulin receptor (KIR) -negative gamma delta T cells and anti phenolic glycolipid antibodies [32, 33]. In a small subset analysis, TB-IRIS was also associated with a peak of non-specific inflammatory cytokines/chemokines (TNF, IL-6, IL-1Beta, IL-10, RANTES and MCP-1) [26]. This observation led to the comment that, like H5N1 influenza infection and experimental anti-CD28 therapy [34, 35], TB-IRIS may be associated with a cytokine release syndrome [36]. We therefore conducted a case-control study to investigate cytokine gene expression and secretion in vitro, and serum cytokine concentrations in vivo to investigate this hypothesis in greater depth.
Materials and Methods
Patient enrolment and Study design
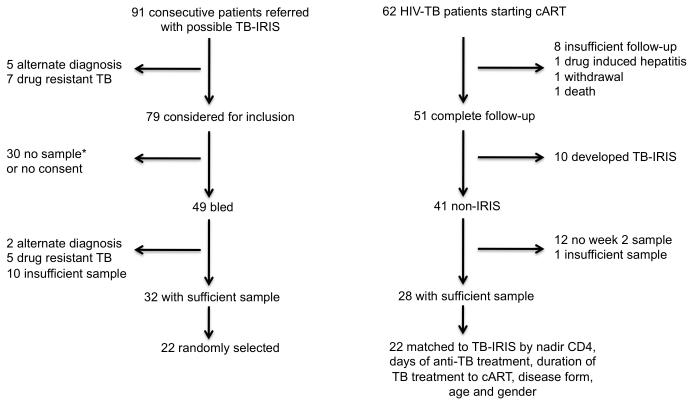
Participants with suspected TB-IRIS were recruited at GF Jooste Hospital, Cape Town, and at the Ubuntu clinic, site B Khayelitsha, South Africa between March 2005 and December 2006. The University of Cape Town Human Research Ethics Committee approved the study (REC 337/2004). TB cases were treated with 6 months of standard first line therapy consisting of isoniazid, rifampicin, pyrazinamide and ethambutol (HRZE) for 2 months followed by HR for 4 months (2HRZE/4HR) while patients with a previous history of TB had streptomycin added to their treatment regimen. All patients were prescribed first line cART combination of stavudine (d4T), lamivudine (3TC), and either efavirenz (EFZ) or nevirapine (NVP). The 22 paradoxical TB-IRIS patients and 22 non-IRIS controls were recruited subject to the following inclusion and exclusion criteria for this study. We included patients who had (1) were cART naive, (2) had clinically or microbiologically confirmed tuberculosis or conformed to WHO definitions for HIV-associated TB and responded to treatment [37] (3) in the case of TB-IRIS had a clear and confirmed diagnosis of paradoxical TB-IRIS based on a validated of consensus case definition definition [14]. (4) Patients with confirmed or suspected rifampicin resistance or multi-drug resistant TB were excluded. Cases and controls were prospectively enrolled subject to inclusion and exclusion criteria and the availability of sufficient sample to perform analyses (Figure 1). For case-control analysis, 22 randomly selected (of 32 available) sample sets drawn from patients at the time of diagnosis with paradoxical TB-IRIS were compared with 22 sample sets from non-IRIS control patients who participated in a prospective study of 62 HIV-TB patients commencing cART [27]. Observation during this study was 12 weeks (84 days) after starting cART and non-IRIS controls were selected from 28 patients with complete follow-up and sufficient sample who did not develop TB-IRIS (Figure 1). Non-IRIS controls were not individually matched to TB-IRIS cases: instead the range of nadir CD4, days of anti-TB treatment, duration of TB treatment to cART, disease form, age and gender of TB-IRIS cases were used to define similar non-IRIS controls. Baseline viral load was not available in all cases and was therefore not factored. The median interval between starting antiretroviral therapy and the onset of TB-IRIS was 14 days (table 1) and [20]. The sample selected from non-IRIS control participants was therefore after 14 days’ cART. The basis for the sample size of 22 cases and controls was dual. Effect size and variance and thus our power to detect differences between groups was estimated from prior analysis of IFN-γ ELISpot responses [27]. The second reason was pragmatic: multiples of 22 accommodate 96 well-based assays including blank and standard wells.
Figure 1. Recruitment of participants to the study.
* Patients in this limb presented to a 24 hour service. If timing meant a blood sample could not be transported to, and processed in, the laboratory within 4 hours, blood was not drawn.
Table 1.
Baseline characteristics of TB-IRIS patients and comparator non-IRIS group
| TB-IRIS | Non-IRIS | p-value | |
|---|---|---|---|
| n | 22 | 22 | NA |
| Median age (Years, IQR) |
31 (23.2 - 52.7) |
35.75 (22.2 - 54.1) |
0.11 |
| Baseline CD4 /μl, IQR |
62 (14.0 -193.0) |
42.5 (5.0 – 302.0) |
0.17 |
| Median days of TB treatment prior to cART |
56 (13.0 -186.0) |
75.5 (29.0 -173.0) |
0.06 |
| Median days of cART to IRIS or to sample |
14 (5.0 -78.0) |
14 (14 - 14) |
0.94 |
| Female n (%) | 68 | 68 | 0.99 |
| Previous TB? | 8 (36) | 3 (14) | |
| TB disease form n (%) |
|||
| Pulmonary or pleural | 12 (55) | 17 (77) | |
| Disseminated | 7 (32) | 4 (18) | |
| Pericardial | 1 (4) | - | |
| Lymphadenopathic | 2 (14) | 1 (4) | |
| Smear positive | 6 | 9 | 0.53 |
| Culture positive | 11 | 3 | 0.02 |
| Smear and culture positive |
1 | 3 | 1.0 |
| Smear and culture negative |
4 | 8 | 0.31 |
Blood processing and cell culture
PBMC were isolated from 30 ml of blood collected in Na-Heparin BD vacutainers using the Ficoll separation method. Following isolation, cells at 2.5 × 106 /ml in RPMI/10% FCS were rested overnight in an incubator at 37°C in 5% C02. The PBMC were restimulated with heat killed H37Rv Mycobacterium tuberculosis for 6 and 24 hours by infecting PBMC at a MOI=1 (H37Rv: PBMC). After re-stimulation, PBMC were harvested and lysed in 350μL of RLT-lysing buffer (Qiagen). Cell lysates from both time points were collected for RNA analysis while only the 24 hour tissue culture supernatants were preserved at −80°C until they were required for further analysis.
