Abstract
AG014699 was the first inhibitor of the DNA-repair enzyme Poly(ADP-ribose) polymerase-1 (PARP-1) to enter clinical trial in cancer patients. In addition to enhancing the cytotoxic effect of DNA-damaging chemotherapies, we have previously shown that AG014699 is vasoactive, thereby having the potential to improve drug biodistribution. The effectiveness of the clinical agent doxorubicin is confounded both by poor tumour penetration and cardiotoxicity elicited via PARP hyperactivation. Here we analysed the impact of AG014699 on doxorubicin tolerance and response in breast (MDA-MB-231) and colorectal (SW620, LoVo) tumour models in vitro and in vivo. As anticipated, AG014699 did not potentiate response to doxorubicin in vitro. In vivo, AG014699 did not influence the pharmacokinetics of doxorubicin, but did ameliorate cardiotoxicity. Both toxicity and extent of amelioration were more pronounced in male versus female mice. AG014699 improved vessel perfusion in both MDA-MB-231 and SW620 tumours yet this did not lead to improved tumour-accumulation of doxorubicin nor enhanced therapeutic response. In contrast, when combined with radiotherapy, AG014699 significantly enhanced response both in vitro and in vivo. Real-time assessment of tumour vessel function and companion histological studies suggest that doxorubicin causes a profound anti-vascular effect that countered the positive effect of AG014699 on perfusion. These data suggest that although AG014699 can enhance response to some chemotherapies via improved delivery, this does not apply to doxorubicin. PARP inhibitors may still be of use to counter doxorubicin toxicity and if the gender effect translates from rodents to humans, this would have greater effect in males.
Keywords: PARP, AG014699, doxorubicin, cardiotoxicity, perfusion
Introduction
The abundant nuclear enzyme Poly(ADP-ribose) polymerase-1 (PARP-1) is activated by DNA breaks and facilitates their repair by loosening chromatin and recruiting repair proteins (1). PARP-1 and its structural homologues, PARP-2 and PARP-3, are the only members of a superfamily of PARP enzymes that actively participate in DNA repair (1,2). Studies with early PARP inhibitors, nicotinamide and 3-aminobenzamide, suggested that they would be useful as anticancer chemosensitisers (3). Subsequent studies showed that PARP inhibitors increase the persistence of DNA damage and cytotoxicity induced by DNA-methylating agents, topoisomerase-I poisons and ionising radiation (4). These observations have been verified using genetic depletion of PARP-1. Potent PARP inhibitors have been developed for clinical trial in cancer patients, the first being AG014699 in 2003 (5). Other companies rapidly followed suit and 9 have PARPi in clinical development (6; www.clincaltrials.gov). BSI-201 impressively enhanced gemcitabine+carboplatin activity in triple-negative breast cancer patients without increasing toxicity (7). There is scant and/or contradictory evidence of the activity of PARPi in combination with these agents in cell-based studies (8-12) but there is evidence of anticancer activity in xenografts (9-12). Similarly, we observed that the PARPi, AG14361, enhanced the anticancer activity of temozolomide against SW620 xenografts but did not increase it’s cytotoxicity against SW620 cells (13). Taken together with the observation that 3-aminobenzamide and nicotinamide increased cisplatin antitumour activity in mice through haemodynamic effects (14), these data suggested that PARPi may improve drug delivery to tumours by altering blood flow. Further investigations revealed that both AG14361 and AG014699 caused arterial relaxation ex vivo and vasodilatation in tumour xenografts (15). Thus the exciting clinical data with BSI-201 could be due to improved drug delivery rather than chemosensitisation. Fluctuations in tumour blood flow have been observed in patients (16) and affect microregional oxygenation and drug delivery (17). Further tumour blood flow can correlate with surgery, chemotherapy and radiotherapy outcome (18). PARPi may therefore improve the activity of all chemotherapeutic drugs limited by poor drug delivery.
Doxorubicin is widely used in the treatment of breast and other cancers http://www.cancerhelp.org.uk/about-cancer/treatment/cancer-drugs/doxorubicin but it exhibits poor tissue penetration, resulting in pronounced gradients of doxorubicin auto-fluorescence with distance from blood microvessels in breast cancer biopsies (19). There is some evidence of enhancement of doxorubicin activity by PARP inhibition in vivo (20,21) but not in vitro (22,23) suggesting that the mechanism could be improved drug delivery. Doxorubicin treatment is limited by dose-limiting cardiotoxicity, in which oxidative damage-induced hyperactivation of PARP has been implicated and PARPi have a protective effect (24-26). The aim of this study was to determine if the clinically active PARPi AG014699 improved the therapeutic index of doxorubicin by both increasing tumour drug delivery and reducing cardiotoxicity. Using a human breast cancer xenograft model (MDA-MB-231), relevant to doxorubicin therapy, and a colon cancer model (SW620) in which we had previously observed AG014699-induced haemodynamic effects, we determined (i) the effect of AG014699 alone and in combination with doxorubicin on tumour blood flow, (ii) the resultant effect on doxorubicin antitumour activity and (ii) doxorubicin-induced cardiotoxicity in male and female mice.
Materials and Methods
Reagents
Chemicals and reagents were from Sigma (Poole, UK) unless otherwise stated. For in vivo evaluation we prepared all agents immediately before administration. Doxorubicin was dissolved in sterile water and AG014699 (Figure 1a) in sterile saline.
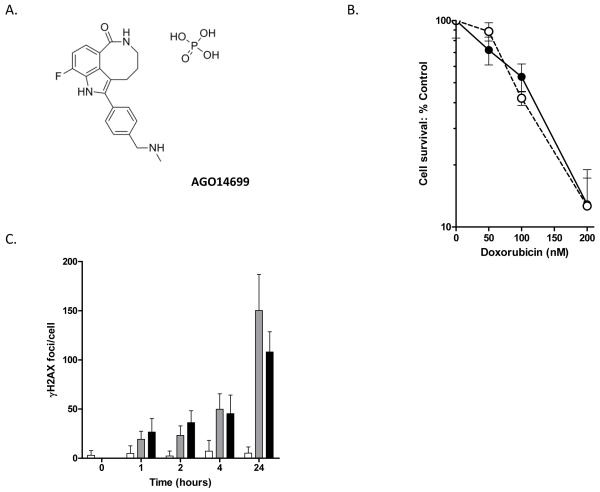
Figure 1. AG014699 (A) does not enhance the response of MDA-MB-231 cells to doxorubicin in vitro.
