Abstract
Neuroblastoma is a common solid tumour of childhood and advanced disease carries a poor prognosis despite intensive multi-modality therapy. Hypoxia is a common feature of solid tumours due to poorly organised tumour-induced neovasculature. Hypoxia is associated with advanced stage and poor outcome in a range of tumour types, and leads to resistance to clinically relevant cytotoxic agents in neuroblastoma and other paediatric tumours in vitro. Resistance to apoptosis is a common feature of tumour cells, and leads to pleiotropic drug resistance, mediated by Bcl-2 family proteins. ABT-737 is a novel small molecule inhibitor of Bcl-2 and Bcl-xL that is able to induce apoptosis in a range of tumour types. Neuroblastoma cell lines are relatively resistant to ABT-737-induced apoptosis in normoxia, but in contrast to the situation with conventional cytotoxic agents, are more sensitive in hypoxia. This sensitisation is due to an increase in ABT-737-induced apoptosis and is variably dependent upon the presence of functional HIF-1α. In contrast to the situation in colon carcinoma and non small cell lung cancer cells hypoxia does not result in down-regulation of the known ABT-737 resistance factor, Mcl-1, nor any other Bcl-2 family proteins. ABT-737 sensitises neuroblastoma cells to clinically relevant cytotoxic agents under normal levels of oxygen, and importantly this sensitisation is maintained under hypoxia, when neuroblastoma cells are resistant to these agents. Thus rational combinations of ABT-737 and conventional cytotoxics offer a novel approach to overcoming hypoxia-induced drug resistance in neuroblastoma.
Keywords: Hypoxia, ABT-737, neuroblastoma, chemotherapy
Introduction
Neuroblastoma is the commonest extracranial solid tumour of childhood. Significant numbers of patients have widely disseminated disease and have poor survival despite intensive multi-agent induction chemotherapy, myeloablative therapy, radiotherapy, surgery and 13-cis retinoic acid (1), and for patients with an inadequate response to induction chemotherapy the outcome is very poor (2).
Hypoxia, a reduction in the level of tissue oxygen, is a common feature of solid tumours because of reduced blood flow in the tumour-induced neovasculature (3). Hypoxia is associated with decreased survival and advanced stage in several types of adult tumour, including squamous cell carcinomas of the head and neck, and carcinoma of the cervix (4). Hypoxia has long been known to decrease the effectiveness of radiotherapy, and more recent studies have shown that cytotoxic agents are also less effective under conditions of low oxygen (5, 6). In neuroblastoma cells hypoxia reduces etoposide, and vincristine–induced apoptosis (7) and leads to resistance to cisplatin (8). Similar effects of hypoxia are seen in the common paediatric tumours rhabdomyosarcoma and Ewing’s sarcoma (9). The principle modulator of tumour cell response to hypoxia is the transcription factor hypoxia-inducible factor 1 (HIF-1) (10). HIF-1 is a heterodimer of HIF-1α and HIF-1β; HIF-1β is constitutively expressed, but HIF-1α levels are kept low through proteasomal degradation in normoxia (11, 12). Under hypoxic conditions HIF-1α is no longer targeted for degradation and is able to dimerise with HIF-1β and transactivate target genes (13). HIF-1α is stabilised in neuroblastoma cell lines in hypoxia, and HIF-1 target genes including VEGF and tyrosine hydroxylase are up-regulated, both in vitro and as xenografts (14). Multiple angiogenic factors, including VEGF, have been shown to be expressed in neuroblastomas in vivo, and higher level expression correlates with advanced disease and poor outcome (15).
Failure of apoptosis is considered a hallmark of cancer (16). Commitment to apoptosis via the mitochondrial pathway is controlled by interactions between anti- and pro-apoptotic Bcl-2 family members which share homology via their BH-3 domains (17). The multi-domain pro-apoptotic Bcl-2 family proteins Bax and Bak are essential for mitochondrial apoptosis and their activity is controlled by the BH-3 only pro-apoptotic Bcl-2 family proteins. Two models have been suggested for this activation of Bax and Bak. In the ‘direct model’, BH-3-only proteins directly activate Bax and Bak, whilst in the ‘indirect model’ BH-3 only proteins bind to anti-apoptotic family members and prevent them from binding to and inhibiting Bak and Bax (18). Activation of Bax and Bak results in release of apoptogens from the mitochondrial inter membrane space and activation of an amplifying cascade of caspase-mediated proteolysis (19). ABT-737 is a novel small molecule that mimics the BH-3 domain of Bad and binds with nanomolar affinity to the hydrophobic pocket of Bcl-2, Bcl-xL and Bcl-w, disrupting their interactions with death-promoting Bcl-2 family members, and releasing these to activate Bax and Bak and engage apoptosis (20) (see Supplemental Figure 1 for the chemical structure of ABT-737 and other drugs used in this study). ABT-737 sensitizes a number of adult cancer cell types to cytotoxic agents (21), and has activity against acute myeloid leukaemia, and small cell lung cancer (SCLC), as a single agent in pre-clinical models (20, 22, 23). ABT-737 binds poorly to Mcl-1 and thus tumour cell expression of Mcl-1 is associated with resistance to ABT-737 (24, 25). ABT-263, an orally bio-available counterpart with similar biological activity, has been evaluated against the paediatric testing panel and has single agent activity against acute lymphoblastic leukaemia (26). ABT-263 is now in clinical trial against adult tumours (27). In this study we have evaluated the efficacy of ABT-737 against neuroblastoma cell lines in hypoxia, both as a single agent and in combination with clinically relevant cytotoxic agents.
Methods
Cell Culture
Both MYCN amplified and MYCN non-amplified neuroblastoma cell lines were used. In addition phenotypically distinct S and N-type subclones of SK-N-SH and LA-N-1 were studied. SH-EP1, SH-SY5Y, LA1-55n and LA1-5s were the kind gift of Dr. Robert Ross (Fordham University, Bronx, NY), NGP cells were a kind gift from Dr Deborah Tweddle (University of Newcastle-upon-Tyne), IMR-32 cells were purchased from ATCC-LGC. All cell lines were authenticated by CRUK in July 2010 using STR profiling. All cell lines were maintained in DMEM-F12 with 10% FCS (Gibco, Invitrogen, Paisley, United Kingdom). SH-EP1 clones stably expressing mouse Bcl-2 or shRNA against Mcl-1 were a kind gift from Professor Simone Fulda (Institut für Experimentelle Tumorforschung in der Pädiatrie, Goethe-Universität, Frankfurt am Main, Germany). For hypoxia experiments, cells were cultured and treated in a sealed modular incubator (InVivo2 Hypoxia workstation 400) flushed with 1% O2, 5% CO2, and 94% N2 (hereafter referred to as hypoxia).