RNA Isolation Procedure and quantitative RT-PCR
RNA was extracted from PBMC lysates using the RNeasy Mini Kit Spin Protocol for isolation of total RNA from Animal Cells (Qiagen, Germany) as per manufacturer’s instructions and stored at −80°C until further use. Primers and probes for RT-PCR were purchased from Applied Biosystems as Predesigned inventoried assay reagents. We used the following TaqMan® Gene Expression Assays: IL-1β, Catalogue Number Hs00174097_m1; IL-2, Hs00174114_m1; IL-4, Hs00174122_m1; IL-5, Hs00174200_m1; IL-6, Hs00985639_m1; IFN-γ, Hs00174143_m1; TNF, Hs00174128_m1; IL-8, Hs01038788_m1; IL-10, Hs00174086_m1; IL-12p40, Hs01011518_m1; lL-13, Hs00174379_m1; IL-15, Hs00542562_m1; IL-17A, Hs00174383_m1; IL-18, Hs01038788_m1; GM-CSF, Hs00171266_m1; TGF-β1, Hs00171257_m1. RNA concentration was determined by Nanodrop and samples diluted to give an RNA working solution of approximately 10ng/μL. RT-PCR was performed using the TaqMan® RNA-Ct 1 Step kit Protocol (Applied Biosystems, Foster City, CA). The reaction mixture prepared using the following outlined procedure: 1 μL of PDAR Assay, 10 μL of 2X buffer, 0.5 μL of RT-enzyme, and 8.5μL of diluted mRNA μL for each reaction. Beta-actin was employed as an endogenous control throughout. RT-PCR was performed under the following universal thermal cycling conditions: Reverse transcription at 48°C for 15 mins., enzyme activation at 95°C for 10mins., denaturation at 95°C for 15sec. (40 cycles), annealing/primer extension at 60°C for 1 min. (40 cycles). To gain an idea of overall transcript abundance the difference in threshold cycle (ΔCt) values (Ct (β-Actin)-Ct (gene of interest)) were compared. Lower ΔCt values therefore reflect greater transcript abundance. The fold induction of genes was calculated by the ΔΔCt method and values normalised by log10 transformation.
Analysis of Tissue culture supernatant and Serum Samples
Assays for IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-13, IL-15, IFN-γ, TNF and GM-CSF were performed in 96-well filter plates, on the Bio-Plex platform (Bio-Rad Laboratories, Hercules, USA), using customized Milliplex™ kits (Millipore, St Charles, Missouri, USA), according to manufacturers instructions.
Interferon-gamma, IL-17A and TGF-β ELISA
Interferon-gamma in culture supernatants was measured as previously described [38] by ELISA using purified anti-human IFN-gamma antibody pairs from BD Pharmingen (Catalogue numbers 551221). IL-17A was determined using the IL-17A ELISA kit (eBioscience) as previously described [39]. TGF-beta1 was measured using a DuoSet ELISA Development System (R&D Systems DY240) according to the manufacturer’s instructions. Briefly, 96 well microplates were coated overnight with 2μg/ml mouse anti-TGF-β1 capture antibody, followed by washing and blocking the wells. Standards and samples were added undiluted (and not acid activated, in order to measure the bioactive TGF-β1) and the plate was incubated overnight at 4°C, followed by washing and addition of 300 ng/ml biotinylated chicken anti-human TGF-β1 detection antibody to all wells for 2 hours at RT. The wells were washed again and streptavidin conjugated to horseradish peroxidase was added for 30 minutes, before a final wash and development of the wells with TMB substrate solution (R&D Systems DY999). Color development was stopped using 2N H2SO4 and optical densities read at 450 nm. The sensitivity of the assay was 16-24 pg/ml.
IL-13 and IFN-γ ELISpot Assays
ELISpot Plus kits (Mabtech AB, Nacka, Sweden) for Human Interleukin-13 (Product Code: 3470-2AW-Plus) and Human IFN- γ (Product Code 3420-2AW) were used for the determination of IL-13 and IFN-γ spot forming cells respectively as previously described [28].
Statistical Analysis
Sample size calculation was based upon existing similar data. For log transformed gene fold induction typical S.D. Range from 0.1-1. The sample size therefore allowed us 80% power to detect a ≥ 2 fold difference between groups when S.D. were small and a 10 fold difference when S.D. were large. The normality of data was assessed by the D’Agostino & Pearson omnibus normality test. Medians are quoted ± IQR and means ± SD. Paired parametric data was analysed by the student’s paired t-test, or repeated measures ANOVA. Paired non-parametric variables were analysed by Wilcoxon signed rank test or Friedman test. Unpaired parametric variables were assessed using the unpaired t-test for parametric data while the Mann Whitney test was used for analysis of unpaired non-parametric data. To factor multiple comparisons p values were multiplied by n-1. GraphPad Prism® Version 5 Software (USA) was used for all statistical tests.
Results
Participant characteristics
22 TB-IRIS cases sampled on the day of presentation (a median of 14 days after commencing cART) and 22 non-IRIS controls sampled after 14 days cART were studied. Case and controls did not differ in baseline CD4, age, gender, tuberculosis disease form, days of anti-TB therapy or days of cART. Cases were more likely to have microbiologically confirmed tuberculosis (Table 1).
Cytokine transcript abundance and fold induction in vitro
PBMC from TB-IRIS and non-IRIS control patients were cultured in the presence or absence of heat killed M. tuberculosis H37Rv (MTB, MOI 1:1) and cultured for 6 and 24 hours. At the end of the culture period supernatant was aspirated, the cells were lysed, RNA extracted and assayed by quantitative RT-PCR. To gain an idea of overall transcript abundance the difference in threshold cycle (ΔCt) values (Ct (β-Actin)-Ct (gene of interest)) were compared. Lower values indicate high transcript abundance of the gene of interest (Tables 2 and 3).
Table 2.
delta CT values for cytokine genes after 6 hours of in vitro culture in the presence or absence of heat killed M. tuberculosis
| Unstimulated | Stimulated | unstimulated vs. stimulated |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | IRIS | IQR | non- IRIS |
IQR | p | IRIS | IQR | non- IRIS |
IQR | p | IRIS | non- IRIS |
| IL-1β | 5.9 | 4.4-6.9 | 5.0 | 3.5-6.6 | 0.193 | −2.6 | −2.9-1.4 | −1.2 | −2.1-0.2 | 0.01 | <0.001 | <0.001 |
| IL-2 | 14.7 | 13.9-16.0 | 14.5 | 13.9-15.1 | 0.474 | 9.3 | 7.4-11.5 | 12.2 | 9.5-13.6 | 0.005 | <0.001 | <0.001 |
| IL-4 | 16.5 | 15.5-17.8 | 15.9 | 14.6-17.2 | 0.136 | 15.0 | 13.9-16.7 | 15.8 | 14.8-16.8 | 0.17 | <0.001 | 0.889 |
| IL-5 | ND | ND | NA | ND | ND | NA | NA | NA | ||||
| IL-6 | 12.1 | 10.4-14.1 | 10.1 | 8.4-12.2 | 0.01 | 2.6 | 1.8-3.4 | 3.5 | 2.0-5.2 | 0.08 | <0.001 | 0.001 |
| IL-8 | 2.9 | 1.4-4.3 | 2.2 | 0.9-2.2 | 0.53 | −1.5 | −3.2-0.0 | −0.6 | −2.9-1.8 | 0.49 | <0.001 | 0.02 |
| IL-10 | 7.2 | 6.4-8.2 | 6.6 | 5.8-7.6 | 0.04 | 5.9 | 3.4-7.1 | 6.1 | 5.0-7.5 | 0.25 | 0.003 | 0.27 |
| IL-12p40 | 16.4 | 15.2-17.4 | 14.7 | 13.1-16.4 | 0.007 | 10.7 | 9.1-11.8 | 11.6 | 9.8-13.2 | 0.25 | <0.001 | <0.001 |
| IL-13 | 18.8 | 17.2-20.3 | 16.4 | 14.7-18.5 | <0.001 | 12.3 | 11.1-13.4 | 14.1 | 13-15.6 | 0.009 | <0.001 | 0.004 |
| IL-15 | 10.5 | 10.0-11.4 | 9.8 | 9.2-10.2 | <0.001 | 7.8 | 7.2-8.8 | 8.3 | 7.8-9.5 | 0.09 | <0.001 | 0.003 |
| IL-17A | 19.8 | 18.7-20.8 | 17.6 | 15.4-19.0 | 0.001 | 13.7 | 12.2-15.7 | 16.3 | 14.4-19.0 | 0.003 | <0.001 | 0.506 |
| IL-18 | 18.2 | 14.6-20.0 | 17.5 | 13.7-19.0 | 0.21 | 14.8 | 11.2-18.3 | 12.2 | 9.7-14.4 | 0.03 | 0.003 | 0.002 |
| IFN-γ | 10.4 | 9.6-11.8 | 9.7 | 9.3-10.8 | 0.039 | 5.3 | 4.2-6.7 | 7.5 | 6.1-8.0 | 0.004 | <0.001 | <0.001 |
| GM-CSF | 13.9 | 12.7-15.1 | 13.5 | 11.7-14.5 | 0.163 | 4.8 | 2.7-6.7 | 6.9 | 4.6-9.4 | 0.038 | <0.001 | 0.001 |
| TGF-β | 3.7 | 3.2-4.1 | 3.3 | 3.1-3.6 | 0.03 | 3.5 | 2.9-4.0 | 3.3 | 3.1-3.9 | 0.71 | 0.17 | 0.61 |
| TNF | 7.8 | 7.3-8.3 | 7.7 | 7.1-8.3 | 0.5 | 2.2 | 1.0-2.2 | 3.6 | 2.5-4.9 | 0.008 | <0.001 | <0.001 |
ND = Not detected, NA = not applicable. p values are uncorrected for multiple comparisons in the table but multiple comparisons we factored in the analysis (see text). Lower values indicate high transcript abundance of the gene of interest.