Clonogenic survival (B) or DNA damage (C) following exposure of cells to doxorubicin for 3h in the presence (open symbols, dashed line) or absence (closed symbols, solid line) of AG014699 (0.4μM). DNA-damage was assessed by counting the number of γH2AX foci in treated cells at different times after doxorubicin (1μM) removal with (closed bars) or without (grey bars) AG014699 and compared with that observed in cells treated with AG014699 alone (open bars).
Cell lines and culture
MDA-MB-231, SW620 and LoVo cells (American Type Culture Collection, Manassas, VA; authenticated by LGC Standards) were maintained in RPMI 1640 containing 10% (v/v) foetal calf serum and 2mM glutamine. Cells were verified as mycoplasma-free (MycoAlert™ Cambrex Bioscience, Rockland USA).
In vitro chemo and radiosensitisation
Chemosensitisation
MDA-MB-231 cells were seeded into replicate wells in 6-well plates. Doxorubicin was added at a range of concentrations in the presence or absence of 0.4μM AG014699 for 3h. Cells were seeded in drug-free medium for colony formation. .
Radiosensitisation
Exponentially growing MDA-MB-231 cells were cultured in medium containing 0.4μM AG014699 or control medium for 1h prior to X-irradiation (2.9Gy/min, Gulmay Medical Ltd., UK) and for a further 24h prior to seeding for colony formation in drug-free medium. Potentially lethal damage repair (PLDR) was measured in confluent G1-arrested MDA-MB-231 cells, exposed to 6Gy γ-irradiation and seeded for colony formation immediately or after a 24hr recovery period. Where indicated, AG014699 (0.4μM) was added 30min prior to irradiation and was present in the recovery incubation.
Analysis
Colonies were stained with crystal violet after 10 to 14 days and counted with an automated colony counter (ColCount, Oxford Optronics Ltd., Oxford, UK). Percent cell survival was calculated by comparison with untreated cells incubated in the presence or absence of AG014699, as appropriate.
γH2AX assay
Cells were seeded on to sterile cover slips in 6-well plates and exposed to doxorubicin (1μM for 3h) or 6Gy radiation with or without AG014699 (0.4μM) for 3h. Cells were washed twice, replenished with fresh medium and duplicate samples harvested at various times thereafter. Cells were formalin-fixed, washed 3x with PBS and blocked using 1% bovine serum albumin (BSA) in PBS for 30mins at room temperature. Cells were permeablised by adding 7% triton X-100 in PBS for 7mins and γH2AX foci revealed using mouse anti-phospho-Histone H2AX (Ser139; 1:500 in 1% BSA/PBS) followed by anti-mouse Alexa-Fluoro-488 (1:1000 in 1% BSA/PBS). 4′,6′-diamidino-2-phenylindole (1:2500 in PBS) was used to reveal nuclei. Samples were mounted (DAKO) and stored in the dark at 4°C until analysis.
In vivo studies
All in vivo studies were approved by the Home Office Inspectorate, local ethics committees and performed under PPL40/3212 (Manchester) and PPL60/3554 (Newcastle) according to UK-CCCR Guidelines (27) and in compliance of The Scientific Procedures Act 1986. Animals were bred in-house and maintained using the highest possible standard of care and priority was given to their welfare .
In vivo pharmacokinetics and toxicity
Pharmacokinetic Studies
Doxorubicin pharmacokinetics were determined following intraperitoneal (i.p.) injection of doxorubicin-HCl, alone or in combination with AG014699 (10mg/kg administered i.p. 30mins prior to doxorubicin) in three studies. Initially plasma pharmacokinetics of 10 mg/kg doxorubicin were determined in Balb-C mice over a 3h time-course. Plasma and tumour pharmacokinetics were assessed using a limited sampling schedule over a 3h time-course in CD-1 nude mice using two models: LoVo (colorectal cancer) and MDA-MB-231. Mice bearing the LoVo xenografts were given 10mg/kg doxorubicin but, because tumour levels were found to be low, mice bearing the MDA-MB-231 xenografts received 20mg/kg. In this latter study plasma and tumour concentrations of AG014699 were also determined.
In all studies mice were bled by cardiac puncture under terminal anaesthesia at various times post-treatment (3 mice/timepoint). Where appropriate, tumours were removed, snap frozen in liquid nitrogen and homogenised in 3 volumes of PBS. Plasma and tumour concentrations of doxorubicin and, where stated, AG014699 were determined by reference to standards prepared in mouse plasma using HPLC (Waters, Watford, Herts UK) with fluorescence detection (λex 475/λ em 555 nm). Briefly samples and standards were spiked with 5μl daunomycin internal standard, acetonitrile precipitated and 50μl supernatant resolved on a Supelcosil LC-CN 5μm 25cm × 4.6 column (Sigma-Aldrich, Supelco, Gillingham, Kent, UK) with a mobile phase of 0.02M phosphate buffer pH3.5 in acetonitrile (5:3 w/w). Pharmacokinetic parameters were calculated using a non-compartmental model with terminal elimination rate estimated by log-linear regression.
Toxicity Studies
Chronic toxicity of Doxorubicin
Male and female CD-1 mice were administered doxorubicin (5mg/kg) ±AG014699 once weekly for three weeks (n=5/group). Body weights and physical condition were monitored. Toxicity was expressed as average body weights as a percentage of the starting body weights.
Acute toxicity of High dose doxorubicin
The acute toxicity of high-dose doxorubicin was assessed in male CD-1 mice treated with 20mg/kg doxorubicin i.p.. Four days after administration there was unexpectedly high toxicity (approximate 15% loss of body weight, failure to groom, hypokinesia) and the study was terminated on grounds of animal welfare. Mice were bled from the tail vein and the level of creatine kinase assessed as a measure of muscle damage.
Troponin 1 and creatine kinase assays
Troponin 1 (Mouse Cardiac Tn-I 96-well ELISA Life Diagnostics, Inc) and Creatine Kinase (QuantiChromTM Creatine Kinase Assay Kit Universal Biologicals (Cambridge) Ltd) were assessed using commercially available ELISA kits. Blood was collected from the tail vein of anaesthetised mice (to avoid mechanical damage to the heart) and the plasma immediately removed by centrifugation and stored frozen at −80°C. Assays were conducted according to the manufacturer’s instructions.