Drug treatment
10mM stock of ABT-737 (Abbott Laboratories, Illinois, USA) was dissolved in DMSO and initial dose response curves for all cell lines were generated using DMSO and a less potent enantiomer (20) as control. The sulforhodamine B (SRB) assay was used to determine total protein reflecting cell number after treatment. Cells were plated in exponential growth phase in 96-well plates and treated for 24-72 hours with varying concentrations of ABT-737 or conventional cytotoxics. Cells were fixed and stained according to standard SRB protocol (28), and absorbance was measured using a microplate reader (Labsystems Multiskan EX, Thermo Scientific) at 540nm.
siRNA treatment
HIF-1α knock-down was achieved using siGENOME SMART pool from Dharmacon Thermo Scientific, control oligos were non-target pool from the same supplier. Cells were treated in 96 or in 6 wells plates as per manufacturer instructions.
Western Blotting
Cells were washed with PBS and lysed into either cell lysis buffer (Cell Signaling) supplemented with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktail I and II (Sigma), or directly into 2x Laemmli sample buffer. Loading was normalised by cell number or by protein concentration which was determined by bicinchoninic acid assay (Thermo Scientific) following the manufacturer’s instructions, both methods gave similar results. Samples were run on appropriate percentage polyacrylamide gels, and transferred to polyvinylidene difluoride membranes (Immobilon, Millipore). Membranes were blocked with 5% (w/v) non fat dried milk/0.05% (v/v) TBS-Tween 20 and probed with primary antibody in 0.05% (v/v) TBS-Tween 20 at 4°C overnight and then with secondary antibody conjugated to horseradish peroxidase in 5% (w/v) milk/0.05% (v/v) TBS-Tween 20 for 1 hour at room temperature. Blots were visualized with the enhanced chemiluminescence system (Amersham) and analyzed on a Fuji LAS-1000 Plus imaging system with AIDA software (Fuji, Bedford, United Kingdom). Primary antibodies used were HIF-1α (BD Pharmingen), carbonic anhydrase IX (gift from Boehringer), GLUT 1 (RD Systems), mouse Bcl-2 (Abcam), human Bcl-2 (Dako), Mcl-1 (BD Pharmingen), Bcl-xL (BD Transduction), Bcl-w (Axxora), Noxa (Imgenex), Bid (RD Systems), actin (AC-40, Sigma), cleaved caspase-3 (Cell Signalling), poly(ADP-ribose) polymerase (PARP; Cell Signalling). Secondary antibodies were either goat anti-mouse horseradish peroxidase or goat anti-rabbit horseradish peroxidase (both from DAKO).
FACS analysis of apoptotic cells
Exponentially growing cells were treated for 18-48 hours with various concentrations of ABT-737 or etoposide as control. Cells were harvested by trypsinisation and stained in 96 wells plate with APC Annexin V and 7-AAD to identify apoptotic cells. Data were collected on BD FACS Array and analysed by FlowJo software.
Drug combination assays
Data from the SRB assay, performed in triplicate, were used to calculate combination index (CI) with CalcuSyn software (Biosoft, Cambridge, UK) (29). This method is based on the multiple drug–effect equation of Chou–Talalay derived from enzyme kinetic models (30). Based on this approach, combination index values <0.9 are considered synergistic, >1.1 are antagonistic, and values 0.9 to 1.1 are nearly additive. The ratios of ABT-737 and cytotoxic drugs were fixed using IC50 values obtained by the SRB assay. Cells were co-treated for 24-72 hours using ABT-737 and the cytotoxic drugs doxorubicin, cisplatin, etoposide, and vincristine. Six drug concentrations were used covering the concentration–effect. Linear correlation coefficients (r) were generated for each concentration response curve to determine the applicability of the data to the method of analysis. In all experiments, r was >0.9.
Statistical analysis
Two-way ANOVA followed by the Bonferroni test was used to establish whether significant differences existed between normoxic and hypoxic conditions over a range of doses. Student’s t test calculations were performed on single dose response data and IC50 calculations.
Results
ABT-737 is more effective in hypoxia in neuroblastoma cell lines
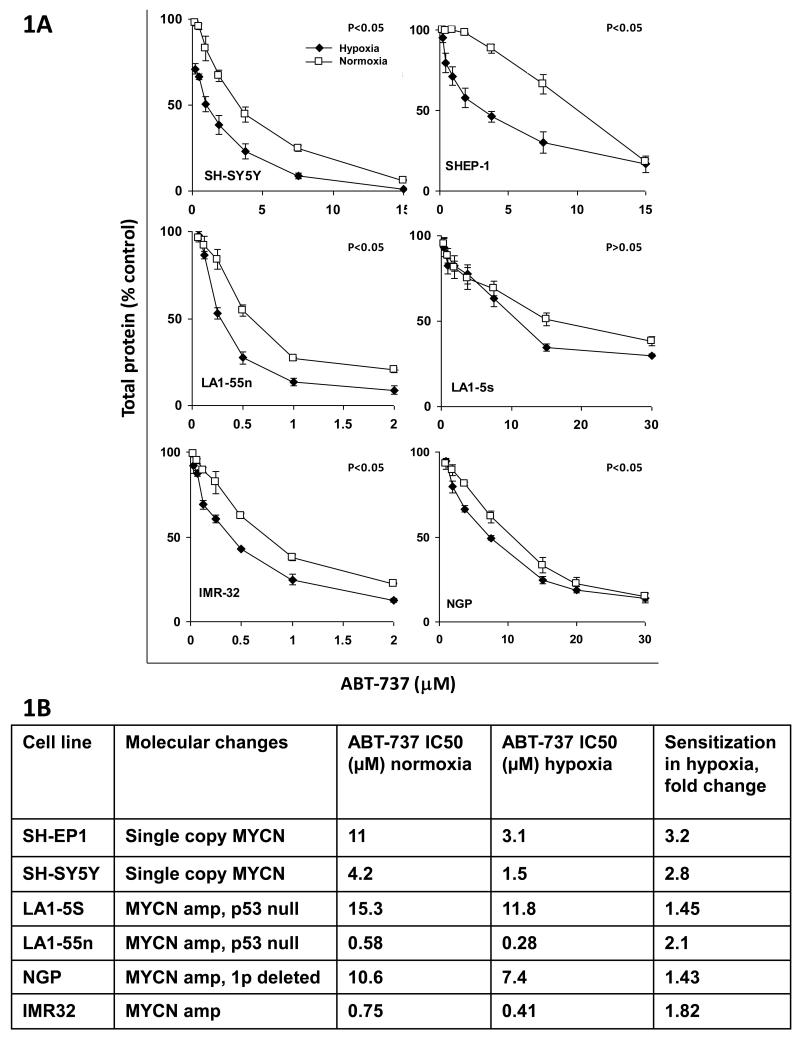
All six neuroblastoma cell lines were relatively resistant to ABT-737 in normoxia, with IC50 values in SRB assay ranging from 0.58μM to 15.3μM (Figure 1A and 1B). There was no correlation between this 26 fold variation in sensitivity to ABT-737 in normoxia and biology; two MYCN amplified cell lines had an IC50 of >10μM and two MYCN amplified lines had IC50 values below 1μM. However in all 6 neuroblastoma cell lines ABT-737 was more effective against cells grown in 1% oxygen (hypoxia) than in 21% oxygen in SRB assay (Figure 1A and 1B). In 5 of 6 cell lines this difference reached statistical significance, whilst in the remaining cell line, LA1-5S, the trend was not significant, and this remained the case whether the difference in the dose response curve between normoxia and hypoxia was analysed (Figure 1A), or whether the IC50 values for ABT-737 in hypoxia or normoxia were compared by Student’s t-test. Despite the wide variation in sensitivity of the neuroblastoma cell lines to ABT-737 in normoxia the degree of sensitisation in hypoxia was relatively consistent, ranging from 1.4 to 3.2 fold. This sensitisation in hypoxia was seen consistently despite the major differences in the biology of the cell lines studied, such that the MYCN non-amplified, non 1P deleted pair, SH-EP1 and SH-SY5Y showed similar sensitisation to the MYCN amplified, 1p deleted lines LA1-55n and NGP.