Table 3.
delta CT values for cytokine genes after 24 hours of in vitro culture in the presence or absence of heat killed M. tuberculosis
| Unstimulated | Stimulated | unstimulated vs. stimulated |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | IRIS | IQR | non- IRIS |
IQR | p | IRIS | IQR | non- IRIS |
IQR | p | IRIS | non- IRIS |
| IL-1β | 6.4 | −1.3-7.8 | 6.7 | 4.0-8.6 | 0.202 | −2.7 | −3.6-1.7 | −0.6 | −1.9-0.2 | <0.001 | < 0.001 | <0.001 |
| IL-2 | 11.5 | 9.0-12.9 | 12.7 | 12.2-13.7 | 0.014 | 8.3 | 7.5-9.4 | 10.0 | 7.0-12.7 | 0.129 | < 0.001 | <0.001 |
| IL-4 | 15.5 | 14.9-16.7 | 16.1 | 15.3-17.1 | 0.409 | 14.4 | 14.1-15.8 | 15.4 | 14.0-16.6 | 0.382 | 0.001 | 0.004 |
| IL-5 | 20.2 | 19.3-20.6 | 18.9 | 18.0-20.0 | 0.003 | 12.1 | 11.4-14.7 | 17.0 | 13.9-20.1 | 0.001 | < 0.001 | 0.13 |
| IL-6 | 12.0 | 4.0-13.8 | 12.4 | 9.7-13.9 | 0.78 | 1.3 | 0.3-3.7 | 4.6 | 3.1-5.9 | <0.001 | < 0.001 | <0.001 |
| IL-8 | 3.0 | −0.2-3.8 | 2.6 | 0.4-5.1 | 0.48 | −3.7 | −4.6-2.1 | −1.5 | −2.8-1.6 | 0.006 | < 0.001 | 0.002 |
| IL-10 | 6.9 | 5.1-8.1 | 6.9 | 6.0-7.1 | 0.74 | 4.9 | 3.1-6.4 | 6.8 | 5.9-8.5 | <0.001 | < 0.001 | 1,0 |
| IL-12p40 | 16.2 | 12.1-17.5 | 16.9 | 13.9-18.1 | 0.20 | 8.1 | 6.5-9.4 | 10.0 | 7.8-13.3 | 0.005 | < 0.001 | <0.001 |
| IL-13 | 17.6 | 13.5-10.3 | 17.6 | 14.8-18.4 | 0.61 | 8.8 | 7.9-10.0 | 11.0 | 9.4-13.7 | 0.002 | < 0.001 | <0.001 |
| IL-15 | 9.6 | 8.6-10.5 | 10.6 | 10.1-11.4 | 0.005 | 8.4 | 7.9-8.7 | 8.6 | 8.0-9.6 | 0.14 | 0.004 | <0.001 |
| IL-17A | 18.3 | 16.3-19.5 | 18.2 | 17.7-19.4 | 0.698 | 12.7 | 11.5-14.1 | 13.9 | 12.8-15.3 | 0.048 | < 0.001 | <0.001 |
| IL-18 | 9.9 | 9.2-10.4 | 10.5 | 9.0-12.6 | 0.14 | 9.9 | 8.9-10.7 | 9.6 | 8.6-11.4 | 0.85 | 0.45 | 0.36 |
| IFNγ | 9.4 | 6.0-10.6 | 9.6 | 8.6-10.7 | 0.259 | 3.3 | 1.8-5.1 | 6.8 | 4.3-8.1 | 0.002 | < 0.001 | <0.001 |
| GM-CSF | 11.8 | 8.6-14.3 | 13.6 | 10.8-15.0 | 0.382 | 2.8 | −0.9-3.7 | 5.0 | 3.9-6.8 | <0.001 | < 0.001 | <0.001 |
| TGF-β | 4.7 | 4.4-5.4 | 4.5 | 4.1-4.9 | 0.1 | 4.6 | 4.3-4.9 | 4.8 | 4.5-5.6 | 0.16 | 0.18 | 0.004 |
| TNF | 7.3 | 2.7-7.90 | 8.2 | 7.7-8.5 | <0.001 | 1.4 | 1.1-2.0 | 3.2 | 2.4-4.9 | <0.001 | < 0.001 | 0.002 |
p values are uncorrected for multiple comparisons in the table but multiple comparisons we factored in the analysis (see text). Lower values indicate high transcript abundance of the gene of interest
At 6 hours the RNA for several of the 16 genes evaluated (IL-13, IL-15 and IL-17A) in unstimulated cells from non-IRIS patients tended to be slightly but significantly higher than TB-IRIS patients (Table 2). Stimulation with MTB increased the abundance of all transcripts studied in both groups with the exception of TGF-β. After Bonferroni (n-1, 15) correction of multiple comparisons, the abundance of IL-17A remained significantly greater in stimulated TB-IRIS cultures (pcorr. = 0.045, table 2).
At 24 hours the RNA for IL-5 in unstimulated cells from non-IRIS patients was significantly higher than TB-IRIS and conversely the levels of IL-2, IL-15 and TNF higher in TB-IRIS (Table 3). Stimulation with MTB increased the abundance of all transcripts studied in both groups with the exception of IL-18 and TGF-β (whose level significantly decreased in non-IRIS). The abundance of IL-1β, IL-5, IL-6, IL-10, IL-13, IL-17A, IFN-γ, GM-CSF and TNF were significantly greater in stimulated TB-IRIS cultures (pcorr. ≤ 0.03, table 3).
