Abstract
Infections caused by multi-resistant Gram positive bacteria represent a major health burden in the community as well as in hospitalized patients. Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium are well-known pathogens of hospitalized patients, frequently linked with resistance against multiple antibiotics, compromising effective therapy. Streptococcus pneumoniae and Streptococcus pyogenes are important pathogens in the community and S. aureus has recently emerged as an important community-acquired pathogen.
Population genetic studies reveal that recombination prevails as a driving force of genetic diversity in E. faecium, E. faecalis, S. pneumoniae, and S. pyogenes and thus, these species are weakly clonal. Although recombination has a relatively modest role driving the genetic variation of the core genome of S. aureus, the horizontal acquistion of resistance and virulence genes plays a key role in the emergence of new clinically relevant clones in this species. In this review we discuss the population genetics of E. faecium, E. faecalis, S. pneumoniae, S. pyogenes, and S. aureus. Knowledge of the population structure of these pathogens is not only highly relevant for (molecular) epidemiological research but also for identifying the genetic variation that underlies changes in clinical behaviour, to improve our understanding of the pathogenic behaviour of particular clones and to identify novel targets for vaccines or immunotherapy.
Keywords: Enterococcus, Streptococcus, Staphylococcus, MLST, evolution, Molecular epidemiology
Introduction
Enterococcus faecium, Enterococcus faecalis, Streptococcus pneunmoniae, Streptococcus pyogenes, and Staphylococcus aureus are low GC Gram-positive bacteria belonging to the phylum Firmicutes. All of these species are human commensals, and as such are part of the normal human microflora having a benign relationship with their host. However, they are also human pathogens capable of infecting a broad range of body sites. The occasional virulence of these species requires a susceptible host, but also reflects a history of selection for specific bacterial variants or clones with enhanced pathogenic potential that are particularly difficult to manage when these clones acquire antibiotic resistance. Despite the fact that enterococci, streptococci and staphylococci all represent important opportunistic pathogens in which resistance has evolved against multiple antibiotics, the epidemiology of resistant clones in these pathogens differ substantially. While antibiotic resistance in S. pneumoniae and S. pyogenes has evolved in clones causing community-acquired infections, multi-resistant S. aureus and enterococci clones are primarily causing infections in hospitalized patients, although community-acquired infections with resistant S. aureus have emerged the last decade. In contrast to S. pneumoniae and S. pyogenes, which are strict human pathogens, S. aureus and enterococci are capable of colonizing and causing infections in a wide range of animals. Consequently, multi-resistant S. aureus and enterococci are also found in non-human reservoirs (e.g. livestock) in which the selective pressure imposed by antibiotic use favours the selection of resistant clones. This review aims to give an overview of the evolution and population dynamics of specific multi-resistant clones in these Gram-positive species, in the context of the differing ecological and epidemiological characteristics of these opportunistic pathogens.
The genus Enterococcus
Enterococci are lactic acid bacteria belonging to the ileal microbiota (Booijink et al., 2010). They represent typical examples of opportunistic pathogens that have long been regarded as relatively harmless commensals colonizing the gastro-intestinal tract of humans and animals. Although considered normal colonizers of the digestive tract, they have also been recognized for more than 100 years as etiological agents of hospital-acquired infections in debilitated patients (Murray, 1990). The perspective on enterococci started to change in the 1980s when these organisms were found to have gained high-level resistance to ampicillin and their growing importance was cemented by the rapid emergence of vancomycin-resistance in the 1990s in hospitals in the US (Rice, 2006). These events catapulted enterococci in the Premier League of multi-drug resistant Gram-positive pathogens, together with pathogens like methicillin-resistance S. aureus and penicillin-resistant S. pneumoniae (Woodford & Livermore, 2009; Nordmann et al., 2007).