Figure 1.
ABT-737 is more effective against neuroblastoma cell lines in hypoxia than in normoxia. A. SRB assays of cell number against ABT-737 concentration in normoxia (21% O2, open squares) compared with hypoxia (1% 02, closed diamonds). P< 0.05 for the comparison between normoxia and hypoxia by 2 way ANOVA for all cell lines except LA1-5S. Treatment durations were 24 hours for LA155n, 48hours for SH-EP1, NGP and IMR32, and 72 hours for SH-SY5Y and LA15S. B. Comparison of SRB IC50 value for ABT-737 between normoxia and hypoxia in neuroblastoma cell lines. P<0.01 for all cell lines by Student t-test.
Sensitisation to ABT-737 in hypoxia is due to increased apoptosis
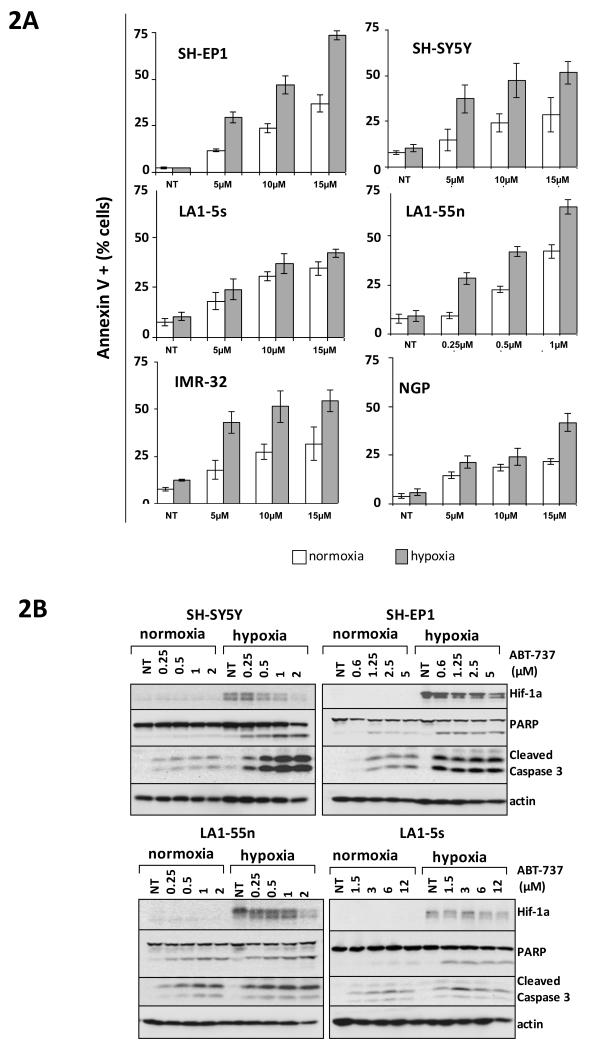
The SRB assay is a measure of protein, and as such will give information only about cell number. To address the mode of hypoxic sensitisation to ABT-737, apoptosis was assayed. One hour exposure to 5μM ABT-737 in MYCN amplified NGP cells lead to an increase in the annexin V positive cell population in hypoxia within 8 hours of exposure to ABT-737 (15.5% of cells annexin V positive in hypoxia compared to 12.2% of cells annexin V positive in hypoxia) and this difference was maintained to 24 hours after ABT-737 exposure (35.9% of cells annexin V positive in hypoxia compared to 17.3% of cells in normoxia) (data not shown). Similar results were seen in the MYCN single copy SH-EP1 cell line where at 8 hours after exposure to 10μM ABT-737 there were 15.8% annexin V positive cells in hypoxia compared to 12.7% in normoxia, and again this difference remained at 24 hours after drug exposure (30.9% annexin V positive in hypoxia compared to 21.3% positive in normoxia), and persisted at 48hrs after drug exposure (56.5% annexin V positive in hypoxia compared to 38.1% positive in normoxia). This increase in ABT-737-induced apoptosis in hypoxia was seen in all 6 neuroblastoma cell lines at 18-48 hrs after ABT-737 exposure (Figure 2A). Immunoblotting for cleaved caspase 3 and cleaved PARP at 18-48 hours after exposure to ABT-737 revealed the same pattern of increased ABT-737-induced apoptosis in hypoxia (Figure 2B). Furthermore inhibition of ABT-737-induced apoptosis with the pan-caspase inhibitor Q-VD-Oph (QVD) ablates the sensitisation of neuroblastoma cells to ABT-737 in hypoxia (Supplemental Figure 2). Thus the sensitisation of neuroblastoma cell lines to ABT-737 in hypoxia is due to an increase in the amount of ABT-737-induced apoptosis in hypoxia.
Figure 2.
ABT-737 is more effective at inducing apoptosis in neuroblastoma cell lines in hypoxia than in normoxia. A. Histograms showing % annexin V positive cells at 18-48 hrs after ABT-737 exposure in all 6 neuroblastoma cell lines in hypoxia and normoxia. B. Representative western blots for HIF-1α, PARP and cleaved caspase 3 in response to 24-72 hr exposure to ABT-737 in varying concentration in normoxia and hypoxia in 4 neuroblastoma cell lines. Actin is shown as a loading control. Blots are representative of 3 independent experiments.
