The crystal structure of the vaccinia virus D13 protein presented by Bahar et al in this issue of Structure displays fused “virus jelly roll” folds, ubiquitous among dsDNA icosahedral viruses. Although D13 is not present in the mature virus its structure suggests its evolutionary descent from an ancient icosahedral ancestor.
The crystal structure of the D13 protein of vaccinia virus reported by Bahar et al. in this issue of Structure (Bahar et al, 2011) expands the connectivity among icosahedral and now non-icosahedral viruses based on the ubiquitous “virus jelly roll” tertiary fold. The literature describing this fold now spans more than 30 years and describes its appearance in viruses infecting all domains of life. Previously the jelly roll fold was only reported in icosahedral capsids but its role in vaccinia virus, described in this paper as well as in earlier biochemical and electron microscopy studies, ties it to other large dsDNA viruses. Vaccinia virus is among the largest mammalian viruses; it contains over 250 genes and has a brick-shaped morphology with dimensions of ~3000 X 2700 X 2500 Å in the mature particle. Vaccinia is a close relative of small pox virus and is the basis for the small pox vaccine. Together with its viral gene product A17, D13 recruits membranes that eventually envelop the virus. Many other structural homologs of D13 play a similar role in that they are associated with an internal viral membrane of icosahedral particles. D13, however, is only transiently associated with the membranes and is not present in the mature particles.
The structural biology of icosahedral viral capsid subunits has been rather monotonous in terms of their tertiary structures. The viral subunit jelly roll fold (Figure 1A) was the only tertiary structure observed in icosahedral capsid proteins for the first 12 years of virus crystallography (Rossmann and Johnson, 1989). The first two virus structures determined at near atomic resolution were single strand (ss) RNA plant viruses with T=3 quasi-symmetry, i.e. the tomato bushy stunt virus and the southern bean mosaic virus. They displayed closely similar shell forming domains, composed of the jelly roll fold, in spite of their low sequence identity. The fold was observed again in the third virus structure reported, that of the satellite tobacco necrosis virus, leading to the, apparently correct, conclusion that icosahedral, ssRNA plant viruses were related through a common ancestor and retained closely similar tertiary structures despite their broad sequence variation. The plot thickened when the T=1 (pseudo T=3) structures of human rhinovirus and poliovirus were determined and the folds of the three different gene products in the icosahedral asymmetric unit, displaying undetectable sequence similarity, all had jelly roll folds. The quaternary structure of the picornaviruses is strikingly similar to the T=3 plant virus structures (Figure 1B). The T=3 capsids have only one type of gene product, which occupies each of the three quasi-equivalent positions, whereas the picornaviruses have different gene products in the three pseudo-equivalent positions. The picornavirus capsids strongly suggested that the gene for the subunit in a T=3 virus triplicated, and the different genes then evolved independently to create very different sequences with closely similar folds that reflected their origin. The addition of a protease, that cleaved the polyprotein of capsid subunits, produced the covalently independent proteins constituting the ubiquitous picornavirus capsid. This hypothesis was supported by the structure of the beanpod mottle virus, a plant comovirus with a picornavirus-like capsid, in which the first two jelly rolls were still a single polypeptide chain, while the subunit occupying the third position was covalently independent. An even earlier stage of the evolution of T=3 capsids to picornavirus capsid was found in the tobacco ringspot virus, in which each of the three jelly rolls in the asymmetric unit are in a single polypeptide chain (Lin and Johnson, 2003). Thus the concept of fused jelly roles was well established in the icosahedral ssRNA virus capsids.
Figure 1. RNA virus jelly rolls.