The digestive tract, the main habitat of Enterococci in humans
Of all Enterococcus species, E. faecalis and E. faecium are the species most frequently found as colonizers of the human gastro-intestinal tract, and are similarly most commonly responsible for infections in hospitalized patients. Undoubtedly, antibiotic resistance has played a pivotal role in the emergence of E. faecalis and E. faecium as nosocomial pathogens. Intrinsic resistance to certain classes of beta-lactam antibiotics (e.g. cephalosporins), low-level resistance to aminoglycosides, lincosamides, streptogramins (in case of E. faecalis) and monobactams provided enterococci with a selective advantage in the hospital environment (Klare et al., 2003). Level of resistance increased over the last 20–30 years due to the ability of E. faecalis and E. faecium to acquire foreign DNA resulting in the rapid accumulation of antibiotic resistance genes (Hegstad et al., 2010). Tetracycline and erythromycin resistance genes were reported to be located on mobile genetic elements as early as the 1970s, signifying the rapid dissemination of resistance traits among enterococci (Courvalin et al., 1974; Clewell et al., 1974). Today, enterococci have acquired resistance against a broad range of antibiotics covering most antimicrobial classes (Klare et al., 2003; Hegstad et al., 2010). Most notably, from a clinical point of view, is the acquired resistance to aminoglycosides, penicillines, especially ampicillin, and vancomycin.
Vancomycin resistance a serious threat
The emergence of vancomycin-resistance by enterococci has become the paradigm of the post-antibiotic era. Acquisition of plasmid-encoded high-level vancomycin resistance, first isolated in 1986 in Europe (Leclercq et al., 1988; Uttley et al., 1988), definitively marked enterococci as one of the most notorious antibiotic resistant bacteria. During the 1990s vancomycin-resistant enterococci (VRE) have disseminated rapidly thoughout hospitals in the US, first in intensive care units (ICU) and subsequently in essentially all hospital wards (National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004, 2004). At that time VRE prevalence rates in hospitals in Europe were still low, while VRE carriage rates in the community, and in meat products via animal reservoirs were high (Bonten et al., 2001). The latter was most probably due to the Europe-wide use of avoparcin, a glycopeptide antibiotic conferring cross-resistance to vancomycin, as an antimicrobial-growth promoter from the early 1970s until 1997, when it was banned in Europe (Goossens, 1998).
Although vancomycin-resistance in enterococci can be mediated by six vancomycin-resistance determinants, the major vancomycin-resistance phenotypes are VanA and VanB (Courvalin, 2006; Boyd et al., 2008). Virtually all VRE recovered from nosocomial infections are also ampicillin resistant. In fact, the emergence of high-level ampicillin resistance, specifically in US hospitals in the early 1980s, preceded the epidemic rise of vancomycin resistance with increasing numbers of high-level ampicillin-resistant enterococci being isolated in US hospitals in the early 1980s (Grayson et al., 1991; Iwen et al., 1997). Acquired high-level resistance to beta-lactam antibiotics in clinical isolates of E. faecium is conferred by mutations of the low-affinity penicillin-binding protein, PBP5, which leads to a lower affinity of this PBP for beta-lactam antibiotics, or by overproduction of PBP5 (Rybkine et al., 1998; Klare et al., 2003). The association between ampicillin and vancomycin resistance phenotypes can sometimes be explained by genetic linkage of high-level ampicillin-resistance and vancomycin-resistance genes (Carias et al., 1998). More frequently this phenotypic linkage probably reflects sequential and independent acquisition of resistance genes resulting in the selective dominance of a small subset of hospital -adapted clones. The progressive increase in high-level ampicillin resistance and vancomycin resistance coincided with the increase of nosocomial infections caused by E. faecium relative to E. faecalis. While up to the 1980s/early 1990s more than 90% of all enterococcal infections were caused by E. faecalis and only 5–10% by E. faecium (Iwen et al., 1997; Murdoch et al., 2002; Treitman et al., 2005) this ratio has gradually changed in favour of E. faecium. Today, between 38–75% of enterococcal infections are caused by E. faecium (Hidron et al., 2008; Markogiannakis et al., 2009; Chiang et al., 2007; Mikulska et al., 2009). The observed ecological shift in enterococcal infections is probably due to the fact that resistance to ampicillin and vancomycin is much more prevalent in E. faecium than in E. faecalis isolates recovered from clinical infections. While to date approximately 90% of E. faecium from healthcare-associated infections (HAI) in the US are resistant to ampicillin and 80% to vancomycin these resistance percentages are significantly lower in E. faecalis (~4% resistance to ampicillin and 7% resistance to vancomycin) (Hidron et al., 2008). Also, globally, the epidemiology of VRE is dominated by E. faecium with vancomycin-resistant E. faecium being isolated from all continents (Tenover & McDonald, 2005; Werner et al., 2008; Camargo et al., 2006; Dahl et al., 2007; Zheng et al., 2007; Lee et al., 2004; Hoshuyama et al., 2008; von Gottberg et al., 2000; Padiglione et al., 2003).
