Abstract
Spigelia anthelmia Linn is used as a herb and is a common annual weed of cultivation in open re-growths, on unused land in towns as well as on road sides. The plant can grow to approximately 30 cm in height. The aim of this study was to screen extracts of Spigelia anthelmia for their anthelmintic activity against an experimental Nippostrongylus braziliensis infection in rats. Acute oral toxicity occurred at a dose of 1,140mg/kg, while anthelmintic trials against Nippostrongylus braziliensis in rats using the aqueous fraction showed a progressive decrease in worm count with increasing dose (10, 13, 16, 20 and 25 mg per kg body weight) (p < 0.05). At 25 mg per kg body weight, the worm count was significantly lower than that at 10mg per kg body weight (p < 0.05).
Keywords: anthelmintic, Spigelia anthelmia, Nippostrongylus braziliensis
Introduction
The local inhabitants of South Western Nigeria often use Spigelia anthelmia Linn (Loganiaceae) for expelling worms. The plant is commonly known as "Aparan", (apa-kill, araworm) and "ewe aran" among the Yorubas people in South Western Nigeria [6].
Ecological conditions occurring in tropical countries are conducive to nematode infections, which are frequent, prevalent and often heavy on domestic animals resulting in substantial losses, including the death of infected livestock [9]. Under most pastoral conditions, endo-parasitism, particularly that due to helminthosis, can result in mortality rates exceeding 50% [4]. Schillhorn [15] reported that helminthosis is the most important killer of small ruminants in Nigeria. Waruiri et al. [18] identified helminthosis to be one of the most significant single impediments to the development of sheep and goat production in the tropics. In Kenya, losses due to helminth parasites constitute 11.8% of the total slaughter for cattle and 46.0% for sheep and goats [7].
The current control methods for internal parasites outside Africa focus on reducing the level of pasture contamination through anthelmintic treatments and/or controlled grazing. In Africa, these methods are limited by the high cost of anthelmintics, their uncertain availability, increased frequency of drug resistance and limited scope in many communal pastoral systems razing [3]. The major control measure against helminthosis in Nigeria is chemotherapy. However, the general availability of these drugs varies, and some drugs of choice are not always available. This calls for studies directed at developing alternative approaches for controlling internal parasites in Africa, which include examining the effectiveness of herbs used locally as anthelmintics. The local people and herdsmen have long recognized the significance of helminthosis, and have made many attempts to control it through the use of herbs. Such herbs are easily accessible and are cost-effective. The cost of treatment with alternative traditional methods is negligible compared with the cost of conventional drugs. In addition to being very inexpensive, alternative herbal preparations have good nutritional value [8,11,13].
The use of traditional medicine in one form or other is widespread throughout the world. These practices are based on beliefs that have existed for hundreds of years before the development and spread of modern medicines and are still in use today. As its name suggests, traditional medicine is part of the tradition of each population or culture handed down from generation to generation. Its acceptance by the population is largely conditioned by cultural factors. Therefore, a great deal of traditional medicine may not be readily transferable from one culture to another [16]. Medicinal plants are a small but important part of the biological heritage of the Earth. Traditional society places a high value on this inheritance, which is expressed through an intimate relationship with nature. It is an undeniable fact that in today's world, herbal medicines play a vital role in health care of large sections of the population, particularly in developing countries, where they often bridge the gap between the availability, and demand for modern medicines [2].
More than 300 species of plants have been used in traditional control of human and animal helminthosis in Africa [6,12]. However, most of these plants have not been studied systematically. It is important to screen local herbs to both supplement proprietary drugs and to provide new chemotherapeutic leads. There is also a need to establish standard doses for herbal preparations and to investigate their toxicity [14,12].
This study screen the aqueous fraction of an ethanolic extract from Spigelia anthelmia (worm weed) for their anthelmintic activity against experimental Nippostrongylus brazilliensis infections in rats, as a prelude to its use in controlling helminthosis in livestock.
Materials and Methods
Collection and preparation of plant material
The entire plant was collected from the Department of Agricultural Engineering Faculty of Agriculture, Ahmadu Bello University, Nigeria between June and August, 2003. Taxonomic identification established by a botanist in the Soil Survey Unit of the Department of Soil Science, Faculty of Agriculture, Ahmadu Bello University, Nigeria. The plant was authenticated by a comparison with the herbarium sample at the National Animal Production Research Institute, Shika, Nigeria. A voucher specimen was deposited and is labeled Specimen number 1.