In vivo efficacy studies
MDA-MB-231 (3×106) or SW620 (1×107) cells were implanted into adult athymic nude mice maintained and handled in isolators under specific pathogen-free conditions. Mice bearing tumours of 200-250mm3 volume were randomized into treatment groups (n=5/group). Doxorubicin was administered once weekly (5mg/kg i.p.) for three weeks or daily for 5d (2mg/kg i.p.) with or without additional AG014699 (10mg/kg i.p.), once daily for 5d. Tumour-localised radiotherapy (X-ray) was administered as five daily 2Gy fractions as described previously (28). AG014699 (10mg/kg i.p.) was administered 30mins prior to each fraction/doxorubicin dose. Tumour volumes and mouse weights were monitored at least 3x weekly. The experiments were terminated before the tumour volume exceeded 1000mm3.
Dorsal window chamber (DWC) studies
DWC surgery has been described previously (28). DWCs were inoculated with MDA-MB-231 or SW620 cells (50μl of a 1×107/ml stock). Once tumours established, mice were anaesthetised and vascular parameters assessed by intra-vital microscopy. Images were taken using bright-field and fluorescence microscopy (Nikon Eclipse E800) prior to and after administration of doxorubicin (10mg/kg i.v.) or AG014699 (1-10mg/kg i.p.). Real-time evaluation of the vascular effects of AG014699 has been described previously (15). Briefly, BSA labelled with Alexa-Fluoro-647 (BSA-647; λex 647nm, 1mg/ml in sterile saline; Molecular Probes, Invitrogen, Paisley, UK) was administered at 0.1ml/mouse i.v. prior to AG014699 and changes in fluorescence (λem 668nm) monitored (Metamorph analysis system).
Histological assessment of vasculature and hypoxia
Mice bearing MDA-MB-231 and SW620 tumours (300-400mm3) were randomly assigned to receive saline (0.1ml/10g), doxorubicin (10mg/kg i.v.), AG014699 (10mg/kg i.p.) or both. 30mins later mice received the hypoxic marker pimonidazole (60mg/kg i.p.; Chemicon International Inc. CA. USA) and after a further 30mins, the perfusion marker Hoechst 33342 (0.1ml of a 6mg/kg stock i.v.). 1min later tumours were excised and rapidly snap-frozen in liquid nitrogen. 8μm cryostat sections were scanned using a NIKON Eclipse E800 fluorescent microscope to determine the number of Hoechst-stained (perfused) vessels. Sections were then co-stained to reveal endothelial structures (rat anti-CD31 Pharmingen, BD Biosciences) and regions of hypoxia (pimonidazole binding, Hypoxyprobe antibody, Chemicon International) as described previously (29). Vessel density was analysed per unit area of the tumour section and hypoxia as the fraction of tumour exhibiting positive staining using a NIKON Eclipse E800 fluorescent microscope (28).
Results
AG014699 doesn’t influence the sensitivity of MDA-MB-231 cells to doxorubicin in vitro but causes sensitisation to ionising radiation in vitro and in vivo
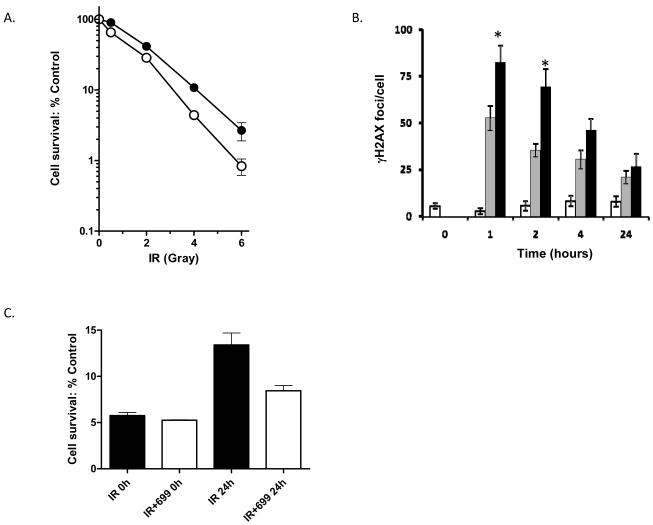
Initial studies were undertaken to confirm that PARP inhibition via AG014699 had no direct impact upon the response of MDA-MB-231 cells to doxorubicin. Cells were treated with increasing concentrations of doxorubicin alone or combined with AG014699 (0.4μM). Cytotoxicity was assessed by clonogenic assay. Untreated cells had a clonogenic potential of 0.69 ± 0.05. AG014699 alone had no significant impact on cell survival and had no effect on the dose response of MDA-MB-231 cells to doxorubicin (Figure 1b). Consistently, the level of DNA damage, determined by the number of γH2AX foci at various time-points after a 3h exposure to doxorubicin (1μM) did not significantly differ between cells treated with doxorubicin alone or with AG014699 (Figure 1c). In contrast when MDA-MB-231 cells were treated with AG014699 in combination with radiation, cytotoxicity was potentiated (Figure 2). In exponentially growing MDA-MB-231 cells AG014699 significantly (p=0.0268) enhanced the radiation response reducing the LD90 of 4.24±0.23Gy for IR alone to 3.53±0.05 for IR+AG014699 (Figure 2a). γH2AX foci were higher in cells co-treated with AG014699 and radiation compared with radiation alone (Figure 2b). To evaluate whether AG014699 also inhibited potentially lethal damage repair (PLDR), confluent MDA-MB-231 cells were exposed to 6Gy with or without AG014699 and seeded for colony formation immediately or after a 24 hr recovery period. AG014699 significantly (p=0.0033) reduced survival compared with radiation treatment alone implying a strong inhibitory effect against PLDR (Figure 2b).
Figure 2. AG014699 enhances the response of MDA-MB-231 cells to radiation treatment in vitro.
Clonogenic survival (A) of cells treated with radiation (closed symbols) ±AG014699 (open symbols) whilst in exponential growth phase. γH2AX foci were analysed to track DNA-damage (B) following radiation alone (6Gy grey bars) versus that observed following treatment with radiation+AG014699 (closed bars) or AG014699 alone (open bars). *p<0.03 versus radiation. Clonogenic survival of cells irradiated (6Gy) whilst growth arrested at confluence and seeded immediately (0h) or 24h later for colony formation (C).
