Abstract
Mesenchymal stem cells (MSCs) have the capabilities for self-renewal and differentiation into cells with the phenotypes of bone, cartilage, neurons and fat cells. These features of MSCs have attracted the attention of investigators for using MSCs for cell-based therapies to treat several human diseases. Because bone marrow-derived cells, which are a main source of MSCs, are not always acceptable due to a significant drop in their cell number and proliferative/differentiation capacity with age, human umbilical cord blood (UCB) cells are good substitutes for BMCs due to the immaturity of newborn cells. Although the isolation of hematopoietic stem cells from UCB has been well established, the isolation and characterization of MSCs from UCB still need to be established and evaluated. In this study, we isolated and characterized MSCs. UCB-derived mononuclear cells, which gave rise to adherent cells, exhibited either an osteoclast or a mesenchymal-like phenotype. The attached cells with mesenchymal phenotypes displayed fibroblast-like morphologies, and they expressed mesenchym-related antigens (SH2 and vimentin) and periodic acid Schiff activity. Also, UCB-derived MSCs were able to transdifferentiate into bone and 2 types of neuronal cells, in vitro. Therefore, it is suggested that the MSCs from UCB might be a good alternative to bone marrow cells for transplantation or cell therapy.
Keywords: differentiation, human, mesenchymal cell, stem cell, umbilical cord blood
Introduction
The blood remaining in the umbilical cord following birth contains hematopoietic precursors and this has become an important alternative source for transplantation of hematopoietic stem cells [2,7,8,11,14]. However, there is controversy as to whether umbilical cord blood (UCB) contains mesenchymal stem cells (MSCs) that are capable of differentiating into cells of different connective tissue lineages such as bone, cartilage and adipose tissues, and these cells are the best candidates for tissue engineering of musculoskeletal tissues [17,20,26]. To date, the most common source of MSCs has been bone marrow, but aspirating bone marrow from the patient is an invasive, painful procedure. In addition, it has been demonstrated that the number of bone marrow MSCs and their ability to differentiate decreases with age [4,15]. Therefore, researchers are looking for alternative sources of MSCs. So far, little success has been reported about the isolation, characterization and differentiation of MSCs from UCB. Erices et al. [5] have reported that UCB-derived mononuclear cells gave rise to 2 adherent cell types, and one of them expressed MSC-related surface antigens. Mareschi et al. [16] reported that under given conditions, it was possible to isolate MSCs from bone marrow, but not from UCB. However, Goodwin et al. [9] have recently reported the multi-lineage differentiation ability of UCB-isolated cells; these cells express bone, fat and neural markers. Kakinuma et al. [13] reported that they could differentiate UCB cells into hepatic progenitor cells. Neither of these reports provided sufficient evidence to fulfill the criteria for qualifying MSCs because both research groups found relatively heterogeneous cells. Wexler et al. [26] have recently reported that UCB is not a rich source of human MSCs, while Romanov et al. [20] also suggested using umbilical cord endothelial cells as an alternative MSC source. Nevertheless, UCB cells have many advantages because of the immaturity of newborn cells compared with adult cells. Furthermore, UCB provides no ethical problems for basic studies and clinical applications. Further, UCB cells can be collected without any harm to the newborn infant. In this study, we examined whether MSCs from UCB could expand their population and if they have the ability to differentiation to bone and neuronal cell types. We also investigated how to purify MSCs from the heterogeneous cell types.
Materials and Methods
Cell collection
UCB cells were obtained from normal full-term and preterm deliveries at Seoul National University Borame Hospital (Seoul, Korea) according to the institutional guidelines. The blood was collected into 250 ml standard blood collection bags (Green Cross, Korea) that contained citrate-phosphate-dextrose anticoagulant.
Cell processing
The buffy coat cells were obtained by centrifugation (400 g for 20 min), and the low-density mononuclear cells (MNC, <1.077 g/ml) were isolated using Ficoll-Paque Plus (Amersham Biosciences, Sweden). The cells were then resuspended in Dulbeco's Modified Eagle low glucose medium (Gibco-BRL, USA) that was supplemented with 10% fetal bovine serum (FBS; Gibco-BRL, USA). The total numbers of nucleated and viable cells were determined with a hemocytometer and by using trypan blue stain [10].
