Abstract
Objective
Lipopolysaccharide or endotoxin constitutes most part of the outer portion of the cell wall in the gram negative bacteria. Sub-clinical endotoxemia could contribute to increased inflammation and mortality in hemodialysis patients. Endotoxin level and clinical effect are determined by its soluble receptor sCD14 and high density lipoprotein. We examine the hypothesis that endotoxin level correlates with mortality.
Methods
In this cohort study, endotoxin levels were measured in 306 long-term hemodialysis patients who were then followed for up to 42 months. Soluble CD14 and cytokines levels were also measured.
Results
The mean (±SD) endotoxin level was 2.31±3.10 EU/ml (min: 0.26 EU/ml, max: 22.94 EU/ml, inter-quartile range: 1.33EU/ml, median: 1.27EU/ml). Endotoxin correlated with C-reactive protein (r = 0.11, p<0.04). On multivariate logistic regression analysis, high body mass index (BMI) and low HDL cholesterol levels were associated with higher endotoxinemia (endotoxin below or above of median). In multivariable Cox regression analysis adjusted for case-mix and nutritional/inflammatory confounders, endotoxin levels in the 3rd quartile vs. 1st quartile was associated with a trend towards increased hazard ratio (HR) for death (HR 1.83, 95% confidence interval: 0.93–3.6, p=0.08).
Conclusions
In this hemodialysis cohort, we found associations between endotoxinemia and CRP, body composition and HDL. A moderately high endotoxin levels tended to correlate with increased mortality than the highest circulating endotoxin level. Additional studies are required to asses the effect of endotoxemia on mortality in dialysis population.
Keywords: Chronic Kidney Disease (CKD), hemodialysis, nutritional status, inflammation, endotoxin, cytokines
Introduction
End Stage Renal Disease (ESRD) patients have increased morbidity and mortality compared to the general population. Infection is the second most important cause of the increased mortality seen in these ESRD patients (1). More than 75% of deaths in these patients is as a result of septicemia (2). The incidence rate of bacterial infections in ESRD patients is one episode per 100 patient months (3, 4). These bacterial infections are often life threatening given the increased susceptibility of uremic patients to infection due to their immune dysfunction(5). While Staphylococcus aureus is the major pathogenic organism (4) responsible for infections in dialysis patients, it has been found that endotoxemia due to gram-negative organisms is also a potential source of inflammation in these ESRD patients.(6)
The Endotoxins (lipopolysaccharide in the outer wall of gram negative bacteria) can generate a complex host response through signaling pathways initiated after attachment of lipopolysaccharide (LPS) to the CD14 antigen on effector cells (7). Initiation of this complex response occurs after binding of the lipopolysaccharide to the lipolysaccharide binding protein (LBP) through a lipid A moiety.(8).CD14 is then activated by the LPS-LBP complex which leads to the activation of the cellular immune complex (9). ESRD patients are exposed to higher levels of endotoxin due to: 1) bacteriolysis in patients suffering from gram-negative sepsis caused by bactericidal systemic antibiotics which release a high volume of endotoxin (10, 11), 2) entry of endotoxin through the intestinal mucosal epithelial by bacterial translocation (12),and 3) potential use of non-ultra pure dialysate for the dialysis (13). Nevertheless, a recent study found that endotoxemia is associated with better survival in peritoneal dialysis patients.(14)
Given that ESRD patients have higher baseline levels of inflammatory markers, (15) we hypothesized that there is a relationship between endotoxin levels and inflammation in these maintenance dialysis patients. In addition, we wanted to examine the impact of the proposed relationship on the nutritional status and mortality in these ESRD patients.
Methods
Patient Population
We studied a population of hemodialysis (HD) patients who were part of the Nutritional and Inflammatory Evaluation in Dialysis (NIED) study (16).The original NIED cohort consisted of more than 3000 MHD outpatients followed for 6 years in eight (8) DaVita maintenance dialysis clinics in Southern CA.(see the NIED study Website at www.Niedstudy.org for more details as well as previous publications (17–21)). To be included in the study, patients had to be at least 18 years old and on outpatient hemodialysis for at least 8 weeks. Patients were excluded if they had an acute infection or had a life expectancy of less than 6 months. The study was approved by the IRB and all subjects gave informed consent prior to being enrolled in the study. A total of 893 long term HD patients were randomly invited and agreed to participate in the NIED study. Out of these subjects, 310 also agreed to undergo an additional substudy including measurement of endotoxin, which led to 306 subjects with endotoxin data, since samples on 4 subjects had top be discarded for contamination. The medical record was thoroughly reviewed for each subject by a collaborating physician in the study. Information such as underlying kidney disease, cardiovascular disease history and other co-morbid illnesses was abstracted. A modified version of the Charlton Co-morbidity Index (i.e. excluding the age and kidney disease components) was used to assess severity of co-morbidities (22, 23). The 306 HD patients were followed for a total of 42 months (March 2004 - September 30, 2007).
Anthropometric and Body Composition Measures
Body weight and anthropometric measurements were performed while patients were on HD or within 5–20 minutes after termination of their hemodialysis treatment. Biceps and triceps skin fold thickness was measured by standard technique using the conventional skin fold caliper (24, 25).
Near Infrared Interactance
To estimate percentage body fat and fat free body mass, near infrared (NIR) interactance was measured at the same time as the anthropometric measurements (26). A commercial NIR interactance sensor with a coefficient of variation of 0.5% for total body fat measurement (portable Furtex 6100; Furtex, Inc, Rockville, VA; www.furtex.com) was used. NIR measurements were performed by placing a Furtex sensor on the upper arm (free of vascular access) for several seconds and entering the required data (data of birth, sex, weight, and height) for each patient. NIR measurements of body fat appear to correlate significantly with other nutritional measures in HD patients (27).
Endotoxin Measurement
We used quantitative Chromogenic Limulus Amebocyte Lysate (LAL) test for endotoxins in plasma (both free and protein-bound forms) using a commercially available kit (QCL-1000, Cambrex bioscience Inc, Walkersville, MD). The minimum detectable level of endotoxin is 0.1 EU/ml.
