Abstract
Monoclonal antibody against kanamycin was prepared, and competitive direct ELISA and immunochromatographic assay were developed using the antibody to detect kanamycin in animal plasma and milk. The monoclonal antibody produced was identified to be IgG1, which has a kappa light chain. No cross-reactivity of the antibody was detected with other aminoglycosides, indicating that the monoclonal antibody was highly specific for kanamycin. Based on competitive direct ELISA, the detection limits of kanamycin were determined to be 1.1 ng/ml in PBS, 1.4 ng/ml in plasma, and 1.0 ng/ml in milk. The concentration of intramuscularly injected kanamycin was successfully monitored in rabbit plasma with competitive direct ELISA. Based on the colloidal gold-based immunochromatographic assay, the detection limits of kanamycin were estimated to be about 6-8 ng/ml in PBS, plasma, and milk. The immunochromatographic assay would be suitable for rapid and simple screening of kanamycin residues in veterinary medicine. Screened positives can be confirmed using a more sensitive laboratory method such as competitive direct ELISA. Therefore, the assays developed in this study could be used to complement each other as well as other laboratory findings. Moreover, instead of slaughtering the animals to obtain test samples, these methods could be applied to determine kanamycin concentration in the plasma of live animals.
Keywords: competitive direct ELISA, immunochromatographic assay, kanamycin, monoclonal antibody
Introduction
Kanamycin, an aminoglycoside antibiotic produced by Streptomyces kanamyceticus, is widely used in veterinary medicine to treat mastitis, bacillary diarrhea, and pneumonia [1]. It is classified as a broad-spectrum antibiotic due to its growth inhibition of Escherichia coli, Pseudomonas aeruginosa, Klebsiella spp., and Proteus spp. [16], and is known to perturb protein synthesis in Gram-negative bacteria by binding to the 30 S subunit of ribosomal RNA, which causes misreading of the genetic code and inhibits translation [6,15]. Kanamycin is a mixture of 3 isomers: kanamycin A, kanamycin B, and kanamycin C. Since the kanamycin components differ markedly in their toxicity, commercial mixtures are required to contain at least 75% kanamycin A and no more than 5% kanamycin B [17].
Despite its impressive clinical effectiveness, kanamycin is potentially ototoxic and nephrotoxic in humans and animals [5]; thus monitoring of the level of its residues in food is essential for the maintenance of public health. For consumer protection, the European Agency for the Evaluation of Medical Products (EMEA) established maximum residue limits (MRL) for edible tissues, and milk: 100 µg/kg for meat, 150 µg/kg for milk, and 100 µg/kg for porcine fat [4]. The MRL of kanamycin in Japan has been set at 250 mg/kg for animal tissue [20]. Therefore, simple and reliable analytical methods are required to monitor kanamycin residue levels in livestock. Various techniques have been developed for the detection of kanamycin residues in milk, urine, blood, and tissues including: microbioassay [13], gas chromatography (GC) [9], high-performance liquid chromatography (HPLC) [11], and enzyme-linked immunosorbent assay (ELISA) [2,10,20]. ELISA has become the most popular method for the detection of chemicals in foods due to its high sensitivity, simplicity, and ability to screen large number of small-volume samples.
In the veterinary fields, however, a more simple and rapid detection method is required. Watanabe et al. [19] reported on a monoclonal-based ELISA and an immunochromatographic assay for monitoring monensin residues in chicken plasma and cattle milk. In addition, several recent studies have reported on a colloidal gold-based immunochromatographic assay. Using this method, Shyu et al. [18] developed a simple and reliable immunochromatographic assay for the detection of ricin, and Putalun et al. [14] developed a one-step immunochromatographic strip test for the detection of sennosides A and B.
In the present study, we produced a monoclonal antibody against kanamycin, and developed a competitive direct ELISA and immunochromatographic assay for the detection of kanamycin in animal plasma and milk; an immunochromatographic assay was developed using colloidal gold-conjugated antibody as a rapid and simple screening method for the detection of kanamycin in veterinary medicine.