Isolation and culture of MSCs
The cells were cultured in growth medium [Dulbeco's Modified Eagle Media-low glucose with the addition of 10% FBS with 2 mmol/l L-glutamine (Gibco-BRL, USA)] and 0.3% penicillin-streptomycin (Gibco-BRL, USA) at 37℃ and a 5% CO2 concentration. The cells were initially plated into a 10 to 175 cm2 tissue culture flask (Nunc, USA) at a density of 1.5 × 106 mononuclear cells/cm2. The cells were transferred to new flask to remove the platelets after 1 day of culture. Half the medium was changed weekly thereafter. The cells were passaged by trypsinization (0.05% trypsin/EDTA solution; Gibco BRL, USA) upon reaching 50% to 60% confluence (5,000-6,000 cells/cm2), and they were replated at a density of 1,000 to 2,000 cells/cm2 [9].
Cytochemical and immunophenotyping of MSCs and characterization of MSCs
Cells in situ were analyzed for the following cytochemical markers: acid phosphatase (AP) and periodic acid-Schiff (PAS). In all cases, the analyses, as well as the selection of positive and negative controls, were performed according to the manufacturer's guidelines (Sigma, USA) [5]. To detect surface antigen, aliquots of fresh UCB or cultured adherent cells were immunolabelled with anti-human antibodies CD51/61 (Pharmingen, USA), SH-2 (Ancell, USA) and vimentin (Chemicon, USA), and the secondary antibodies: FITC anti-mouse IgG diluted 1 : 100 (Zymed, USA).
Osteogenic potential of MSCs
Once sufficient numbers of cells were grown from UCB, the cells were plated at 1,500 to 4,000 cells/cm2 in growth medium. Osteogenesis medium (growth medium with the addition of 0.1 µmol/l dexamethasone [Sigma, USA], 0.05 mmol/l ascorbic acid-2-phosphate [Sigma, USA] and 10 mmol/l β-glycerophosphate [Sigma, USA]) was applied 24 h after plating [9,12]. The medium was changed every 3 to 4 days. Osteogenesis was assessed on day 14. The presence of hydroxyapatite [(Ca10(PO4)6(OH)2)] nodules was visualized with a 2% silver nitrate solution (Sigma, USA).
Neural differentiation of MSCs
The cells were plated at 1,000 to 2,000 cells/cm2 in complete medium with the addition of 10 ng/ml basic fibroblast growth factor (bFGF; Roche, Switzerland), 10 ng/ml human epidermal growth factor (hEGF; Roche, Switzerland) and 10 ng/ml human neural growth factor (hNGF; Invitrogen, USA) for 14 days. To confirm the expression of neural related antigen, rabbit polyclonal antibodies were used against neuron-specific enolase (NSE; Chemicon, USA) and glial fibrillary acidic protein (GFAP; Chemicon, USA). For the immunocytochemical NSE and GFAP labeling, cells (cord blood passage 2) were rinsed with PBS and then fixed with 3.7% formaldehyde in PBS for 10min at room temperature. They were then treated with ice cold 100% methanol for 10min, 100% acetone for 5min, and then 0.4% Triton X-100 in PBS for 10min with triple PBS rinses between each treatment. The samples were treated with 2% horse serum (Gibco-BRL, USA) and 2% goat serum (Zymed, USA) in PBS containing 4% BSA (PBS/BSA) for 100min at 37℃ to block the non-specific binding of primary antibodies. The antibodies were diluted in PBS/BSA plus 2% horse sera or 2% goat sera at 1 : 200 for NSE and 1 : 200 for GFAP, respectively. The primary antibodies were incubated with the cells for 1 h at 37℃. The samples were rinsed three times with PBS. The following fluorescent secondary antibodies were added concurrently: FITC and TRITC anti-rabbit antibodies (Zymed Laboratories, USA) that were diluted 1 : 200 in PBS/BSA plus 2% horse sera and 2% goat sera, respectively, for 45min at 37℃. The slides were rinsed with PBS and then mounted in Gelvatol (Lab Vision, USA). The fluorescence was visualized using a fluorescent microscope.
