Abstract
Acute rhabdomyolysis is a clinical and laboratory syndrome resulting from the breakdown of skeletal muscle, with the release of intracellular contents into the circulatory system, which can cause potentially lethal complications. Here, we present the case of a patient who developed acute rhabdomyolysis after consumption of meloxicam for jaw pain and experienced generalized myalgias in the context of an acute febrile illness with generalized urticaria. Further investigation indicated elevated muscle enzymes and acute renal failure. Serological analysis revealed that the patient was positive for Ross River virus (RRV) IgM. Genetic studies to detect CYP2C9 polymorphisms were negative. Meloxicam was discontinued. He responded to conservative measures within 2 weeks. Oral aspirin challenge was negative, suggesting a drug-specific effect of meloxicam rather than a class effect. Our case indicates a causative role for meloxicam and/or acute RRV in rhabdomyolysis.
Keywords: RRV infection, meloxicam, rhabdomyolysis
INTRODUCTION
Acute rhabdomyolysis is a clinical and laboratory syndrome resulting from the breakdown of skeletal muscle, with the release of intracellular contents into the circulatory system, which can cause potentially lethal complications. There are a number of possible factors that may lead to acute rhabdomyolysis, and many patients present with multiple causes. Here we present a patient who developed acute rhabdomyolysis after consumption of meloxicam for jaw pain and experienced generalized myalgias in the context of an acute febrile illness with generalized urticaria.
CASE REPORT
A 74-year-old, previously healthy male presented to the emergency department with an acute febrile illness, rash, generalized myalgias, and jaw pain for 3 days. Examination showed generalized urticarial rash with no evidence of lymphadenopathy, mucosal lesions, proximal muscle weakness, or arthropathy.
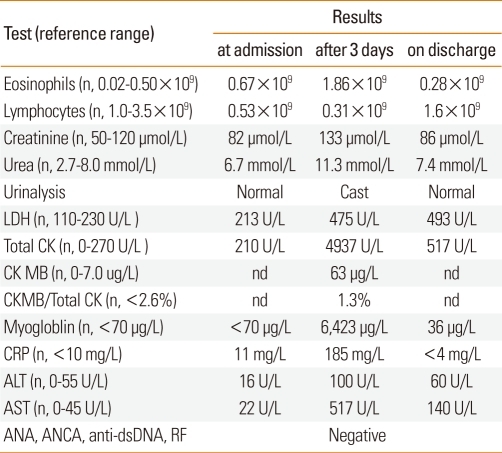
The patient had taken meloxicam for the jaw pain for 3 days prior to presentation. Three days later, he developed proximal muscle weakness of the lower limbs. Laboratory tests revealed mild impairment of renal function, eosinophilia, and lymphopenia (Table 1). Muscle enzymes were markedly elevated, and serum myoglobin was detected. Viral serology revealed a positive response for IgM antibody against Ross River virus (RRV), suggestive of an acute infection, with negative IgM for hepatitis viruses A, B, and C, Epstein-Barr Virus, and Cytomegalovirus. Autoimmune serologies were also negative, including antinuclear antibody, extractable nuclear antigens, and antineutrophil cytoplasmic antibody (Table 1). Needle muscle biopsy of the left vastus lateralis performed at the time of proximal muscle weakness showed acute muscle necrosis with features of mitochondrial damage. Skin biopsy revealed features consistent with urticaria along with mild eosinophilic and neutrophilic infiltration without any evidence of vasculitis. Chest X-ray, electrocardiogram, and renal ultrasound were normal. The patient was treated with intensive fluid resuscitation, and normalization of his muscle enzymes and renal function occurred over the course of 1 week. Oral aspirin challenge (1-day protocol with incremental doses of ≤600 mg aspirin and a cumulative dose of 985 mg) was undertaken to seek a safe alternative, non-steroidal anti-inflammatory drug (NSAID). This was well tolerated, and no changes in clinical or laboratory parameters, including renal function or muscle enzymes, were detected after the procedure. Genetic tests to detect CYP2C9 polymorphisms were negative.