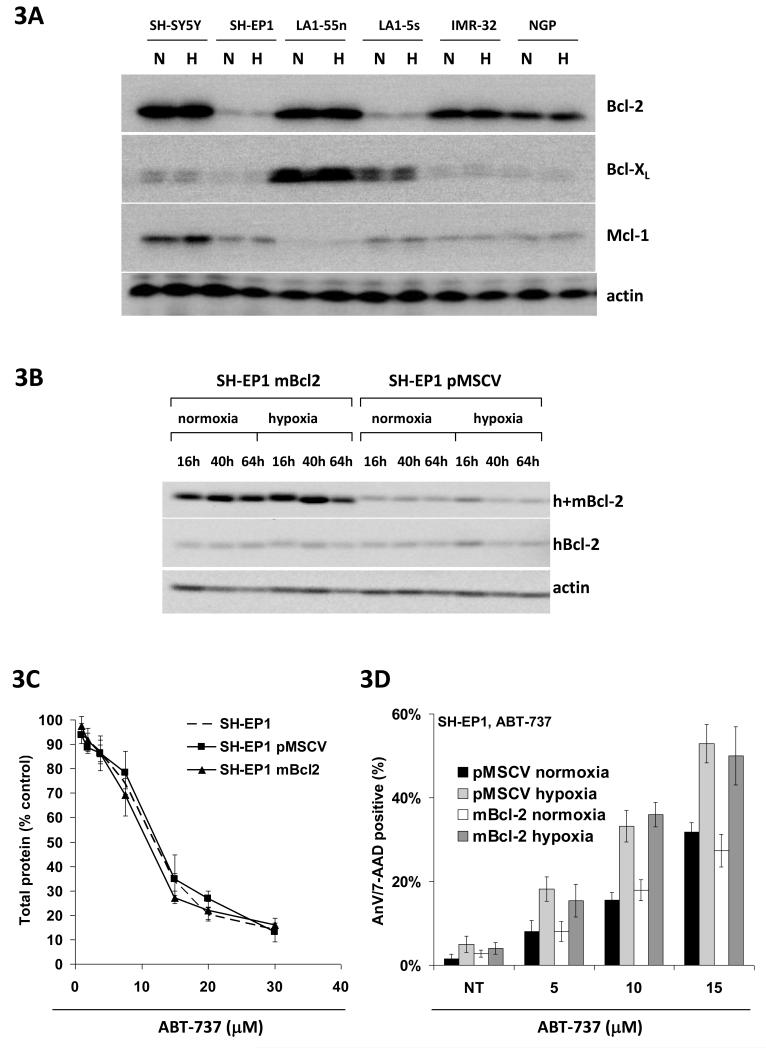
Differential expression of Bcl-2 family proteins does not account for differences in sensitivity to ABT-737 in neuroblastoma cell lines
Because of the wide variation in sensitivity to ABT-737 across the 6 neuroblastoma cell lines expression levels of Bcl-2 and Bcl-xL, known targets for ABT-737, and Mcl-1, a known resistance marker for ABT-737, were studied. As can be seen in Figure 3A there was considerable variation in expression of these proteins across the cell line panel. To some extent protein levels of the ABT-737 target Bcl-2 did seem to correlate with sensitivity to ABT-737, such that the two neuroblastoma cell lines with the lowest expression of Bcl-2, LA1-5S and SH-EP1, were the two most resistant to ABT-737. However NGP cells have a very similar IC50 to ABT-737 to SH-EP 1 cells in normoxia (10.6μM compared to 11μM) but very different levels of expression of Bcl-2 protein. Less correlation was seen with levels of Bcl-xL protein; although the highest expressors of Bcl-xL were the most sensitive cell line (LA1-55n), the most resistant cell line (LA1-5S) also expressed far more Bcl-xL than the remaining cell lines. With regard to Mcl-1 protein levels the pattern was similar, thus the most sensitive cell line (LA1-55n) had the lowest level of Mcl-1, but the cell line with the highest expression of Mcl-1 was not the most resistant to ABT-737 (SH-SY5Y).
Figure 3.
Differential expression of Bcl-2 family proteins does not account for differences in sensitivity to ABT-737 in neuroblastoma cell lines. A. Western blot showing variation in expression of the ABT-737 targets Bcl-2 and Bcl-xL, and the recognised resistance marker Mcl-1 across the 6 neuroblastoma cell lines. Note the lack of change in protein level between normoxia (N) and after 48 hrs in hypoxia (H). Actin is shown as a loading control. Blots are representative of 3 independent experiments. B. Expression of mouse Bcl-2 protein in stably transfected SH-EP1mBcl-2 cells in comparison with vector only controls (SH-EP1pMSCV). No difference in human Bcl-2 levels are seen with an antibody that recognises only human Bcl-2 (hBcl-2), but higher expression of Bcl-2 is seen in SH-EP1mBcl2 cells with an antibody that recognises both human and mouse Bcl-2 (h+mBcl-2). No changes in levels of either human or mouse Bcl-2 were seen over a 64 hour exposure to hypoxia. Actin is shown as a loading control. Blots are representative of 3 independent experiments. C. Increased expression of Bcl-2 does not sensitise SH-EP1 cells to ABT-737. The response of wild type SH-EP1 cells, vector-only controls (SH-EP1pMSCV) and SH-EP1 cells stably over-expressing mouse Bcl-2 (SH-EP1mBcl-2) to ABT-737 in SRB assay in normoxia is identical. D. Increased expression of Bcl-2 does not sensitise SH-EP1 cells to ABT-737-induced apoptosis. No difference in % annexin V/7-AAD positive cells was seen after 24 hr exposure to ABT-737 at varying concentrations between vector-only controls (SH-EP1pMSCV) and SH-EP1 cells stably over-expressing mouse Bcl-2 (SH-EP1mBcl-2), in either normoxia or hypoxia.
To test whether differences in expression of the ABT-737 target Bcl-2 could account for differences in sensitivity to ABT-737 the responses of SH-EP1 cells stably over-expressing mouse Bcl-2 were investigated. ABT-737 is able to bind to and inhibit mouse Bcl-2 with a similar level of efficacy to human Bcl-2 (Saul Rosenberg, Abbott Laboratories, personal communication). SHEP-Bcl2 cells express detectably higher levels of Bcl-2 than vector transfected controls (SHEP-pMSCV), and this is not due to any difference in their levels of human Bcl-2 (Figure 3B). However this clear increase in level of Bcl-2 protein has no effect upon the response of SH-EP1 cells to ABT-737 in normoxia, in which no difference can be seen between wild type SH-EP1 cells, SH-EP1 cells expressing mouse Bcl-2 and SH-EP1 cells containing vector alone in SRB assay (Figure 3C). In addition overexpression of Bcl-2 had no effect upon the induction of apoptosis by ABT-737 in SH-EP1 cells in normoxia as measured by flow cytometry for annexin V, or on the sensitisation of SH-EP1 cells to ABT-737-induced apoptosis in hypoxia (Figure 3D). Thus levels of Bcl-2 do not modulate sensitivity to ABT-737 in normoxia in these neuroblastoma cell lines.
