Abstract
Background
Few equations for calculating body fat percentage (BF%) from field methods have been developed in South Asian children.
Objective
To assess agreement between BF% derived from primary reference methods and that from skinfold equations and bio-impedance analysis (BIA) in Indian children.
Methods
We measured BF% in two groups of Indian children. In Pune, 570 rural children aged 6-8 years underwent dual-energy X-ray absorptiometry (DXA) scans. In Mysore 18O was administered to 59 urban children aged 7-9 years. We conducted BIA at 50kHz and anthropometry including subscapular and triceps skinfold thicknesses. We used the published equations of Wickramasinghe, Shaikh, Slaughter and Dezenburg to calculate BF% from anthropometric data and the manufacturer’s equation for BIA measurements. We assessed agreement with values derived from DXA and DLW using Bland Altman analysis.
Results
Children were light and thin compared to international standards. There was poor agreement between the reference BF% values and those from all equations. Assumptions for Bland Altman analysis were not met for Wickramasinghe, Shaikh and Slaughter equations. The Dezenberg equations under-predicted BF% for most children (mean difference in Pune −13.4, LOA −22.7, −4.0 and in Mysore −7.9, LOA −13.7 and −2.2). The mean bias for the BIA equation in Pune was +5.0% and in Mysore +1.95% and the LOA were wide; −5.0, 15.0 and −7.8, 11.7 respectively.
Conclusions
Currently available skinfold equations do not accurately predict BF% in Indian children. We recommend development of BIA equations in this population using a 4-compartment model.
Keywords: body composition, India, children, bio-impedance, skinfold
Introduction
According to a recent WHO report the prevalence of type 2 diabetes in India is predicted to be 79 million (approximately 7% of the population) by 2030. The increase in childhood overweight and obesity (Kumar et al., 2007; Lobstein et al., 2004; World Health Organisation, 2007b) is thought to contribute to this public health problem.
A review by Misra (Misra et al., 2007) concluded that abdominal adiposity and excess body fat are important risk factors for development of insulin resistance in South Asian children. A UK study found that children of South Asian origin are more sensitive to the adverse metabolic effects of obesity at lower percentage body fat (BF%) than Caucasians (Whincup et al., 2002), moreover these effects occur earlier in childhood in those of South Asian origin (Bhardwaj et al., 2008). Measurement of body composition in South Asian children is therefore likely to be important in identifying groups and individuals at risk of metabolic disease including type 2 diabetes.
The 4 compartment model (4C) of assessing body composition is considered to be one of the most accurate methods available. This approach requires specialised equipment, e.g. Air Displacement Plethysmography; Under-Water Weighing; Dual Energy X-ray Absorptiometry (DXA) thus restricting its use to laboratory settings. For larger scale research or screening purposes more portable field methods are desirable. At present the most commonly used methods are BMI, skinfold measurements and bio-impedance analysis (BIA). All are inexpensive and relatively quick to perform but are not direct measures of BF%. BMI is a measure of weight relative to height and therefore does not give any information on body composition. Skinfold and BIA data require equations to calculate BF% from thickness and bioelectrical impedance measurements respectively. These equations have been shown to be sensitive to the ethnicity of the population (Dezenberg et al., 1999; Morrison et al., 2001). In addition, due to the higher levels of risk of metabolic disorders at a lower threshold of BF% among South Asians (Misra and Khurana, 2009), it has been recommended that risk assessment studies should use population-specific skinfold and BIA equations (Bhat et al., 2005). Recently equations have been developed in Indian adults using primary reference methods (Goel et al., 2008) for this purpose. We are currently aware of two skinfold equations developed in South Asian children, both using a two-compartment (2C) model (Shaikh and Mahalanabis, 2004) and none for converting BIA measurements to BF%. The objective of the current study was to compare BF% values derived from published skinfold and BIA equations with measurements obtained using DXA and 18O dilution in free-living children from two regions of India.
Method
The study was conducted at two centres in India, a rural area near Pune in Western India and the city of Mysore in the southern state of Karnataka. In Pune, DXA scans were used to measure fat mass (FM) and fat free mass (FFM) while in Mysore, isotope (18O) dilution was used to measure total body water (TBW) from which FM and FFM were calculated, DXA and 18O dilution were considered to be reference methods for the purpose of this study. The anthropometry and BIA data collection for both centres were carried out using equipment supplied by the same manufacturers and all measurers received standardised training and followed the same protocol.