Other Laboratory Tests
Pre-dialysis and post-dialysis blood samples were obtained on a mid-week day that coincided with the day that the required quarterly blood tests were done at the DaVita dialysis facilities. Single pooled Kt/V was used to represent the weekly dialysis dose. All laboratory studies were performed by DaVita Laboratories (Deland, FL) using automated methods. Serum high sensitivity C-reactive protein (CRP) was measured using a turbidometric immunoassay (WPCI, Osaka, Japan; normal range<3.0mg/L) (28, 29). Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) levels were measured with using immunoassay kits (R&D Systems, Minneapolis, Minn., USA; units: pg/ml; normal range: IL-6: < 9.9 pg/ml, TNF- α : < 4.7 pg/ml) (30, 31). The C-reactive protein (CRP), TNF-α and IL-6 levels were measured in the General Clinical Research Center Laboratories at Harbor UCLA. Serum Transthyretin (pre-albumin) was measured by immunoprecipitation and the plasma homocysteine concentration was measured by high performance liquid chromatography (HPLC) in the Harbor-UCLA Clinical Laboratories.
Statistical Methods
Pearson’s correlation coefficient(r) was used for analyses of linear associations. Multivariate logistic regression analysis was performed to obtain adjusted p-values controlled for case-mix and other covariates. Death hazard ratios (HRs) were obtained using Cox proportional hazard models controlling for the relevant covariates.
We performed incremental levels of multivariate adjustment where: (A) Case-mix variables including age, gender, race (African-American), diabetes mellitus, and dialysis vintage were included. (B) Malnutrition-inflammation complex syndrome (MICS) variables included such as albumin, creatinine, hemoglobin, total iron binding capacity, normalized protein catabolic rate, white blood count and; normalized protein catabolic rate (nPCR) [also known as normalized protein nitrogen appearance (nPNA)]; and body mass index. (C) Additional adjustment was done for three inflammatory markers (CRP, IL-6, and TNFα) in a fully adjusted Cox regression model.
We expected significant confounding in the unadjusted models where relevant confounders such as age and gender were not taken into account. In fact, while the results from the adjusted models may have been over-adjusted (possibly due to inclusion of biological intermediates that are along the causal pathway from predictor to outcome variable), we make our inferences based on models adjusted for case-mix. Because of uncertainty regarding which final model is in fact the most parsimonious, we include 3 levels of adjustment in the presented data so that the full spectrum of results can be appreciated. The data analysis was done using STATA version 11.1 (STATA Corporation, College Station, TX).
Results
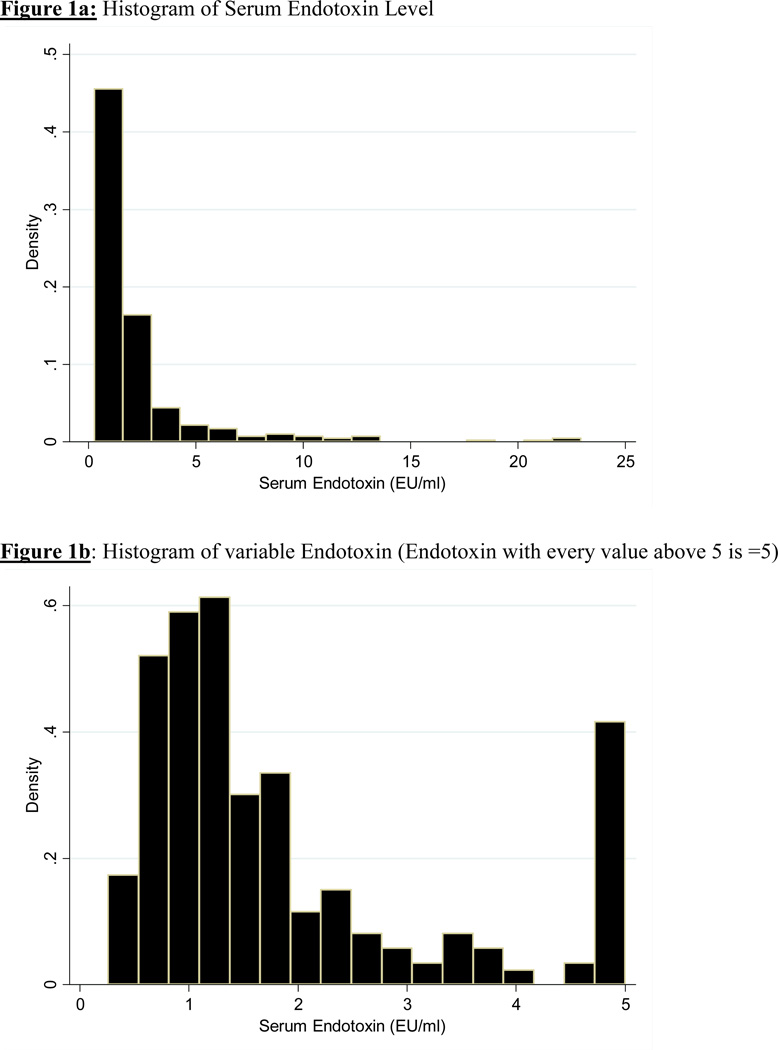
The mean (±SD) endotoxin level was 2.31±3.10 EU/ml (min: 0.26 EU/ml, max: 22.94 EU/ml, inter-quartile range: 1.33 EU/ml, median: 1.27 EU/ml). Baseline demographic, clinical, and laboratory values in the 306 MHD patients studied are shown in Table 1. The patients mean age (±SD) was 55±15 years; 48% of patients were women (n=149), 30% (n=92) were African-American and 57% were diabetic. The dialysis vintage was 50±35 months (median + inter-quartile range: 45+ 44 months). Mean endotoxin level was 2.3±3.1 EU/ml (median: 1.27 EU/ml). Figure 1 shows the distribution of endotoxin levels. After ranking subjects according to their serum endotoxin level, we categorized them into quartiles with 75–77 patients in each group. Table 1 lists relevant demographic, clinical and laboratory measures across quartiles of endotoxin levels. Older patients were more likely to be in the higher quartiles of endotoxin levels. No other significant trend was seen in the demographic, physical or biochemical variables as it relates to increasing quartile of endotoxin levels.
Table 1.