Extraction
Immediately after collection, the plant was sorted, cleaned and dried in the open air for one week. The dried material (500 g) was pulverized into powder using a Lab mill machine in clean containers and labeled for easy identification. The plant was extracted using the cold method reported by Wagner et al. [17].
The filtrate was evaporated to dryness in vacuo at 60℃ using a Buchi rotary evaporator coupled to a thermo regulator. The residue was then partitioned using chloroform and water. The aqueous phase was further partitioned using ethylacetate [17], and the aqueous fraction was concentrated in a water bath. The solid extract obtained was removed and stored in labeled beakers at 4℃ until required.
Experimental animals
Albino rats (Wistar strain) weighing between 80~200 g were purchased from the animal house at the National Institute for Trypanosomiasis Research (NITR), Vom Substation, Vom Plateau State, Nigeria. The animals were allowed two weeks to acclimatize to the laboratory conditions. They were provided with a ration containing commercial poultry feed growers mash (Silver feeds company, Nigeria), groundnut cake and maize bran at a ratio of 1 : 0.5 : 2 respectively, as well as water from the Ahmadu Bello University water supply ad libitum. All the rats were dewormed using albendazole (Concept Pharmaceuticals, India) at 7.5 mg/kg in order to establish a worm-free colony. Sawdust was used as bedding and was changed every three days. The rats were identified by marks on their tails and cages.
Safety tests
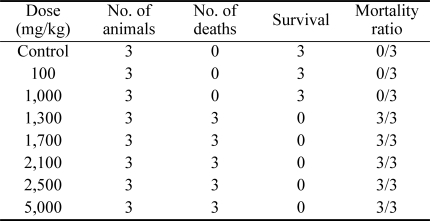
Acute toxicity studies were carried out using the method reported by Lorke [10]. Fifteen rats were divided into 5 groups consisting of three rats each. The rats in groups 1, 2, 3 and 4 were dosed intraperitoneally with an aqueous extract of Spigelia anthelmia at a rate of 100, 1,000, 2,500 and 5,000 mg/kg body weight, respectively. The fifth group, which was used as the control, received distilled water at 5 ml/kg body weight. The animals were observed for 72 h. The highest dose that did not cause death and the lowest dose that caused death were selected and used as guidelines for further acute toxicity studies. Studies were then carried out using three groups containing three rats each. These three groups were administered the doses of 1,300, 1,700 and 2,100 mg/kg body weight, respectively. The control group received distilled water at a rate of 5 ml/kg body weight. The animals that survived were monitored for two weeks to determine if there were any delayed toxic effects. The test was then terminated after another two weeks. All animals were then sacrificed and post mortem examination was conducted. The median lethal dose (LD50) was calculated using the method reported by Lorke [10].
Screening test
The screening was carried out using the method described by Cavier [5]. The trials commenced on the 10th day after infecting each of 35 rats with 200 larvae (L3) of Nippostrongylus brazilliensis. On days 10, 11 and 12 days post-infection, the aqueous fraction of the extract was tested on five groups of rats, each containing five rats. Each rat in the respective group received 10, 13, 16, 20 and 25 mg/kg body weight orally using an 18-gauge needle fitted with a plastic cannula. Five other rats were dosed with water at a rate of 5 ml/kg body weight and used as the control.
The results were analyzed using the method reported by Cavier [5]. The percentage deparasitization was calculated using the following formula:
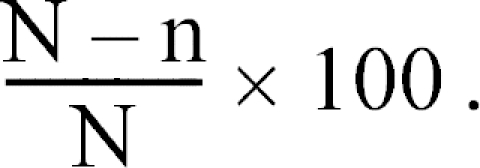
Where
N = average number of worms found in the control animals and
n = average number of worms found in the groups of treated animals.
The result of the chemotherapeutic study was subjected to statistical analysis using Statistical Packages for the Social Science (SPSS) version 10. A p value <0.05 was considered significant.