Participants
Pune
All children enrolled in the Pune Maternal Nutrition Study (PMNS) were eligible for the present study. The PMNS is a prospective population study of the relationship of maternal diet and nutritional status to CVD risk markers in the offspring. It has been described in detail previously (Yajnik et al., 2003). Briefly, 814 pregnant women from 6 villages (total population of 35,000) in a rural area near Pune were enrolled in the study and there were 762 live singleton births. Of these, 704 children attended for follow up between the ages of 6-8 years.
Mysore
Children were recruited from the Parthenon birth cohort study (17). In brief, 663 women receiving care at the ante-natal clinic of the Holdsworth Memorial Hospital (HMH), Mysore gave birth to live singleton babies without major congenital malformations. Anthropometry of the offspring was performed at birth and at least annually thereafter. Of the 630 children who remained in the cohort in 2006, 60 children (30 male) aged 7-9 years were recruited into the current study. The cohort were divided into fifths according to BMI at their most recent follow up and approximately 12 children from each fifth were selected.
Procedure
In Pune a total body DXA scan was performed between December 2000 and January 2003 at the Diabetes Unit, KEM hospital using a DPX-IQ240 pencil beam machine (Lunar Corp., Madison, WI). The scans were analysed using Paediatric software version 4.7 to yield FM and FFM values for each child. The cuts were positioned to measure total bone mineral, FM and FFM for the following anatomical regions; head, arms, legs, trunk, spine and pelvis (Ganpule et al., 2006).
The majority of children underwent anthropometry and BIA on the same day as the scan but in some cases this was not possible, the data analysis relates to children whose DXA, anthropometry and BIA measurements were made within a one week period.
In Mysore, each child consumed doubly labelled water to measure their total energy expenditure, reported elsewhere (Krishnaveni et al., 2009) BIA and anthropometry were performed on the same day. For the measurement of TBW reported here, each child was administered 1.5g/kg body weight of 10% H218O (Taiyo Nippon Sanso Corporation, Tokyo, Japan). The isotope dose was prepared at St John’s Research Institute, Bangalore and delivered to the HMH Epidemiology Research Unit, Mysore where it was stored in a refrigerator for no longer than one month until administration. Basal urine samples were collected prior to administration of the isotope dose. The child was asked to drink the dose using a straw; once the child had finished the container was rinsed using bottled water and given to the child to drink, this process was repeated to ensure complete consumption of the dose. The time at completion was noted down. Post-dose urine samples were collected after 6h, corresponding to the time of equilibration, and on days 7 and 14. The exact time of collection was noted and the samples were stored at −20°C in sealed containers until analysis at St John’s Research Institute, Bangalore.
The urine samples were analysed for their 18O enrichment using the principle of isotopic equilibration (Kurpad et al., 1997), on a continuous flow isotope ratio mass spectrometer (Europa Scientific Ltd, Crewe, UK). The CV of repeated measurements of 18O was <0.01%. Pool sizes for each of the isotopes were calculated from the isotope dose and the zero-time enrichment of the isotope in body water. This value was obtained from the Y-intercept of the plot of the log-transformed urinary isotopic washout data against time in the original double-labelled water experiment (Coward, 1990). The calculation also took into account the measurement of the enrichment of the dose, as well as dilution factors during administration.
Anthropometry
The children wore light clothing and no footwear. Height was measured to the nearest 0.1cm using a wall-mounted stadiometer (Microtoise, CMS instruments, London). Weight was measured to the nearest 100g with electronic weighing scales placed on a level surface (Salter, UK). Body mass index (BMI) was calculated as: Weight (kg)/ Height (m)2.
Mid upper arm circumference (MUAC) was measured to the nearest 0.1cm at the halfway point between the acromion and the olecranon. Triceps and subscapular skinfold thicknesses were measured to the nearest 0.1mm using Harpenden ‘John Bull’ callipers (CMS Instruments, London). Three readings were taken and the mean used in the analysis. All measurements were made on the left side of the body. In Pune the measurements were made on the right side if the child was left-handed. There were four measurers in Pune and five in Mysore: mean inter-observer CVs were 5.4% and 6.8% for triceps; 7.4% and 7.7% for subscapular skinfolds respectively, mean intra-observer CVs were <2.0% and 4.0% for triceps; <2.0% and 4.6% for subscapular skinfolds respectively.
Bio-impedance Analysis
Whole body impedance at 50kHz was measured using a Quadscan 4000 analyser or a Multiscan 5000 (both Bodystat, Isle of Mann, British Isles). In Pune, BIA was measured after at least 5 hours (overnight) fasting and with an empty bladder. In Mysore the measurements were done after the child had provided the basal urine sample. Any jewellery and metal accessories were removed, children were asked to lie supine for 5 minutes before starting the measurements. One electrode was attached at the level of the ulnar head at the wrist and the other just behind the knuckles. On the foot, the two electrodes were attached at the level of the medial and lateral malleoli and just behind the toes, respectively. Impedance (Ω) at 50kHz and BF% measurements were recorded. In Mysore the CV in the impedance measurement was <1%, in Pune this was not measured.