AG014699 had no effect on the pharmacokinetics of doxorubicin in vivo
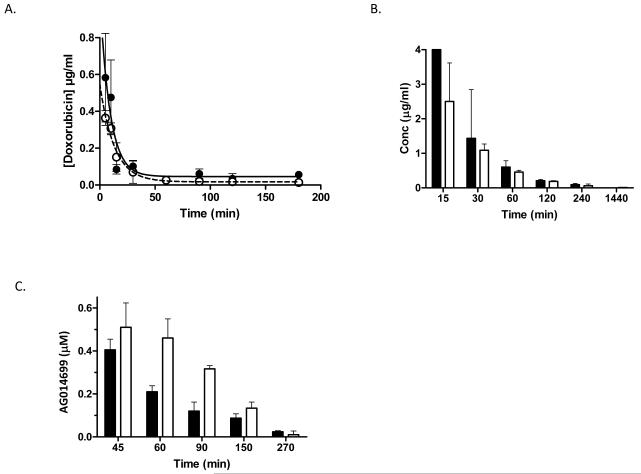
We investigated whether AG014699 affected the plasma pharmacokinetics of doxorubicin in female Balb-C mice by administering doxorubicin (10mg/kg i.p.) alone or 30min after AG014699 (10mg/kg i.p.). Plasma levels of doxorubicin were determined by HPLC. Pharmacokinetic parameters were determined using non-compartmental analyses. The plasma elimination of doxorubicin was rapid and well described by a monoexponential decay curve both when administered alone and when given in combination with AG014699. AG014699 did not markedly affect any measured parameters (Figure 3; supplementary Table 1). A second limited sampling study was undertaken in CD-1 nude mice. As in the Balb-C model AG014699 did not influence the plasma pharmacokinetics of doxorubicin. In contrast doxorubicin did appear to increase the concentration of AG014699 in the plasma, largely as a result of decreased clearance of AG014699 (Figure 3; supplementary Tables 1 and 2).
Figure 3. AG014699 does not affect the plasma pharmacokinetics of doxorubicin.
Doxorubicin, detected by HPLC using fluorescence detection, in plasma from Balb-C mice (A) treated with a single i.p. dose of doxorubicin (10mg/kg) either alone (solid symbols) or 30min after a single i.p. dose of AG014699 (10mg/kg; open symbols). (B) Limited sampling study in CD-1 nude mice in which AG014699 (white bars indicate doxorubicin 20mg/kg+AG014699 10mg/kg) had no effect on doxorubicin (black bars) plasma pharmacokinetics. (C) Plasma AG014699 after administration alone (10mg/kg; black bars) or with doxorubicin (20 mg/kg white bars)
Male mice exhibit worse toxicity than females in response to doxorubicin which is ameliorated by AG014699
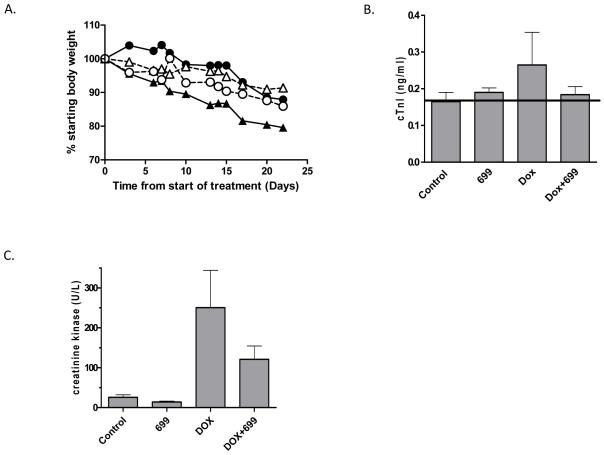
Previous studies have suggested that doxorubicin toxicity is worse in male versus female rodents (30, 31). Further PARP hyper-activation in response to doxorubicin-induced oxidative stress has been implicated as a key mediator of normal tissue damage (24). To assess chronic toxicity, male and female mice were administered doxorubicin once weekly (5mg/kg) either alone or in combination with AG014699 (10mg/kg) for three weeks and toxicity monitored by assessing body weight. All drug-treated mice lost weight. The greatest loss was in male mice treated with doxorubicin alone (20%). Co-treatment with AG014699 resulted in only 10% weight loss, indicating reduced toxicity. In contrast, in female mice doxorubicin only caused a 10% reduction in body weight, which was unaffected by co-administration of AG014699. The weight of control mice receiving saline or mice treated with AG014699 alone was stable or increased over the same time-frame (data not shown). Troponin 1 levels were monitored in the plasma of the male mice as a direct marker of cardiac damage. Doxorubicin treatment alone increased plasma troponin 1 (Figure 4b). However, the addition of AG014699 reduced plasma troponin 1 towards that observed in controls or animals treated with AG014699 alone (Figure 4b). Acute toxicity was also monitored following high dose doxorubicin (20mg/kg). Body weight was monitored for 4d post-treatment. Doxorubicin alone resulted in a 13% weight-loss. When combined with single (day 1) or multiple (dailyx4) dosing with AG014699 (10mg/kg) weight-loss was 8%, suggesting that AG014699 partially ameliorated toxicity (data not shown). Troponin 1 could not be detected in plasma upon sacrifice at day 4. However creatine kinase was assessed as a potential marker of (cardiac) muscle damage. Again, consistent with the weight change data, AG014699 modulated the increase in creatine kinase levels compared to that observed with doxorubicin treatment alone (Figure 4c).
Figure 4. Doxorubicin is more toxic in male rather than female mice and AG014699 reduces this toxicity.
Male (triangles) and female (circles) mice were administered doxorubicin (5mg/kg) with (open symbols, dashed lines) or without (closed symbols, solid lines) AG014699 once per week for three weeks (A and B). Data show average body weights (n=5/group) as a percentage of the body weights at the start of the dosing period (A) and plasma troponin 1 levels at the end of the experiment in male mice (B). Acute toxicity (4 days post 20mg/kg doxorubicin) is also reduced by AG014699. (C) Shows the plasma levels of creatine kinase following treatment in male mice. In each experiment, AG014699 was administered at 10mg/kg 30min prior to doxorubicin.
AG014699 increases the IR response but not the response of MDA-MB-231 or SW620 xenografts to doxorubicin treatment in vivo despite improving tumour perfusion
To confirm that MDA-MB-231 in vitro radio-sensitisation translated to in vivo radio-potentiation, MDA-MB-231 tumours were established in nude mice and treated with 5 daily fractions of 2Gy alone or combined with AG014699 (10mg/kg; 30min prior to each IR fraction). Control tumours reached 3 times the treatment volume in 13±1d. AG014699 alone had no effect on tumour regrowth (14±1d; Figure 5a). Radiation significantly delayed tumour growth to 33±4 days which was further delayed by the addition of AG014699 (43±2; p=0.05 versus radiotherapy alone; Figure 5a). Similarly dephosphorylated-AG014699 significantly enhanced radiation-induced tumour growth delay in LoVo xenografts (tumour growth delay radiation alone 32 ± 1 day; radiation+ AG14447 41 ± 2 days; p=0.002 supplementary figure 1).