Materials and Methods
Materials
Kanamycin sulfate, gentamicin sulfate, neomycin sulfate, streptomycin sulfate, keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), horseradish peroxidase (HRP), goat anti-mouse IgG-horseradish peroxidase conjugate, o-phenylenediamine dihydrochloride, hydrogen peroxide, Freund's complete adjuvant, Freund's incomplete adjuvant, polyoxyethylene-sorbitan monolaurate (Tween 20), and colloidal gold particle were purchased from Sigma-Aldrich (USA). Polyethylene glycol 1,500 (PEG 1,500), microtiter plates, and microculture plates (96- and 24-well plates) were obtained from Gibco BRL (USA). Monoclonal antibody isotyping kit was obtained from Pierce (USA). BALB/c mice and rabbits were purchased from Charles River (Korea). High flow nitrocellulose membrane was obtained from Millipore (USA).
Preparation of conjugates
Kanamycin was conjugated with KLH according to the procedure described by Lewis et al. [12] using EDC. Kanamycin-BSA and kanamycin-HRP conjugate were prepared using the method of Haasnoot et al. [7].
Mouse immunization
The kanamycin-KLH conjugate was prepared by emulsifying 100 µg antigen conjugate in 100 µl PBS with equal volume of Freund's complete adjuvant using syringes. The emulsion was injected intraperitoneally into BALB/c female mice (8-10 weeks old). After 2 weeks, the mice were injected intraperitoneally every two weeks with the emulsified mixture of the immunogen and Freund's incomplete adjuvant. A final boosting of 100 µg antigen conjugate in 100 µl PBS, without adjuvant, was performed to stimulate the blast cell proliferation 4 days before fusion as described previously [3]. Ten days after each injection, blood was collected from the retrobulbar plexus of each mouse, and antibody titers were determined by indirect ELISA using serially diluted serum and goat anti-mouse IgG conjugated with peroxidase.
Preparation of monoclonal antibody
Hybridoma cell lines were produced by the fusion of myeloma cells (Sp2/0) and spleen cells obtained from immunized mice using PEG 1,500 as described previously [8]. Twelve days after the fusion, competitive indirect ELISA was performed to screen for antibody-producing cells using culture supernatant. A stable hybridoma cell producing antibody with the highest binding capacity and sensitivity to kanamycin was selected, and cloned to 0.5 cells/well by limit dilution. The cultured hybridoma cells (5 × 106 cell) were injected intraperitoneally into the mice, and monoclonal antibody was prepared from the ascites fluid of each mouse as described previously [8].
Isotype determination
The isotype class and subclass of the secreted antibody were determined using a mouse monoclonal antibody isotyping kit. Each well of the microtiter plates was coated with 100 µl kanamycin-BSA conjugate (5 µg/ml) in 50 mM sodium-bicarbonate buffer (pH 9.6) and incubated for 3 h at 37℃. Unbound conjugate was removed from the plate with washing solution (0.02% Tween 20 in PBS), and each well was blocked with 200 µl blocking solution (1% skim milk in 50 mM sodium-bicarbonate buffer, pH 9.6) at 37℃ for 1 h. After incubation with 50 µl cell culture supernatant, 50 µl each of anti-mouse IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, kappa light chain, or lambda light chain produced in rabbit was individually added to each well and incubated for 1 h at 37℃. After washing, all wells were then further incubated with 50 µl goat anti-rabbit IgG conjugated with peroxidase for 1 h. After washing, 100 µl ABTS (2,2-azino-di[3-ethyl benzthiazoline sulfonic acid]) substrate solution was added to each well. Subsequently, the plates were incubated for 30 min at room temperature, and the color developed was measured at 405 nm using an ELISA reader.