Ethical Permission
Ethical permission was obtained from the KEM Hospital Ethical Committee, Pune and the Mysore Holdsworth Memorial Hospital Research Ethics Committee. Informed consent was obtained from parents.
Data Analysis
BF% was calculated from skinfolds using the equations shown in table 1. Where the equations yielded FM, weight measured by weighing scales was used to calculate BF%. For the 18O analysis, FFM was calculated from total body water in kg (TBW) using recently published hydration factors (HF) (Wells et al., 2010). BF% was then calculated using the equation BF % = 100 × (Weight − TBW/HF) / Weight.
Table 1.
Skinfold and Bio-impedance equations used for deriving Body Fat Percentage, including details of methodolgy of equation development.
| First Author | Equation1 | Reference Method | n | Age (years) | Ethnicity |
|---|---|---|---|---|---|
| Wickramasinghe | FM (boys) = 0.68*A + 0.246*T + 0.383*SS − 1.61 − 3.45 FM (girls) = 0.680*A + 0.246*T + 0.383*SS − 3.45 |
D2O dilution | 188 | 5-15 | South Asian |
| Shaikh | BF% (boys) = 5.304 + 0.269*T + 0.50*SS + 0.685*M − 0.063*A BF% (girls) = 7.017− 0.053*T + 0.201*SS + 0.765*M + 0.052*A |
Bio-impedance (validated with D2O dilution) | 184 | 1-5 | South Asian |
| Slaughter | BF% (boys) = 1.21*(T+SS) − 0.008*(T+SS)2 − 1.7 BF% (girls) = 1.33*(T+SS) − 0.013*(T+SS)2 − 2.5 |
Photon Absorptiometry, D2O dilution & Hydrostatic Weighing | 310 | 8-29 | Caucasian African American |
| Dezenberg | FM (boys) = 0.342*W + 0.256*T − 6.501 FM (girls) = 0.342*W + 0.256*T − 5.664 |
DXA | 202 | 4-10.9 | Caucasian African American |
| Bio-impedance | Bodystat BF% = [(W − TBW/HF)/W ]*100 | ||||
| Kushner | TBW = 0.59*(H2/R) + 0.065*W + 0.004 | D2O dilution | 59 | 0-66 | Caucasian |
| Lohman | HF (7-8 years) males = 0.768, females = 0.776 | D2O dilution | 11 | 7-11y | No information |
BF% = body fat %, T= triceps skinfold (mm), SS = subscapular skinfold (mm), M= mid upper arm circumference (cm), A=age (months), FM= fat mass (kg), W=weight (kg), TBW = total body water (kg), H = height (cm), R = Resistance (Ω).
T- tests were used to investigate the differences in BF% between boys and girls. Where weight was a term in an equation, we used that measured by scales. DXA FM and BF% were based on the weight as measured by the DXA machine. Scatter plots of BF% from the Wickramasinghe (Wickramasinghe et al., 2008), Shaikh (Shaikh and Mahalanabis, 2004), Slaughter (Slaughter et al., 1988), Dezenberg (Dezenberg et al., 1999) and BIA equations against the DXA (for Pune) and DLW (for Mysore) were produced to provide a visual assessment of their comparability. We did a Bland Altman (Bland and Altman, 1986) analysis to quantify the agreement between predicted BF% and the reference methods in each cohort. We calculated the mean bias and 95% limits of agreement (LOA) for the equations where the bias and variability were uniform throughout the range of BF%. The data were analysed using STATA statistical software version 10 (StataCorp, College Station, TX).
Results
In Pune, of 704 children who attended for follow up, 71 did not have their DXA and anthropometry measurements made within one week and 73 had incomplete data, leaving 560 in the final analysis. In Mysore one child spilt the DLW and a negative BF% result was derived from 18O analysis for another, this left data from 58 children in the analysis. Anthropometric, bio-impedance and calculated BF% data for all children are shown in table 2. On average the children in both groups were light and had a low BMI for age compared to WHO reference data (World Health Organisation, 2007a). All children from Pune and girls from Mysore were short for age relative to WHO data. Girls in both centres had higher skinfold and bio-impedance measurements than boys. Median BF% from DXA and 18O was significantly higher in girls than boys in both groups (p<0.001).
Table 2.