Results
Establishment of primary culture
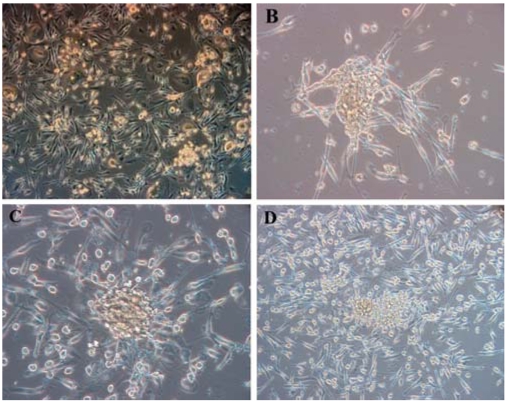
The whole cord blood mononuclear fraction was isolated and then cultured. Attached cells were observed at 5-7 days after the initial plating. The floating cells were removed from the changed medium and then the attached cells were subsequently passaged. Low-glucose medium and an acidic environment facilitated the elimination of the hematopoietic progenitor cells [9]. After 4 weeks of culture, the UCB-derived MSCs were recognizable as adherent cells with a fibroblast-like appearance (Fig. 1).
Fig. 1.
Initially adherent mesenchymal-like cells grew as spindle-shaped or stellate-shaped cells that developed into multi-polar fibroblastoid cells. They gradually reached confluency at about 30 days. A; Primary culture day 14. B & C; Primary culture day 21. D; Primary culture day 30. B, C and D shows cell clusters. A & D: ×100, B & C: ×200.
Characteristics of adherent cells for MSCs culture
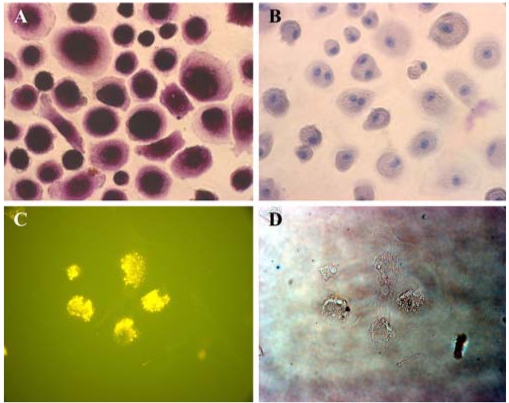
There are 2 types of adherent cells from the UCB: osteoclast-like cells and mesenchymal-like cells. The morphology of the osteoclast-like cells was heterogeneous and elongated or oval/round shape with smooth borders, and in certain cases the cells showed cytoplasmic extensions. These cells were usually in contact with each other; however, the most remarkable feature was the presence of multinucleated cells with nuclei congregated around a central area. These cells were positive for AP activity, but they were negative for PAS (Fig. 3A). Osteoclast-related antigen CD51/61 (vitronectin receptor) was also expressed (Fig. 3C). The initially adherent mesenchymal-like cells grew as spindle-shaped cells, which developed into multi-polar fibroblastoid cells. These cells then gradually reached confluency at about 30 days. Cytochemical analysis demonstrated that the mesenchymal-like cells were positive for PAS (Fig. 2C), but they were negative for AP activity. The cells showed immunophenotypic marker positivity for mesenchym-related antigens SH2 and vimentin (Fig. 2A).
Fig. 3.
Osteoclast-like cells were positive for AP activity, but they were negative for PAS. Osteoclast-related antigen CD51/61 (the vitronectin receptor) was also expressed. A; There was AP staining with the osteoclast-like cells. B; Negative control (counterstained with hematoxylin). C; Immunofluorescence assay for CD51/61 with osteoclast like cells. D; Phase contrast. ×200.
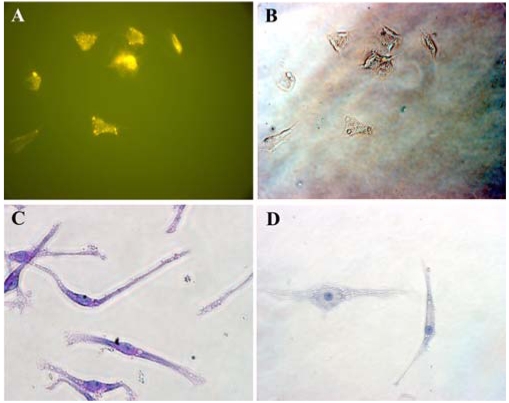
Fig. 2.