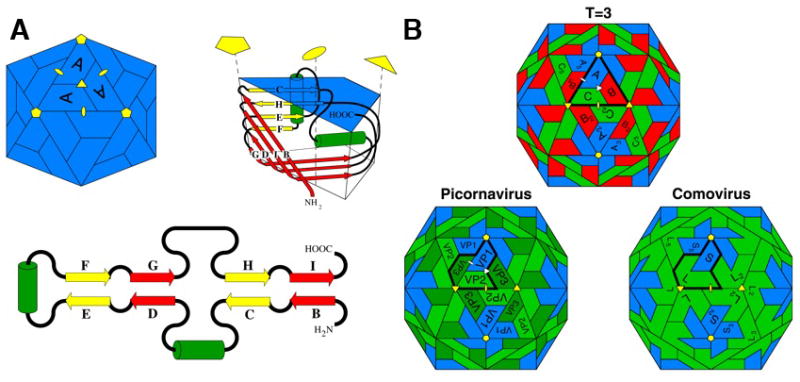
(A) A diagram of the viral jelly roll tertiary structure observed in many ssRNA icosahedral virus capsids (top right), its topology (lower) and its disposition in a T=1 icosahedral particle (top left). β-strands (represented by yellow or red arrows that correspond to their sheets in the folded structure) and positions of insertions between strands are shown in the topological representation. The green cylinders represent helices that are usually conserved. The C–D, E–F, and G–H loops often contain significant insertions. The fold is named after the procedure a baker would use to prepare a jelly roll, i.e. making an extended piece of dough, folding it back on itself, as in the topological figure, and then rolling it up as in the tertiary structure figure.
(B) The relationship between capsids formed by 180 jelly rolls arranged with icosahedral symmetry. Top: a T=3 capsid (typical of ssRNA plant viruses) formed by one type of gene product. Positions A, B, and C are occupied by subunits with identical amino acid sequences. Lower left: The picornavirus capsid has 3 different gene products, all with jelly roll folds, but without a detectable sequence similarity, occupying the positions Vp1, Vp2 and Vp3. These are equivalent, in terms of the quaternary structure, to A, C and B in the T=3 virus. Lower right: The comovirus capsid in which VP2 and VP3 are fused by a linker to form a single polypeptide chain made of two jelly rolls. Picorna virus capsids probably evolved from a T=3 capsid in which the subunit gene triplicated to form three jelly rolls that evolved independently. Eventually they were separated by post-translational proteolysis. The comovirus capsid appears to be an intermediate in this process.
The structure of the adenovirus hexon subunit reported by Burnett and colleagues (Roberts et al., 1986) revealed another way, in which the jelly roll was incorporated into a virus capsid. Adenovirus is a 1000 Å dsDNA virus with a pseudo T=25 capsid (Figure 2). Its main structural proteins are 60 identical subunits that form the 12 pentamers and 720 identical subunits that form 240 pseudo hexamers. The subunits forming pseudo hexamers are two jelly rolls that are fused together. Trimers of these subunits form a pseudo-hexagonal base and these hexamers interact with each other to form the pseudo equivalent shell. The outer portion of the subunit is clearly trimeric, as the N-terminal jelly roll has two elaborate insertions between the strands of the beta sheet, whereas the C-terminal jelly has much smaller insertions.
Figure 2. Structure-based relationships of dsDNA virus jelly rolls.

Based on structural superpositions, the dsDNA jelly rolls organize as shown. In each case, the tertiary structure is shown as well as the quaternary structure of the capsids in which they appear. This fold is exceptionally versatile in generating capsids with large pseudo-equivalent surface lattices. Both D13 and the adenovirus subunit are associated with viruses infecting mammalian hosts and they both have elaborate insertions between strands of the jelly rolls compared to the other viruses in this category. However, the largest insertion in D13 is in the C-terminus jelly roll, while the largest insertions in the adenovirus subunit are in the N-terminal jelly roll. The canonical dsDNA fused jelly rolls is shown for STIV with the strands labeled in the established manner.