Cross-reactivity
To determine the specificity of the kanamycin antibody, cross-reactivity of the antibody with other aminoglycosides (gentamicin, neomycin, and streptomycin) was determined by competitive indirect ELISA. The cross-reactivity at B/B0 value of 50% (CR50) was calculated as described previously [10].
Competitive direct ELISA
Competitive direct ELISA was developed using monoclonal antibody and kanamycin-HRP conjugate. Each well of the microtiter plates was coated with 100 µl aliquots of kanamycin antibody (diluted 1/2,000 in PBS) and incubated for 3 h at 37℃. Unbound antibody was removed from the plate with the washing solution (0.02% Tween 20 in PBS), and each well was blocked with 200 µl of blocking solution (1% skim milk in PBS) at 37℃ for 1 h. Kanamycin standards (50 µl each; 1 to 1,000 ng/ml) were added to each well, and incubated with 50 µl diluted kanamycin-HRP conjugate (diluted 1/2,000 in PBS) for 1 h at 37℃. After removing the unbound kanamycin and kanamycin-HRP conjugate with the washing solution, 100 µl of substrate solution was added to each well, which was then incubated for 20 min at 37℃. Absorbance was measured at 490 nm using an ELISA reader (Emax; Molecular Devices, USA).
To prepare the calibration curves of kanamycin in the plasma and milk, kanamycin stock standard solutions (1,000 µg/ml) were prepared by dissolving kanamycin in the rabbit plasma or bovine milk. The solutions were further diluted with the plasma or milk to 0, 10, 20, 50, 100, 250, 500, 1,000, 2,500, 5,000, and 10,000 ng/ml, which were then diluted 10-fold in PBS. The standard curves of kanamycin in the plasma and milk were established by competitive direct ELISA. The detection limits were determined as the mean background levels plus three times the standard deviation.
Monitoring of blood concentration
Kanamycin was administered intramuscularly to rabbits at 20 mg/kg/day for 3 consecutive days. Blood samples were collected from the ear vein of each rabbit 2, 4, 6, 8, 10, and 12 h after the last injection of kanamycin, and were centrifuged (2,000 × g) for 10 min to obtain plasma. Plasma samples were diluted 10-fold in PBS and subjected to competitive direct ELISA to determine the kanamycin concentration in the blood.
Immunochromatographic assay
Colloidal gold (40 nm in diameter) was conjugated with anti-kanamycin monoclonal antibody as described previously [18]. Briefly, 20 µg of purified monoclonal antibody was added to 1 ml colloidal gold solution (pH 8.0). After incubation at room temperature for 10 min, the conjugates were blocked with 1% BSA solution for 30 min. The gold-labeled antibody were supplied, ready to use, in 0.2 M Tris-HCl buffer (pH 8.7) containing 1% Tirton X-100 at an optical density of 10 at 540 nm. Since it was quite concentrated, gold-labeled antibody was diluted 10-fold and was measured at 540 nm using spectrophotometer.
One microliter (3 µg kanamycin-BSA) of kanamycin-BSA (3 mg/ml) conjugate was applied to one end of the nitrocellulose membrane strip (HF 135, 25 × 4.5 mm). After drying, the lower edge of the test strip was dipped into the well containing a mixture of 50 µl diluted sample (5-fold in 0.2 M Tris-HCl, pH 8.7, 1% Triton X-100) and 2 µl colloidal gold-labeled monoclonal antibody. After the mixture of sample and the gold-labeled antibody moved up the membrane, the color intensities decreased with increasing concentration of kanamycin in sample solutions was observed.