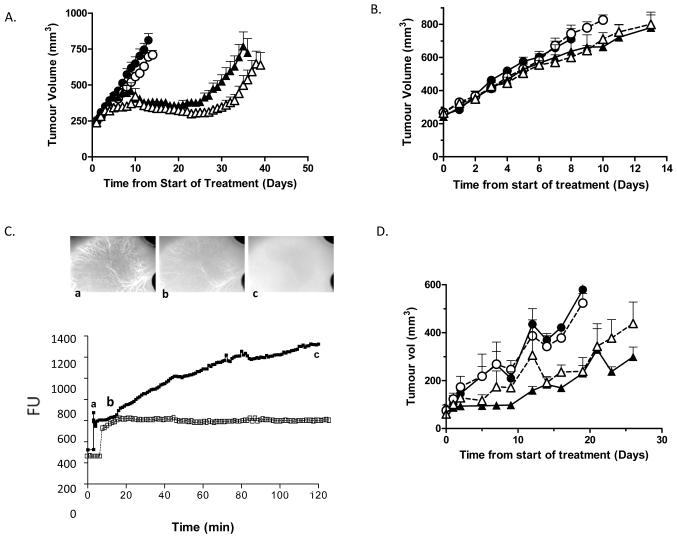
Figure 5. AG014699 enhances the radiation response of MDA-MB-231 xenografts but does not potentiate the response of MDA-MB-231 or SW620 tumours to doxorubicin in spite of causing significant improvements in vessel perfusion.
Mice bearing MDA-MB-231 xenografts were administered radiotherapy (2Gy fractions dailyx5; [A]) or doxorubicin (5mg/kg once weekly; [B]) ± AG014699 (10mg/kg dailyx5) and tumour volume monitored daily. Shown are average volumes ±sem (n=5/group). C) MDA-MB-231 tumours were established in DWC. Mice were administered BSA-647 (i.v. [depicted in image a]) and tumour accumulation of fluorescence monitored in real-time. AG014699 (10mg/kg; closed symbols) or saline (open symbols) were administered once a plateau was achieved [image b]. AG014699 caused significant accumulation of Alexa-BSA [image c] whilst saline had no effect. D) Mice bearing SW620 tumours received doxorubicin (5mg/kg) ±AG014699 (10mg/kg) once weekly. Data show average tumour volumes post-treatment ±sem (n=5/group). A, B and D: Closed circles, control; open circles, AG014699 alone; closed triangles, IR (A) or doxorubicin (B,D) alone; open triangles IR (A) or doxorubicin (B,D) plus AG014699.
We have previously demonstrated that AG014699 is vasoactive in colorectal xenografts (15). We hypothesised that an AG014699-mediated improvement in vascular perfusion would enhance the efficacy of doxorubicin in vivo. Female cba nude mice bearing MDA-MB-231 tumour xenografts were treated with doxorubicin (5mg/kg) once weekly alone or with AG014699 (10mg/kg dailyx5). Tumour growth delay (4x treatment volume) was 17±4d in doxorubicin treated mice compared with 14±3d in controls. AG014699 had no effect on tumour growth alone, nor did it enhance the growth delay observed when combined with doxorubicin (Figure 5b). The weight loss observed over the duration of the experiment was approximately 5% in doxorubicin and doxorubicin/AG014699 treated mice. The inability of AG014699 to enhance the effect of doxorubicin was not “mouse-strain specific” as we undertook a similar study with female CD-1 nude mice bearing MDA-MB-231 tumours treated weekly with doxorubicin (5 mg/kg) ±AG014699 (10 mg/kg). Doxorubicin caused a modest delay in tumour growth (20±6d) compared to controls (14±3d). AG014699 had no significant effect either alone (15±3d) or in combination with doxorubicin (22±4d).
To ensure that the previously reported vasoactivity of AG014699 was not restricted to colorectal xenografts, MDA-MB-231 tumours were established in dorsal window chambers (DWC) to allow real-time assessments of vessel function following AG014699 administration. Mice were administered Alexa-fluoro-conjugated BSA-647 and fluorescence uptake in to the tumours assessed as an indicator of tumour perfusion. AG014699 treatment (10mg/kg) led to a marked enhancement in tumour fluorescence (Figure 5c) in the MDA-MB-231 model as previously observed in colorectal tumours. In contrast, when mice received saline, fluorescence did not rise above the plateau achieved before administration (Figure 5c). There was a dose dependency to the fold enhancement in fluorescence observed over the basal plateau which rose from 1.5 with 1mg/kg AG014699, to 1.7 at 3mg/kg and 2.2 at 10mg/kg (data not shown). The lack of correlation between vascular effects and doxorubicin response could still have been model dependent. We therefore evaluated doxorubicin efficacy with and without AG014699 treatment in male and female mice bearing SW620 xenografts. In accordance with the MDA-MB-231 data, AG014699 did not potentiate doxorubicin response either on the weekly or daily x5 schedule in either gender (Figure 5d and data not shown).