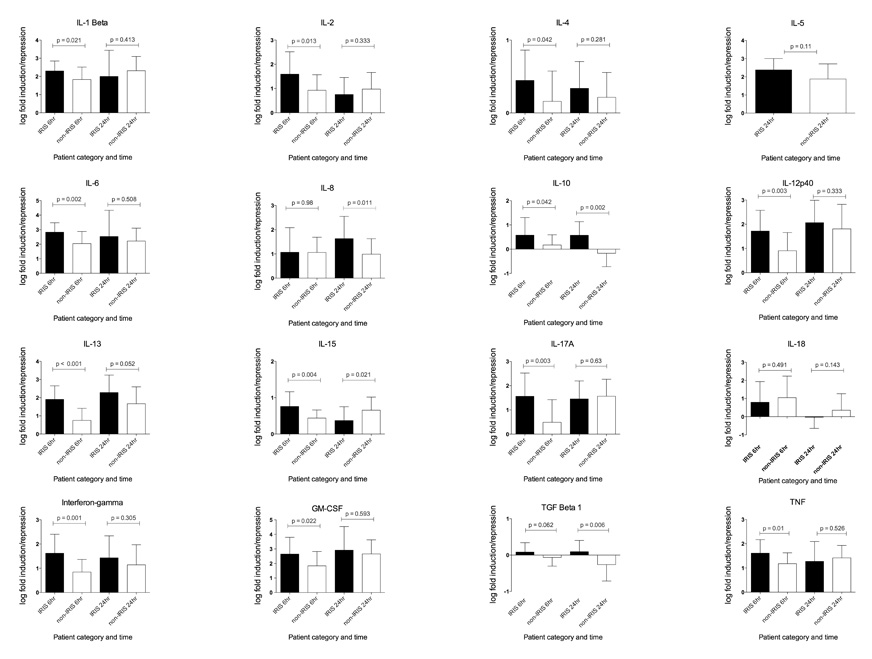
The fold induction of genes in stimulated relative to unstimulated cultures at the same time point was calculated by the ΔΔCt method and values normalised by log10 transformation (Figure 2). In TB-IRIS patients at 6 hours IL-1β, IL-6 and GM-CSF were more than 100 fold induced and IL-2, IL-8, IL-12p40, IL-13, IL-17A, IFN-γ and TNF more than 10-fold. Induction was higher in TB-IRIS than non-IRIS for IL-1β, IL-2, IL-4, IL-6, IL-10, IL-13, IL-15, IL-17A, IFN-γ, GM-CSF and TNF (p ≤ 0.05). After Bonferroni correction of p values the differences in IL-6, IL-12p40, IL-13, IL-17A and IFN-γ (pcorr ≤ 0.05) remained significant.
Figure 2. Average log fold induction of cytokine genes by heat killed M. tuberculosis in TB-IRIS and non-IRIS patients.
PBMC from 22 TB-IRIS and 22 non-IRIS control patients were cultured in the presence or absence of heat killed M. tuberculosis H37Rv for 6 and 24 hours. At the end of the culture period the cells were lysed, RNA extracted and used in quantitative RT-PCR. The fold induction of genes was calculated by the ΔΔCt method and values normalised by log10 transformation. At 6 hours, induction was significantly higher in TB-IRIS than non-IRIS for IL-1β, IL-2, IL-4, IL-6, IL-10, IL-13, IL-15, IL-17A, IFN-γ, GM-CSF and TNF (p ≤ 0.05). At 24 hours significant differences between TB-IRIS and non-IRIS were present for IL-8, IL-10, IL-15 (higher in non-IRIS) and TGF-β1. p values are uncorrected in this figure with significant associations shown in red
At 24 hours the fold induction in TB-IRIS patients tended to be similar to the 6 hour time point (with the exception of IL-2, IL-15 and IL-18 which showed a reduction). However fewer differences between TB-IRIS and non-IRIS were observed due to increases that occurred between 6 and 24 hours in the latter group. Significant differences between TB-IRIS and non-IRIS persisted however for IL-8, IL-10, IL-15 and TGF-β1. TGF-β1 mRNA levels however tended to be minimally influenced by the presence of MTB and all fold values were close to baseline.
Cytokines secreted into cell culture supernatant
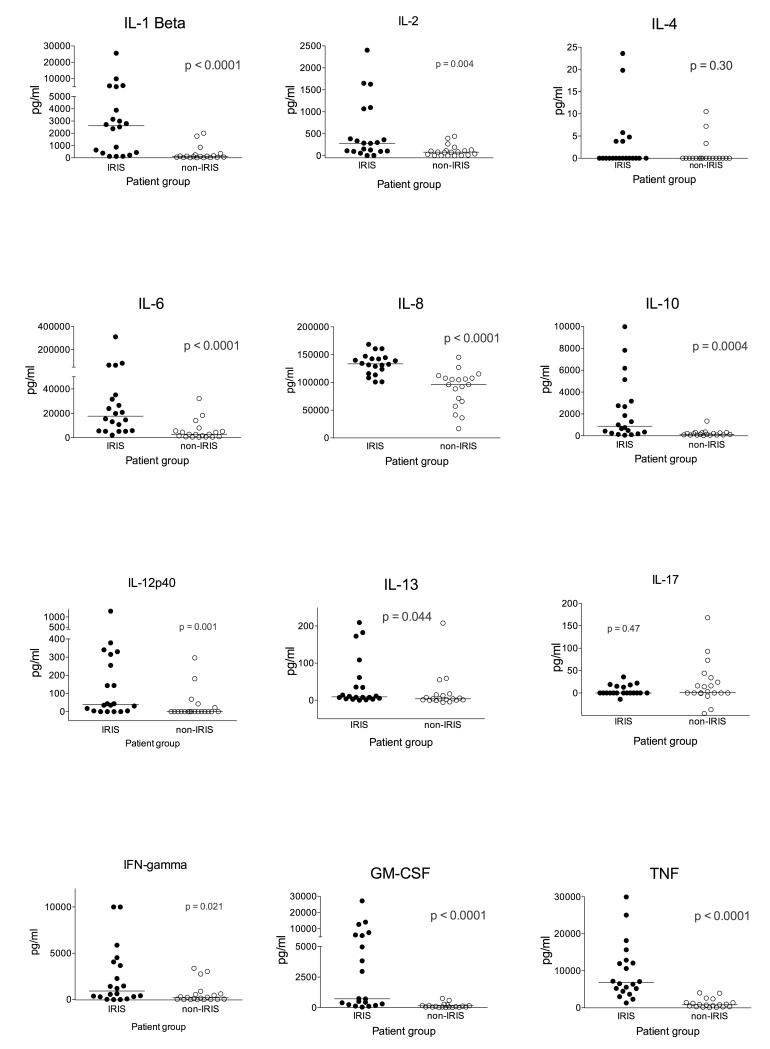
The corresponding tissue culture supernatants for 20 IRIS and 19 non-IRIS patients were assayed for cytokine content by multiplex analysis (with the exception of IL-17A and TGF-β which were analysed by ELISA). Beads for IL-18 were unavailable and as transcript levels did not differ between groups this cytokine was not analysed further. IL-5 and IL-15 protein were undetectable except in small quantities in 3 samples in the case of IL-5 (data not shown). IL-4 and IL-17, whose levels were close to the lower detection limits, did not differ between TB-IRIS and non-IRIS (Figure 3). Constitutive secretion of TGF-β was similar in IRIS and non-IRIS groups, the concentration tended to decrease slightly on restimulation in both groups. Otherwise supernatant cytokine concentrations were consistently and significantly higher in TB-IRIS. After correction of p-values for multiple comparisons the largest and significant fold differences were in IL-12p40, IL-1β, GM-CSF, TNF, IL-10, IL-6, IL-2 and IL-8 (pcorr ≤ 0.04). Of these cytokines only IL-2 is exclusively of lymphoid origin, the others being predominantly the products of myeloid cells.