Table 1.
Summary of the laboratory findings of our patient
n, normal; nd, not determined; ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic antibody; dsDNA, double-stranded deoxyribonucleic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatinine kinase; CRP, C-reactive protein; LDH, lactate dehydrogenase; RF, rheumatoid factor.
The chronology of events suggested a diagnosis of meloxicam-induced rhabdomyolysis in the context of acute RRV infection. Accordingly, the patient was advised to avoid meloxicam in the future.
DISCUSSION
Necrosis of muscle (rhabdomyolysis) is accompanied by the release of muscle contents, including myoglobin, creatine phosphokinase (CK), potassium, aldolase, lactate dehydrogenase, and glutamic-oxaloacetic transaminase, into the serum. Serum CK is the most sensitive enzyme marker of muscle injury.1 There are a number of possible factors that may lead to acute rhabdomyolysis, and many patients present with multiple causes. The most common causes are exertion, crush injury, seizures, alcohol, viral infections, muscle enzyme deficiencies, electrolyte abnormalities, endocrinopathies, drug abuse, and statins.2 Rhabdomyolysis is commonly associated with myoglobulinuria, which can lead to acute renal failure. Weakness, myalgias, and tea-colored urine are the cardinal clinical manifestations of rhabdomyolysis.
Drugs are frequently implicated in rhabdomyolysis and may directly or indirectly impair muscle metabolism by altering the balance between energy production and expenditure.3 Sarcolemmal injury and increased permeability through the activation of phospholipase A, leading to the efflux of intracellular contents and the influx of sodium and calcium, are also proposed mechanisms of drug-induced muscle damage.4-6 The most common causative drugs include antipsychotics and antidepressants, sedative hypnotics, statins, drugs of addiction, and antihistamines.7
There are case reports of rhabdomyolysis induced by NSAIDs8,9; however, rhabdomyolysis due to meloxicam has not been previously reported. Certain CYP2C9 polymorphisms change the metabolism of this class of drug, and thus have been shown to increase the risk for rhabdomyolysis. This phenomenon has most extensively been reported with celecoxib, a selective cyclooxygenase-2 inhibitor.10,11 In our case, meloxicam was the putative cause of our patient's complications, based on the chronological sequence of events (i.e., development of symptoms within 3 days of consumption) and the absence of other myotoxic drugs. The initial presentation of rash and eosinophilia is suggestive of an allergic reaction to meloxicam. The most definitive test for allergy, re-challenge with meloxicam, was deemed inappropriate due to the severity of the initial reaction. Aspirin challenge confirmed that the reaction was a drug-specific effect, rather than a class effect of pharmacological inhibition of arachidonic acid metabolism.
Viral infections may also precipitate rhadomyolysis. Clinical presentation can vary in severity from mild myalgias to overt muscle injury and acute renal failure in severe cases. Viruses implicated in such a reaction include influenza virus A/B, parainfluenza virus, coxsackie virus, Epstein-Barr virus, herpes simplex virus, adenovirus, and cytomegalovirus.12 Viruses may damage muscles directly through the invasion of muscle fibers or indirectly through the activation of the immune response.13-15 Mosquito-borne alphaviruses, such as RRV, have been reported to target bone, joint, and skeletal muscle tissue in a mouse model.16,17 Moreover, histological analyses have demonstrated that RRV infection results in severe inflammation of these tissues by infiltrating macrophages, NK cells, and CD4+ and CD8+ T lymphocytes.