Doxorubicin causes acute vascular changes that counteract the positive effects of AG014699 on vessel perfusion
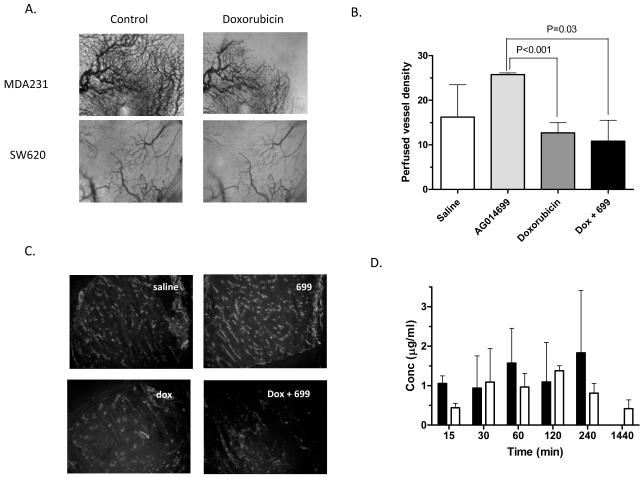
Failure of AG014699 to enhance doxorubicin could be based upon several mechanisms. We decided to evaluate whether doxorubicin itself caused changes in vascular function and whether this could impair the influence of AG014699 on perfusion. MDA-MB-231 xenografts were established in DWCs. Doxorubicin (10mg/kg) was administered and vessels monitored at various time-points thereafter under trans-illumination. Doxorubicin caused an acute vessel shutdown, consistent with a pronounced anti-vascular effect (Figure 6a). These acute vascular changes were also apparent in SW620 tumours (Figure 6a). To complement these real-time observations a histological study was undertaken. SW620 tumours were treated with either saline, AG014699 (1mg/kg), doxorubicin (10mg/kg) or doxorubicin plus AG014699 and excised 60min later. The hypoxia marker pimonidazole and the perfusion marker Hoechst 33342 were administered 30 and 1min prior to tumour excision respectively. The extent of tumour hypoxia did not vary significantly from that observed in control tumours (13±3% tumour area). The perfused vessel density increased upon AG014699 treatment (Figure 6b; 26±0.4mm−2 compared with 16±7 in controls). Doxorubicin alone caused a slight reduction in vascular perfusion and, in combination, ablated the enhanced perfusion associated with AG014699 treatment (Figure 6b). Similarly in MDA-MB-231 tumours AG014699 increased perfusion from 6±5mm−2 to 13±7mm−2, which was impaired upon combination with doxorubicin (9±4mm−2). We determined the effect of AG014699 on the distribution of doxorubicin to tumour tissue in female CD-1 nude mice bearing MDA-MB-231 tumours using a limited sampling schedule (Figure 6d). AG014699 did not affect the levels of doxorubicin detected within the MDA-MB-231 tumours. However, doxorubicin did increase AG014699 within tumours, consistent with our previous observations that doxorubicin decreases AG014699 clearance resulting in elevated levels within tissue and plasma (supplementary Table 2). Similar observations were made in mice bearing LoVo colorectal tumours (data not shown).
Figure 6. Doxorubicin causes acute vascular effects that counteract the improved perfusion observed with AG014699 alone.
MDA-MB-231 and SW620 tumours were established in DWC and vessels observed immediately before or 30min after administration of doxorubicin (10mg/kg) (A). Histological assessment of tumour perfusion following AG014699 and doxorubicin treatment determined by assessing the number Hoechst-stained (perfused) vessels 1h after drug treatment in SW620 tumours (B). Shown in are average values ±sd (n=5/group). Representative images of Hoechst distribution are shown in (C). AG014699 does not enhance tumour uptake of doxorubicin (D). MDA-MB-231 tumour bearing mice were treated with doxorubicin alone (20mg/kg; closed bars) or doxorubicin plus AG014699 (10mg/kg; open bars). Doxorubicin levels were determined by HPLC with fluorescence detection (n=3/timepoint).
Discussion
Therapeutic inhibition of the DNA-repair enzyme PARP-1 has emerged as an exciting field in anti-cancer therapeutics. We have previously shown that PARPi can improve tumour perfusion, and potentially drug delivery, which would explain why PARPi can enhance the in vivo therapeutic tumour response in models where little sensitization is observed in vitro. In this study we aimed to evaluate the benefit of combining the clinical PARPi, AG014699, with doxorubicin, a chemotherapy agent whose anticancer activity is known to be limited by poor tumour distributions and whose dose limiting toxicity appears dependent on hyperactivation of PARP (24).
We anticipated that PARP inhibition would have little direct effect on doxorubicin cytotoxicity in vitro. Accordingly neither doxorubicin-induced cytotoxicity nor DNA damage were potentiated by AG014699 in MDA-MB-231 breast cancer cells. These data are consistent with previously published observations using earlier less potent PARPi (22). In contrast, AG014699 significantly enhanced the in vitro response to radiotherapy, illustrating that a potentiating effect can be observed in the MDA-MB-231 model when an appropriate DNA-damaging agent is used.
We hypothesized that in vivo, combining AG014699 with doxorubicin would be beneficial in two ways; firstly, by ameliorating cardiotoxicity and secondly, by allowing greater tumour accumulation of doxorubicin and hence greater efficacy. We first established that AG014699 had no effect on the pharmacokinetics of doxorubicin that could have influenced the outcomes of both studies. Interestingly, although AG014699 did not alter the plasma pharmacokinetics of doxorubicin, doxorubicin altered the clearance of AG014699 resulting in higher plasma concentrations in mice treated with the combination versus AG014699 alone. Potentially doxorubicin-induced nephrotoxicity (32,33) impairs renal clearance of AG014699. Cardiotoxicity studies were undertaken using both male and female mice as previous literature has demonstrated that the detrimental effects of doxorubicin are more prevalent in males (30,31). In keeping with these findings, chronic dosing with doxorubicin caused greater weight loss in male compared with female mice. This was associated with increased plasma concentrations of troponin 1, a marker of cardiac muscle damage. Both weight loss and troponin 1 levels were ameliorated by co-administration of AG014699. Since doxorubicin-induced cardiotoxicity is proposed to be due to PARP hyperactivation, a recent demonstration that PARP activity is higher in male mice and humans than in females (34) is highly pertinent to our observations. Similar findings were observed upon acute dosing of doxorubicin using creatine kinase which appeared a more sensitive read-out for toxicity than troponin over the 4d timeframe used. Previous data have shown that nephrotoxicity occurs before cardiotoxicity in rodents (33) and, similar to cardiotoxicity, involves peroxidation (32,35) and presumably therefore PARP hyperactivation. Creatine kinase possibly reflects this nephrotoxicity or more generalised toxicity (32). Importantly, the observation that AG014699 prevents the rise in plasma creatine kinase indicates a protective effect of PARPi against various normal toxicities beyond cardiotoxicity.
Doxorubicin is clinically used in breast cancer so we analysed the effect of doxorubicin with and without combined treatment with AG014699 in MDA-MB-231 tumours. Surprisingly, we saw no enhancement of response despite AG014699 alone resulting in markedly improved perfusion in the MDA-MB-231 model observed using real-time intravital microscopy. In our previous studies, such marked vascular responses were sufficient to enhance the therapeutic response of SW620 colorectal xenografts to temozolomide, yielding prolonged tumour regression and cure in 40% of treated animals despite having no sensitizing effect in vitro (36). We analysed whether the ability of AG014699 to potentiate the response to doxorubicin was model and/or gender dependent by repeating the efficacy studies in both male and female mice bearing SW620 tumours using multiple treatment protocols. Although previous data has suggested that SW620 tumours are unresponsive to doxorubicin (37), in our studies we observed a significant response at the dose used (5mg/kg weekly). However, as with the MDA-MB-231studies no improvement was observed in the SW620 model when doxorubicin was combined with AG014699. This led us to assume that there was a common mechanism that ablated the potential impact of AG014699 vascular effects on doxorubicin biodistribution in both models.