Results
Monoclonal antibody production
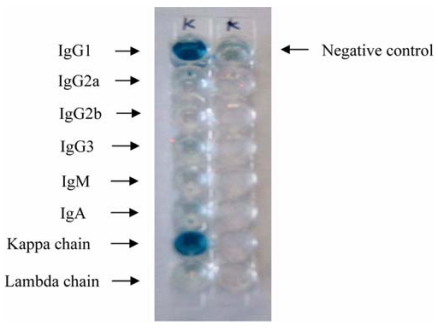
Monoclonal antibody against kanamycin was prepared as described in Materials and Methods. After purification, the isotype class and subclass of the antibody were determined using a mouse monoclonal antibody isotyping kit. Based on the results of the assay, the monoclonal antibody of kanamycin was confirmed to be an IgG1, which has a kappa light chain (Fig. 1).
Fig. 1.
Determination of monoclonal antibody isotype. Rabbit antisera specific for mouse IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, kappa light chain, and lambda light chain were added to each well, and detected with goat anti-rabbit IgG conjugated with peroxidase. The negative control included only pre-immune serum.
Cross-reactivity
The purified monoclonal antibody did not show any cross-reactivity with other aminoglycosides (gentamicin, neomycin, and streptomycin) except kanamycin, indicating that the monoclonal antibody was highly specific for kanamycin (Table 1).
Table 1.
Cross-reactivity (CR50) of the monoclonal antibody of kanamycin with aminoglycosides
Competitive direct ELISA
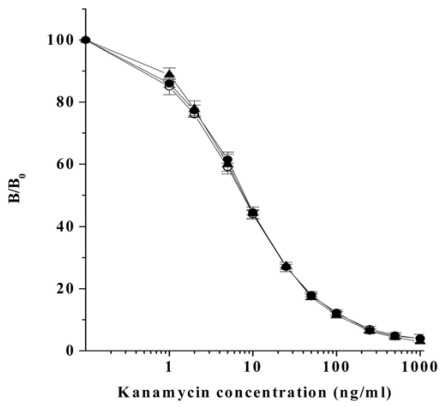
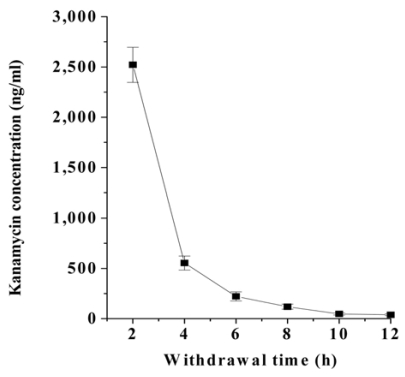
To determine the detection limits of kanamycin in the plasma and milk, standard curves of kanamycin in PBS, rabbit plasma, and bovine milk were constructed by competitive direct ELISA (Fig. 2). Detection limits of kanamycin determined by the ELISA method are summarized in Table 2. The detection limits, which were defined as the mean background levels plus three times the standard deviation, were 1.1 ng/ml in PBS, 1.4 ng/ml in plasma, and 1.0 ng/ml in milk, respectively (Table 2). Concentration of intramuscularly injected kanamycin was successfully monitored in rabbit plasma through competitive direct ELISA. The kanamycin concentration in blood sharply increased to 2,500 ng/ml after the intramuscular administration, for up to 2 h, and then rapidly decreased to less than 500 ng/ml 4 h after withdrawal (Fig. 3).
Fig. 2.
Standard curve of kanamycin in PBS (○), rabbit plasma (●), and bovine milk (▲) constructed through competitive direct ELISA. B and B0 are the absorbance at 490 nm in the presence or absence of free kanamycin. Each value shows the mean (±SD) of B/B0 (n = 4).
Table 2.
Detection limits of kanamycin determined by competitive direct ELISA
*Mean background levels were obtained from the standard curves of kanamycin in PBS, rabbit plasma, and bovine milk constructed by competitive direct ELISA.
**Detection limits: the mean background levels plus three times standard deviation (Bo+3 SD).
Fig. 3.
