Abstract
Eimeria (E.) tenella (local isolate) sporozoites were adapted on the chorioallantoic membrane (CAM) of 10-12 days chicken embryos and completed its life cycle in 6~7 days at 39℃ and 70 per cent humidity. Only 23 embryos (4.6%) were found dead from 1~4 day post inoculation of sporozoites with mild lesions on CAM with no gametocytes but few sporozoites in chorioallantoic fluid (CAF). On 5~7 day post inoculation, 432 embryos (86.4%) were found dead with severe haemorrhages on CAM and CAF contained uncountable number of gametocytes. After seven days post inoculation, 45 embryos (9%) were found to be alive. Some oocysts were also detected in the CAF on 6~7 days post inoculation. In the histological sections of the CAM, there were abundant small dark colored rounded bodies of gametes; distributed extensively in tissues of CAM on 5~7 days post inoculation of sporozoites. In some cases, cluster of small mature and immature relatively large bodies were seen in increasing numbers on 5~6 days post inoculation.
Keywords: Eimeria tenella, chorioallantoic membrane, adaptation, chicken embryos
Introduction
Members of genus Eimeria are considered species and site specific [4]. Limited studies have been conducted on the growth of Eimeria species on other than normal hosts [4~8]. Sporozoites of E. tenella adapted successfully on chorioallantoic membrane (CAM) of chicken embryos [1]. Present paper reports some observations on the adaptation of E. tenella (local isolates) on chicken embryos through chorioallontoic membrane.
Materials and Methods
Sporulated oocysts of E. tenella (local isolate) maintained at Immunoparasitology Laboratory, Department of Veterinary Parasitology, University of Agriculture, Faisalabad, Pakistan were used in the present studies.
Sporulated oocysts were subjected to ex-sporocystation to release sporozoites [9]. Their concentration was maintained at 1.8 × 103~2 × 103 per 0.1 ml, in phosphate buffered saline (PBS; pH 7.2) and were inoculated (0.1 ml each) into 10-12 days chicken embryos (500 divided into 10 batches) through CAM. Embryos were maintained at 39℃ and 70% of humidity [1]. Candling was performed daily to record the observations.
Chorioallantoic fluid (CAF) containing the gametocytes (macrogametoeytes and microgametoeytes) was collected from dead embryos. CAM from dead embryos having haemorrhages was preserved in 70% alcohol for histopathological studies [2].
Results
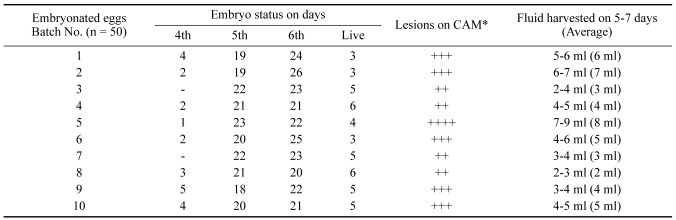
E. tenella (local isolate) sporozoites were adapted on the CAM of 10~12 days chicken embryos and completed its life cycle in 6~7 days at 39℃ and 70% of humidity. The results of embryo status from 1 to 7 days post inoculation and haemorrhages on the CAM were recorded (Table 1).
Table 1.
Observations on adaptation of E. tenella (local isolates) on chicken embryos
*: ++; mild hemorrhages, +++; moderate hemorrhages, ++++; severe hemorrhages.
Only 23 embryos (4.6%) were found dead from 1 to 4 days post inoculation of sporozoites with mild lesions on CAM and quantity of fluid harvested was minimum (0.5-1 ml) and no gametocytes but few sporozoites were seen in CAF. On 5 to 7 days post inoculation, 432 (86.4%) embryos were found dead with severe hemorrhages (+++) on CAM and maximum CAF (7-9 ml) was harvested with uncountable number of gametocytes. After seven days post inoculation, 45 (9%) embryos were found to be alive. The average volume of CAF harvested from 5 to 7 days post inoculation was 4.7 ml per embryo. The gametocytes were found to be entangled with the embryo mass (urates and blood). Embryos having severe hemorrhages and maximum CAF was found to contain more numerous gametocytes but were uncountable on either case. Some oocysts were also detected in the CAF on 6~7 days post inoculation.
In the histological sections of the CAM, there were abundant small dark colored rounded bodies of gametes; distributed extensively in tissues of CAM on 5~7 days post inoculation of sporozoites. In some cases, cluster of small mature and immature relatively large bodies were seen in increasing numbers on day 5~6 days post inoculation.