We concentrated on vascular effects and found that doxorubicin produced a profound, acute impact on tumour vascularisation. Real-time imaging suggested acute vessel shutdown that seemed focused on smaller, potentially less mature vessels. Histological assessment revealed that AG014699 alone increased the number of perfused vessels, but when combined with doxorubicin, the positive effect of AG014699 was completely ablated in SW620 tumours and significantly reduced in MDA-MB-231 xenografts. Consequently tumour levels of doxorubicin were not improved by AG014699 co-treatment as would have been anticipated with a classic enhancement in delivery. These studies are consistent with previous preclinical studies demonstrating that doxorubicin decreases tumour blood flow (38) and blunts the response of isolated aortas to the endothelium-dependent vasodilator, acetylcholine (39). In contrast, AG014699 did potentiate radiotherapy response that could be attributed both to improved oxygenation and increased cytotoxicity in the presence of a PARP inhibitor.
Taken together these data suggest that although AG014699 can enhance perfusion and improve the therapeutic response of some chemotherapies via improved tumour delivery, this cannot be extrapolated to all agents. Doxorubicin appears to have a direct effect on tumour vessels that confounds the ability of AG014699 to enhance perfusion. We have previously shown that AG014699 can inhibit the function of myosin light chain kinase, potentially preventing vasoconstriction (15). Whether the doxorubicin effect is via this same pathway is as yet unknown, but it is certainly rapid, and observed within 30mins. In the context of doxorubicin, PARP-inhibitors would still have utility in ameliorating dose-limiting toxicity. Recent studies demonstrate that women have lower PARP activity than men, analogous to the gender differences in mice (30,31,34). If the gender influences on cardiotoxicity translate from rodent to humans, PARP inhibitors would have greater utility in males.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of Pfizer for the provision of AG014699 used in these studies.
Financial support: This work was principally supported by a grant from Cancer Research UK jointly to K.J.W. and N.J.C (C7820/A8200). Further contributions were made by EU FP7 Metoxia Grant agreement no. 222741 to KJW.
Footnotes
Disclosure of Potential Conflicts of Interest:
NJC receives grant support from Pfizer and NJC and KJW are named inventors on patents relating to AG014699.
References
- 1.Hakame A, Wong H-K, Dantzer F, Schreiber V. The expanding field of poly(ADP-ribosyl)ation reactions. EMBO Reports. 2008;9:1094–100. doi: 10.1038/embor.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boheler C, Gauthier LR, Mortusewicz O, Biard DS, Saliou J-M, Bresson A, Sanglier-Cianferani S, et al. Poly (ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. PNAS. 2011;108:2783–8. doi: 10.1073/pnas.1016574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durkacz BW, Omidiji O, Gray DA, Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980;283:593–6. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- 4.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. Nature Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plummer R, Jones C, Middleton M, Wilson R, Evans J, Olsen A, et al. Phase I Study of the Poly(ADP-Ribose) Polymerase Inhibitor, AG014699, in Combination with Temozolomide in Patients with Advanced Solid Tumors. Clin Cancer Res. 2008;14:7917–23. doi: 10.1158/1078-0432.CCR-08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvert H, Azzariti A. The clinical development of inhibitors of poly(ADP-ribose) polymerase. Ann Oncol. 2011;22(Suppl 1):i53–9. doi: 10.1093/annonc/mdq667. [DOI] [PubMed] [Google Scholar]
- 7.O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 8.Bowman KJ, White A, Golding BT, Griffin RJ, Curtin NJ. Potentiation of anticancer agent cytotoxicity by the potent poly(ADP-ribose) polymerase inhibitors, NU1025 and NU1064. Br J Cancer. 1998;78:1269–77. doi: 10.1038/bjc.1998.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob DA, Bahra M, Langrehr JM, Boas-Knoop S, Stefaniak R, Davis J, et al. Combination therapy of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide and gemcitabine shows strong antitumor activity in pancreatic cancer cells. J Gastroenterol Hepatol. 2007;22:738–48. doi: 10.1111/j.1440-1746.2006.04496.x. [DOI] [PubMed] [Google Scholar]
- 10.Miknyoczki SJ, Jones-Bolin S, Prichard S. Chemopotentiation of temozlomide, irinotecan and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol Cancer Ther. 2003;2:371–82. [PubMed] [Google Scholar]
- 11.Racz I, Tory K, Gallyas F, Jr, Berente B, Osz E, Jaszlits L, et al. BGP-15 - a novel poly(ADP-ribose) polymerase inhibitor - protects against nephrotoxicity of cisplatin without compromising its antitumour activity. 2002;63:1099–111. doi: 10.1016/s0006-2952(01)00935-2. [DOI] [PubMed] [Google Scholar]
- 12.Chan G, Pan Q-C. Potentiation of the antitumour activity of cisplatin in mice by 3-aminobenzamide and nicotinamide. Cancer Chemother Pharmacol. 1988;22:303–7. doi: 10.1007/BF00254236. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, et al. Preclinical evaluation of a novel poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor, AG14361, with significant anticancer chemo- and radio-sensitization activity. JNCI. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 14.Bernges F, Zeller WJ. Combination effects of poly(ADP-ribose) polymerase inhibitors and DNA damaging agents in ovarian tumor cell lines - with special reference to cisplatin. J Cancer Res Clin Oncol. 1996;122:665–70. doi: 10.1007/BF01209029. [DOI] [PubMed] [Google Scholar]