Descriptive statistics
| Measure, median (IQR) | Pune | Mysore | ||
|---|---|---|---|---|
| Boys (n=296) | Girls (n=264) | Boys (n=29) | Girls (n=29) | |
| Age (years) | 6.2 (6.1,6.3) | 6.2 (6.1,6.3) | 8. 7 (8.5,8.9) | 8.9 (8.5,9.0) |
| Weight (kg) | 16.3 (15.2,17.6) | 15.8 (14.7,17.1) | 23.8 (22.5,26.3) | 24.0 (22.0,25.4) |
| WHO Weight for age * | 20.7 (18.9,22.7) | 20.5 (18.6,22.8) | 27.0 (24.5,29.9) | 27.4 (24.5,30.7) |
| Height (cm) | 110.0 (107.4,113.0 ) |
110.0 (107.0,112.7 ) |
130.4 (126.3,132.8 ) |
126.5 (124.1,129.7 ) |
| WHO Height for age * |
116.4
(113.1,119.8 ) |
116.1
(112.6,119.6 ) |
130.4
(126.4,134.3 ) |
131.0
(126.0,134.0 ) |
| BMI (kg/m2) | 13.5 (12.9,14.1) | 13.1 (12.5,13.8) | 14.3 (13.4,15.4) | 14.1 (13.6,16.0) |
| WHO BMI for age * | 15.3 (14.5,16.3) | 15.3 (14.3,16.4) | 15.9 (14.9,17.1) | 15.9 (14.8,17.3) |
| Triceps skinfold (mm) | 5.9 (5.2,6.7) | 6.4 (5.6,7.4) | 6.8 (5.7,8.3) | 9.7 (8.1,13.5) |
| Subscapular skinfold (mm) | 4.7 (4.3,5.2) | 5.1 (4.6,5.9) | 5.4 (4.8,6.2) | 7.6 (6.5,11.0) |
| Bio-impedance (Ω at 50kHz) | 837 (788,883) | 886 (827,945) | 778 (749,834) | 904 (846,957) |
| BF% from: | ||||
| DXA/ DLW | 17.9 (14.9,20.9) | 20.8 (17.9,24.3) | 21.6 (18.2,24.0) | 28.3 (24.0,31.4) |
| Wickramasinghe equation | 14.6 (12.9,17.4) | 27.5 (25.0,30.6) | 19.3 (17.6,21.3) | 35.8 (31.8,40.7) |
| Shaikh equation | 15.0 (14.2,15.8) | 23.2 (22.7,23.8) | 15.0 (14.1,16.5) | 27.5 (26.2,28.3) |
| Slaughter equation | 10.3 (9.1,11.6) | 11.2 (10.0,12.6) | 11.5 (10.1,14.5) | 16.6 (13.9,23.1) |
| Dezenberg equation | 3.6 (0.9,6.5) | 8.9 (6.1,11.6) | 15.0 (11.4-17.4) | 21.2 (18.0-25.1) |
| Bio-impedance equation | 23.2 (20.2,26.1) | 26.3 (23.4,28.7) | 23.6 (19.5,25.9) | 33.6 (28.5,36.3) |
World Health Organization reference data, 2007
Scatterplots showing BF% derived from the reference methods plotted against BF% derived from the skinfold equations showed very similar patterns in the relationship between the two variables in both Pune and Mysore (figure 1). The relationships between BF% from skinfold and BIA equations and that from the reference method are described below:
Figure 1.
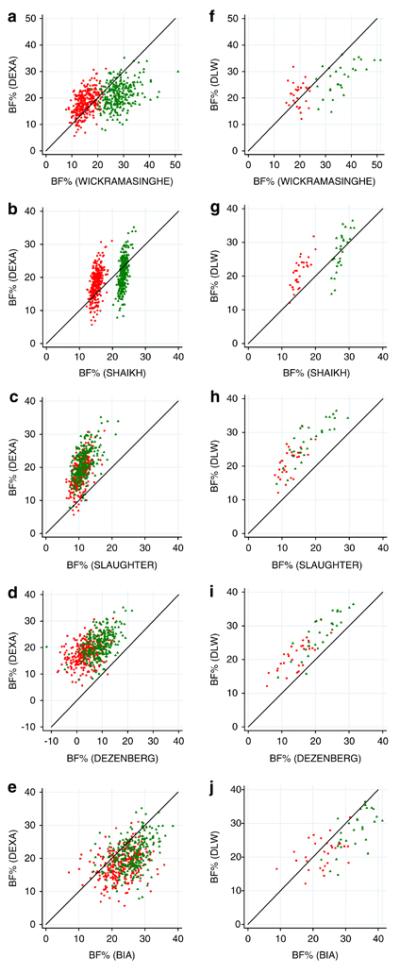
Scatterplots of body fat percentage calculated using published equations against that from a reference method. ‘BF% Wickramasinghe’,’BF% Shaikh’, BF% Slaughter, ‘BF% Dezenberg’ refer to body fat percentage calculated using the respective published equation; ‘BF% DXA’, body fat percentage derived from dual-energy X-ray absorptiometry scan; ‘BF% DLW’, body fat percentage derived from 18O dilution administered as doubly labelled water. Figures a-e relate to data from Pune (n=570), figures f-j relate to data from Mysore (n=58).