Molecular epidemiology of E. faecium
Molecular epidemiological studies of VRE outbreaks in the US using pulsed-field gel electrophoresis (PFGE) not only reported intra- and inter-hospital dissemination of single clones (Handwerger et al., 1993; Boyce et al., 1994; Moreno et al., 1995; Dunne & Wang, 1997) but the presence of polyclonal VRE in single hospitals suggested simultaneous spread of multiple VRE clones (Bonten et al., 1996; Mato et al., 1996). However methods like PFGE, that index rapidly evolving variation, may give misleading results for epidemiological typing in rapidly recombining and evolving bacterial genera such as Enterococcus (Paulsen et al., 2003; Willems et al., 2005; Ruiz-Garbajosa et al., 2006). The observation that isolates belonging to the same clone may differ in up to 7 PFGE bands (Morrison et al., 1999) illustrates that PFGE is too discriminatory to study the long term and global epidemiology of enterococci.
The first insights into the genetic relatedness of a large sample of E. faecium isolates came from a study in 2000 when 255 vancomycin-resistant E. faecium from different ecological niches and geographic locations were genotyped by amplified fragment length polymorphism (AFLP) (Willems et al., 2000). Since AFLP combines the analysis of small conserved and variable DNA regions distributed over the whole genome, this technique will identify clusters of related isolates that would not be detected by PFGE. In this study, 84% of isolates recovered from hospitalized patients from different parts of the world clustered together in a single genogroup C, distinct from 75% of isolates from non-hospitalized persons and 100% of the isolates from pigs (genogroup A), 92% of poultry isolates (genogroup B) and 70% of veal calf isolates (genogroup D). The existence of a specific hospital subpopulation in E. faecium was also demonstrated by comparative genomic hybridisation (CGH) of 97 E. faecium isolates from diverse ecological and geographic background and representing both vancomycin-resistant and –susceptible isolates to an E. faecium mixed whole genome array. Clustering of CGH data based on the presence or absence of a hybridisation signal grouped 75% of the globally dispersed hospital outbreak and clinical isolates into a single hospital clade. Furthermore, this clade contained only 7% of the community (human, animal and environmental) isolates (Leavis et al., 2007). This finding indicated that the genetic content of hospital isolates differed from non-hospital isolates.
Genetic evolution of hospital-associated E. faecium
The question is whether relatedness-based on shared DNA fragments (AFLP) or genes/genetic elements (CGH) also means that hospital isolates share a common evolutionary history. Whole genome sequencing of 7 E. faecium strains, representing four clinical isolates from 4 hospitals in three different countries on two continents, one carrier isolate from a hospitalized patient and two carrier isolates from two non-hospitalized persons, provided a detailed analysis on diversity and evolutionary relatedness of E. faecium isolates (van Schaik et al., 2010). The observation that a neighbour-joining tree based on shared gene content and a maximum likelihood tree based on concatenated alignment of 649 orthologous proteins showed a similar topology, with the community isolates clearly distinct from the hospital isolates, is consistent with the hypothesis that hospital isolates not only share DNA content but are also descended from a comparatively recent common ancestor.