Therapeutic index
The therapeutic index, which is a measure of the drug's safety, was calculated using the following formula:
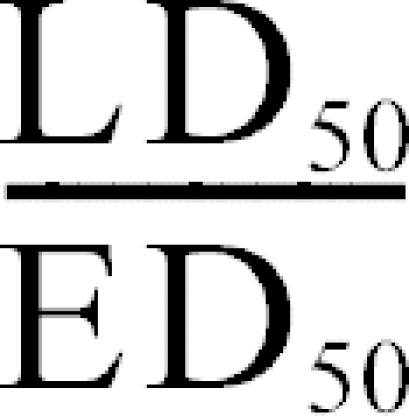
Results
LD50 determination and pathological findings
The median lethal dose (LD50) of the extract was estimated to be 1,140 mg/kg body weight using the formula reported by Lorke [10] (Table 1). At post mortem, the dead animals showed an enlarged and congested liver, hemorrhagic enteritis, an enlarged and congested spleen, petechial hemorrhagic lesions in the lungs and congested kidneys. There was no visible gross lesion in the heart.
Table 1.
The acute toxicity induced by the intraperitoneal administration of the aqueous fraction of Spigelia anthelmia Linn extract in rats
The calculated therapeutic index for Spigelia anthelmia Linn was 54.28.
Screening test
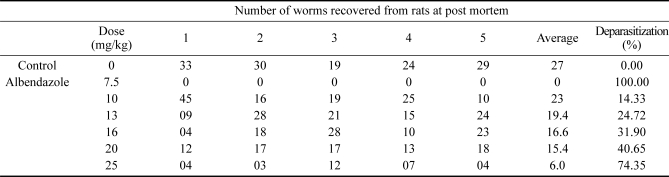
Table 2 shows the efficacy of the screening test for Spigelia anthelmia Linn in rats. The results show that the aqueous portion of Spigelia anthelmia Linn is effective against adult Nippostrongylus braziliensis in rats at nontoxic doses. The extract caused significant deparasitization of 74.7% at a dose of 25 mg/kg body weight (p < 0.05).
Table 2.
Results of the chemotherapeutic trials
The calculated median effective dose (ED50) for the extract was 21 mg/kg body weight.
The worm counts obtained from the rats given doses of 10 mg/kg, 13 mg/kg, 16 mg/kg, 20 mg/kg and 25 mg/kg ranged from 10~45, 9~28, 4~28, 12~18 and 3~12 respectively, indicating a progressive decrease in the worm count with increasing dose. The mean worm count decreased from 23 at 10 mg/kg to only 6 at 25 mg/kg body weight (Table 2) (p < 0.05) The mean percentage deparasitization increased from 14.33 for the rats given the extract at 10 mg/kg bodyweight to 74.34 for those given 25 mg/kg body weight.
Table 3 shows the Analysis of variance (ANOVA) table for the worm count. The table shows a significant difference between the doses given. Significantly higher numbers of worms were recorded at the lowest dose of 10mg/kg compared with 25 mg/kg body weight (p < 0.05). This suggests that the aqueous portion of Spigelia anthelmia has good anthelmintic efficacy.
Table 3.
The descriptive statistics of the aqueous extract of Spigelia anthelmia
Note: asignificant (p < 0.05), bNon-significant (p > 0.05), abNon complete manifestation, Data expressed as mean ± SD.
Discussion
The LD50 of the ethanol extract of Spigelia anthelmia Linn plant suggests that this extract is only slightly toxic to rats. The signs of toxicity observed were similar to those reported by Wagner et al. [17].
The result of the acute toxicity studies carried out in this study is in sharp contrast to the reports by Dalziel [6] and Oliver [14] who showed that this plant is toxic in its fresh state via the oral route and is capable of causing death in domestic animals within one to two hours. Wagner et al. [17], reported an LD50 of 222 mg/kg body weight in rats.
The significant deparasitization of 74.7% caused by the aqueous fraction of the Spigelia anthelmia Linn extract at a dose of 25mg/kg body weight is similar to the deparasitization of 75% reported by Ibrahim [8] through the oral administration of Annona senegalensis at 20 g per kg body weight against the same parasite in rats. This level of deparasitization is higher than the respective deparasitization of 69, 60 and 58% caused by the oral administration of 12.5 g/kg, 20 g/kg and 20 g/kg of Cassia occidentalis Linn, Annogeissus leocarpus Hochst and Diosperos mespiliformis Hochst in rats infected with Nippostrongylus braziliensis [8] However, Adegbulugbe [1] reported 96% deparasitization after administering the seeds of Carica papaya to rats orally at a dose of 100 mg/kg body weight, which is much higher than that obtained in this study. However, it should be noted that the dose used in their study was four times higher than that used in the current study.