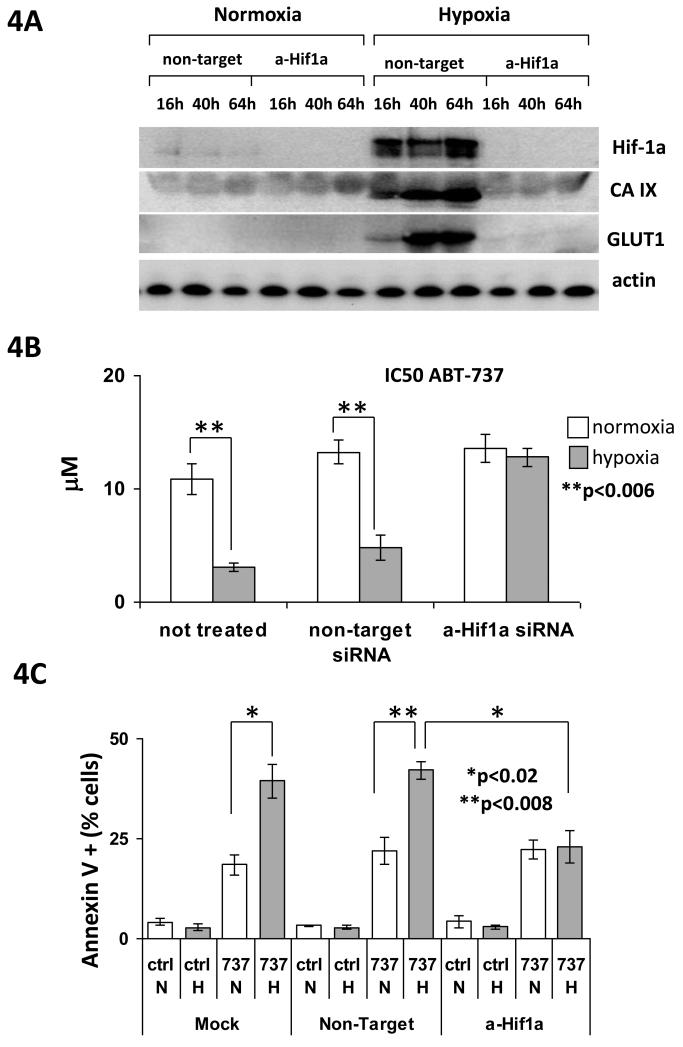
Sensitisation of neuroblastoma cells to ABT-737 in hypoxia is variably dependent on HIF-1α
HIF-1 is the central regulator of cellular response to hypoxia, and we and others have previously shown that resistance to cytotoxic agents in hypoxia in neuroblastoma cell lines is dependent upon HIF-1α (7, 8). Our previous studies with ABT-737 in the colon carcinoma cell line HCT-116 had shown that sensitisation to ABT-737 in hypoxia did not depend upon the presence of functional HIF-1 (31). To evaluate the importance of HIF-1 in the sensitisation of neuroblastoma cells to ABT-737 in hypoxia SH-EP1 cells with HIF-1α transiently down-regulated by siRNA were generated. These cells showed no detectable protein expression of HIF-1α over the timecourse of either SRB or annexin V assay, and no detectable protein expression of the known HIF-1 transcriptional targets carbonic anhydrase IX (CAIX) and glucose transporter 1 (GLUT1), indicating lack of functional HIF-1 (Figure 4A). Loss of functional HIF-1 had no effect upon the response of SH-EP1 cells to ABT-737 in normoxia but, unlike the situation in the colon carcinoma cell line HCT-116, in SH-EP 1 cells loss of functional HIF-1 lead to loss of sensitisation to ABT-737 in hypoxia in SRB assay (Figure 4B). In addition whilst the loss of functional HIF-1 had no effect on ABT-737-induced apoptosis in normoxia it significantly reduced ABT-737-induced apoptosis in hypoxia, and lead to the loss of the previously highly significant difference between ABT-737-induced apoptosis in normoxia and hypoxia (Figure 4C). However in three other neuroblastoma cell lines, SHSY5Y, IMR-32 and LA1-55n, knockdown of HIF1α with siRNA, whilst leading to functional inhibition of the HIF-1 pathway as measured by GLUT-1 expression, did not prevent sensitisation to ABT-737 in hypoxia (Supplemental Figure 3). Thus in some neuroblastoma cell lines sensitisation to ABT-737 in hypoxia seems to be dependent upon functional HIF-1α, whilst in others it is not.
Figure 4.
Sensitisation of neuroblastoma cells to ABT-737 in hypoxia is variably dependent on HIF-1alpha. A. SH-EP1 cells transiently transfected with siRNA to HIF-1α show no protein expression of HIF-1α over a 64 hr timecourse in hypoxia, and no hypoxia-induced up-regulation of the known HIF-1 transcriptional targets CA IX and GLUT-1. Actin is shown as a loading control. Blots are representative of 3 independent experiments. B. Sensitisation to ABT-737 by 48 hr exposure to hypoxia is lost in SH-EP 1 cells transiently transfected with siRNA to HIF-1α. The mean IC50 to ABT-737 calculated from 3 separate SRB assays is shown in normoxia and hypoxia for wild type, non-target siRNA transfected, and HIF-1α siRNA transfected SH-EP1 cells. The difference between the IC50 in SRB in normoxia and hypoxia was significant by Student t-test (p<0.006) for wild type and non-target controls, but no difference was seen between normoxia and hypoxia in HIF-1α siRNA transfected SH-EP1 cells. C. Sensitisation of SH-EP1 cells to ABT-737-induced apoptosis is lost in SH-EP 1 cells transiently transfected with siRNA to HIF-1α. % of annexin V/7-AAD positive cells (mean of 3 independent experiments) in mock treated, non-target siRNA transfected, and HIF-1α siRNA transfected SH-EP1 cells is shown after 24 hr exposure to ABT-737 at the normoxic IC50 concentration in normoxia and hypoxia. The increase in apoptosis in hypoxia in mock and non-target transfected SH-EP1 cells is significant by Student t-test (p<0.02), and the reduction in ABT-737-induce apoptosis in hypoxia in HIF-1α siRNA transfected SH-EP1 cells in comparison with non-target transfected cells is also significant by Student t-test (p<0.008).