 Male
Male  Female
Female
• Wickramasinghe Equation
This equation tended to under-estimate boys’ and overestimate girls’ BF% in both centres compared to the reference values (figures 1a&f).
• Shaikh Equation
This predicted girls’ BF% to be approximately 10% higher than boys, but within each sex it estimated values in a narrow range for all children (figures 1b&g) when compared with the reference methods.
• Slaughter Equation
This tended to under-estimate BF%, except in the least adipose children from Pune (figure 1c) where the equation placed the children within a narrower range of BF% than DXA. In Mysore the median value for BF% derived using the Slaughter equation was approximately 10% lower than that measured by the reference method (figure 1h). The distance between the Slaughter values and the line of equality was greatest at the middle of the range of DLW values (approximately 25-35%).
• Dezenberg Equation
This underestimated BF% for both groups of children (figures 1d&i) compared with the reference methods. Among the Pune children, there were 58 (10.3%) with negative values for BF%.
• BIA Bodystat equation
This tended to over-estimate BF%, especially in boys (figures e&j). There was a wide degree of scatter for both boys and girls which indicated considerable error occurring across the range of BF%.
The agreement between the reference methods and the Dezenberg equation was better in terms of the LOA (mean difference in Pune −13.4, LOA −22.7, −4.0 and in Mysore −6.5, LOA agreement −12.3 and −0.6), but there was a systematic underprediction in both groups of children. There was a significant positive bias in values derived from the BIA equation (+5.0% in Pune (LOA, −5.0, 15.0 and +3.4% in Mysore, LOA −6.7, 13.5). In the case of the Wickramasinghe, Shaikh and Slaughter equations the bias was not uniform throughout the range of the data. For this reason a Bland Altman analysis of the agreement between BF% calculated by the equations with the primary reference method was not considered to be meaningful.
Discussion
The objective of the present study was to compare BF% derived from field methods, using published equations, with BF% from reference methods in two groups of Indian children aged 6-9 years. These commonly used equations to calculate BF% from skinfold and BIA measurements yielded values that had poor agreement with those derived from reference techniques showing both imprecision and bias.
An important finding was that the results were remarkably similar in the two groups of children from different regions of India. This indicates that Indian children may be different from children of other ethnicities in terms of body composition. It also suggests that despite the limitations of the reference methods used there is a similar relationship between field and reference methods in both centres.
Below is a discussion of the findings relating to each equation.
• Wickramasinghe Equation
The Wickramasinghe equation tended to overpredict for girls and underpredict for boys. It is likely that this is due to the age range of children in whom the equation was developed, (5-15 years). A study in Caucasian children has shown the difference in fat distribution between boys and girls increases with sexual maturity (Taylor et al., 2009). The difference in mean BF% between the boys and girls in the Wickramasinghe study was 11.5% whereas in the present study it was 2.9% for Pune and higher for the slightly older Mysore children (7.2%).
• Shaikh Equation
BF% calculated by the Shaikh equation ranged in a linear fashion from underestimation to overestimation. The equation calculated the BF% of all children to be within a range of less than 10% while, according to the reference methods, the values for both Mysore and Pune children range from <10% to 40%. The Shaikh study participants were pre-school children who may have had a narrower range of BF% than older children, and perhaps more importantly the equation was developed using BIA as the reference method.
• Slaughter Equation
The Slaughter equation, which was developed in Caucasian and African American children living in the USA, tended to underestimate BF% for the vast majority of the Indian children (Slaughter et al., 1988). The authors found that there was less than one percent difference between the groups, so ethnicity was not adjusted for in their equation. A study in the UK in 30 children found that BF% was underestimated by this equation when compared to a 4C model (Wells et al., 1999). The Slaughter equation was published over twenty years ago, since when children in most populations have become more adipose. The large under-estimation found in our study is likely to be due to differences in body composition between South Asians and people of other ethnicities. An equation for calculating BF% from BMI developed for use in Caucasian adults was shown to underestimate BF% in Asian adults (Deurenberg-Yap et al., 2002) and it has been previously shown in both children (Krishnaveni et al., 2005; Lovegrove, 2007) and adults (Lovegrove, 2007) that Indians tend to have a higher BF% than Caucasians for a given BMI.