Baseline Demographic, Clinical and Laboratory Values in Total and According to Quartiles of Endotoxin in 306 Maintenance Hemodialysis Patients.
| Variable | Quartile 1 N=77 (0.26–0.90) |
Quartile 2 N=76 (0.91–1.26) |
Quartile 3 N=77 (1.28–2.23) |
Quartile 4 N=76 (>=2.24) |
p-for- trend |
|---|---|---|---|---|---|
| Demographic | |||||
| Age(yr) | 52.2±16.4 | 53.5±14.9 | 57.3±13.5 | 58.1±13.3 | 0.03 |
| Women (%) | 31(40%) | 41(54%) | 36(47%) | 39(51%) | 0.35 |
| Race(%African American) | 20(26%) | 31(41%) | 20(26%) | 21(28%) | 0.13 |
| Diabetes Mellitus | 42(55%) | 37(49%) | 46(60%) | 49(65%) | 0.20 |
| Modified Charlson comorbidity | 1.7±1.7 | 2.0±1.7 | 1.9±1.5 | 1.7±1.2 | 0.42 |
| Score | |||||
| Crude Mortality Rate* | 16(21%) | 17(22%) | 21(27%) | 17(22%) | 0.79 |
| Primary Insurance(%Medicare) | 25(40%) | 37(60%) | 32(52%) | 35(57%) | 0.14 |
| Body Composition | |||||
| Body Mass Index(kg/m2) | 25.5±6.7 | 25.3±6.3 | 26.5±5.8 | 26.9±6.0 | 0.29 |
| Triceps Skinfold (mm) | 16.2±10.6 | 17.9±11.3 | 15.9±7.9 | 18.0± 10.1 | 0.48 |
| Biceps Skinfold (mm) | 8.9±7.9 | 9.6±8.0 | 8.7±5.6 | 9.7±7.0 | 0.81 |
| Midarm Circumfrance (cm) | 30.5±6.4 | 30.4±5.7 | 30.8 ±5.6 | 31.4±5.89 | 0.77 |
| Hemodialysis Treatment | |||||
| Dialysis Vintage (mo) | 43.0±28.6 | 59.0±38.4 | 49.8± 32.7 | 48.4± 38.5 | 0.04 |
| Dialysis Dose(single-pool Kt/V) | 1.75± 0.31 | 1.68 ±0.25 | 1.70 ±0.32 | 1.65±0.22 | 0.19 |
| nPCR(g/kg/d) | 1.13 ±0.28 | 1.03 ±0.19 | 1.06 ±0.23 | 1.11 ±0.27 | 0.08 |
| Erythropoietin dose(1000 U/wk) | 14.9±12.6 | 17.5±17.7 | 11.7± 96.5 | 13.1± 15.9 | 0.09 |
| Biochemical Measurement | |||||
| Serum endotoxin | 0.67±0.15 | 1.10±0.11 | 1.67±0.25 | 5.82±4.67 | <0.01 |
| soluble CD14 (ug/ml) | 7.2±2.2 | 7.5±3.0 | 7.5±2.3 | 6.8±2.1 | 0.21 |
| lbumin(g/dl) | 4.0± 0.4 | 4.0±0.40 | 4.0±0.3 | 3.9±0.3 | 0.27 |
| Prealbumin (mg/dl) | 29.7±9.2 | 28.9±8.3 | 30.0±9.1 | 28.5±7.4 | 0.71 |
| creatinine(mg/dl) | 10.4±3.0 | 10.1±2.7 | 9.3± 2.5 | 9.9±2.6 | 0.13 |
| TIBC (mg/dl) | 205.4±35.5 | 201.9±33.5 | 206.1± 36.8 | 209.6± 35.8 | 0.61 |
| Calcium(mg/dl) | 9.5±0.6 | 9.7±0.6 | 9.5±0.6 | 9.7±0.6 | 0.26 |
| Phosphorus(mg/dl) | 5.6±1.4 | 5.4±1.4 | 5.4±1.3 | 5.4±1.3 | 0.14 |
| Alkaline Phos (u/l) | 124.8±71.9 | 134.8±63.6 | 131.5±100 | 116.3 ±70.5 | 0.48 |
| Ferritin (ng/ml) | 656.7 ±427.6 | 634.6±350.6 | 612.4± 351.5 | 604.5± 383.5 | 0.84 |
| Total Homocysteine(umol/l) | 24.7±9.5 | 26.2±7.94 | 25.2± 6.6 | 26.5 ±8.2 | 0.54 |
| LDL cholesterol(mg/dl) | 74.7±27.9 | 80.8±36.1 | 80.9± 30.3 | 80.6± 35.0 | 0.62 |
| HDL Cholesterol(mg/dl) | 38.1±14.4 | 38.3±13.7 | 34.4 ±11.4 | 35.7± 11.4 | 0.22 |
| Total Cholesterol(mg/dl) | 152.0±31.2 | 166.9± 61.6 | 171.2± 57.8 | 149.8 ±34.9 | 0.55 |
| Triglycerides(mg/dl) | 134.3±88.5 | 161.2±154.1 | 155.4 ±88.6 | 160.9 ±155.6 | 0.56 |
| C-reactive Protein(mg/dl) | 4.2±5.3 | 5.1±5.6 | 5.2±6.0 | 5.4± 5.6 | 0.62 |
| Interleukin 6(pg/ml) | 12.4±19.1 | 10.5±10.2 | 9.1±11.1 | 10.2 ±12.4 | 0.52 |
| Tumor Necrosis Factor(pg/ml) | 2.5±0.9 | 2.6±1.3 | 2.5± 0.85 | 2.4±0.9 | 0.59 |
| Blood Hemoglobin(g/dl) | 12.0±0.9 | 12.0±0.9 | 12.3± 0.9 | 12.1±0.8 | 0.40 |
| White blood Cells(103 cells/ul) | 7.0±2.4 | 6.8±2.2 | 6.8±1.7 | 6.8±1.6 | 0.84 |
Note: Values expressed as mean SD or percentage. P values for dialysis dose (vintage),ferritin level, C-reactive protein level, interleukin 6 level, and tumor necrosis factor α level are based on the logarithmic values of these measures. Conversion factors for units: albumin in g/dl to g/L,x10;creatinine in mg.dl to umol/L,x88.4;calcium in mg/dl to mmol/L,x0.2495;phosphorus in mg/dl to mmol/L,x0.3229; homocysteine in umol to mg/L,x0.1352;total,low density lipoprotein, and high-density lipoprotein cholesterol in mg.dl to mmol/L,x0.2586;triglycerides in mg/dl to mmol/L,x0.01129;hemoglobin in g/dl to g/L,x10.Ferritin in ng/ml and ug/L and white blood cell count in 103 /ul and 109/l require no coversion.