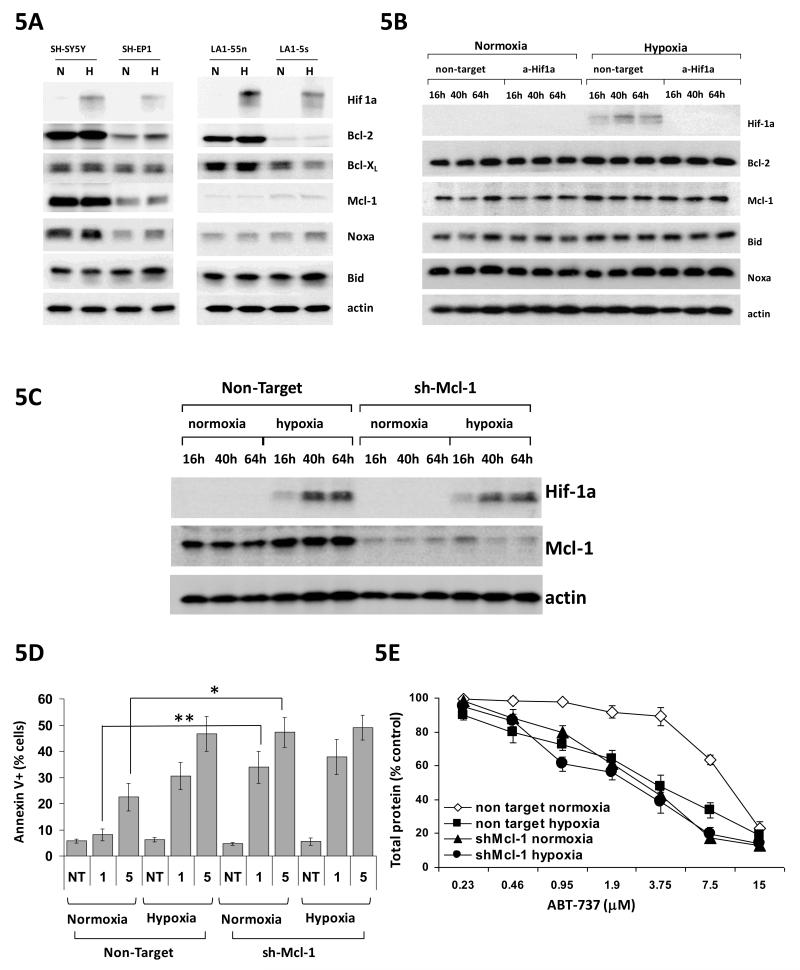
Changes in Mcl-1 protein level do not account for sensitisation of neuroblastoma cells to ABT-737 in hypoxia
In colon carcinoma and NSCLC cell lines Mcl-1 down-regulation at the translational level accounts for sensitisation to ABT-737 in hypoxia (31). In neuroblastoma cell lines there was no detectable change in the protein level of Mcl-1 in hypoxia (Figure 5A), nor of protein levels of other potential mediators of sensitisation to ABT-737 (Bcl-2, Bcl-xL, Bid and Noxa). In addition there was no change in levels of these proteins after down-regulating HIF-1alpha with siRNA, under which conditions hypoxic sensitisation to ABT-737 is lost in SH-EP1 cells (Figure 5B). Stable down-regulation of Mcl-1 by shRNAi in SH-EP1 cells does lead to sensitisation to ABT-737 in normoxia as might be expected given its importance as a resistance marker for ABT-737 (Figure 5C-E). However reduction in Mcl-1 protein levels by shRNAi has no effect on the response of SH-EP 1 cells to ABT-737 in hypoxia measured either by SRB assay (Figure 5E) or annexin V assay (Figure 5D) despite differences in levels of Mcl-1 protein between the non-target and anti Mcl-1 transfected cells being as great in hypoxia as in normoxia (Figure 5C). Thus although reduction of Mcl-1 levels in SH-EP1 cells can sensitise to ABT-737 in normoxia, the same degree of reduction in Mcl-1 protein level does not sensitise SH-EP1 cells to ABT-737 in hypoxia. In three other neuroblastoma cell lines, SH-SY5Y, LA-1-55n and IMR-32, down-regulation of Mcl-1 with siRNAi also sensitised cells to ABT-737 in normoxia but similarly failed to prevent sensitisation to ABT-737 in hypoxia (Supplemental Figure 4). Taken together with the lack of any change in Mcl-1 protein levels in hypoxia, or after loss of hypoxic sensitisation after removal of functional HIF-1 in SH-EP1 cells, this suggests that, unlike in colon carcinoma and NSCLC cell lines, Mcl-1 down-regulation is not the mechanism for hypoxic sensitisation to ABT-737 in neuroblastoma cells.
Figure 5.
Changes in Mcl-1 protein level do not account for sensitisation of neuroblastoma cells to ABT-737 in hypoxia. A. No changes in levels of Bcl-2, Bcl-XL, Mcl-1, noxa or Bid were seen in 4 neuroblastoma cell lines after 48 hr exposure to hypoxia. Actin is shown as a loading control. Blots are representative of 3 independent experiments. B. No changes in protein levels of Bcl-2, Mcl-1, Bid and Noxa were seen over a 64 hr timecourse after transfection of SH-EP1 cells with either non-target or HIF-1α siRNA, despite the dramatic loss of sensitisation to ABT-737 in hypoxia following transfection with HIF-1α siRNA. Actin is shown as a loading control. Blots are representative of 3 independent experiments. C. Reduction in protein expression of Mcl-1 in SH-EP1 cells over a 64 hr timecourse in SH-EP1 clone stably expressing short hairpin RNA against Mcl-1 in comparison with non-target short hairpin. Actin is shown as a loading control. Blots are representative of 3 independent experiments. D. Reduction in protein level of Mcl-1 increases ABT-737-induced apoptosis in normoxia as shown by a significant increase in % of annexin V/7-AAD positive cells 24 hrs after treatment with either 1 or 5μM ABT-737 (* p<0.02 at 1μM and **p<0.05 at 5μM by Student t-test). No increase in ABT-737-induced apoptosis is seen in hypoxia. E. Reduction in protein level of Mcl-1 sensitises SH-EP 1 cells to ABT-737 in normoxia, but not in hypoxia in SRB assay. Compare the dose response curve for cell expressing non-target hairpin (open diamonds) with cells stably expressing shRNA which targets Mcl-1 (closed triangles).
ABT-737 sensitises neuroblastoma cell lines to conventional cytotoxic agents in normoxia and hypoxia
Neuroblastoma cell lines are relatively resistant to ABT-737 as a single agent, with IC50 values in the range of 0.58 to 15.3 μM. However it is likely that, in the clinic, ABT-737 would be used in combination with conventional cytotoxic agents. Neuroblastoma, Ewing’s sarcoma and rhabdomyosarcoma cell lines are all resistant to conventional cytotoxic agents in hypoxia. Given the sensitisation of neuroblastoma cell lines to ABT-737 in hypoxia the ability of ABT-737 to sensitise to conventional cytotoxic agents in normoxia and hypoxia was evaluated using SRB assay and formal combination analysis was performed using the combination index. Vincristine, etoposide, cisplatin and doxorubicin were chosen as agents that have wide use in the clinical management of neuroblastoma. In general the combination of ABT-737 and etoposide, when given simultaneously, was synergistic in all 4 cell lines in normoxia with combination index (CI) values ranging from 0.122 for LA-15S cells to 0.91 for LA-155n cells (Table 1). Under hypoxia the synergy between etoposide and ABT-737 was either maintained, in LA1-5S (0.12 in normoxia and 0.27 in hypoxia) or enhanced, in SH-EP1, SH-SY5Y and LA1-55n cells (0.79 to 0.37; 038 to 015; 0.91 to 0.82 respectively). Synergy was also observed between ABT-737 and doxorubicin in SH-EP1, SH-SY5Y and LA1-5S cells, with additivity seen between ABT-737 and doxorubicin in LA-155n cells. Again in hypoxia this was either maintained or improved, so that synergy between ABT-737 and doxorubicin was seen in all 4 cell lines in hypoxia, with the CI value in LA155n cells falling from 0.99 in normoxia to 0.51 in hypoxia. Synergy was seen between ABT-737 and cisplatin in SH-EP1, SH-SY5Y and LA155n cells and additivity was seen between ABT-737 and cisplatin in LA-15S cells in normoxia. Under hypoxia synergy was seen between cisplatin and ABT-737 in all 4 cell lines. Synergy between ABT-737 and vincristine was seen only in LA-15S cells in normoxia and slight antagonism was seen between vincristine and ABT-737 in SH-SY5Y and LA1-55n cells in normoxia. However in all 4 cell lines in hypoxia the CI value was reduced and synergy was seen in SH-EP1, SH-SY5Y and La1-5S cells, and additivity in LA1-55n cells.
Table 1.