Depletion profile of kanamycin in rabbit plasma after intramuscular administration of kanamycin. Kanamycin was administered intramuscularly to rabbits at 20 mg/kg/day for 3 consecutive days. Blood samples were collected from the ear vein of each rabbit 2, 4, 6, 8, 10, and 12 h after the last injection of kanamycin. Plasma samples were diluted 10-fold in PBS and subjected to the competitive direct ELISA. Each value shows the mean (±SD) of kanamycin concentration (n = 4).
Immunochromatographic assay
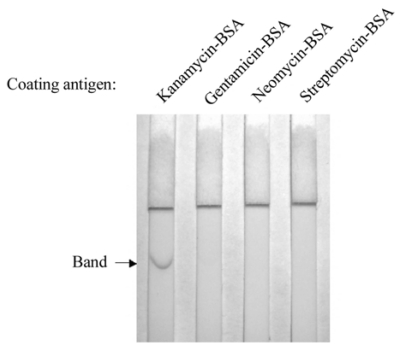
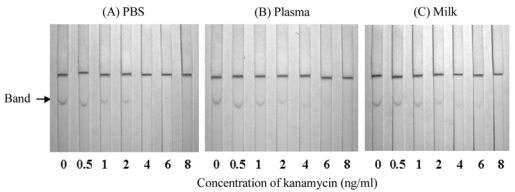
The gold-labeled monoclonal antibody of kanamycin did not show any cross-reactivity with the other aminoglycosides tested; as revealed by the immunochromatographic assay (Fig. 4). Rabbit plasma, PBS, and bovine milk samples spiked with kanamycin (0, 0.5, 1, 2, 4, 6, and 8 ng/ml) were tested with the immunochromatographic assay (Fig. 5). The color intensity gradually decreased with increasing concentration of kanamycin, and disappeared completely at 6 or 8 ng/ml of kanamycin in the samples. Therefore, as a result of the immunochromatographic assay the detection limits were estimated to be about 6-8 ng/ml of kanamycin in PBS, plasma, and milk.
Fig. 4.
Cross-reactivity of the monoclonal antibody of kanamycin with aminoglycosides in immunochromatographic assay. Three microgram of kanamycin-BSA, gentamicin-BSA, neomycin-BSA, or streptomycin-BSA conjugate was applied to each strip of a nitrocellulose membrane. After the gold-labeled antibody moved up the membrane, the intensity of the red color band on each membrane strip was observed.
Fig. 5.
Immunochromatographic assay for the detection of kanamycin. A series of dilutions (0, 0.5, 1, 2, 4, 6, and 8 ng/ml) of kanamycin were prepared in PBS (A), rabbit plasma (B), and bovine milk (C).
Plasma samples collected from rabbits 2, 4, 6, 8, 10, and 12 h after intramuscular injection of kanamycin (20 mg/kg/day for 3 consecutive days) were subjected to immunochromatographic assay (Fig. 6). No color development was observed in all of the strips studied; this suggested that plasma samples contained higher than 6 ng/ml of kanamycin. Subsequent ELISA gave the accurate level of kanamycin concentration (Fig. 6).
Fig. 6.
Comparison of immunochromatographic assay with competitive direct ELISA. Kanamycin was administered intramuscularly to rabbits at 20 mg/kg/day for 3 consecutive days as described in Materials and Methods. Blood samples were collected from rabbits 2, 4, 6, 8, 10, and 12 h after the last injection of kanamycin. Plasma samples were subjected to immunochromatographic assay. Subsequent competitive direct ELISA determined the kanamycin concentration in plasma samples.
Discussion
We produced a monoclonal antibody that was highly specific for kanamycin based on both an ELISA and an immunochromatographic assay. The specificity of the antibody can be explained by the differences in the molecular structure of the aminoglycosides. All aminoglycosides consist of two or more amino sugars joined by a glycosidic linkage to a hexose nucleus, which is either streptose (found in streptomycin) or 2-deoxystreptamin (characteristic of all other aminoglycosides) [16]; the aminoglycoside families are distinguished by the amino sugars attached to the nucleus. In either kanamycin or gentamicin, two amino sugars are attached to 2-deoxystreptamin, whereas neomycin has three amino sugars attached. In addition, kanamycin reacts with the antibody specific to its structure, because the molecular structure of the attached sugars is quite different from those of neomycin, gentamicin, and streptomycin [7]. These identified structural differences enable each antibody to recognize its own specific antigen.