Cytochemical analysis shows that the mesenchymal-like cells were positive for PAS, but they were negative for AP activity. The immunophenotyping of these cells showed positivity for mesenchym-related antigen SH2. A; SH-2 (Endoglin) staining with the mesemchymal-like cells. B; Phase contrast image, C; PAS staining with mesenchymal-like cells. D; Negative control (counterstained with hematoxylin). ×200.
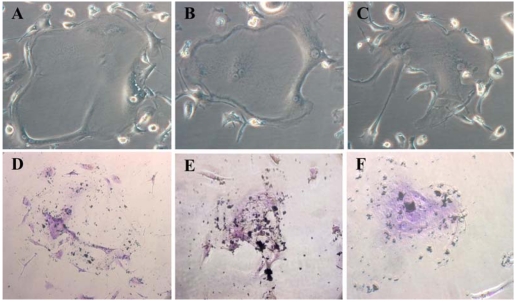
Cord blood MSCs exhibit osteogenic potential
To induce osteogenic differentiation from the MSCs, we used an osteogenic induction medium that consisted of β-glycerol phosphate, ascorbic acid and dexamethasone. The UCB MSCs formed hydroxyapatite, suggesting that MSCs have osteogenic potential (identified by Von-Kossa staining) (Fig. 4). However, unlike bone marrow MSCs, the UCB MSCs did not show cuboidal morphology, which is a typical aspect of osteoblast-enriched cultures derived from bone marrow.
Fig. 4.
Expression of the bone phenotype after exposure of UCB mesenchymal-like cells to differentiation stimuli. A, B & C; UCB MSCs' was morphologically changed (day 14), Phase contrast, ×200. D, E & F; UCB MSCs' osteogenesis as confirmed by calcium accumulation, Von-Kossa staning, ×200.
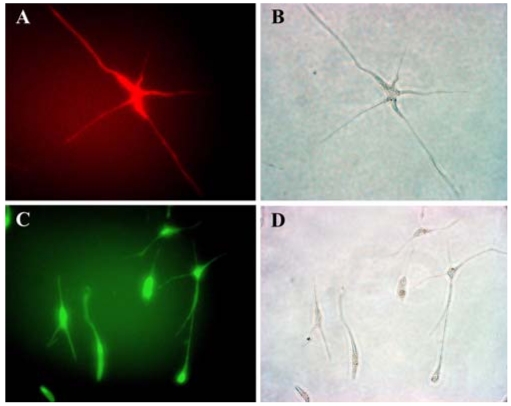
Cord blood MSCs express neural-specific antigens
To evaluate whether cord blood MSCs have an ability to differentiate into neuronal cells, we cultured UCB MSCs in neurogenic medium. When exposed to hEFG/hFGF/hNGF for 2 weeks, the UCB MSCs expressed neural-specific antigen and they showed some morphological features of neural cells such as long multi-polar extensions and branching ends. After neuronal differentiation, the UCB MSCs expressed NSE (Fig. 5A) and GFAP (Fig. 5D), which are cytoskeletal proteins in neurons and astrocytes, respectively. Therefore, the MSCs in human umbilical cord blood can expand in vitro and differentiate into non-mesenchymal cells.
Fig. 5.
Pattern of the expression of neural specific antigens in UCB mssenchymal-like cells. A; Stained with NSE. B; Phase contrast image. C; Stained with GFAP. D; Phase contrast. (×200).