ABT-737 sensitises neuroblastoma cell lines to conventional cytotoxics agents in both normoxia and hypoxia. Combination index (CI) values are shown for the combination of ABT-737, at its IC50 dose in normoxia and hypoxia, and vincristine, cisplatin, etoposide and doxorubicin in 4 neuroblastoma cell lines. CI values of 1 indicate additivity, values below 1 indicate synergy; the lower the value the greater the synergy.
| Cell line | Combination with ABT-737 |
IC50 normoxia |
IC50 hypoxia |
|---|---|---|---|
| SH-EP1 | etoposide | 0.79333 | 0.36945 |
| SH-EP1 | doxorubicin | 0.8162 | 0.88215 |
| SH-EP1 | cisplatin | 0.55949 | 0.66894 |
| SH-EP1 | vincristine | 1.06206 | 0.60202 |
| SH-SY5Y | etoposide | 0.38021 | 0.14637 |
| SH-SY5Y | doxorubicin | 0.51517 | 0.17208 |
| SH-SY5Y | cisplatin | 0.90007 | 0.20182 |
| SH-SY5Y | vincristine | 1.22763 | 0.52357 |
| LA1-5s | etoposide | 0.122 | 0.27131 |
| LA1-5s | doxorubicin | 0.6305 | 0.76332 |
| LA1-5s | cisplatin | 1.04588 | 0.61367 |
| LA1-5s | vincristine | 0.73087 | 0.69199 |
| LA1-55n | etoposide | 0.90738 | 0.81952 |
| LA1-55n | doxorubicin | 0.99311 | 0.51199 |
| LA1-55n | cisplatin | 0.89014 | 0.86183 |
| LA1-55n | vincristine | 1.246 | 0.91284 |
Discussion
Hypoxia is a near universal feature of solid tumours and is associated with advanced stage and poor prognosis in a range of adult tumour types. Less is known about the significance of hypoxia in paediatric tumour types although studies are emerging to suggest that hypoxia leads to the same drug resistance in childhood cancer cells that has been reported in adult tumour types (7-9). Data also suggest that hypoxia is a feature of neuroblastoma and contributes to poor prognosis and drug-resistance (14, 15). Thus therapeutic strategies that target hypoxic areas of tumour, and are able to re-sensitise hypoxic tumour cells to clinically relevant cytotoxic agents would be of considerable interest in the treatment of this poor prognosis tumour.
Activity of the orally bioavailable ABT-737 analogue, ABT-263, against neuroblastoma xenografts in the paediatric pre-clinical testing panel was limited (26), and in agreement with this we found that all 6 neuroblastoma cell lines studied were relatively resistant to ABT-737. However all 6 biologically variable neuroblastoma cell lines were more sensitive to ABT-737 under 1% oxygen (Figure 1), and this was due to increased ABT-737-induced apoptosis (Figure 2). Protein levels of the known ABT-737 targets Bcl-2 and Bcl-xL, as well as those of the known resistance factor Mcl-1, have all been reported to be important in determining cellular sensitivity to ABT-737 (20, 22, 24, 25). Previous study has suggested that neuroblastoma cell lines can be classified according to their Bcl-2 family protein dependence, and that those which are Bcl-xL or Bcl-w dependent are very sensitive to ABT-737 (32). In our neuroblastoma cell line panel no consistent relationship between the levels of Bcl-2, Bcl-xL and Mcl-1 and the cellular response to ABT-737 in SRB assay could be observed, although it is noticeable that the two most sensitive cell lines were the two with the lowest protein levels of Mcl-1 (LA1-55n and IMR-32). We hypothesised that the relatively low protein level of the ABT-737 target Bcl-2 might account for the relative drug resistance of the two S-type neuroblastoma cell lines SH-EP1 and LA-15S. However overexpression of mouse Bcl-2 in SH-EP1 cells had no effect on the response of SH-EP1 cells to ABT-737 in SRB assay in normoxia, nor on the induction of apoptosis by ABT-737 in normoxia and hypoxia (Figure 3C, D). This data strongly suggests that protein levels of Bcl-2 are not a determinant of neuroblastoma cell response to ABT-737.
We have recently reported sensitisation of small cell lung cancer (SCLC) and colorectal carcinoma (CRC) cell lines to ABT-737 in hypoxia in vitro and in xenograft (31) and in these cell types this sensitisation was not dependent upon a functional HIF-1 pathway. In neuroblastoma cells we have previously shown that hypoxia-induced drug resistance is dependent upon functional HIF-1 (7). In the current study reduction in protein levels of HIF-1α by siRNA results in an inactive HIF-1 pathway in SH-EP1 neuroblastoma cells in hypoxia, and results in loss of hypoxia-induced sensitisation to ABT-737, which is due to a reduction in ABT-737-induced apoptosis in hypoxia (Figure 4B, 4C). However loss of a functional HIF-1 pathway in SHSY-5Y, LA15S and IMR-32 cells after siRNA to HIF-1α does not prevent sensitisation to ABT-737 in hypoxia (Supplemental Figure 3). Thus in some neuroblastoma cell lines sensitisation to ABT-737 in hypoxia in neuroblastoma cells is dependent upon a functional HIF-1 pathway, whilst in others, as in SCLC and CRC cell lines, it is not.
A number of Bcl-2 family proteins have been reported to be up or down regulated in hypoxia, including Bid, Mcl-1 and Noxa (5, 33-35). Our previous work reported hypoxia-induced down-regulation of Mcl-1 in SCLC and CRC cell lines, and suggested that this was due to reduced protein translation in hypoxia (31). In SCLC and CRC cell lines this reduction in Mcl-1 protein levels is not dependent upon the presence of functional HIF-1, despite the mcl-1 promoter containing an HRE (31). However changes in Mcl-1 protein levels in hypoxia seem to be variable between cell types. In hepatocellular carcinoma and tracheobronchial cells Mcl-1 is up-regulated in hypoxia (34, 35), whilst in mouse embryonic fibroblasts hypoxia has no effect on Mcl-1 levels (36). In the current study we did not observe any changes in the protein level of Mcl-1 in hypoxia in any of the 4 neuroblastoma cell lines studied (Figure 5A). Furthermore we also failed to observe any changes in the protein level of Mcl-1 after the HIF-1 pathway was inactivated with siRNA to HIF-1α, despite the loss of sensitisation of SH-EP1 cells to ABT-737 in hypoxia in this setting (Figure 5B). Reduction of protein levels of Mcl-1 with shRNA clearly sensitised SH-EP1, SH-SY5Y, LA155n and IMR-32 cells to ABT-737 in normoxia (Figure 5C-E, Supplemental Figure 4), as would be expected given the importance of Mcl-1 in resistance to ABT-737 in other cell types and as previously reported in neuroblastoma (24, 32, 37). However the degree of Mcl-1 protein reduction by shRNA in SH-EP1 cells was as marked in hypoxia as in normoxia, yet this change in Mcl-1 protein level failed to have any effect on ABT-737-induced apoptosis in SH-EP1 cells in hypoxia (Figure 5B, C), and failed to prevent sensitisation of the other three neuroblastoma cell lines to ABT-737 in hypoxia (Supplemental Figure 4). This data, together with the lack of change in Mcl-1 protein levels in neuroblastoma cells in hypoxia, and the lack of change in Mcl-1 protein levels following siRNA-mediated loss of HIF-1α in SH-EP1 cells, indicates that Mcl-1 is not involved in hypoxia-induced sensitisation of neuroblastoma cells to ABT-737.