Abbreviations: Kt/V, dialysis dose; nPCR, normalized Protein Catabolic rate
Mortality pertains to a maximum of 33 months.
LDL: Low density lipoprotein
HDL: High Density Lipoprotein
TIBC: Total Iron Binding Capacity
Figure 1.
a: Histogram of Serum Endotoxin Level
b: Histogram of variable Endotoxin (Endotoxin with every value above 5 is =5)
Factors Correlated with Endotoxin Level
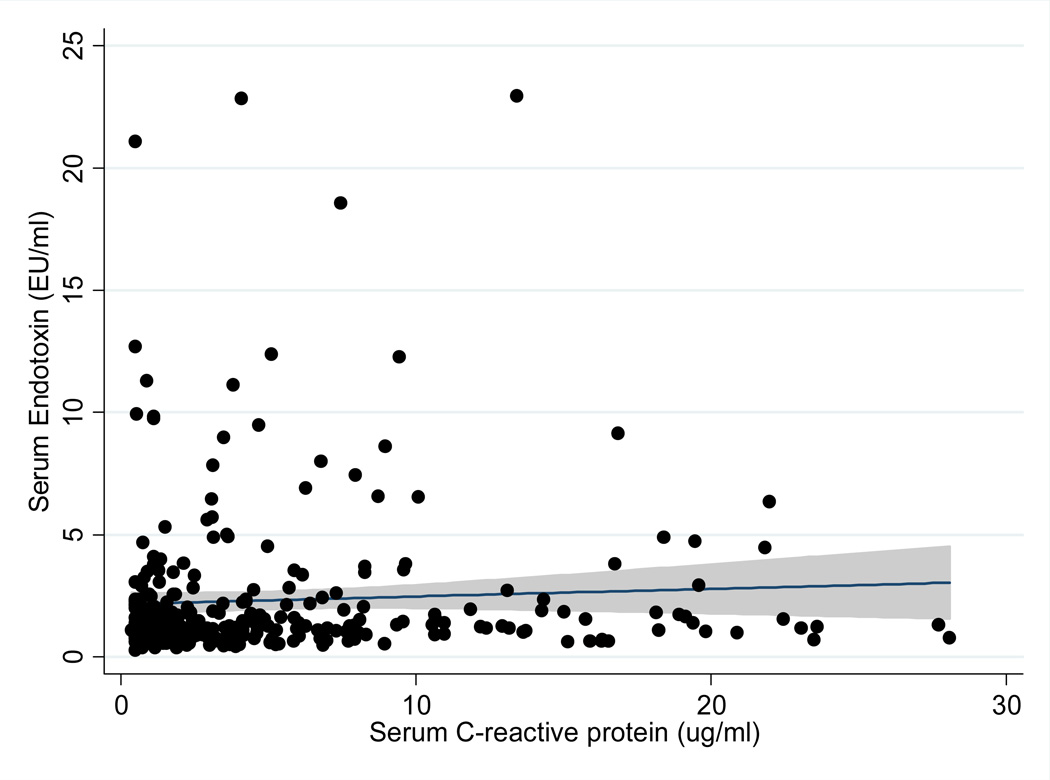
Table 2 shows the unadjusted and adjusted correlations between Endotoxin levels and relevant nutritional, inflammatory and biochemical variables. There was a statistically significant correlation between endotoxin level and CRP level (p-value <0.05) after adjustment for relevant covariates. This positive correlation was further supported by the scatter plot shown in figure 2 (r=0.11, P<0.05) No correlation was seen between the other markers of inflammation and Endotoxin level. We defined the endotoxin level is equal 5 µg/ml when it was more than 5 µg/ml in our further analyses. Table 3 shows the results of univariate (unadjusted) and multivariate logistic regression analysis of the association of the variables of interest and Endotoxin level. Body mass Index and HDL cholesterol level were associated with endotoxin levels after multivariate logistic regression analysis. There was a 5% increased odds of higher endotoxin levels for each 1kg/m2 increase in body mass index. There was a 3% decrease in endotoxin levels for each 1mg/dl decrease in HDL cholesterol level.
Table 2.
Bivariate (unadjusted) and partial (adjusted) correlation coefficients between soluble endotoxin and relevant variables in 306 maintenance hemodialysis patients.
| Variable | Pearson Correlation Co-efficient |
P | Adjusted Correlation Co-efficient* |
P |
|---|---|---|---|---|
| Age | 0.04 | 0.54 | −0.05 | 0.44 |
| Dialysis Vintage (log Scale) | −0.03 | 0.56 | 0.00 | 0.94 |
| Body Mass Index | 0.05 | 0.34 | 0.03 | 0.57 |
| Normalized Protein | 0.09 | 0.12 | 0.07 | 0.26 |
| Catabolic Rate | ||||
| Calcium | 0.02 | 0.79 | 0.04 | 0.55 |
| Phosphorus | −0.06 | 0.32 | 0.05 | 0.39 |
| Intact PTH (logScale) | 0.03 | 0.61 | 0.05 | 0.35 |
| Alkaline | 0.04 | 0.54 | 0.06 | 0.32 |
| Phosphatase | ||||
| Albumin | 0.05 | 0.42 | 0.05 | 0.40 |
| Pre-Albumin | −0.01 | 0.89 | −0.01 | 0.87 |
| TIBC | 0.01 | 0.87 | −0.03 | 0.62 |
| Ferritin | 0.05 | 0.37 | 0.05 | 0.41 |
| Creatinin | −0.02 | 0.73 | 0.01 | 0.89 |
| IL6(LogScale) | −0.04 | 0.46 | −0.06 | 0.32 |
| TNF(Log Scale) | −0.02 | 0.75 | −0.01 | 0.88 |
| CRP (log Scale) | 0.07 | 0.2 | 0.11# | 0.04 |
| LDL | −0.02 | 0.74 | 0.00 | 0.99 |
| HDL | −0.06 | 0.30 | −0.06 | 0.30 |
| Cholesterol | −0.05 | 0.70 | −0.05 | 0.73 |
| Triglycerides | −0.04 | 0.54 | −0.05 | 0.47 |
| Blood hemoglobin | −0.01 | 0.89 | −0.02 | 0.68 |
| White blood cells | −0.01 | 0.84 | −0.04 | 0.45 |
| Percentage | 0.01 | 0.84 | 0.04 | 0.55 |
| lymphocytes | ||||
| Zemplar | 0.02 | 0.74 | 0.05 | 0.42 |
| Ktv | −0.02 | 0.70 | −0.03 | 0.66 |
| Precalcitonin | −0.10 | 0.30 | −0.15 | 0.15 |
| CD14 (ug/ml) | −0.04 | 0.50 | −0.00 | 0.98 |
In adjusted analysis age, sex, diabetes, log Interleukin-6, log TNF alpha, log Vintage were included as covariates.