Our patient had taken meloxicam in the context of an acute infection with RRV, and the subsequent rapid development of rhabdomyolysis suggested a causative role for meloxicam and/or RRV infection. As we did not perform a meloxicam challenge, it is unclear whether meloxicam was the primary cause or it exacerbated RRV-induced muscle injury.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Ellinas PA, Rosner F. Rhabdomyolysis: report of eleven cases. J Natl Med Assoc. 1992;84:617–624. [PMC free article] [PubMed] [Google Scholar]
- 2.Grob D. Rhabdomyolysis and drug-related myopathies. Curr Opin Rheumatol. 1990;2:908–915. doi: 10.1097/00002281-199002060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kakulas B. Experimental myopathies. In: Walton JN, editor. Disorders of voluntary muscle. New York: Churchill Livingstone; 1981. pp. 393–400. [Google Scholar]
- 4.Knochel JP. Mechanisms of rhabdomyolysis. Curr Opin Rheumatol. 1993;5:725–731. doi: 10.1097/00002281-199305060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Rubin BB, Liauw S, Tittley J, Romaschin AD, Walker PM. Prolonged adenine nucleotide resynthesis and reperfusion injury in postischemic skeletal muscle. Am J Physiol. 1992;262:H1538–H1547. doi: 10.1152/ajpheart.1992.262.5.H1538. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis -- an overview for clinicians. Crit Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delrio FG, Park Y, Herzlich B, Grob D. Case report: diclofenac-induced rhabdomyolysis. Am J Med Sci. 1996;312:95–97. doi: 10.1097/00000441-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Knobloch K, Rossner D, Gössling T, Lichtenberg A, Richter M, Krettek C. Rhabdomyolysis after administration of diclofenac. Unfallchirurg. 2005;108:415–417. doi: 10.1007/s00113-004-0874-z. [DOI] [PubMed] [Google Scholar]
- 10.Tang C, Shou M, Rushmore TH, Mei Q, Sandhu P, Woolf EJ, Rose MJ, Gelmann A, Greenberg HE, De Lepeleire I, Van Hecken A, De Schepper PJ, Ebel DL, Schwartz JI, Rodrigues AD. In-vitro metabolism of celecoxib, a cyclooxygenase-2 inhibitor, by allelic variant forms of human liver microsomal cytochrome P450 2C9: correlation with CYP2C9 genotype and in-vivo pharmacokinetics. Pharmacogenetics. 2001;11:223–235. doi: 10.1097/00008571-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kirchheiner J, Störmer E, Meisel C, Steinbach N, Roots I, Brockmöller J. Influence of CYP2C9 genetic polymorphisms on pharmacokinetics of celecoxib and its metabolites. Pharmacogenetics. 2003;13:473–480. doi: 10.1097/00008571-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Nauss MD, Schmidt EL, Pancioli AM. Viral myositis leading to rhabdomyolysis: a case report and literature review. Am J Emerg Med. 2009;27:372.e5–372.e6. doi: 10.1016/j.ajem.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Takada T, Takagi D, Takeyama N, Kitazawa Y. Acute renal failure due to rhabdomyolysis associated with echovirus 9 infection: a case report and review of literature. Jpn J Med. 1989;28:237–242. doi: 10.2169/internalmedicine1962.28.237. [DOI] [PubMed] [Google Scholar]
- 14.Naylor CD, Jevnikar AM, Witt NJ. Sporadic viral myositis in two adults. CMAJ. 1987;137:819–821. [PMC free article] [PubMed] [Google Scholar]
- 15.Konrad RJ, Goodman DB, Davis WL. Tumor necrosis factor and coxsackie B4 rhabdomyolysis. Ann Intern Med. 1993;119:861. doi: 10.7326/0003-4819-119-8-199310150-00024. [DOI] [PubMed] [Google Scholar]
- 16.Morrison TE, Whitmore AC, Shabman RS, Lidbury BA, Mahalingam S, Heise MT. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J Virol. 2006;80:737–749. doi: 10.1128/JVI.80.2.737-749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seay AR, Griffin DE, Johnson RT. Experimental viral polymyositis: age dependency and immune responses to Ross River virus infection in mice. Neurology. 1981;31:656–660. doi: 10.1212/wnl.31.6.656. [DOI] [PubMed] [Google Scholar]