Discussion
The inoculation of sporozoites (1.8 × 103~2.0 × 103) into the CAM produced the uncountable number of gametocytes and few oocysts in the CAF on 5~7 days post inoculation. During that period, 4.6% death in embryos was observed from day 1 to 4 that may be not associated with parasitemia and due to the toxic substances [3]. From day 5 to 7, 86.4% were found dead and 9% were found alive. Embryos having severe haemorrhages and maximum CAF were found to contain more numerous gametocytes but were uncountable on either case.
Adaptation of E. tenella on CAM of Japanese quail's embryos have also been conducted successfully [7] but its development through histopathological technique have not been studied. In the histological sections of the CAM, there were abundant small dark colored rounded bodies of gametes; distributed extensively in tissues of CAM on 5~7 days post inoculation of sporozoites.
The gametes were found to be penetrated deep into the tissues of CAM for development and later they may form into zygote (oocyst). Similar observations have also been recorded previously [4]. A few cluster of relatively larger bodies observed in the present findings might be the schizonts [4,10]. In the present studies, gametes like bodies were observed 5 ~7 days of post-inoculation of sporozoites into chicken embryos while in the natural host (chicken) the gametes were observed 3~4 days post infection [10]. Schizonts and gametes recovered 5~7 days post inoculation of sporozoites of E. tenella (local isolate) into the CAM in contrary to observations recorded in previous studies where schizonts and gametes recovered 7~9 days and 7~11 days post inoculation of sporozoites into CAM [4], respectively. This variation may be due to strain difference as local isolate of E. tenella was used. Numerous merozoites were also observed on the CAM on 6~7 days post inoculation.
The results of the present study will pave a way to use the egg adapted gametocytes as an antigen for vaccine preparation against coccidiosis in poultry.
Acknowledgments
The funds for this project were sponsored by Higher Education Commission, Islamabad, Government of Pakistan under Merit Scholarships Scheme.
References
- 1.Akhtar M, Ayaz MM, Hayat CS, Ashfaque M, Hussain I. Adaptation of Eimeria tenalla (local isolate) sporozoites in chicken's embryos. Pakistan Vet J. 2002;22:40–41. [Google Scholar]
- 2.Bankroft JD, Stevens A. Theory and practice of histological techniques. Ames: The lowa State University Press; 1990. pp. 246–247. [Google Scholar]
- 3.Brackett S, Bliznick A. The reproductive potential of five species of coccidian of the chicken as demonstrated by oocyst production. J Parasitol. 1951;3:133–139. [PubMed] [Google Scholar]
- 4.Long PL. Development of Eimeria tenalla in avian embryos. Nature. 1965;208:509–510. doi: 10.1038/208509a0. [DOI] [PubMed] [Google Scholar]
- 5.Long PL. The growth of some species of Eimeria in avian embryos. Parasitology. 1966;56:575–581. doi: 10.1017/s0031182000069055. [DOI] [PubMed] [Google Scholar]
- 6.Long PL. Eimeria tenalla reproduction, pathogenicity and Immunogenicity of a strain maintained in chicken embryos by serial passage. J Comp Pathol. 1972;82:429–437. doi: 10.1016/0021-9975(72)90042-4. [DOI] [PubMed] [Google Scholar]
- 7.Nakai Y, Tsuchiya H, Takahashi S. Cultivation of Eimeria tenalla in Japanese quail embryos (Coturnix coturnix japoruca) J Parasitol. 1992;78:1024–1026. [PubMed] [Google Scholar]
- 8.Shirley MW, Mcdonald V, Ballingall S. Eimeria species from chicken: from merozoites to oocysts in embryonated eggs. Parasitology. 1981;83:257–259. doi: 10.1017/s0031182000085279. [DOI] [PubMed] [Google Scholar]
- 9.Speer CA, Hammond DM, Maht JL, Roberts WL. Structure of oocysts and sporocysts walls and excystation of sporozoites of Isospora canis. J Parasitol. 1973;59:35–40. [PubMed] [Google Scholar]
- 10.Vetterling JM, Doran DJ. Schizogony and gametogony in the life cycle of poultry coccidian Eimeria acervulina Tyzzer, 1929. J Parasitol. 1966;52:1150–1157. [PubMed] [Google Scholar]