Bold values have significant P-value.
Figure 2.
Scatter Plot, Regression Line, and 95% confidence intervals reflecting correlation between serum levels of endotoxin and value of serum CRP.
Table 3.
Linear logistic regression estimated odds ratios for endotoxinemia (endotoxin level above vs. below of median) in 306 maintenance hemodialysis patients
| Variable | Logistic Regression | P | Adjusted Logistic Regression* |
P |
|---|---|---|---|---|
| Age(each 10 year increase in age) | 1.02 (1.01–1.04) | 0.004 | 1.02 (1.01–1.04) | 0.10 |
| Gender (women vs. men) | 0.92 (0.59–1.45) | 0.73 | 1.13 (0.68–1.88) | 0.64 |
| Dialysis Vintage(log Scale) (each 1 month unit increase) | 0.93 (0.72–1.20) | 0.59 | 1.12 (0.82–1.52) | 0.45 |
| Body Mass Index (each 1 kg/m2 increase) | 1.04 (1.0–1.08) | 0.07 | 1.05# (1.01–1.10) | 0.04 |
| Normalized Protein Catabolic Rate (each 1 g/kg/d unit increase) | 1.03 (0.41–2.59) | 0.95 | 1.06 (0.36–3.09) | 0.92 |
| Calcium (each 1 mg/dl unit increase) | 1.05 (0.73–1.52) | 0.79 | 1.40 (0.87–2.10) | 0.18 |
| Phosphorus (each 1 mg/dl unit increase) | 0.90 (0.76–1.07) | 0.25 | 0.95 (0.78–1.16) | 0.63 |
| Alkaline Phosphatase (each 1 mg/dl unit increase) | 1.0 (1.0–1.0) | 0.52 | 1.00 (1.00–1.01) | 0.87 |
| Albumin (each 1 mg/dl unit increase) | 0.92 (0.48–1.74) | 0.80 | 0.92 (0.40–2.08) | 0.83 |
| Pre-albumin (each 1 mg/dl unit increase) | 1.0 (0.97–1.03) | 0.96 | 1.00 (0.97–1.04) | 0.78 |
| TIBC (each 1 mg/dl unit increase) | 1.0 (1.0–1.0) | 0.30 | 1.0 (1.0–1.0) | 0.55 |
| Ferritin (each 1 mg/dl unit increase) | 1.0 (1.0–1.0) | 0.4 | 1.0 (1.0–1.0) | 0.40 |
| Creatinine (each 1 mg/dl unit increase) | 0.92 (0.86–1.0) | 0.06 | 0.92 (0.81–1.04) | 0.17 |
| IL6 (log Scale) (each 1 mg/dl unit increase) | 0.88 (0.68–1.14) | 0.35 | 0.71 (0.51–1.00) | 0.05 |
| TNF (log Scale) (each 1 mg/dl unit increase) | 0.79 (0.42–1.47) | 0.45 | 0.92 (0.46–1.85) | 0.82 |
| CRP (log Scale) (each 1 mg/dl unit increase) | 1.07 (0.88–1.31) | 0.5 | 1.12 (0.87–1.4) | 0.39 |
| LDL (each 1 mg/dl unit increase) | 1.0 (1.0–1.01) | 0.43 | 1.0 (1.0–1.0) | 0.93 |
| HDL (each 1 mg/dl unit increase) | 0.98 (0.96–1.0) | 0.05 | 0.97 (0.95–1.0) | 0.02 |
| Cholesterol (each 1 mg/dl unit increase) | 1.0 (0.99–1.0) | 0.98 | 1.0 (0.98–1.01) | 0.66 |
| Blood hemoglobin (each 1 mg/dl unit increase) | 1.2 (0.94–1.61) | 0.13 | 1.2 (0.92–1.65) | 0.16 |
In adjusted analysis age, gender, diabetes, albumin, body mass index, creatinine, hemoglobin, total ironbinding capacity, normalized protein catabolic rate and logarithm of 3 inflammatory markers. Log interleukin6, log tumor necrosis factor alpha, log C-reactive protein were included as covariates
Serum Endotoxin and Survival
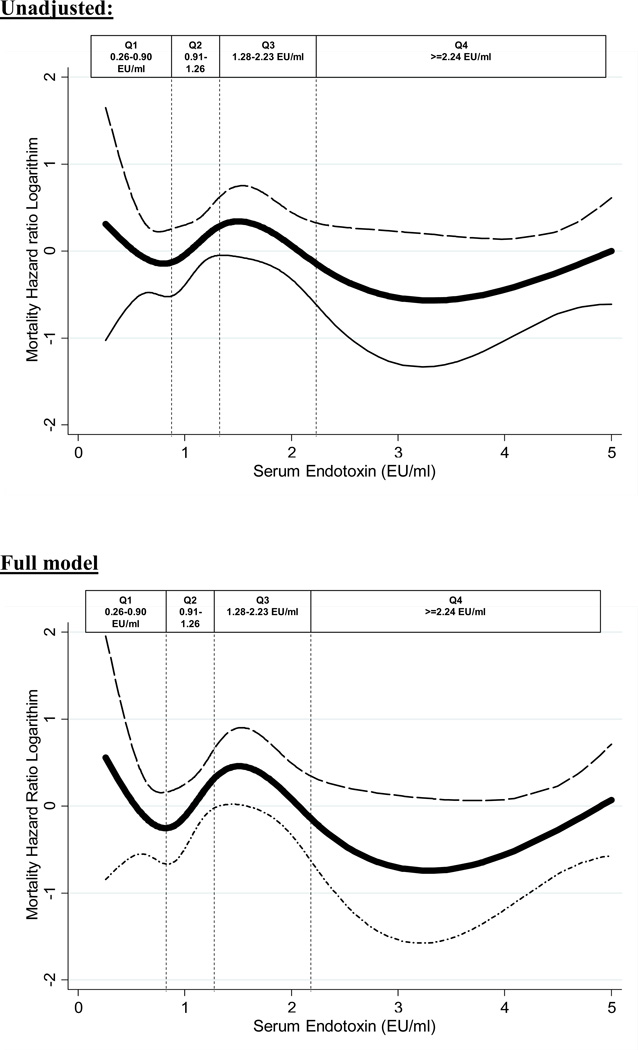
During the 42 months follow-up, 58(20%) subjects died, 33(11%) received a renal transplant and 26(8%) were lost to follow up. The hazard ratio for mortality is shown in Table 4. Hazard ratio for mortality was not significant across the quartiles of increasing endotoxin levels. However, there was a trend towards the 3rd quartile of endotoxin levels (1.28–2.23 EU/ml) being associated with an 83% increase in mortality [i.e. HR 1.83 (0.93, 3.60), p-value 0.08]. Cubic spline plots shown in Figure 3 further illustrate the nature of the relationships shown in Table 4.