Discussion
The presence of mesenchymal stem/progenitor cells in cord blood has recently been identified [5]. However, these cells are known to poorly proliferate in vitro culture system. Therefore, some researchers are skeptical about the presence of human mesenchymal progenitor cells in umbilical cord blood [20,26]. Despite the fact that bone marrow represents the main available source of MSCs, the use of bone marrow-derived cells is not always acceptable due to the high degree of viral infection and the significant drop in cell number and proliferative/differentiation capacity when the cells age. UCB cells have many advantages because of the immaturity of newborn cells compared with adult cells. In this study, we showed that UCB-derived mononuclear cells, when cultured in medium containing 10% FBS, were able to generate adherent cells. In addition, we were able to induce successful proliferation of mesenchymal-like cells (higher than 80% confluency). However, we found that the nature of adherent cells was not the same for all cases: the adherent cells exhibited either an osteoclast-like or a mesenchymal-like phenotype, and each was characterized by the following features. First, approximately 40% of the cord blood collections gave rise to cultures of adherent cells that displayed the morphology and characteristics of multinucleated osteoclast-like cells. These cells expressed markers for osteoclasts such as a strong tartrate-resistant acid phosphatase activity and the expression of CD51/CD61 [5,23,25]. Second, almost 60% of the cord blood cells gave rise to an adherent layer that was initially formed by individual cells (70-80%) or colonies of cells (20-30%), which rapidly gave rise to a well established layer of fibroblastoid cells, and these cells showed rapid growth. The adherent cells expressed mesenchymal progenitor-related antigens SH2 and vimentin [5]. The MSCs were also positive for PAS, and MSCs are known to express antigens such as SH2, SH3, SH4, ASMA, MAB 1470, CD13, CD29, CD49e and CD54 [1,3,4,19]. Our object for this study was to focus on whether mesenchymal cell can be propagated in vitro and then purified into 2 types of cells (mesenchymal cell & osteoclast cell). Therefore, in order to purify MSCs, we tried to isolate the MSCs from osteoclasts because there is the time difference to become detached between the MSCs and osteoclasts during the subculture, but these two types of cells could not be clearly isolated. A further study is needed to clearly exclude the osteoclasts from MSCs. Lee et al. [15] also tried negative selection to sort the MSCs according to the negative lineage markers (CD3, CD7, CD19, CD38 and glycophorin-A), yet the resulting cell population was still heterogeneous. Therefore, further investigations should be conducted on refining methods to purify and expand MSCs from UCB. Nevertheless, we obtained several findings from the present study. First of all, we got a high ratio of MSCs among the attached cells from much fresher UCB samples, and this suggest that this factor is more important than the ratio gap of MSCs between the preterm samples and full-term samples. Second, we also tried to sort mesenchymal cells via a Magnetic Cell Separation System (MACS; Miltenyi Biotec GmbH, Germany). Even though the number of mesenchymal cells was much higher than the number of osteoclast cells after MACS sorting, the proliferation of mesenchymal cells was inhibited because the osteoclast cells grew faster than the mesenchymal cells, indicating that a high number of osteoclast cells can inhibit the proliferation of mesenchymal cells (data not shown). Therefore, in a further study, we need to find media that can inhibit osteoclast cell proliferation with not affecting the proliferation of mesenchymal cells after performing selection with the right markers for mesenchymal cells. This is a key to isolating MSCs from UCB and to maintain their good condition for MSCs proliferation. However, in present study, we used FBS for our research, and we need to find methods to expand MSCs ex vivo without using FBS for clinical use and performing cell therapy. Lee et al. [15] also used the same culturing method as ours in their study. We also demonstrated that MSCs are capable of differentiating with bone and neural features in vitro. In the presence of dexamethasone, β-glycerol phosphate and ascorbate, the MSCs expressed bone cell traits such as formation of mineralizing colonies [3,12]. Moreover, MSCs exposed to a defined neurogenic medium for 2 week were shown to have some features of neural cells in culture, such as long multi-polar extensions and branching ends that stained positive with NSE for the neurons, and they stained positive for GFAP in the astrocytes. The immunophenotype and functional properties displayed by cord-blood-derived MSCs very closely resembled the characteristics observed for bone marrow-derived mesenchymal progenitor cells [3,6,19]. Yet in order to get more evidence on the multipotency of mesenchymal stem cells in human cord blood, the gene expression for specific markers of neuronal or osteogenic cells should be investigated. It has been shown that mature osteoclast or their progenitors are circulating in umbilical cord blood [5,21]. The presence of mesenchymal progenitor cells in cord blood is rational because it can be hypothesized that both hematopoietic and mesenchymal progenitors are traveling, via cord blood, from early fetal haematopoietic sites to the newly formed bone marrow [18,24]. In this study, despite the relatively low number of harvested UCB cells that could be developed into MSCs, like was also shown by other researchers, our results suggest that cord blood might be rich in mesenchymal progenitors, the same as the case for haematopoietic progenitors [22,27]. It was thought that the time from sampling to primary culture, the proper technique and the maintenance temperature may be important factors to collect MSCs. Based on their large ex vivo expansion capacity, as well as on their differentiation potential, cord blood-derived mesenchymal cells may be an attractive cell source for cellular or gene transfer therapy for treating such incurable disease as neuro-degenerative disease.