Figure 3. Cytokine content of tissue culture supernatants.
Culture supernatants arising from 20 IRIS and 19 non-IRIS controls were assayed by Luminex. IL-4 and IL-17, whose levels were close to lower detection limits, did not differ between TB-IRIS and non-IRIS. Otherwise levels were consistently and significantly higher in TB-IRIS. After correction of p-values for multiple comparisons the largest and significant fold differences were in IL-12p40, IL-1β, GM-CSF, TNF, IL-10, IL-6, IL-2 and IL-8. p values are uncorrected in this figure with significant associations shown in red
Internal validity of luminex determination of IFN-γ and IL-13
Luminex analysis is a convenient and powerful technology but has on occasions been reported to correlate poorly with ELISA (arbitrarily defined as gold standard). There was insufficient sample to secondarily test all analytes. However the same supernatant and cells were additionally assayed for IFN-γ secretion by both ELISA and ELISpot using the same stimulus, MTB H37Rv. Correlation between ELISA and Luminex values was very strong (Spearman r = 0.82, p < 0.0001), although Luminex consistently rendered higher values. IFN-γ ELISpot also correlated positively and significantly with both ELISA (r = 0.49, p = 0.005) and Luminex (r = 0.5, p = 0.003). Furthermore there was similar association between TB-IRIS and elevation of IFN-γ by ELISA (325, IQR 90-750 pg/ml versus 57, IQR 0-213 pg/ml, p = 0.005) and by ELISpot (730, IQR 240-1665 SFC/106 PBMC versus 20, IQR 0-362 SFC/106 PBMC, p = 0.001). IL-13 ELISpot (using MTB H37Rv) was also performed on PBMC from a subset 16 IRIS and 10 non-IRIS patients. Like the ELISA (Figure 3) the commonest value was 0 SFC/106 PBMC with no significant difference between IRIS and non-IRIS groups (p = 0.38).
Cytokines in serum samples
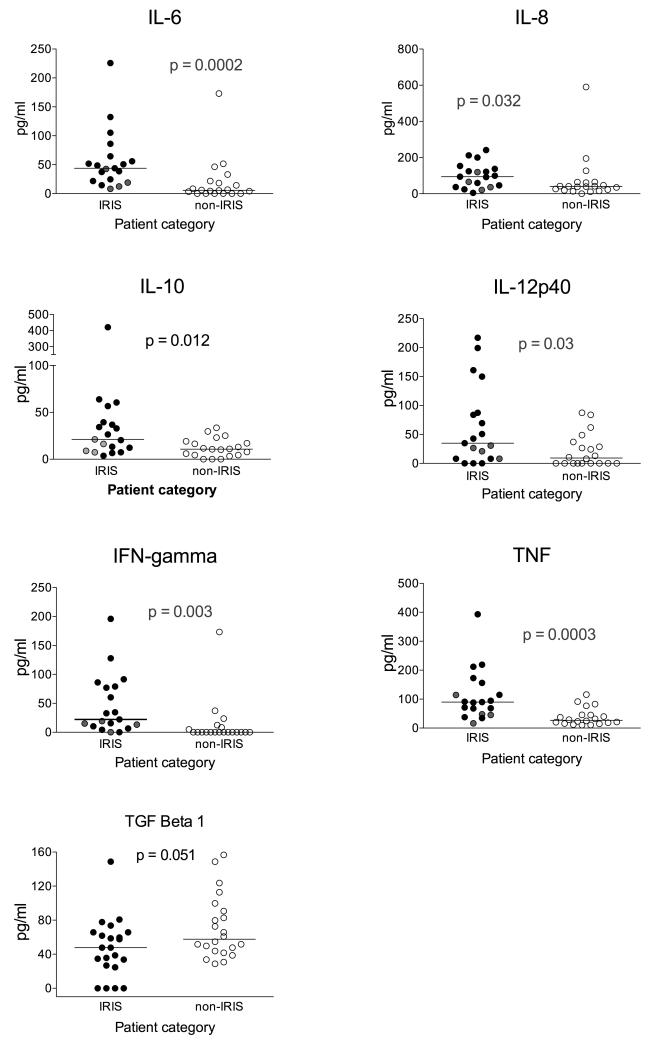
Based upon the quantitative RT-PCR and supernatant results we next assayed by luminex the concentration of the eleven most consistently discriminatory cytokines in serum samples taken from the same patients at the same time point. Serum concentrations of IL-1β, IL-2, IL-13 and GM-CSF were consistently close to the lower limit of assay detection and did not significantly differ between TB-IRIS and non-IRIS groups (data not shown). Serum concentrations of IL-6, IL-8, IL-10, IL-12p40, IFN-γ and TNF were significantly higher in the serum of TB-IRIS patients whilst TGF-β showed a trend towards higher values in non-IRIS (Figure 4). After correction of p-values for multiple comparisons the largest and significant fold differences were in TNF, IL-6, and IFN-γ (pcorr ≤ 0.02).
Figure 4. Serum cytokine concentrations.
Luminex assays of the serum concentration of 19 IRIS and 20-non-IRIS controls for the most consistently discriminatory cytokines taken from the same patients at the same time. After correction of p-values for multiple comparisons the largest and significant fold differences were in TNF, IL-6, and IFN-γ. Four TB-IRIS patients (shown in grey circles) were classified as having localized (usually lymphadenopathic) disease. In these patient a clear trend towards lower serum cytokine concentrations was seen with 22/24 (92%, 95% CL 74-97%) cytokine values falling on or below the median. p values are uncorrected in this figure.
Stratification by localized or disseminated disease
Most TB-IRIS patients in this study had either TB-IRIS with pronounced systemic signs or disseminated TB (≥ 2 organs involved, table 1). However four patients were classified as having disease localized to one anatomic site without systemic involvement. In these patients a clear trend towards lower serum cytokine concentrations was seen with 22/24 (92%, 95% CL 74-97%) cytokine values falling on or below the median (Figure 4). Thus the elevation or otherwise of cytokines appears to relate partially to TB-IRIS extent.
Effect of steroid therapy on cytokines in serum samples
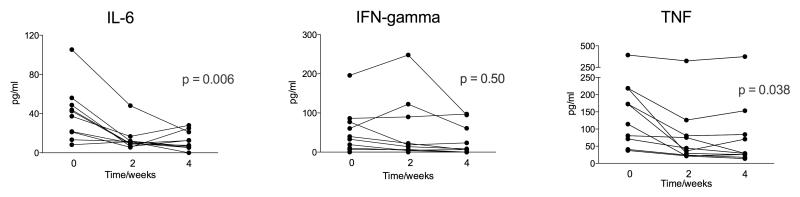
Expert opinion favours the use of adjunctive corticosteroid therapy in some cases of TB-IRIS, an opinion recently provided greater evidence by our randomized placebo-controlled trial of prednisone in TB-IRIS that showed this therapy was associated with more rapid resolution of symptoms (Meintjes G et al. accepted for publication). To better explore cause and effect we therefore analysed serum concentrations of IFN-γ, IL-6 and TNF in a subset of 10 TB-IRIS trial participants who received corticosteroid therapy for 4 weeks (1.5mg/kg daily for 2 weeks then 0.75 mg/kg for 2 weeks). The concentrations of IL-6 (p = 0.006) and TNF (p = 0.038) significantly declined whereas no effect on IFN-γ concentrations was observed (Figure 5).
