Abstract
Propofol (2,6-diisopropylphenol) is an ultrashort-acting sedative agent with sedative and amnestic effects that is used not only for anesthesia but also for sedation during minor outpatient procedures and endoscopic examinations. Rare cases of anaphylaxis following propofol administration have been reported in the medical literature. Documentation of anaphylaxis is often lacking because the cause and effect relationship is often hard to prove. Only a minority of patients get referred for allergy testing to confirm the offending drug. Here we report a 74-year-old woman who had an anaphylactic reaction with severe oropharyngeal edema and bronchospasm for a few minutes after receiving propofol during endoscopic examination. An allergy skin test was positive for both propofol and soybean. Soybean in the intralipid is one component of propofol, and we concluded that this anaphylaxis was caused by soybean.
Keywords: Bronchial spasm, angioedema, anaphylaxis, propofol
INTRODUCTION
Propofol (2,6-diisopropylphenol) is a substituted isopropylphenol compound that causes a depression in consciousness.1 Propofol is thought to be a relatively safe intravenous anesthetic, and rare cases of anaphylaxis following propofol administration have been reported in the medical literature.2 Here we report a patient who experienced an anaphylactic reaction with severe oropharyngeal edema and bronchospasm for 1 minute after receiving propofol during endoscopic examination. The case was confirmed by an allergic skin test to be a type I hypersensitive reaction with oropharyngeal angioedema and bronchospasm, due to repeat exposure to propofol.
CASE REPORT
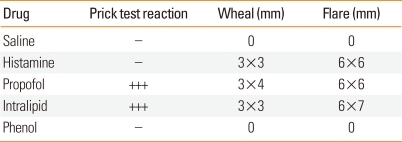
A previously healthy 74-year-old woman, 143 cm in height and 49 kg in weight, visited our hospital to undergo esophagogastroduodenoscopy (EGD) for a check-up. She experienced abdominal discomfort to soybean without skin lesion and asthma, but she had no other remarkable medical history including allergy. An EGD procedure using 15 mg propofol was performed twice in this patient prior to this admission without any side effects. Her initial blood pressure (BP) was 130/70 mmHg and heart rate was regular, ranging from 80 to 90 beats per minute. We did not use lidocaine for oropharyngeal anesthesia, and all of the medical team involved in her exam used vinyl gloves. Under standard monitoring, sedation was induced with 15 mg intravenous propofol (Propofol Inj., Jeil Pharm, Seoul, Korea). The patient was then sedated and we began the exam. Approximately 1 minute following administration of intravenous propofol, stridor was heard and oxygen saturation fell to 56% on pulse oximetry. We immediately applied inhaled oxygen (6 L/min) via nasal prong, but oxygen saturation did not increase. We decided to stop the exam, and tried to remove the endoscope. However, the endoscope became stuck in her throat. Thus, we removed the endoscope by compulsion. Ten seconds after endoscope removal, severe wheezing was heard and her oxygen saturation fell to 56%. The larynx was observed using a laryngoscope for endotracheal intubation. At that time, marked swelling and severe edema of the epiglottis extending to the arytenoids cartilage was detected. Epinephrine (1 mg) was immediately administered subcutaneously, together with 125 mg intravenous methylprednisolone infusion. Because the patient's symptoms did not improve after 1 minute, 1 mg epinephrine was administered intravenously. After a further 1 minute, her oxygen saturation recovered to 98% and the wheezing subsided. The patient's BP never went below 130/70 mmHg during the event. She recovered smoothly from anesthesia without signs of airway obstruction and was transferred to the general ward. Blood samples were drawn immediately after the event. Tryptase level (normal range, 0-13.5 µg/L) was 4.65 µg/L at 5 minutes and 6.61 µg/L at 2 hours. Arterial blood gas analysis showed pH 7.404, pCO2 42.5 mmHg, pO2 119.6 mmHg, HCO3- 26 mEq/L, and SaO2 98.3%. Total IgE was 111 kU/L. The patient underwent skin-prick testing 14 days after the event. The skin-prick tests of 55 common inhalant allergens were negative. However, propofol and 20% intralipid (SMOFlipid®, Fresenius Kabi, Bad Homburg, Germany) showed an immediate reaction (Table). Thus, we suspected the soybean in intralipid, a component of propofol, as the cause of the anaphylaxis, because she had a food allergy history to soybean. She was informed of the results and of the risk for anaphylaxis if re-exposed to propofol or nutritional supplements containing soybean in the future.