After intramuscular administration, the kanamycin concentration in blood sharply increased for up to 2 h, and then rapidly decreased 4 h after withdrawal. When aminoglycosides are administered into the body cavity, which contain serosal surfaces, extremely rapid and complete absorption takes place; whereas slow absorption is observed when it is administered orally or rectally [17]. In addition, Isoherranen and Soback [9] reported that aminoglycosides bind readily to tissue proteins and macromolecules with ionic bonds; while such binding is less evident with plasma proteins. They also showed that aminoglycoside accumulation in the renal proximal tubules was several-fold higher than in the plasma or other tissues, and that the half-life of aminoglycosides was 2-3, and 30-700 h, in plasma and tissues, respectively. Therefore, due to the longer and more variable half-life of aminoglycosides in the tissues compared to plasma, we plan a future study to determine the time-dependent concentration of kanamycin in milk, blood, and tissues after administration of kanamycin to cows with mastitis. The ELISA method developed in this study could be applied to determine the aminoglycoside concentration in the plasma of live animals.
In veterinary medicine, a simple and rapid detection method for aminoglycoside levels is required. Recently, several studies have focused on colloidal gold-based immunochromatographic assays [14,18]. The application of immunogold detection has several advantages. First, the nanoparticles of colloidal gold show better mobility than other materials in a porous nitrocellulose membrane. Second, the colloidal gold particles are less susceptible to aggregation during the preparation. Finally, a gold-labeled antibody improves assay sensitivity [18]. In the present study, a compromise was made between the sensitivity and the non-specific binding of antigen-antibody reactions in the immunochromatographic assay. On the basis of the findings, that the pH and the composition of the developing solution were important for better resolution, we optimized the assay conditions for better sensitivity without any cross-reactivity or non-specific binding.
In conclusion, due to its rapid and simple application, the colloidal gold-based immunochromatographic assay can be applied to the detection of kanamycin in veterinary medicine. For greater accuracy, however, detection should be supported by a more sensitive laboratory method such as the competitive direct ELISA method. The assays developed in this study could complement each other and provide a useful tool for veterinary medicine. Moreover, instead of slaughtering animals, to obtain test samples, the methods developed in the present study can be applied to determine kanamycin concentration in the plasma of live animals.
Acknowledgments
This study was supported by Korea Research Foundation Grant (KRF-005-E00076/KRF-005-E00078).
References
- 1.Cleveland CB, Francke DE, Heller WM, Kepler JA, Provost GP, Reilly MJ. AHFS Drug Information. Bethesda: American Society of Hospital Pharmacists Press; 1990. pp. 51–67. [Google Scholar]
- 2.DeCastro AF, Place JD, Lam CT, Patel C. Determination of kanamycin concentration in serum by substrate-labeled fluorescent immunoassay. Antimicrob Agents Chemother. 1986;29:961–964. doi: 10.1128/aac.29.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon DE, Warner RL, Ram BP, Hart LP, Pestka JJ. Hybridoma cell line production of specific monoclonal antibody to the mycotoxins zearalenone and a-zearalenol. J Agric Food Chem. 1987;35:122–126. [Google Scholar]