Figure 5. Effect of steroid therapy on cytokines in serum samples.
Serum concentrations of IFN-γ, IL-6 and TNF were determined in a subset of 10 TB-IRIS who received corticosteroid therapy for 4 weeks. The concentrations of IL-6 and TNF significantly declined whereas no effect on IFN-γ concentrations was observed.
Discussion
We have conducted a case-control analysis of 22 TB-IRIS patients sampled on the day of clinical presentation compared with 22 similar controls in order to determine whether dysregulated cytokine responses might contribute to pathology and symptoms in this condition. Stimulation of PBMC with M. tuberculosis increased the abundance of the majority of transcripts with IL-1β, IL-5, IL-6, IL-10, IL-13, IL-17A, IFN-γ, GM-CSF and TNF being significantly greater in stimulated TB-IRIS cultures at either the 6 or 24 hour time points. MTB stimulated gene induction in vitro was significantly greater in TB-IRIS patients for IL-6, IL-10, IL-12p40, IL-13, IL-17A and IFN-gamma. In tissue culture supernatants, the concentrations of IL-12p40, IL-1Beta, IL-2, IL-6, IL-8, IL-10, IL-12p40, IFN gamma, GM-CSF and TNF were higher in TB-IRIS patients. In serum, significantly higher concentrations in TB-IRIS patients were observed for TNF, IL-6, and IFN-gamma and the serum concentrations of IL-6 and TNF decreased during prednisone therapy of TB-IRIS. Thus many pro- and anti-inflammatory cytokine transcript and protein levels are elevated in TB-IRIS patients strongly suggesting that cytokine release contributes to pathology and symptoms in this condition. IL-6 and TNF were elevated under all conditions and decreased in serum during corticosteroid therapy such that blockade of these cytokines may be a novel and rational approach to immunomodulation in TB-IRIS.
The cytokine release syndrome (sometimes referred to as a cytokine storm or hypercytokinaemia) occurs in a number of infectious and non-infectious diseases including graft versus host disease (GVHD) [40], acute respiratory distress syndrome (ARDS) [41], sepsis [42], H5N1 influenza [34] and the systemic inflammatory response syndrome (SIRS) [43] . The experimental drug TGN1412 also caused serious acute illness likely to be driven by cytokines when given to six participants in a Phase I trial [35]. The syndrome does not appear to have a quantitative definition but is characterised as including fever, hypotension, increased endothelial permeability, oedema and vasodilatation. Multiple cytokine mediators of inflammation are described as being elevated. TB-IRIS can be of acute onset especially the unmasking form and fatal ARDS is described [44-46]. Patients with paradoxical TB-IRIS frequently have prolonged fever and tachycardia and appear to be at increased risk of venous thromboembolism [20, 47]. Whilst the concentrations of serum cytokines that we report are not as high for example as those reported in the TGN1412 (anti-CD28) trial or in H5N1 we suggest that the exaggerated cytokine responses we have observed in TB-IRIS patients compared to similar patients who do not experience the syndrome may contribute substantially to pathogenesis.
Antitretroviral therapy effectively suppresses HIV-1 replication and thereby allows recovery of CD4 lymphocyte numbers and function with the most rapid CD4 rise occurring early in therapy [28, 48]. It is thus logical to investigate whether dysregulated CD4 responses contribute to TB-IRIS. Very large antigen specific Th1 CD4 expansions accompany cART mediated immune restoration in both TB-IRIS patients and similar co-infected persons who do not develop the syndrome [26, 27]. In keeping with these observations we observed an increase in IFN-γ transcript abundance, fold induction and both secreted and serum cytokine in TB-IRIS patients (Tables 2,3; Figures 3,4). We also documented increased IL-12p40 fold induction and secreted cytokine and also increased IL-2 in tissue culture supernatants from TB-IRIS patients. However our observations suggest that lymphocyte subsets other than Th1 may also contribute. Thus transcripts from the ‘Th2’ genes IL-5 and IL-13 were elevated in TB-IRIS patients although that did not translate into elevated protein concentrations. It is also interesting to note that the transcript of IL-17A was, after correction for multiple comparisons, the only significantly elevated in TB-IRIS patients at 6 hours (table 2); a difference reflected at 24 hours and in fold induction. Again protein concentrations were low and did not differ between groups. IL-17 is markedly pro-inflammatory and has been implicated in the early protective immune response to TB [49]. In humans IL-17 secretion in response to TB appears mediated by a distinct T cell subset with phenotypic characteristics of long-lived central memory cells [39]. The lack of protein secretion we observed may have been due to IFN-γ mediated suppression of IL-17 in vitro [50]. IL-17 is indirectly chemotactic for neutrophils [51] and the cold abscesses that occur in TB-IRIS are characterised by the presence of neutrophils [52]. Further work on the potential for early IL-17 production to contribute to inflammation in TB-IRIS may therefore be of interest.
It is also of interest that elevation of cytokines predominantly of myeloid (e.g. IL-1β, IL-6, IL-8 and IL-12p40) or of dual myeloid/lymphoid (e.g. TNF, GM-CSF and IL-10) origin characterise TB-IRIS. Separating cause and effect in observational studies is difficult but there is a report of unmasking TB-IRIS in which fatal bronchiolitis obliterans was associated with macrophage infiltration [46]. cART partially restores all aspects of immune function and others have speculated on the role of the macrophage in this condition [53]. It is certainly striking that we have observed TB-IRIS after as few as 3 days cART: well before CD4 restoration can be substantial. It has been previously noted that the numbers of both myeloid and plasmacytoid dendritic cells increase during cART [54]. We were also interested to note consistent elevation of immunoregulatory IL-10: the hypothesis that a deficiency of regulation may contribute to TB-IRIS is attractive but again not supported by elevation, rather than suppression, of IL-10.
Whilst ours is one of the largest studies of the pathogenesis of TB-IRIS to date, it remains apparent from the distribution of results we obtained that this is a heterogeneous condition and we caution against causal interpretations of association in observational studies. The purpose of the study was to evaluate differences at presentation of TB-IRIS comparing results to similar participants who did not experience the condition. Prediction of TB-IRIS did not form part of the scope of the study: we are pursuing this in other studies. We do however have the advantage of preliminary data that suggests corticosteroid therapy was associated with a decrease in serum TNF and IL-6 concentrations (Figure 5). It is known that this therapy leads to more rapid symptom resolution in TB-IRIS (Meintjes G et al. in press). Not all cases of TB were culture confirmed although all did conform to WHO guidelines on the diagnosis of HIV associated TB [37] and all showed response to anti-TB therapy prior to cART. TB-IRIS remains a diagnosis of exclusion: no patients with known drug resistant TB were included and the case definition we employed for TB-IRIS has been twice independently validated [14, 19, 24]. CD4 counts were not re-evaluated at the time of TB-IRIS (and after the corresponding time interval in non-IRIS controls), thus the relationship of cytokine release to the increase in CD4 could not be evaluated. Luminex analysis is a relatively new technology that permits a much greater number of analytes to be assayed than hitherto possible. We based our reagent selection on experience when comparing luminex with ELISA [55, 56] and internal validity at least of IFN-γ estimations by both ELISpot and ELISA was good. Multiple comparisons have also been made but we have restricted our conclusions to results upon which stringent Bonferroni correction was applied and also the biological plausibility of being elevated in all assays that we have performed.