Table.
Results of skin-prick testing
+++, ratio (allergen/histamine),1≤R<2.
DISCUSSION
The most common drugs that trigger IgE-mediated anaphylaxis are neuromuscular blocking agents and antibiotics, including penicillin and cephalosporins, during the perioperative period.2 Neuromuscular blocking agents are involved in perioperative IgE-mediated anaphylaxis in 50-70% of cases according to epidemiological studies.2-5
Propofol is an alkylphenol derivative (2,6-diisopropylphenol) marketed as an oil water emulsion using soybean oil (10%), and egg lecithin (1.2%) as the emulsifying agent. Lecithin is a highly purified phosphatide found in egg yolk, but is not the allergenic determinant.6 Five major allergens, Gal d 1-5, have been characterized in hen eggs.7 Chicken serum albumin (Gal d 5) is the major allergen in egg yolk.8 There is no confirmed report of propofol-induced anaphylaxis by allergy testing in egg-allergic patients.9,10
The formulation of propofol as well as its active component contain soybean oil. Soy allergy is one of the most common food allergies in childhood, affecting approximately 0.4% of preschool children. It is considered an early-onset food allergy with most patients developing soy tolerance by late childhood.11 Soy allergy is occasionally present during adulthood. Refined soy oil, such as that present in propofol, is safe for people with soy allergy because the allergenic proteins are removed during the refining process. Thus, it is unlikely that the soy oil present in propofol will induce allergy, as the dose of protein contained in refined soy oil is too small to provoke a reaction.12 The few documented IgE-mediated anaphylactic reactions to propofol have been shown to be elicited by the isopropyl or phenol groups rather than the lipid vehicle.13,14
Although IgE-mediated anaphylactic reactions to propofol are rare, the incidence of allergic reaction during anesthesia is in the range of 1:10,000 to 1:20,000, and 1.2% of cases of perioperative anaphylactic shock are attributable to propofol.15 For this reason, the product was reformulated as an emulsion containing soybean oil and purified egg phosphatide (extracted from egg yolk) to reduce allergenicity. Propofol allergy is often attributed to the presence of a diisopropyl side chain or phenol group or soybean rather than to egg allergy.13 Allergic reactions to propofol on first exposure are usually due to the isopropyl groups that may act as epitopes and that are present in various medications and cosmetics.14 Allergic reactions to propofol upon re-exposure are usually due to the phenol molecule.16,17
In Korea, Shin et al.18 and Chung et al.19 reported anaphylaxis after exposure to propofol, but they could not determine which component caused the anaphylaxis, and their patients experienced anaphylaxis after first propofol exposure. In this case, we proved through skin-prick testing, without increasing tryptase level, that the soybean component of propofol was an important cause of anaphylaxis during an anesthetic procedure. To the best of our knowledge, this is the first case report of anaphylaxis after re-exposure to propofol in Korea. Referral of these cases to an allergy clinic is important for subsequent anesthetic exposure.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Shafer A, Doze VA, Shafer SL, White PF. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology. 1988;69:348–356. doi: 10.1097/00000542-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Dewachter P, Mouton-Faivre C, Castells MC, Hepner DL. Anesthesia in the patient with multiple drug allergies: are all allergies the same? Curr Opin Anaesthesiol. 2011;24:320–325. doi: 10.1097/ACO.0b013e3283466c13. [DOI] [PubMed] [Google Scholar]