• Dezenberg Equation
The Dezenberg equation underestimated BF% to an even greater extent than the Slaughter equation. There were several equations published as a result of the Dezenberg study, one of which contained an abdominal skinfold term. Indians have been shown to have relatively increased abdominal fat, including subcutaneous as well as intra-abdominal fat, compared with Caucasians (Raji et al., 2001). We did not collect abdominal skinfolds in the present study and it would have been interesting to see how well the equation performed in our population if we had this information. We have shown that triceps skinfold measurements alone are not adequate predictors of BF% in Indian children.
• BIA Equation
The Bodystat BIA equations showed the least bias, but wide LOA. Therefore they may be useful for measuring differences between populations or changes at the group level but not differences or changes at the level of the individual. It is possible that the electrolyte content of the extracellular fluid varies by ethnicity but if this was the case one would expect to see a more systematic bias between the reference method and BIA. Our study used a BIA machine that did not yield separate reactance and resistance values, it was therefore not possible for us to check the relationship between these parameters as has been done in other studies (Rush et al., 2003). A potential advantage of BIA over skinfolds in the South Asian population is that it is likely to better reflect differences in centrally stored truncal fat.
• Limitations of the study
A limitation of the current study is that we did not measure abdominal skinfold, which would have allowed us to investigate the accuracy of other equations, for example Huang et al (Huang et al., 2003) developed an equation incorporating abdominal skinfold in Latin American children whose BF% values were underpredicted by ‘Caucasian’ equations. However, when considering this skinfold as a tool for large studies it should be pointed out that it is a technically difficult measurement and children in particular may be less compliant. A second limitation is that both reference methods assume a constant hydration of FFM. It is known that hydration of FFM decreases with age (Lohman, 1989; Wells et al., 2010) and there is evidence that hydration of FFM varies by ethnicity in Singaporean adults of Indian, Chinese and Malay descent (Deurenberg-Yap et al., 2001) but to the best of our knowledge no studies have assessed differences between South Asian children and those of other ethnicity. Thirdly, the DXA technique has not been validated against animal carcass measurements or a 4 C model in this population as has been done in previous studies (Dezenberg et al., 1999; Goran et al., 1996). DXA was designed to measure bone density which it does with accuracy and precision. BF% measurements from DXA tend to be precise but the accuracy has been questioned. When compared with a 4C model a study in young healthy females reported an under/ overestimation of BF% by DXA of up to 28% at the individual level (Wong et al., 2002). The relationship between BF% from DXA and BF% from a 4C model has been shown to vary according to BF%, with DXA underestimating BF% in those with lower BF% and overestimating in those with higher BF% (Sopher et al., 2004). A further study in 5-21 year olds found that the bias of BF% measurements obtained from DXA varies according to gender, size and BF% (Williams et al., 2006). The authors propose that the distribution of fat may influence the accuracy of DXA, which is of particular relevance to the Indian population as there is evidence that their fat is more centrally distributed than that of white Caucasians (Krishnaveni et al., 2005).
• Implications
The main implication of the findings in this study is that the currently available skinfold and bio-impedance equations are not suitable for measuring absolute values of BF% in Indian children at the individual level. Due to the tendency of Indians to store fat centrally we would suggest that skinfolds, which are an indicator of subcutaneous fat, may not best reflect the adiposity of Indian children. We would recommend that a validation study using a 4C model of body composition analysis is carried out in a large representative sample of Indian children in order to assess the ability of skinfold measurements to accurately predict BF%, and to develop suitable equations for predicting BF% from BIA measurements which may be more useful in this population. Tanner staging should be carried out to assess sexual maturity and if necessary a ‘maturity term’ or separate equations for different stages of puberty should be developed.
The availability of valid equations for converting field measurements to BF% will allow effective monitoring of children’s body composition and more accurate evaluation of programmes aimed at reducing risk of metabolic disease in the South Asian population.
Acknowledgements
SK contributed to data analysis, interpretation of results and drafted the manuscript; GVK, SRV, HGL and DSB carried out newborn and childhood data collection and contributed to manuscript preparation; AKW and AMG carried out the statistical analysis of data, contributed to interpretation of results and manuscript preparation; RK carried out the calculations related to 18O dilution; CHDF designed the study, coordinated analysis, interpretation and drafting of the paper; CSY designed the study and contributed to interpretation of the results; AVK coordinated 18O dilution analysis and related calculations, contributed to interpretation of results and drafting of manuscript.
This work was funded by the Medical Research Council and the Wellcome Trust.
Footnotes
The authors declare no conflicts of interest.