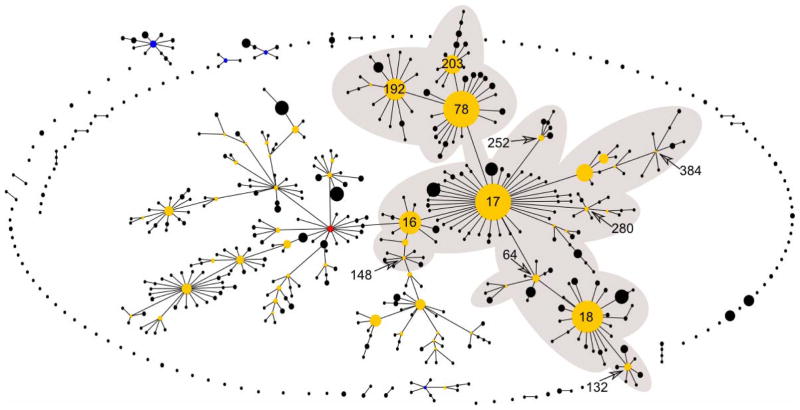
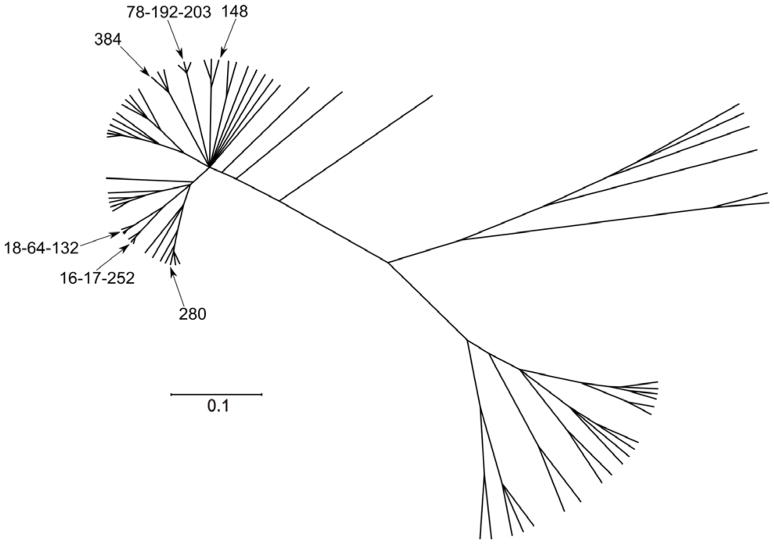
More in depth analysis of the evolutionary relatedness of E. faecium genotypes on a population level came from multilocus sequence typing (MLST) data (Homan et al., 2002). The first E. faecium population-wide study using MLST characterized a global collection of human (hospital and community-acquired) and non-human (animals and the environment) E. faecium and defined 175 sequence types (STs). STs were grouped with the eBURST algorithm. eBURST, which divides an MLST data set of any size into groups of related isolates and clonal complexes, predicts the founding (ancestral) genotype of each clonal complex, and computes the bootstrap support for the assignment (Feil et al., 2004). This clustering indicated that the majority of the globally representative hospital isolates were genotypically and evolutionary closely related and belonged to a single clonal-complex (CC), CC17 (Willems et al., 2005). However, the E. faecium population structure based on all STs (n=554) available in the MLST database (http://efaecium.mlst.net/ accessed on July 19th 2010) inferred by eBURST resulted in one major large straggly CC, which includes the previously designated CC17, 18 minor CCs and 110 singletons, with sixty-nine percent of the E. faecium STs in the database clustering in the major CC (Fig. 1). Recently, Turner and co-workers showed that eBURST links, i.e. eBURST inference on recent evolutionary descent, in populations in which >25% of STs belong to a single large straggly group, may not be accurate (Turner et al., 2007). Consistent with an inaccurate eBURST-based clustering is the observation that major hospital-associated subgroup founders (ST17, ST18, and ST78) that are linked together using eBURST belong to different genetic lineages in a neighbour-net tree or ClonalFrame (Didelot & Falush, 2007) based phylogentic tree constructed from a concatenation of the seven MLST housekeeping genes (Willems, 2010) (Fig. 2)(Willems & van Schaik, 2009). Also preliminary analysis of the population genetics of E. faecium using a recent Bayesian modeling approach (BAPS software) (Tang et al., 2009) demonstrated that the major hospital clones ST78 and its single locus variants ST192 and ST203 group in a different BAPS group than ST17 and ST18 (Jukka Corander, Personal communication, 2010). This demonstrates that hospital-derived E. faecium strains have not recently evolved from a single common ancestor and consequently the designation of CC17 as a hospital-selected clonal complex may well be erroneous. Instead, clinical and outbreak-associated isolates, collectively referred to as hospital-derived isolates, form a polyclonal E. faecium subpopulation harbouring evolutionary distinct clones. Despite the apparent lack of a common evolutionary history, hospital-derived clones are, based on AFLP, MLST, and comparative genomics (see above), genetically and evolutionary distinct from non-hospital isolates. This is also illustrated by the fact that the seven major ampicillin-resistant hospital-derived clones represented by ST16, ST17, ST18, ST78, ST117, ST192 and ST203, accounting for the majority (56%) of all 910 hospital-derived isolates, are only found sporadically among non-hospital isolates (41/513) (http://efaecium.mlst.net/). Furthermore, these clones have spread globally among hospitalized patients and can therefore be considered as highly successful and high-risk clones for dissemination of antibiotic resistance in the hospital environment thus contributing most to the hospital-burden of E. faecium (Werner et al., 2008; Deplano et al., 2007; Panesso et al., 2010; Camargo et al., 2006; Zheng et al., 2007; Lester et al., 2008; Klare et al., 2005; Damani et al., 2010; Bonora et al., 2007; Ko et al., 2005; Top et al., 2008; Khan et al., 2008; Koh et al., 2006; Valdezate et al., 2009; Billstrom et al., 2010; Hsieh et al., 2010; Ergani-Ozcan et al., 2008; Caplin et al., 2007; Galloway-Pena et al., 2009; Freitas et al., 2010; Willems et al., 2005; Libisch et al., 2008; Coque et al., 2005; Homan et al., 2002).