Although the fused jelly rolls in the dsDNA virus capsids are technically equivalent to the subunits that are fused together in beanpod mottle virus, a careful examination suggests that the dsDNA fused jelly roll is on an evolutionary path that is different from that of the ssRNA virus jelly rolls. First, the disposition of the jelly rolls relative to the particle surface is different between the dsDNA viruses and the ssRNA viruses. Hexamers or pseudo hexamers in T=3 and picornavirus virus capsids have the long dimension of the jelly rolls approximately tangential to the particle surface, whereas all the dsDNA virus jelly rolls have the long dimension roughly radial relative to the spherical particle. Second the ssRNA fused subunits appear as “beads on a string”, with discrete jelly rolls linked by an extended connector. The fused jelly rolls in the dsDNA viruses appear as a single domain with beta sheet that are formed between the F strand of the N-terminal jelly roll and the B’ strand on the C-terminal jelly roll, resulting in a continuous beta sheet that extends through both jelly rolls. Fused jelly rolls have appeared in the subunit structure of the dsDNA PRD1 bacteriophage, where they also formed trimers that are highly similar to adenovirus trimers (Bamford et al., 2005). Like adenovirus, PRD1 has a T=25 pseudo-equivalent surface lattice, but PRD1 contains an internal membrane to which the fused jelly rolls are attached. The fused jelly rolls are also seen in numerous large dsDNA, icosahedral viruses (Figure 2), in which they are associated with internal membranes, including the algal-infecting virus PBCV, the sulfolobus infecting STIV and the PM2 bacteriophage (Abrescia et al., 2008; Bamford et al., 2005).
The D13 crystal structure now reported here reveals a role for fused jelly rolls that is related to, but different, from those previously observed (Bahar et al, 2011). The vaccinia viral gene products A17 and D13 recruit the virus membrane that eventually envelops the mature particle. The novel aspect of D13 is that it is not present in the mature virus, but serves as an intermediate in the assembly of the virus membrane and is released from the membrane prior to particle maturation. D13 does not interact directly with membranes; rather it binds to gene product A17 that resides in the recruited membrane. D13 contributes to the crescent-shaped membrane morphology that precedes assembly of the particles through the formation hexagonal arrays on the outer surface of the membranes (Hyun et al., 2007). As the crescents coalesce, a spherical particle envelope is formed. D13 is released at this stage through the action of a virally encoded protease, I7, that cleaves A17 at the N-terminal and C-terminal regions (Bisht et al., 2009). Only the N-terminal cleavage is required to release D13, allowing normal maturation of the particles into the enveloped brick-shaped mature virions. The structure presented by Bahar et al clearly ties D13 in with the other fused double jelly roll structures in terms of tertiary and quaternary structure (Figure 2) and strongly suggests an evolutionary history for D13 that links it to the established class of dsDNA subunits found in many large dsDNA viruses.
References
- Abrescia NG, Grimes JM, Kivela HM, Assenberg R, Sutton GC, Butcher SJ, Bamford JK, Bamford DH, Stuart DI. Mol Cell. 2008;31:749–761. doi: 10.1016/j.molcel.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Bahar MW, Graham SC, Stuart DI, Grimes JM. Structure. 2011;(this issue) doi: 10.1016/j.str.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford DH, Grimes JM, Stuart DI. Curr Opin Struct Biol. 2005;15:655–663. doi: 10.1016/j.sbi.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Bisht H, Weisberg AS, Szajner P, Moss B. J Virol. 2009;83:9140–9150. doi: 10.1128/JVI.00875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun JK, Coulibaly F, Turner AP, Baker EN, Mercer AA, Mitra AK. J Virol. 2007;81:11075–11083. doi: 10.1128/JVI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Johnson J. Adv Virus Res. 2003;62:167–239. doi: 10.1016/s0065-3527(03)62004-x. [DOI] [PubMed] [Google Scholar]
- Roberts MM, White JL, Grütter MG, Burnett RM. Science. 1986;232:1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- Rossmann MG, Johnson JE. Ann Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]