References
- BHARDWAJ S, MISRA A, KHURANA L, GULATI S, SHAH P, VIKRAM NK. Childhood obesity in Asian Indians: a burgeoning cause of insulin resistance, diabetes and sub-clinical inflammation. Asia Pac. J. Clin. Nutr. 2008;17(Suppl 1):172–175. [PubMed] [Google Scholar]
- BHAT DS, YAJNIK CS, SAYYAD MG, RAUT KN, LUBREE HG, REGE SS, et al. Body fat measurement in Indian men: comparison of three methods based on a two-compartment model. Int. J. Obes. (Lond) 2005;29:842–848. doi: 10.1038/sj.ijo.0802953. [DOI] [PubMed] [Google Scholar]
- BLAND JM, ALTMAN DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- COWARD A, Prentice AM. Calculation of pool sizes and flux rates. In the doubly labeled water method for measuring energy expenditure; Technical recommendations for use in humans. A consensus report by the IDECG working group. Vienna: 1990. pp. 48–68. Ref Type: Report. [Google Scholar]
- DEURENBERG-YAP M, CHEW SK, DEURENBERG P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes. Rev. 2002;3:209–215. doi: 10.1046/j.1467-789x.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- DEURENBERG-YAP M, SCHMIDT G, VAN STAVEREN WA, HAUTVAST JG, DEURENBERG P. Body fat measurement among Singaporean Chinese, Malays and Indians: a comparative study using a four-compartment model and different two-compartment models. Br. J Nutr. 2001;85:491–498. doi: 10.1079/bjn2000276. [DOI] [PubMed] [Google Scholar]
- DEZENBERG CV, NAGY TR, GOWER BA, JOHNSON R, GORAN MI. Predicting body composition from anthropometry in pre-adolescent children. Int. J Obes. Relat Metab Disord. 1999;23:253–259. doi: 10.1038/sj.ijo.0800802. [DOI] [PubMed] [Google Scholar]
- GANPULE A, YAJNIK CS, FALL CH, RAO S, FISHER DJ, KANADE A, et al. Bone mass in Indian children--relationships to maternal nutritional status and diet during pregnancy: the Pune Maternal Nutrition Study. J. Clin. Endocrinol. Metab. 2006;91:2994–3001. doi: 10.1210/jc.2005-2431. [DOI] [PubMed] [Google Scholar]
- GOEL K, GUPTA N, MISRA A, PODDAR P, PANDEY RM, VIKRAM NK, et al. Predictive equations for body fat and abdominal fat with DXA and MRI as reference in Asian Indians. Obesity (Silver. Spring) 2008;16:451–456. doi: 10.1038/oby.2007.55. [DOI] [PubMed] [Google Scholar]
- GORAN MI, DRISCOLL P, JOHNSON R, NAGY TR, HUNTER G. Cross-calibration of body-composition techniques against dual-energy X-ray absorptiometry in young children. Am. J. Clin. Nutr. 1996;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- HUANG TT, WATKINS MP, GORAN MI. Predicting total body fat from anthropometry in Latino children. Obes. Res. 2003;11:1192–1199. doi: 10.1038/oby.2003.164. [DOI] [PubMed] [Google Scholar]
- KRISHNAVENI GV, HILL JC, VEENA SR, LEARY SD, SAPERIA J, CHACHYAMMA KJ, et al. Truncal adiposity is present at birth and in early childhood in South Indian children. Indian Pediatr. 2005;42:527–538. [PubMed] [Google Scholar]
- KRISHNAVENI GV, VEENA SR, KURIYAN R, KISHORE RP, WILLS AK, NALINAKSHI M, et al. Relationship between physical activity measured using accelerometers and energy expenditure measured using doubly labelled water in Indian children. Eur. J. Clin. Nutr. 2009;63:1313–1319. doi: 10.1038/ejcn.2009.95. [DOI] [PubMed] [Google Scholar]
- KUMAR S, MAHABALARAJU DK, ANUROOPA MS. Prevalence of obesity and its influencing factor among affluent school children of Davangere city. Indian Journal of Community Medicine. 2007;32:15–17. [Google Scholar]
- KURPAD AV, BORGONHA S, SHETTY PS. Measurement of total energy expenditure by the doubly labelled water technique in free living Indians in Bangalore city. Indian J. Med. Res. 1997;105:212–219. [PubMed] [Google Scholar]
- LOBSTEIN T, BAUR L, UAUY R. Obesity in children and young people: a crisis in public health. Obes. Rev. 2004;5(Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- LOHMAN T. Assessment of Body Composition in Children. Pediatric Exercise Science. 1989;1:19–30. doi: 10.1123/pes.1.1.19. [DOI] [PubMed] [Google Scholar]
- LOVEGROVE JA. CVD risk in South Asians: the importance of defining adiposity and influence of dietary polyunsaturated fat. Proc. Nutr Soc. 2007;66:286–298. doi: 10.1017/S0029665107005514. [DOI] [PubMed] [Google Scholar]