Synergy between ABT-737 and conventional cytotoxic agents in normoxia has been reported in a range of adult tumour types (21), and ABT-737 is able to reverse 13-cis-retinoic acid-induced resistance to cytotoxic agents in neuroblastoma cell lines (38). Our previous work in SCLC and CRC had shown that this synergy was maintained, and occasionally enhanced, in hypoxia (31). In the current study this enhancement of the interaction between ABT-737 and conventional clinically relevant cytotoxic agent was more pronounced. Thus although a degree of synergy between ABT-737 and cytotoxic agents was seen across all 4 neuroblastoma cell lines in normoxia, it was almost invariably more marked in hypoxia (Table 1). In particular in the situations where no synergy was observed between ABT-737 and cytotoxic agent in normoxia, such as with vincristine in SH-SY5Y cells, where the relationship was antagonistic (CI 1.22), synergy was seen in hypoxia (CI 0.52). Indeed in hypoxia there was no combination between ABT-737 and cytotoxic with a CI value of above 0.91 in any of the neuroblastoma cell lines. Thus in addition to being more effective as a single agent against neuroblastoma cells in hypoxia, ABT-737 is also better able to sensitise neuroblastoma cell lines to conventional cytotoxic agents in hypoxia.
In conclusion this study shows that ABT-737 is more effective against neuroblastoma cell lines under hypoxia than normoxia, and importantly is better able to synergise with conventional cytotoxic agents under hypoxia, when neuroblastoma cells are more resistant to these agents. This data have potential significance for the future use of Bcl-2 family targeting drugs against neuroblastoma suggesting that combination with conventional cytotoxics may offer a possible strategy for improving the survival of children with this poor prognosis disease. However further work will be needed to investigate these combinations in animal models.
Supplementary Material
Acknowledgements
We are grateful to Dr Ali Raoof for Supplemental Figure 1.
This study was supported by Cancer Research UK core funding to the Paterson Institute (Grant number: C147). Martin Brandenburg received a CRUK PhD student stipend.
Footnotes
The authors have no conflict of interests to declare.
References
- 1.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. New Eng J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 2.Katzenstein HM, Cohn SL, Shore RM, Bardo DM, Haut PR, Olszewski M, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event free survival in high risk neuroblastoma. J Clin Oncol. 2004;22:3909–15. doi: 10.1200/JCO.2004.07.144. [DOI] [PubMed] [Google Scholar]
- 3.Harris AL. Hypoxia-a key regulatory element in tumour growth. Nature Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 4.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 5.Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters B, Wilson C, et al. Hypoxia-mediated down-regulation of Bid and Bax in tumours occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875–89. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown LM, Cowen RL, Debray C, Eustace A, Erler JT, Sheppard FC, et al. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol Pharmacol. 2006;69:411–8. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- 7.Hussein D, Estlin EJ, Dive C, Makin GWJ. Chronic hypoxia promotes hypoxia-inducible factor-1alpha-dependent resistance to etoposide and vincristine in neuroblastoma cells. Mol Cancer Ther. 2006;5:2241–50. doi: 10.1158/1535-7163.MCT-06-0145. [DOI] [PubMed] [Google Scholar]
- 8.Das B, Yeger H, Tsuchida R, Torkin R, Gee MF, Thorner PS, et al. A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1α through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma. Cancer Res. 2005;65:7267–75. doi: 10.1158/0008-5472.CAN-04-4575. [DOI] [PubMed] [Google Scholar]
- 9.Kilic M, Kasperczyk H, Fulda S, Debatin K-M. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene. 2007;26:2027–38. doi: 10.1038/sj.onc.1210008. [DOI] [PubMed] [Google Scholar]
- 10.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 12.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-l alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O-2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 14.Jogi A, Ora I, Nilsson H, Makino Y, Poellinger L, Axelson H, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA. 2002;99:7021–6. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–8. [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 17.Youle RJ, Strasser A. The Bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 18.Galonek HL, Hardwick JM. Upgrading the Bcl-2 network. Nat Cell Biol. 2006;8:1317–9. doi: 10.1038/ncb1206-1317. [DOI] [PubMed] [Google Scholar]
- 19.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 20.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 21.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–7. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 22.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–8. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 25.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lock R, Carol H, Houghton PJ, Morton CL, Kolb EA, Gorlick R, et al. Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1181–9. doi: 10.1002/pbc.21433. [DOI] [PubMed] [Google Scholar]
- 27.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 28.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 29.Chou T, Hayball MP. CalcuSyn, Windows software for dose effect analysis. Biosoft; Cambridge: 1996. [Google Scholar]
- 30.Chou T, Talalay P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J Biol Chem. 1977;252:6438–42. [PubMed] [Google Scholar]
- 31.Harrison LRE, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denenny O, et al. Increased sensitivity of human lung and colorectal cancer cells to the BH-3 mimetic ABT-737 in hypoxia via down-regulation of Mcl-1. J Clin Invest. 2011;121:1075–87. doi: 10.1172/JCI43505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldsmith KC, Lestini BJ, Gross M, Ip L, Bhumbla A, Zhang X, et al. BH3 response profiles from neuroblastoma mitochondria predict activity of small molecule Bcl-2 family antagonists. Cell Death Diffn. 2010;17:872–82. doi: 10.1038/cdd.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med. 2004;199:113–24. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piret JP, Minet E, Cosse JP, Ninane N, Debacq C, Raes M, et al. Hypoxia-inducible factor-1-dependent overexpression of myeloid cell factor-1 protects hypoxic cells against tert-butyl hydroperoxide-induced apoptosis. J Biol Chem. 2005;280:9336–44. doi: 10.1074/jbc.M411858200. [DOI] [PubMed] [Google Scholar]
- 35.Liu XH, Yu EZ, Li YY, Kagan E. HIF-1alpha has an anti-apoptotic effect in human airway epithelium that is mediated via Mcl-1 gene expression. J Cell Biochem. 2006;97:755–65. doi: 10.1002/jcb.20683. [DOI] [PubMed] [Google Scholar]
- 36.Brunelle JK, Shroff EH, Perlman H, Strasser A, Moraes CT, Flavell RA, et al. Loss of Mcl-1 protein and inhibition of electron transport chain together induce anoxic cell death. Mol Cell Biol. 2007;27:1222–35. doi: 10.1128/MCB.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lestini BJ, Goldsmith KC, Fluchel MN, Liu X, Chen NL, Goyal B, et al. Mcl1 downregulation sensitizes neuroblastoma to cytotoxic chemotherapy and small molecule Bcl-2 family antagonists. Cancer Biol Ther. 2009;8:1587–95. doi: 10.4161/cbt.8.16.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadjidaniel MD, Reynolds CP. Antagonism of cytotoxic chemotherapy in neuroblastoma cell lines by 13-cis-retinoic acid is mediated by the antiapoptotic Bcl-2 family proteins. Mol Cancer Ther. 2010;9:3164–74. doi: 10.1158/1535-7163.MCT-10-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.