- 15.Ali M, Telfer BA, McCrudden C, O’Rourke M, Thomas HD, Kamjoo M, et al. Vasoactivity of AG014699, a clinically active small molecule inhibitor of poly(ADP-ribose) polymerase: a contributory factor to chemopotentiation in vivo? Clin Cancer Res. 2009;15:6106–12. doi: 10.1158/1078-0432.CCR-09-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pigott KH, Hill SA, Chaplin DJ, Saunders MI. Microregional fluctuations in perfusion within human tumours detected using laser Doppler flowmetry. Radiother Oncol. 1996;40:45–50. doi: 10.1016/0167-8140(96)01730-6. [DOI] [PubMed] [Google Scholar]
- 17.Tannock IF, Lee CM, Tunggal JK, Cowan DSM, Egorin MJ. Limited penetration of anticancer drugs through tumour tissue: A potential cause of resistance of solid tumours to chemotherapy. Clin Cancer Res. 2002;8:878–84. [PubMed] [Google Scholar]
- 18.Durand RE, Aquino-Parsons C. Clinical relevance of intermittent tumour blood flow. Acta Oncologica. 2001;40:929–36. doi: 10.1080/02841860152708206. [DOI] [PubMed] [Google Scholar]
- 19.Lankelma J, Dekker H, Luque FR, Luykx S, Hoekman K, van der Valk P, et al. Doxorubicin gradient in human breast cancer. Clin Can Res. 1999;5:1703–7. [PubMed] [Google Scholar]
- 20.Joseph I, Ferguson D, Palma J, Refici M, Godzicki L, Rodriguez L, et al. Poly(ADP-ribose) polymerase inhibitor, ABT-472 enhances antitumour activity of doxorubicin in human xenograft models and protects against drug-induced cardiac toxicity. EJC. 2004 Suppl2#8 Abs 473. [Google Scholar]
- 21.Mason KA, Valdecanas D, Hunter NR, Milas L. INO-1001, a novel inhibitor of poly(ADP-ribose) polymerase, enhances tumor response to doxorubicin. Investigational New Drugs. 2008;26:1–5. doi: 10.1007/s10637-007-9072-5. [DOI] [PubMed] [Google Scholar]
- 22.Bowman KJ, Newell DR, Calvert AH, Curtin NJ. Differential effects of the poly(ADP-ribose) polymerase (PARP) inhibitor NU1025 on topoisomerase I and II inhibitor cytotoxicity. Br J Cancer. 2002;84:106–12. doi: 10.1054/bjoc.2000.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holl V, Coelho D, Weltin D, Hyun JW, Dufour P, Bischoff P. Modulation of the antiproliferative activity of anticancer drugs in hematopoietic tumor cell lines by the poly(ADP-ribose) polymerase inhibitor 6(5H)-phenanthridinone. Anticancer Research. 2000;20:3233–41. [PubMed] [Google Scholar]
- 24.Pacher P, Liadet L, Bai P, Virah L, Mabley JG, Hasko G, et al. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. JPET. 2002;300:862–7. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 25.Szenczi O, Kemecsei P, Holthuijsen MFJ, vanRiel NAW, van der Vusse GJ, Pacher P, et al. Poly(ADP-ribose) polymerase regulated myocardial calcium handling in doxorubicin-induced heart failure. Biochem Pharmacol. 2005;69:725–32. doi: 10.1016/j.bcp.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C. Beneficial effect of a novel ultrapotent PARP inhibitor in murine models of heart failure. Int J Molecular Med. 2006;17:369–75. [PMC free article] [PubMed] [Google Scholar]
- 27.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–77. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams KJ, Telfer BA, Shannon AM, Babur M, Stratford IJ, Wedge SR. Combining radiotherapy with AZD2171, a potent inhibitor of vascular endothelial growth factor signaling: pathophysiologic effects and therapeutic benefit. Mol Cancer Ther. 2007;6:599–606. doi: 10.1158/1535-7163.MCT-06-0508. [DOI] [PubMed] [Google Scholar]
- 29.Lunt SJ, Cawthorne C, Ali M, Telfer BA, Babur M, Smigova A, et al. The hypoxia-selective cytotoxin NLCQ-1 (NSC 709257) controls metastatic disease when used as an adjuvant to radiotherapy. Br J Cancer. 2010;103:201–8. doi: 10.1038/sj.bjc.6605753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kratz F, Ehling G, Kauffmann HM, Unger C. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl) hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin binding prodrug of the anticancer agent doxorubicin. Human & Experimental Toxicology. 2007;26:19–35. doi: 10.1177/0960327107073825. [DOI] [PubMed] [Google Scholar]
- 31.Yan X, Stone JR, Schuldt AJT, Okoshi K, Okoshi MP, Nakayama M, et al. Doxorubicin induces severe cardiac dysfunction in NRG-1 knockout mice. Journal of Cardiac Failure. 2003;9(Suppl. S18) [Google Scholar]
- 32.El-Shitany NA, El-Haggar S, El-desoky K. Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food and Chemical Toxicology. 2008;46:2422–8. doi: 10.1016/j.fct.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Robert J. Preclinical assessment of anthracycline cardiotoxicity in laboratory animals: Predictiveness and pitfalls. Cell Biol Toxicol. 2007;23:27–37. doi: 10.1007/s10565-006-0142-9. [DOI] [PubMed] [Google Scholar]
- 34.Zaremba T, Thomas HD, Cole M, Coulthard SA, Plummer ER, Curtin NJ. Poly(ADP-ribose) polymerase-1 (PARP-1) pharmacogenetics, activity and expression analysis in cancer patients and healthy volunteers. Biochem J. 2011;436:671–9. doi: 10.1042/BJ20101723. [DOI] [PubMed] [Google Scholar]
- 35.Mimnaugh EG, Trush MA, Gram TE. A possible role for membrane lipid peroxidation in anthracycline nephrotoxicity. Biochemical Pharmacology. 1986;35:4327–35. doi: 10.1016/0006-2952(86)90713-6. [DOI] [PubMed] [Google Scholar]
- 36.Thomas HD, Calabrese CR, Batey MA, Canan S, Hostomsky Z, Kyle S, et al. Preclinical selection of a novel poly(ADP-ribose) polymerase (PARP) inhibitor for clinical trial. Mol Cancer Ther. 2007;6:945–56. doi: 10.1158/1535-7163.MCT-06-0552. [DOI] [PubMed] [Google Scholar]
- 37.Ramachandran C, Nair PKR, Alamo A, Cochrane CB, Escalon E, Melnick SJ. Anticancer effect of amooranin in human colon carcinoma cell line in vitro and in nude mice xenografts. Int J Cancer. 2006;119:2443–54. doi: 10.1002/ijc.22174. [DOI] [PubMed] [Google Scholar]
- 38.Durand RE, LePard NE. Tumour blood flow influences combined radiation and doxorubicin treatments. Radiotherapy & Oncology. 1997;42:171–9. doi: 10.1016/s0167-8140(96)01878-6. [DOI] [PubMed] [Google Scholar]
- 39.Ulu N, Buikema H, van Gilst WH, Navis G. Vascular dysfunction in adriamycin nephrosis:different effects of adriamycin exposure and nephrosis. Nephrology Dialysis Transplantation. 2008;23:1854–60. doi: 10.1093/ndt/gfm911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.