- MISRA A, KHURANA L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr. Relat Disord. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- MISRA A, KHURANA L, VIKRAM NK, GOEL A, WASIR JS. Metabolic syndrome in children: current issues and South Asian perspective. Nutrition. 2007;23:895–910. doi: 10.1016/j.nut.2007.08.018. [DOI] [PubMed] [Google Scholar]
- MORRISON JA, GUO SS, SPECKER B, CHUMLEA WC, YANOVSKI SZ, YANOVSKI JA. Assessing the body composition of 6-17-year-old Black and White girls in field studies. Am J Hum. Biol. 2001;13:249–254. doi: 10.1002/1520-6300(200102/03)13:2<249::AID-AJHB1035>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- RAJI A, SEELY EW, ARKY RA, SIMONSON DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J. Clin. Endocrinol. Metab. 2001;86:5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- RUSH EC, PUNIANI K, VALENCIA ME, DAVIES PS, PLANK LD. Estimation of body fatness from body mass index and bioelectrical impedance: comparison of New Zealand European, Maori and Pacific Island children. Eur. J Clin Nutr. 2003;57:1394–1401. doi: 10.1038/sj.ejcn.1601701. [DOI] [PubMed] [Google Scholar]
- SHAIKH S, MAHALANABIS D. Empirically derived new equations for calculating body fat percentage based on skinfold thickness and midarm circumference in preschool Indian children. Am J Hum. Biol. 2004;16:278–288. doi: 10.1002/ajhb.20030. [DOI] [PubMed] [Google Scholar]
- SLAUGHTER MH, LOHMAN TG, BOILEAU RA, HORSWILL CA, STILLMAN RJ, VAN L, et al. Skinfold equations for estimation of body fatness in children and youth. Hum. Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- SOPHER AB, THORNTON JC, WANG J, PIERSON RN, JR., HEYMSFIELD SB, HORLICK M. Measurement of percentage of body fat in 411 children and adolescents: a comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics. 2004;113:1285–1290. doi: 10.1542/peds.113.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR RW, GRANT AM, WILLIAMS SM, GOULDING A. Sex Differences in Regional Body Fat Distribution From Pre- to Postpuberty. Obesity. (Silver. Spring) 2009 doi: 10.1038/oby.2009.399. [DOI] [PubMed] [Google Scholar]
- WELLS JC, FULLER NJ, DEWIT O, FEWTRELL MS, ELIA M, COLE TJ. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904–912. doi: 10.1093/ajcn/69.5.904. [DOI] [PubMed] [Google Scholar]
- WELLS JC, WILLIAMS JE, CHOMTHO S, DARCH T, GRIJALVA-ETERNOD C, KENNEDY K, et al. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am. J Clin. Nutr. 2010;91:610–618. doi: 10.3945/ajcn.2009.28428. [DOI] [PubMed] [Google Scholar]
- WHINCUP PH, GILG JA, PAPACOSTA O, SEYMOUR C, MILLER GJ, ALBERTI KG, et al. Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ. 2002;324:635. doi: 10.1136/bmj.324.7338.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKRAMASINGHE VP, LAMABADUSURIYA SP, CLEGHORN GJ, DAVIES PS. Assessment of body composition in Sri Lankan children: validation of a skin fold thickness equation. Ceylon Med J. 2008;53:83–88. doi: 10.4038/cmj.v53i3.247. [DOI] [PubMed] [Google Scholar]
- WILLIAMS JE, WELLS JC, WILSON CM, HAROUN D, LUCAS A, FEWTRELL MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am. J Clin. Nutr. 2006;83:1047–1054. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- WONG WW, HERGENROEDER AC, STUFF JE, BUTTE NF, SMITH EO, ELLIS KJ. Evaluating body fat in girls and female adolescents: advantages and disadvantages of dual-energy X-ray absorptiometry. Am. J Clin. Nutr. 2002;76:384–389. doi: 10.1093/ajcn/76.2.384. [DOI] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANISATION . The WHO Child Growth Standards. Geneva: 2007a. Ref Type: Data File. [Google Scholar]
- WORLD HEALTH ORGANISATION . The world health report 2007 - A safer future: global public health security in the 21st century. WHO Press; Geneva: 2007b. Ref Type: Report. [Google Scholar]
- YAJNIK CS, FALL CH, COYAJI KJ, HIRVE SS, RAO S, BARKER DJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes. Relat Metab Disord. 2003;27:173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]