Acknowledgments
This work was supported by the Seoul R&BD Program (10548) and the Brain Korea 21 Program for Veterinary Science.
References
- 1.Bendall LJ, Kortlepel K, Gottlieb DJ. Human acute myeloid leukemia cells bind to bone marrow stroma via a combination of beta-1 and beta-2 integrin mechanisms. Blood. 1993;82:3125–3132. [PubMed] [Google Scholar]
- 2.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 5.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 6.Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82:66–76. [PubMed] [Google Scholar]
- 7.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, Cooper S, English D, Kurtzberg J, Bard J, Boyse EA. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, Fernandez M, Chastang C Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7:581–588. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez-Rodriguez M, Reyes-Maldonado E, Mayani H. Characterization of the adherent cells developed in Dexter-type long-term cultures from human umbilical cord blood. Stem Cells. 2000;18:46–52. doi: 10.1634/stemcells.18-1-46. [DOI] [PubMed] [Google Scholar]
- 11.Han IS, Ra JS, Kim MW, Lee EA, Jun HY, Park SK, Kwon BS. Differentiation of CD34+ cells from human cord blood and murine bone marrow is suppressed by C6 beta-chemokines. Mol Cells. 2003;15:176–180. [PubMed] [Google Scholar]
- 12.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 13.Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, Yasumizu T, Teraoka H. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217–227. doi: 10.1634/stemcells.21-2-217. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Koh SK, Song SU, Shin SH, Choi GS, Kim WC, Lee MH, Seoh JY, Park SK, Fraser JK. Ex vivo expansion and clonality of CD34+ selected cells from bone marrow and cord blood in a serum-free media. Mol Cells. 2002;14:367–373. [PubMed] [Google Scholar]
- 15.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 16.Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099–1100. [PubMed] [Google Scholar]
- 17.Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48:913–927. doi: 10.1002/(sici)1097-4636(1999)48:6<913::aid-jbm22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Peault B. Hematopoietic stem cell emergence in embryonic life: developmental hematology revisited. J Hematother. 1996;5:369–378. doi: 10.1089/scd.1.1996.5.369. [DOI] [PubMed] [Google Scholar]
- 19.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 21.Roux S, Quinn J, Pichaud F, Orcel P, Chastre E, Jullienne A, De Vernejoul MC. Human cord blood monocytes undergo terminal osteoclast differentiation in vitro in the presence of culture medium conditioned by giant cell tumor of bone. J Cell Physiol. 1996;168:489–498. doi: 10.1002/(SICI)1097-4652(199609)168:3<489::AID-JCP1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Shields LE, Andrews RG. Gestational age changes in circulating CD34+ hematopoietic stem/progenitor cells in fetal cord blood. Am J Obstet Gynecol. 1998;178:931–937. doi: 10.1016/s0002-9378(98)70526-5. [DOI] [PubMed] [Google Scholar]
- 23.Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 24.Tavassoli M, Minguell JJ. Homing of hemopoietic progenitor cells to the marrow. Proc Soc Exp Biol Med. 1991;196:367–373. doi: 10.3181/00379727-196-43201. [DOI] [PubMed] [Google Scholar]
- 25.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 27.Wyrsch A, dalle Carbonare V, Jansen W, Chklovskaia E, Nissen C, Surbek D, Holzgreve W, Tichelli A, Wodnar-Filipowicz A. Umbilical cord blood from preterm human fetuses is rich in committed and primitive hematopoietic progenitors with high proliferative and self-renewal capacity. Exp Hematol. 1999;27:1338–1345. doi: 10.1016/s0301-472x(99)00059-4. [DOI] [PubMed] [Google Scholar]