- 4.European Agency for the Evaluation of Medical Products (EMEA) London: 2003. Regulation No. EMEA/MRL/886/03-FINAL. [Google Scholar]
- 5.Finitzo-Hieber T, McCracken GH, Jr, Roeser RJ, Allen DA, Chrane DF, Morrow J. Ototoxicity in neonates treated with gentamicin and kanamycin: results of a four-year controlled follow-up study. Pediatrics. 1979;63:443–450. [PubMed] [Google Scholar]
- 6.Fourmy D, Yoshizawa S, Puglisi JD. Paromomycin binding induces a local conformational change in the A-site of 16S rRNA. J Mol Biol. 1998;277:333–345. doi: 10.1006/jmbi.1997.1551. [DOI] [PubMed] [Google Scholar]
- 7.Haasnoot W, Stouten P, Cazemier G, Lommen A, Nouws JFM, Keukens HJ. Immunochemical detection of aminoglycosides in milk and kidney. Analyst. 1999;124:301–305. doi: 10.1039/a807846g. [DOI] [PubMed] [Google Scholar]
- 8.Harlow ED, Lane D. Antibodies: A Laboratory Manual. New York: Cold Spring Harbor Laboratory; 1988. pp. 196–214. [Google Scholar]
- 9.Isoherranen N, Soback S. Chromatographic methods for analysis of aminoglycoside antibiotics. J AOAC Int. 1999;82:1017–1045. [PubMed] [Google Scholar]
- 10.Kitagawa T, Fujiwara K, Tomonoh S, Takahashi K, Koida M. Enzyme immunoassays of kanamycin group antibiotics with high sensitivities using anti-kanamycin as a common antiserum: reasoning and selection of a heterologous enzyme label. J Biochem. 1983;94:1165–1172. doi: 10.1093/oxfordjournals.jbchem.a134461. [DOI] [PubMed] [Google Scholar]
- 11.Kubo H, Kobayashi Y, Nishikawa T. Rapid method for determination of kanamycin and dibekacin in serum by use of high-pressure liquid chromatography. Antimicrob Agents Chemother. 1985;28:521–523. doi: 10.1128/aac.28.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JE, Nelson JC, Elder HA. Radioimmunoassay of an antibiotic: gentamicin. Nat New Biol. 1972;239:214–216. doi: 10.1038/newbio239214a0. [DOI] [PubMed] [Google Scholar]
- 13.Maitra SK, Yoshikawa TT, Guze LB, Schotz MC. Determination of aminoglycoside antibiotics in biological fluids: a review. Clin Chem. 1979;25:1361–1367. [PubMed] [Google Scholar]
- 14.Putalun W, Morinaga O, Tanaka H, Shoyama Y. Development of a one-step immunochromatographic strip test for the detection of sennosides A and B. Phytochem Anal. 2004;15:112–116. doi: 10.1002/pca.752. [DOI] [PubMed] [Google Scholar]
- 15.Ren YG, Martinez J, Kirsebom LA, Virtanen A. Inhibition of Klenow DNA polymerase and poly(A)-specific ribonuclease by aminoglycosides. RNA. 2002;8:1393–1400. doi: 10.1017/s1355838202021015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martindale W, Reynolds JEF. The Extra Pharmacopoeia. 30th ed. London: Pharmaceutical Press; 1993. pp. 109–113. [Google Scholar]
- 17.Riviere JE, Spoo JW. Aminoglycoside antibiotics. In: Adams HR, editor. Veterinary Pharmacology and Therapeutics. 8th ed. Ames: Iowa State University Press; 2001. pp. 841–867. [Google Scholar]
- 18.Shyu RH, Shyu HF, Liu HW, Tang SS. Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon. 2002;40:255–258. doi: 10.1016/s0041-0101(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Satake A, Matsumoto M, Kido Y, Tsuji A, Ito K, Maeda M. Monoclonal-based enzyme-linked immunosorbent assay and immunochromatographic rapid assay for monensin. Analyst. 1998;123:2573–2578. doi: 10.1039/a805362f. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Satake A, Kido Y, Tsuji A. Production of monoclonal antibody and development of enzyme-linked immunosorbent assay for kanamycin in biological matrices. Analyst. 1999;124:1611–1615. doi: 10.1039/a906026j. [DOI] [PubMed] [Google Scholar]