- 3.Kroigaard M, Garvey LH, Gillberg L, Johansson SG, Mosbech H, Florvaag E, Harboe T, Eriksson LI, Dahlgren G, Seeman-Lodding H, Takala R, Wattwil M, Hirlekar G, Dahlén B, Guttormsen AB. Scandinavian Clinical Practice Guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007;51:655–670. doi: 10.1111/j.1399-6576.2007.01313.x. [DOI] [PubMed] [Google Scholar]
- 4.Harper NJ, Dixon T, Dugué P, Edgar DM, Fay A, Gooi HC, Herriot R, Hopkins P, Hunter JM, Mirakian R, Pumphrey RS, Seneviratne SL, Walls AF, Williams P, Wildsmith JA, Wood P, Nasser AS, Powell RK, Mirakhur R, Soar J Working Party of the Association of Anaesthetists of Great Britain and Ireland. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009;64:199–211. doi: 10.1111/j.1365-2044.2008.05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebo DG, Fisher MM, Hagendorens MM, Bridts CH, Stevens WJ. Anaphylaxis during anaesthesia: diagnostic approach. Allergy. 2007;62:471–487. doi: 10.1111/j.1398-9995.2007.01347.x. [DOI] [PubMed] [Google Scholar]
- 6.MacPherson RD. Pharmaceutics for the anaesthetist. Anaesthesia. 2001;56:965–979. doi: 10.1046/j.1365-2044.2001.02216.x. [DOI] [PubMed] [Google Scholar]
- 7.Tey D, Heine RG. Egg allergy in childhood: an update. Curr Opin Allergy Clin Immunol. 2009;9:244–250. doi: 10.1097/ACI.0b013e32832b1f00. [DOI] [PubMed] [Google Scholar]
- 8.Clark AT, Skypala I, Leech SC, Ewan PW, Dugué P, Brathwaite N, Huber PA, Nasser SM British Society for Allergy and Clinical Immunology. British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy. Clin Exp Allergy. 2010;40:1116–1129. doi: 10.1111/j.1365-2222.2010.03557.x. [DOI] [PubMed] [Google Scholar]
- 9.Hofer KN, McCarthy MW, Buck ML, Hendrick AE. Possible anaphylaxis after propofol in a child with food allergy. Ann Pharmacother. 2003;37:398–401. doi: 10.1345/aph.1C227. [DOI] [PubMed] [Google Scholar]
- 10.Tashkandi J. My patient is allergic to eggs, can i use propofol? A case report and review. Saudi J Anaesth. 2010;4:207–208. doi: 10.4103/1658-354X.71581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savage JH, Kaeding AJ, Matsui EC, Wood RA. The natural history of soy allergy. J Allergy Clin Immunol. 2010;125:683–686. doi: 10.1016/j.jaci.2009.12.994. [DOI] [PubMed] [Google Scholar]
- 12.Bradley AE, Tober KE, Brown RE. Use of propofol in patients with food allergies. Anaesthesia. 2008;63:439. doi: 10.1111/j.1365-2044.2008.05505.x. [DOI] [PubMed] [Google Scholar]
- 13.de Leon-Casasola OA, Weiss A, Lema MJ. Anaphylaxis due to propofol. Anesthesiology. 1992;77:384–386. doi: 10.1097/00000542-199208000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Laxenaire MC, Mata-Bermejo E, Moneret-Vautrin DA, Gueant JL. Life-threatening anaphylactoid reactions to propofol (Diprivan) Anesthesiology. 1992;77:275–280. doi: 10.1097/00000542-199208000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Gangineni K, Scase AE, Fearn J. Propofol and peanut allergy. Anaesthesia. 2007;62:1191. doi: 10.1111/j.1365-2044.2007.05337.x. [DOI] [PubMed] [Google Scholar]
- 16.L'Hocine L, Boye JI. Allergenicity of soybean: new developments in identification of allergenic proteins, cross-reactivities and hypoallergenization technologies. Crit Rev Food Sci Nutr. 2007;47:127–143. doi: 10.1080/10408390600626487. [DOI] [PubMed] [Google Scholar]
- 17.Tsai MH, Kuo PH, Hong RL, Yang PC. Anaphylaxis after propofol infusion for Port-A-Cath insertion in a 35-year old man. J Formos Med Assoc. 2001;100:424–426. [PubMed] [Google Scholar]
- 18.Shin CH, Lee YH, Kim YM, Park SH, Sung IY, Choi SW, Park SE. Severe oropharyngeal angioedema caused by propofol: a case report. Korean J Anesthesiol. 2006;50:S68–S70. [Google Scholar]
- 19.Chung IK, Jeon SW, Kim SU, Sohn JW, Jung DW, Jung MK, Kim SK. A case of propofol-induced anaphylaxis. Korean J Med. 2010;79:549–552. [Google Scholar]