Table 4.
Hazard Ratio of 33 month mortality according to quartiles of Endotoxin in 306 maintenance hemodialysis patients.
| Endotoxin Quartiles | Q1 (n=77) HR(%CI) (0.26–0.90) |
Q2 (n=76) HR(%CI) (0.91–1.26) |
Q3 (n=76) HR(%CI) (1.28–2.23) |
Q4 (n=77) HR(%CI) (>=2.24) |
|---|---|---|---|---|
| Unadjusted | 0.97 (0.49–1.92) P=0.09 |
1(Reference) | 1.40 (0.75–2.7) P=0.28 |
0.89 (0.45–1.75) P=0.73 |
| Case-mix* | 1.16 (0.58–2.3) P=0.67 |
1(Reference) | 1.60 (0.58–2.3) P=0.2 |
0.88 (0.44–1.76) P=0.73 |
| Previous + MICS# | 1.07 (0.51–2.22) P=0.85 |
1(Reference) | 1.64 (0.84–3.2) P=0.14 |
0.82 (0.40–1.69) P=0.60 |
| Previous + inflammation†(full mode) | 1.07 (0.50–2.28) P=0.86 |
1(Reference) |
1.83 (0.93–3.6) P=0.08 |
0.84 (0.40–1.75) P=0.64 |
Abbreviation: CI, confidence interval; HR, Hazard Ratio; MICs, Malnutrition-inflammation-cachexia syndrome.
Case-Mix variables includes age, sex, race/ethnicity, diabetes, and log vintage
MICS variables includes values for albumin, creatinine, hemoglobin, total iron-binding capacity, normalized protein catabolic rate, and body mass index.
Full Model consists of case mix and MICS and logarithm of 3 inflammatory markers: C-reactive protein, interleukin 6, and tumor necrosis factor-α
Figure 3.
Cubic Spline exhibiting the association between Serum Endotoxin (adjusted variable with >5 =5) level and mortality level in 306 MHD patients.
Discussion
In 306 maintenance hemodialysis patients, we found that circulating endotoxin level was associated with higher CRP levels and BMI but lower HDL level. Whereas we did not find an incremental association between elevated circulating endotoxin levels and mortality in maintenance hemodialysis patients, we did find that moderately high (3rd quartile) but not the highest (4th quartile) circulating endotoxin levels (endotoxin greater than 1.265 EU/ml and less than 2.237 EU/ml) tended to be associated with increasing mortality (83% higher) compared to mortality in the lowest quartile of endotoxin levels. Though this trend was not statistically significant, it appeared robust when adjustment was made for case-mix and other nutritional and inflammatory measures, including serum IL-6 and TNF-a. A recent study by McIntyre et al has shown significant association between higher circulating level of endotoxin with higher mortality rate in hemodialysis patients (32).
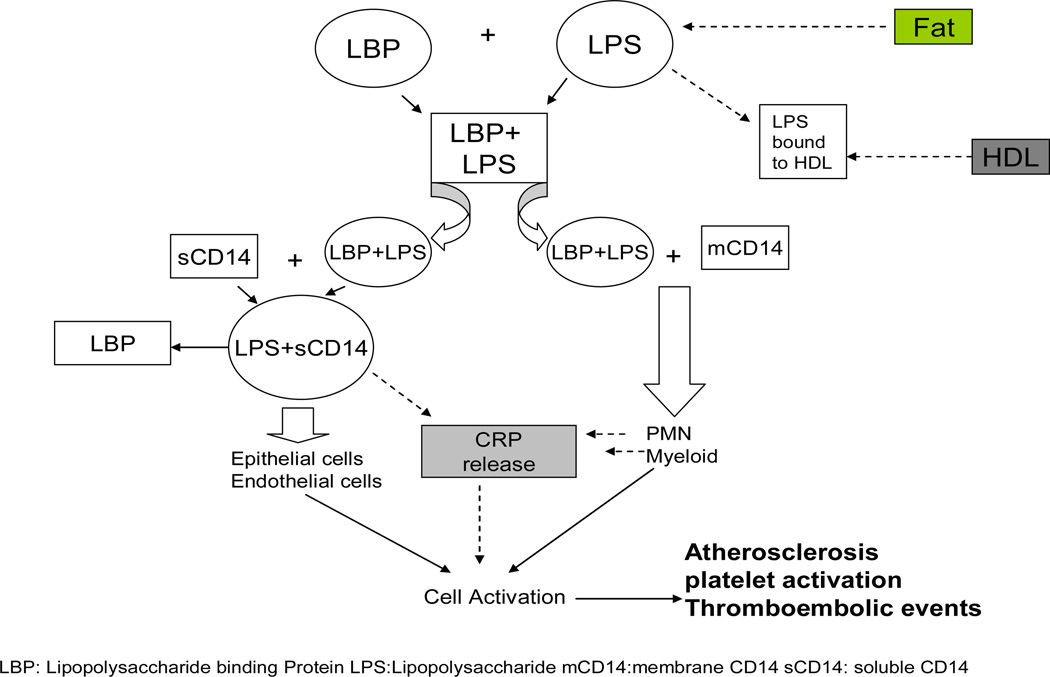
CKD patients have higher prevalence of inflammation (33) which is an independent risk factor for cardiovascular events through promotion of atherosclerosis (34). Infection being the 2nd most common cause of death in hemodialysis patients (1), bacterial infections especially by gram negative bacteria serves as a major contributor (35). Endotoxin (Lipid A), a glucosamine based phospholipid, is the hydrophobic anchor of lipopolysaccharide and makes up the outer monolayer of the outer membranes of most gram-negative bacteria (36). As it is biologically active portion of lipopolysaccharide molecule (37), it contributes in the activation of the host immune cells like macrophages etc, and results in the release of inflammatory mediators(38). This activation of cascade occurs through the combination of lipopolysaccharide (LPS) with lipopolysaccharide binding protein (LBP) and then interaction of this complex with CD14 (cell surface antigen). CD14 has two forms namely soluble and myeloid, and these two forms interact with LPS-LBP complex through two different pathways as highlighted in Figure 4.