That we identified IL-6 and TNF secretion as elevated in all circumstances (Figure 6) and potentially amenable to immune modulation is consistent with the finding that polymorphism in these genes may associate with the risk of IRIS [57] and the elevation of IL-6 in other forms of IRIS [58, 59]. IL-6 is a key driver of the acute phase response and we have previously documented that the C-reactive protein (CRP) is invariably elevated at presentation of TB-IRIS [20]. TNF has consistently been associated with both protection and pathology in TB [60-62]. Recent case reports have documented a beneficial effect of TNF blockade on paradoxically deteriorating TB in HIV-1 uninfected patients [63, 64]. However a clinical study of anti-TNF antibodies in TB-IRIS would face a number of difficult issues because it is recognized this therapy has a prolonged half-life and also leads to the reactivation of TB [61]. A phase II study in patients with known drug sensitive TB undergoing severe TB-IRIS in whom corticosteroid therapy was either ineffective or contraindicated might be justifiable. But in most environments in which TB-IRIS is encountered the new knowledge that the condition is contributed to by antigen load and attendant exaggerated cytokine release encourages reinvestigation of the possibility of co-existent TB drug resistance, optimization of anti-TB therapy, and intensified supportive care together with the judicious use of adjunctive corticosteroid therapy [52].
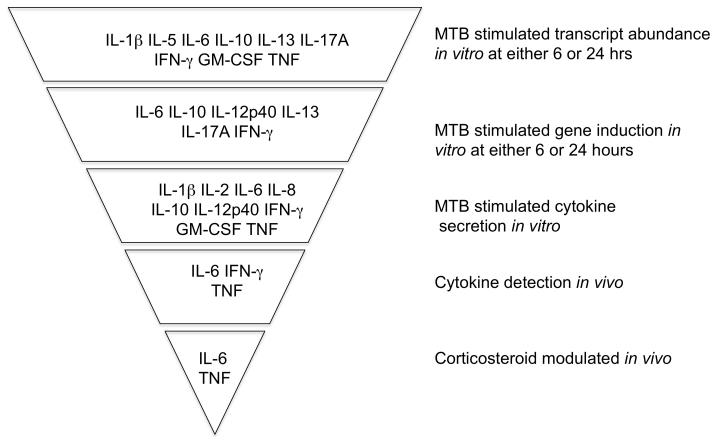
Figure 6. Summary of results and implication of TNF and IL-6 in the pathogenesis of TB-IRIS.
Whilst many pro- and anti-inflammatory cytokine transcript and protein levels were elevated in TB-IRIS patients according to experimental circumstances only IL-6 and TNF were elevated in all circumstances. Thus blockade of IL-6 or TNF may be a rational approach to immunomodulation in this condition.
Acknowledgements
This work was supported by the Wellcome Trust (references 084323, 081667, 088316, 084670, 083226). Additional support was provided by the Medical Research Councils of the UK and South Africa, by the European Union (Sante/2006/105-061) and by the European and Developing Countries Clinical Trials Partnership (EDCTP 060613). Priscilla Mouton and Musaed Abrahams are especially thanked for assistance with recruitment, and clinical assessment of, study participants. Anali Conessa Botella is thanked for helpful comments on the manuscript.
References
- 1.Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007;370(9604):2030–2043. doi: 10.1016/S0140-6736(07)61262-8. [DOI] [PubMed] [Google Scholar]
- 2.Lawn S, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Global tuberculosis control - Epidemiology, Strategy, financing. WHO; Geneva: 2009. WHO/HTM/TB/2009.411. [Google Scholar]
- 4.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 5.Brinkhof MW, Egger M, Boulle A, May M, Hosseinipour M, Sprinz E, Braitstein P, Dabis F, Reiss P, Bangsberg DR, Rickenbach M, Miro JM, Myer L, Mocroft A, Nash D, Keiser O, Pascoe M, van der Borght S, Schechter M. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007;45(11):1518–1521. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Karim Q Abdool. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS. 1992;6(11):1293–1297. doi: 10.1097/00002030-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Breen RA, Smith CJ, Bettinson H, Dart S, Bannister B, Johnson MA, Lipman MC. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59(8):704–707. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, Mvondo D Mvondo, Longuet P, Leport C, Vilde JL. Determinants of Immune Reconstitution Inflammatory Syndrome in HIV Type 1-Infected Patients with Tuberculosis after Initiation of Antiretroviral Therapy. Clin Infect Dis. 2004;39(11):1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 10.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr., Hamill RJ. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19(4):399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 11.Manosuthi W, Kiertiburanakul S, Phoorisri T, Sungkanuparph S. Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J Infect. 2006;53(6):357–363. doi: 10.1016/j.jinf.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21(3):335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 13.Burman W, Weis S, Vernon A, Khan A, Benator D, Jones B, Silva C, King B, LaHart C, Mangura B, Weiner M, El-Sadr W. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11(12):1282–1289. [PubMed] [Google Scholar]
- 14.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, John L, van der Loeff MS, Reiss P, Lynen L, Janoff EN, Gilks C, Colebunders R. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tansuphasawadikul S, Saito W, Kim J, Phonrat B, Dhitavat J, Chamnachanan S, Pitisuttithum P. Outcomes in HIV-infected patients on antiretroviral therapy with tuberculosis. Southeast Asian J Trop Med Public Health. 2007;38(6):1053–1060. [PubMed] [Google Scholar]
- 16.Serra FC, Hadad D, Orofino RL, Marinho F, Lourenco C, Morgado M, Rolla V. Immune reconstitution syndrome in patients treated for HIV and tuberculosis in Rio de Janeiro. Braz J Infect Dis. 2007;11(5):462–465. doi: 10.1590/s1413-86702007000500004. [DOI] [PubMed] [Google Scholar]
- 17.Baalwa J, Mayanja-Kizza H, Kamya MR, John L, Kambugu A, Colebunders R. Worsening and unmasking of tuberculosis in HIV-1 infected patients after initiating highly active anti-retroviral therapy in Uganda. Afr Health Sci. 2008;8(3):190–195. [PMC free article] [PubMed] [Google Scholar]
- 18.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158(1):157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 19.Manosuthi W, Van Tieu H, Mankatitham W, Lueangniyomkul A, Ananworanich J, Avihingsanon A, Siangphoe U, Klongugkara S, Likanonsakul S, Thawornwan U, Suntisuklappon B, Sungkanuparph S. Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2009;23(18):2467–2471. doi: 10.1097/QAD.0b013e32832f7b59. [DOI] [PubMed] [Google Scholar]
- 20.Meintjes G, Rangaka MX, Maartens G, Rebe K, Morroni C, Pepper DJ, Wilkinson KA, Wilkinson RJ. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis. 2009;48(5):667–676. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olalla J, Pulido F. Paradoxical response to antitubercular treatment in patients with human immunodeficiency virus infection. Enfermedades infecciosas y microbiologia clinica. 2001;19(2):88–90. doi: 10.1016/s0213-005x(01)72574-8. [DOI] [PubMed] [Google Scholar]
- 22.Michailidis C, Pozniak AL, Mandalia S, Basnayake S, Nelson MR, Gazzard BG. Clinical characteristics of IRIS syndrome in patients with HIV and tuberculosis. Antivir Ther. 2005;10(3):417–422. doi: 10.1177/135965350501000303. [DOI] [PubMed] [Google Scholar]
- 23.Pepper DJ, Marais S, Maartens G, Rebe K, Morroni C, Rangaka MX, Oni T, Wilkinson RJ, Meintjes G. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48(11):e96–107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]
- 24.Haddow LJ, Moosa MY, Easterbrook PJ. Validation of a published case definition for tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(1):103–108. doi: 10.1097/QAD.0b013e32832ec1f4. [DOI] [PubMed] [Google Scholar]