Figure 1.
Population snapshot based on all unique STs of the entire E. faecium MLST database accessed on 19/7/2010 and visualized using eBURST. The shaded area indicates the previously designated CC17. ST16, ST17, ST18, ST64, ST78, ST132, ST148, ST192, ST203, ST252, ST280, and ST384, representing multidrug resistant hospital isolates and belonging to the previously designated CC17 are indicated.
Figure 2.
Phylogenetic analysis based on nucleotide sequences of the E. faecium MLST genes of 73 STs representing major subgroup founders (>3 SLV links) and singletons with no double locus variant links to CCs. The phylogenetic tree was based on all trees sampled after the burn-in periode obtained using ClonalFrame after 100,000 iterations, including 50,000 burn-in. ST17, ST18, ST78, ST192 and ST203, representing multidrug resistant hospital isolates are indicated.
There is one other reservoir known where these typical hospital clones seem to reside. Damborg and co-workers showed in two recent publications that ampicillin-resistant E. faecium clones that are frequently associated with infections in hospitalized patients were present in 48 of 63 dogs (Damborg et al., 2008; Damborg et al., 2009). It remains to be investigated whether this has resulted from anthropo-zoonotic transfer or whether dogs form a risk for zoonotic transfer of these clones to hospitalized patients.
Acquisition of adaptive elements by hospital-association E. faecium
The success of these hospital clones most probably is due to the cumulative acquisition of adaptive elements like genes or genetic elements encoding antibiotic resistance determinants (e.g. high-level ampicillin-and quinolone-resistance) (Leavis et al., 2003), and also genes linked to virulence. Well characterised virulence genes in E. faecium include (Leavis et al., 2006; Willems et al., 2005; Coque et al., 2002; Galloway-Pena et al., 2009; Hegstad et al., 2010)esp and other genes on the esp pathogenicity island, hyl and several genes encoding surface proteins (Willems et al., 2001; Coque et al., 2002; Rice et al., 2003; Leavis et al., 2004; Vankerckhoven et al., 2004; Coque et al., 2005; Klare et al., 2005; Camargo et al., 2006; Galloway-Pena et al., 2009; Leavis et al., 2007; Hendrickx et al., 2007). Character evolution analysis based on CGH data identified IS elements, specifically IS16, to be associated with hospital-derived isolates. It has been argued that the acquisition of IS elements might facilitate the subsequent acquisition of virulence determinants like the esp gene (Leavis et al., 2007), and the availability of multiple whole genome sequences in the near future will greatly help to explore this possibility. The process of cumulative acquisition of adaptive elements, combined with the progressive increase in fitness, has been termed genetic capitalism (Baquero, 2004).
What biological characteristics of E. faecium allowed for the rapid evolutionary development of hospital-selected E. faecium clones that started to dominate enterococcal hospital epidemiology from the 1980s is not known. It is interesting to note however, that these hospital clones are enriched in particular insertion sequence (IS) elements (Leavis et al., 2007). Transposable elements (TEs), like IS elements, may dramatically increase the rate of molecular evolution thereby significantly increasing genome restructuring and adaptability to changing requirements, as in hospital environments (Oliver & Greene, 2009). This may have promoted genetic diversification in specific subpopulations and the evolutionary development of the successful E. faecium clones mentioned above.