Figure 4.
Cellular mechanism of lipopolysaccharide action and activation of the cytokine system and interaction with lipoproteins.
A possible explanation for the paradoxical effect of higher concentration of endotoxin on mortality can be explained through endotoxin action at receptor level shown in (Figure 4). Raj et al found that increased soluble CD14 level was associated with higher death risk in CKD patients (21). Endotoxin mediates its effect after binding with CD14 to specific receptors resulting in activation of a cascade of inflammatory cytokines(7). In lower concentrations it activates the immune system to combat infection without causing overt damage while very high concentration suggests that they are not bound 50 pg/ml or greater were at increased risk for development of atherosclerosis (39). Lack of association with inflammatory cytokines apart from CRP, in our study, further supports this explanation. Indeed a recent study found that endotoxemia is associated with better survival in peritoneal dialysis patients.(14)
Another plausible explanation for this association is that in our study population, the mean level of endotoxin was 2.31±3.10 EU/ml and sCD14 was 7.24±2.45 ug/ml. sCD14 values across the quartiles of endotoxin level showed that group with higher mortality risk had higher sCD 14 as compared to the other two groups. Further analysis showed no significant correlation was found between the endotoxin and CD14. So this finding further suggests that, to manifest its effect, endotoxin requires a certain amount of sCD14 in the blood to show maximum activation of the inflammatory cascasde Table 1.
Data also suggested that older patients had higher level of endotoxin and this increment had significant association (Table 1) but no significant correlation was found between endotoxin and age (Table 2). The only significant positive correlation that was found in this study is between CRP levels and endotoxin levels when adjusted for case-mix variables (Table 2). Szeto et al found a similar association between CRP and Serum endotoxin levels in peritoneal dialysis patients. Their study also found a negative correlation between serum albumin and serum endotoxin which we did not find in our study (6). Our findings are similar to those of Goncalves et al who reported that there were no association between endotoxin levels and circulating cytokines (40).
An inverse association was also found between HDL and endotoxin levels in after both unadjusted and adjusted linear regression analyses. This finding correlated with the fact that HDL levels decline more than any other lipoproteins in septic patients (41). LPS is detoxified in the circulation by incorporation into lipoproteins (LDL, VLDL, TGL and HDL)(42, 43).
Selection bias during study enrollment resulting in a younger maintenance HD cohort is one of the major limitations of this study. However, because mortality rate in the original NIED study cohort was lower than in the baseline dialysis population (16), it might be argued that the strength of the association seen is much lower than would be seen in a more randomly selected sample of dialysis patients. The strength of our study relates to the fact that participants were selected randomly without prior knowledge of their inflammation status. Further, we had a fairly large sample size with comprehensive clinical and laboratory evaluation. We were able to do body composition measurement, obtain detailed information on co-morbid illnesses and measure levels of pro-inflammatory cytokines.
Conclusion
In our study of 306 MHD patients who were followed for up to 3.5 years, increasing endotoxin levels was not associated with increased mortality. This possibly is due to complex interaction of endotoxin with its receptors and signaling cascade. Additional studies are necessary to assess the relationship between endotoxin concentration and other long-term outcomes in these maintenance hemodialysis patients.
Acknowledgment
The authors are thankful to dietitians and other teammates in DaVita Wild West and Gold Coast Divisions for supporting the study and the staff at Harbor-UCLA GCRC Core Laboratories for the management of blood samples and measuring inflammatory markers.
Funding Source
The study was supported by Dr. Kalantar-Zadeh’s research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106, R21 DK078012, and K23 DK61162), a research grant from DaVita Clinical Research and a philanthropic grant from Mr. Harold Simmons. Dr. Raj is supported by the National Institute of Health Grants AG21560 and R01DK073665-01A1. Dr. Miklos Zsolt Molnar received grants from the National Research Fund (NKTH-OTKA-EU 7KP-HUMAN-MB08-A-81231), was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (2008–2011), and is recipient of the Hungarian Eötvös Scholarship (MÖB/66-2/2010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in part as an abstract during the annual meeting of the American Society of Nephrology, Oct 27–30, 2009, San Diego, CA.
Relevant Potential Conflict of Interest
KKZ, CPK and/or JDK have received recent grants and/or honoraria from Genzyme, Inc, the manufacturer of Sevelamer (Renagel™ and Renvela™) and doxercalciferol (Hectoral™), Abbott laboratories, the manufacturer of Paricalcitol (Zemplar™) and calcitriol (Calcijex™), Fresenius Medical Care, the distributor of calcilum acetate (PhosLo™), Shire Pharmaceuticals, the manufacturer of lanthanum carbonoate (Fosrenol™), and/or Amgen, Inc, the manufacturer of Cinacalcet hydrochloride (Sensipar™ or Mimpara™).