- 25.Hengel RL, Allende MC, Dewar RL, Metcalf JA, Mican JM, Lane HC. Increasing CD4+ T cells specific for tuberculosis correlate with improved clinical immunity after highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2002;18(13):969–975. doi: 10.1089/088922202760265632. [DOI] [PubMed] [Google Scholar]
- 26.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20(2):F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 27.Meintjes G, Wilkinson KA, Rangaka MX, Skolimowska K, van Veen K, Abrahams M, Seldon R, Pepper DJ, Rebe K, Mouton P, van Cutsem G, Nicol MP, Maartens G, Wilkinson RJ. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178(10):1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson KA, Seldon R, Meintjes G, Rangaka MX, Hanekom WA, Maartens G, Wilkinson RJ. Dissection of regenerating T-Cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2009;180(7):674–683. doi: 10.1164/rccm.200904-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, Huffam S, Oelrichs R, Sophea P, Saphonn V, Kaldor J, Cooper DA, Chhi Vun M, French MA. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200(11):1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 30.Tan DB, Yong YK, Tan HY, Kamarulzaman A, Tan LH, Lim A, James I, French M, Price P. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9(5):307–316. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 31.Seddiki N, Sasson SC, Santner-Nanan B, Munier M, van Bockel D, Ip S, Marriott D, Pett S, Nanan R, Cooper DA, Zaunders JJ, Kelleher AD. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009;39(2):391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 32.Bourgarit A, Carcelain G, Samri A, Parizot C, Lafaurie M, Abgrall S, Delcey V, Vicaut E, Sereni D, Autran B. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cells. J Immunol. 2009;183(6):3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 33.Simonney N, Dewulf G, Herrmann JL, Gutierrez MC, Vicaut E, Boutron C, Leportier M, Lafaurie M, Abgrall S, Sereni D, Autran B, Carcelain G, Bourgarit A, Lagrange PH. Anti-PGL-Tb1 responses as an indicator of the immune restoration syndrome in HIV-TB patients. Tuberculosis (Edinb) 2008;88(5):453–461. doi: 10.1016/j.tube.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 34.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 36.Ruhwald M, Ravn P. Immune reconstitution syndrome in tuberculosis and HIV-co-infected patients: Th1 explosion or cytokine storm? AIDS. 2007;21(7):882–884. doi: 10.1097/QAD.0b013e3280b079c8. [DOI] [PubMed] [Google Scholar]
- 37.WHO . Improving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis among adults and adolescents: Recommendations for HIV-prevalent and resource-constrained settings. Geneva: 2006. [Google Scholar]
- 38.Connell TG, Shey MS, Seldon R, Rangaka MX, van Cutsem G, Simsova M, Marcekova Z, Sebo P, Curtis N, Diwakar L, Meintjes GA, Leclerc C, Wilkinson RJ, Wilkinson KA. Enhanced Ex vivo stimulation of Mycobacterium tuberculosis-specific T cells in HIV-infected persons via antigen delivery by the Bordetella pertussis adenylate cyclase vector. Clin Vaccine Immunol. 2007;14(7):847–854. doi: 10.1128/CVI.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180(3):1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H, Lehmacher W, Ruckdeschel G, Gleixner B, Riedner C, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75(4):1011–1016. [PubMed] [Google Scholar]
- 41.Belperio JA, Keane MP, Lynch JP, 3rd, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med. 2006;27(4):350–364. doi: 10.1055/s-2006-948289. [DOI] [PubMed] [Google Scholar]
- 42.Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290. doi: 10.1016/S1473-3099(09)70066-0. [DOI] [PubMed] [Google Scholar]
- 43.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38(12):1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Goldsack NR, Allen S, Lipman MC. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex Transm Infect. 2003;79(4):337–338. doi: 10.1136/sti.79.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177(7):680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23(1):143–145. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- 47.Pepper DJ, Rebe K, Morroni C, Wilkinson RJ, Meintjes G. Clinical deterioration during antitubercular treatment at a district hospital in South Africa: the importance of drug resistance and AIDS defining illnesses. PLoS ONE. 2009;4(2):e4520. doi: 10.1371/journal.pone.0004520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277(5322):112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 49.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4(+) T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 50.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 51.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- 52.Marais S, Wilkinson RJ, Pepper DJ, Meintjes G. Management of patients with the immune reconstitution inflammatory syndrome. Current HIV/AIDS reports. 2009;6(3):162–171. doi: 10.1007/s11904-009-0022-z. [DOI] [PubMed] [Google Scholar]
- 53.Van den Bergh R, Vanham G, Raes G, De Baetselier P, Colebunders R. Mycobacterium-associated immune reconstitution disease: macrophages running wild? Lancet Infect Dis. 2006;6(1):2–3. doi: 10.1016/S1473-3099(05)70302-9. author reply 4-5. [DOI] [PubMed] [Google Scholar]
- 54.Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24(6):647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 55.Chegou NN, Black GF, Kidd M, van Helden PD, Walzl G. Host markers in Quantiferon supernatants differentiate active TB from latent TB infection : preliminary report. BMC pulmonary medicine. 2009;9(1):21. doi: 10.1186/1471-2466-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Djoba Siawaya JF, Roberts T, Babb C, Black G, Golakai HJ, Stanley K, Bapela NB, Hoal E, Parida S, van Helden P, Walzl G. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS One. 2008;3(7):e2535. doi: 10.1371/journal.pone.0002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price P, Morahan G, Huang D, Stone E, Cheong KY, Castley A, Rodgers M, McIntyre MQ, Abraham LJ, French MA. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS. 2002;16(15):2043–2047. doi: 10.1097/00002030-200210180-00009. [DOI] [PubMed] [Google Scholar]
- 58.Stone SF, Price P, Brochier J, French MA. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy. J Infect Dis. 2001;184(8):1073–1077. doi: 10.1086/323599. [DOI] [PubMed] [Google Scholar]
- 59.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3(1):21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 60.Bekker LG, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor-alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178(2):580–584. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]
- 61.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson RJ, DesJardin LE, Islam N, Gibson BM, Kanost RA, Wilkinson KA, Poelman D, Eisenach KD, Toossi Z. An increase in expression of a M. tuberculosis mycolyl transferase gene (fbpB) occurs early after infection of human monocytes. Mol Microbiol. 2001;39:813–821. doi: 10.1046/j.1365-2958.2001.02280.x. [DOI] [PubMed] [Google Scholar]
- 63.Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47(10):e83–85. doi: 10.1086/592695. [DOI] [PubMed] [Google Scholar]
- 64.Wallis RS, van Vuuren C, Potgieter S. Adalimumab treatment of life-threatening tuberculosis. Clin Infect Dis. 2009;48(10):1429–1432. doi: 10.1086/598504. [DOI] [PubMed] [Google Scholar]