In conclusion, the aqueous portion of Spigelia anthelmia had an acceptable anthelmintic efficacy. The calculated median effective dose (ED50) for the extract was 21 mg/kg body weight. The therapeutic index obtained in this study suggests that the extract is quite safe even at higher doses. This study confirmed the anthelmintic activity of Spigelia anthelmia Linn. Moreover, the extract was found to have high anthelmintic activity against Nippostrongylus braziliensis in rats. However, further secondary screening of the plant in higher livestock species will be needed.
References
- 1.Adegbulugbe AO. Toxicity and anthelmintic activity of extracts of the seeds of Carica papaya Linn. Nigeria: Department of Veterinary Physiology and Pharmacology, Ahmadu Bello University; 1997. p. 45. DVM Thesis. [Google Scholar]
- 2.Akerele O. Medicinal plants and primary health care: an agenda for action. Fitoterapia. 1988;59:355–363. [Google Scholar]
- 3.Bakunzi FR, Serumaga-Zake PAE. The effect of strategic anthelmintic treatment on internal parasites in communally grazed sheep in a semi-arid area as reflected in the faecal nematode egg count. Trop Anim Health Prod. 2000;32:295–302. doi: 10.1023/a:1005264906954. [DOI] [PubMed] [Google Scholar]
- 4.Barger IA. Helminth parasites and animal production. In: Symons LEA, Donald AD, Dineen JK, editors. Biology and Control of Endoparasites. Sydney: Academy Press; 1982. pp. 133–155. [Google Scholar]
- 5.Cavier R. Chemotherapy of Helminthiasis. Vol. 1. Oxford: Pergamon Press; 1973. pp. 215–436. [Google Scholar]
- 6.Dalziel JM. The Useful Plants of West Tropical Africa. London: Crown Agents; 1937. p. 612. [Google Scholar]
- 7.Githigia SM, Kimoro CO, Mwangi GM, Gichanya J. Prevalence and economic significance of Oesophagostomum and other helminth parasites of ruminants surveyed in selected abattoirs around Nairobi, Kenya. Bull Anim Health Prod Afr. 1995;43:29–33. [Google Scholar]
- 8.Ibrahim MA. Evaluation of the activities of some African traditional anthelmintic herbs against Nippostrongylus braziliensis In rats. Nigeria: Department of Veterinary Physiology and Pharmacology, Ahmadu Bello University; 1984. p. 119. M. Sc. Thesis. [Google Scholar]
- 9.Kuil H. Gastrointestinal nematodes of sheep in the Zaria area of Northern Nigeria. Monograph, Institute of Tropicxal Diseases, Urtretch and Department of Parasitology and Entomology. Zaria: Ahmadu Bello University; 1970. pp. 1–47. [Google Scholar]
- 10.Lorke DA. New approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 11.Mbaria JM, Maitho TE, Mitema ES, Muchiri DJ. Comparative efficacy of pyrethrum marc with albendazole against sheep gastrointerstinal nematodes. Trop Anim Health Prod. 1998;30:17–22. doi: 10.1023/a:1005005208588. [DOI] [PubMed] [Google Scholar]
- 12.Nwude N, Ibrahim MA. Plants used in traditional veterinary medical practice in Nigeria. J Vet Pharmacol Ther. 1980;3:261. [Google Scholar]
- 13.Okon ED. Effect of parturition on faecal strongyle egg output in Nigerian goats. Bull Anim Health Prod Afr. 1980;28:155–158. [PubMed] [Google Scholar]
- 14.Oliver B. Nigeria's usefull plants. Nigerian Field. 1959;24:160–182. [Google Scholar]
- 15.Schillhorn-van Veen TW. Small ruminants health problems in Northern Nigeria with emphasis on the helminthosis. Nigerian Vet J. 1973;2:26–31. [Google Scholar]
- 16.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. Chichester: Wiley; 1982. pp. 142–145. [Google Scholar]
- 17.Wagner H, Seegert K, Odenthal KP, Esposito Avella M, Villarreal E, Solis P, Gupta MP. Preliminary pharmacologic evaluation of Spigelia anthelmia aerial parts. Int J Pharmacognosy. 1993;31:7–14. [Google Scholar]
- 18.Waruiri RM, Mbuthia PG, Njiro SM, Ngatia TA, Weda EH, Ngotho JW, Kanyati PN, Munyua WK. Prevalence of gastrointestinal parasites and lungworms in wild and domestic ruminants in game ranching farm in Kenya. Bull Anim Health Prod Afr. 1995;43:253–259. [Google Scholar]