References
- 1.Mailloux LU, Bellucci AG, Wilkes BM, Napolitano B, Mossey RT, Lesser M, Bluestone PA. Mortality in dialysis patients: analysis of the causes of death. Am J Kidney Dis. 1991;18(3):326–335. doi: 10.1016/s0272-6386(12)80091-6. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney international. 2000;58(4):1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 3.Kessler M, Hoen B, Mayeux D, Hestin D, Fontenaille C. Bacteremia in patients on chronic hemodialysis. A multicenter prospective survey. Nephron. 1993;64(1):95–100. doi: 10.1159/000187285. [DOI] [PubMed] [Google Scholar]
- 4.Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9(5):869–876. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 5.Cohen G, Haag-Weber M, Horl WH. Immune dysfunction in uremia. Kidney Int Suppl. 1997;62:S79–S82. [PubMed] [Google Scholar]
- 6.Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3(2):431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. Science. 4975. Vol. 249. New York, NY: 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein; pp. 1431–1433. [DOI] [PubMed] [Google Scholar]
- 8.Tobias PS, Soldau K, Ulevitch RJ. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. The Journal of biological chemistry. 1989;264(18):10867–10871. [PubMed] [Google Scholar]
- 9.Tobias PS, Ulevitch RJ. Lipopolysaccharide-binding protein and CD14 in the lipopolysaccharide-dependent activation of cells. Chest. 1994;105(Suppl)(3):48S–50S. doi: 10.1378/chest.105.3.48s. [DOI] [PubMed] [Google Scholar]
- 10.Holzheimer RG. The significance of endotoxin release in experimental and clinical sepsis in surgical patients--evidence for antibiotic-induced endotoxin release? Infection. 1998;26(2):77–84. doi: 10.1007/BF02767765. [DOI] [PubMed] [Google Scholar]
- 11.Mock CN, Jurkovich GJ, Dries DJ, Maier RV. Clinical significance of antibiotic endotoxin-releasing properties in trauma patients. Arch Surg. 1995;130(11):1234–1240. doi: 10.1001/archsurg.1995.01430110092017. discussion 1240-1231. [DOI] [PubMed] [Google Scholar]
- 12.Van Leeuwen PA, Boermeester MA, Houdijk AP, Ferwerda CC, Cuesta MA, Meyer S, Wesdorp RI. Clinical significance of translocation. Gut. 1994;35(Suppl)(1):S28–S34. doi: 10.1136/gut.35.1_suppl.s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arizono K, Nomura K, Motoyama T, Matsushita Y, Matsuoka K, Miyazu R, Takeshita H, Fukui H. Use of ultrapure dialysate in reduction of chronic inflammation during hemodialysis. Blood purification. 2004;22(Suppl 2):26–29. doi: 10.1159/000081870. [DOI] [PubMed] [Google Scholar]
- 14.Szeto CC, Kwan BC, Chow KM, Lai KB, Pang WF, Chung KY, Leung CB, Li PK. Endotoxemia is associated with better clinical outcome in incident chinese peritoneal dialysis patients: a prospective cohort study. Perit Dial Int. 2010;30(2):178–186. doi: 10.3747/pdi.2008.00242. [DOI] [PubMed] [Google Scholar]
- 15.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Seminars in dialysis. 2002;15(5):329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 16.Colman S, Bross R, Benner D, Chow J, Braglia A, Arzaghi J, Dennis J, Martinez L, Baldo DB, Agarwal V, Trundnowski T, Zitterkoph J, Martinez B, Khawar OS, Kalantar-Zadeh K. The Nutritional and Inflammatory Evaluation in Dialysis patients (NIED) study: overview of the NIED study and the role of dietitians. J Ren Nutr. 2005;15(2):231–243. doi: 10.1053/j.jrn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Shantouf RS, Budoff MJ, Ahmadi N, Ghaffari A, Flores F, Gopal A, Noori N, Jing J, Kovesdy CP, Kalantar-Zadeh K. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31(5):419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(12):2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(4):683–692. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, Kopple JD. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56(2):338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj DS, Shah VO, Rambod M, Kovesdy CP, Kalantar-Zadeh K. Association of soluble endotoxin receptor CD14 and mortality among patients undergoing hemodialysis. Am J Kidney Dis. 2009;54(6):1062–1071. doi: 10.1053/j.ajkd.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, Nissenson A. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18(10):2781–2788. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 23.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. The American journal of medicine. 2000;108(8):609–613. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 24.Nelson EE, Hong CD, Pesce AL, Peterson DW, Singh S, Pollak VE. Anthropometric norms for the dialysis population. Am J Kidney Dis. 1990;16(1):32–37. doi: 10.1016/s0272-6386(12)80782-7. [DOI] [PubMed] [Google Scholar]
- 25.Williams AJ, McArley A. Body composition, treatment time, and outcome in hemodialysis patients. J Ren Nutr. 1999;9(3):157–162. doi: 10.1016/s1051-2276(99)90056-0. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Dunne E, Nixon K, Kahn K, Lee GH, Kleiner M, Luft FC. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant. 1999;14(1):169–175. doi: 10.1093/ndt/14.1.169. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. The American journal of clinical nutrition. 2006;83(2):202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. The New England journal of medicine. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 29.Erbagci AB, Tarakcioglu M, Aksoy M, Kocabas R, Nacak M, Aynacioglu AS, Sivrikoz C. Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta cardiologica. 2002;57(3):197–204. doi: 10.2143/AC.57.3.2005389. [DOI] [PubMed] [Google Scholar]
- 30.Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17(9):1684–1688. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 31.Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annual review of immunology. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK. Circulating Endotoxemia: A Novel Factor in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Clin J Am Soc Nephrol. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panichi V, Migliori M, De Pietro S, Taccola D, Bianchi AM, Norpoth M, Metelli MR, Giovannini L, Tetta C, Palla R. C reactive protein in patients with chronic renal diseases. Renal failure. 2001;23(3–4):551–562. doi: 10.1081/jdi-100104737. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney international. 1999;55(2):648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, Hsu WH, Chen HJ, Chen W, Shih CM, Hsia TC, Tu CY. Different bacteriology and prognosis of thoracic empyemas between patients with chronic and end-stage renal disease. Chest. 2007;132(2):532–539. doi: 10.1378/chest.07-0005. [DOI] [PubMed] [Google Scholar]
- 36.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annual review of biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rietschel ET, Kirikae T, Schade FU, Ulmer AJ, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke HD, Kusumoto S, et al. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology. 1993;187(3–5):169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- 38.Bosshart H, Heinzelmann M. Targeting bacterial endotoxin: two sides of a coin. Annals of the New York Academy of Sciences. 2007;1096:1–17. doi: 10.1196/annals.1397.064. [DOI] [PubMed] [Google Scholar]
- 39.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. Journal of the American College of Cardiology. 1999;34(7):1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 40.Goncalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AE, Lima EG, Fuerbringer R, Sauthier SM, Riella MC. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21(10):2788–2794. doi: 10.1093/ndt/gfl273. [DOI] [PubMed] [Google Scholar]
- 41.Gordon BR, Parker TS, Levine DM, Saal SD, Wang JC, Sloan BJ, Barie PS, Rubin AL. Low lipid concentrations in critical illness: implications for preventing and treating endotoxemia. Critical care medicine. 1996;24(4):584–589. doi: 10.1097/00003246-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(24):12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feingold KR, Funk JL, Moser AH, Shigenaga JK, Rapp JH, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infection and immunity. 1995;63(5):2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]