Shared hospital and community clones in E. faecalis
As in E. faecium, the genome of E. faecalis is also infested by TEs. The E. faecalis V583 genome has 38 IS elements and more than a quarter of the genome consists of probable mobile or foreign DNA (Paulsen et al., 2003; Manson et al., 2010). The extreme high abundance of TEs in E. faecalis and the advanced pheromone system in E. faecalis that enables not only spread of plasmid but also transfer of large genomic regions including virulence and resistance genes (Manson et al., 2010), may have increased genome plasticity and species adapatability to changing conditions in hospital environment (Oliver & Greene, 2009). Comparison of gene tree topologies of individual MLST genes indicate recombination rates in E. faecalis that are even higher than in E. faecium (Ruiz-Garbajosa et al., 2006; Willems et al., 2005). Recent multi-genome analysis of E. faecalis and E. faecium showed that both have an open pan-genome indicating that both organisms can efficiently acquire and integrate foreign DNA in their gene pool (Nelson et al., 2010; van Schaik et al., 2010).
It is interesting to note that high prevalent E. faecium hospital clones are virtually absent in the community (Werner et al., 2011), while this is not the case in E. faecalis. The seven most prevalent E. faecalis clones among clinical and outbreak-associated isolates (n=355), based on MLST (Ruiz-Garbajosa et al., 2006), ST6, ST9, ST16, ST21, ST28, ST40, and ST87, account for only 37% of the hospital-derived isolates (http://efaecalis.mlst.net/), while this is 56% for the seven most prevalent hospital-derived E. faecium clones (see above). These clones are also found frequently in the community, including farm animals and food products (in 17% of 351 isolates (http://efaecalis.mlst.net/) (Ruiz-Garbajosa et al., 2006), while in E. faecium major hospital-derived clones are more rarely found in the community (8% of 513 non-hospital isolates; see also above (McBride et al., 2007; Larsen et al., 2010; Freitas et al., 2009; Lopez et al., 2010; Burgos et al., 2009). This indicates that in E. faecalis clones are more often shared between hospitalized patients and other reservoirs. In line with this result is the finding based on CGH, and confirmed by PCR, that genes involved in the virulence of E. faecalis, such as gelatinase, cytolysin, the enterococcal surface protein Esp and aggregation substance, can also be frequently found in nonclinical E. faecalis strains, such as isolates from food or healthy babies (Semedo et al., 2003; Solheim et al., 2009; van Schaik & Willems, 2010). This suggests that there is no clear distinction between pathogenic and non-pathogenic clones in E. faecalis. Nevertheless, some E. faecalis clones, like ST6, ST9, ST28 and ST40, represented by at least 10 isolates in the database (http://efaecalis.mlst.net/) are clearly enriched (>70% of isolates) among hospitalized patients and are distributed world wide (McBride et al., 2007; Nallapareddy et al., 2005; Ruiz-Garbajosa et al., 2006; Freitas et al., 2009; Solheim et al., 2009; Damani et al., 2010; Lopez et al., 2010; Lester et al., 2010; Kawalec et al., 2007; Sun et al., 2009; McBride et al., 2009).
Streptococcus pneumoniae
The pneumococcus (S. pneumoniae) is the leading cause worldwide of community-acquired pneumonia. Other disease manifestations associated with pneumococcal infection include common, mild self-limiting infections such as acute otitis media, but extend to rare cases of invasive disease associated with high mortality, such as meningitis (Durbin, 2004). Its clinical burden is concentrated among the very old and very young, and it is estimated to be responsible for more than 800,000 deaths per annum in children under 5 years of age (O’Brien et al., 2009). Despite this high toll, the vast majority of pneumococci are found in asymptomatic nasopharyngeal carriage, the prevalence of which varies by age and region (Crook et al., 2004). The carriage state is responsible for transmission, and is the stage of pneumococcal life history at which interventions such as antibiotics and vaccines exert their selective pressure.
Pneumococci with reduced susceptibility to penicillin were first noted in a clinical setting in the 1960s (Kislak et al., 1965), but the risk posed by increasing resistance was not heeded until much later. By the mid 1980s penicillin resistant pneumococci had been reported worldwide (Appelbaum, 1987), and by the 1990s were responsible for >50% cases of invasive disease in some samples (Jacobs, 2003; Fenoll et al., 1998). More recently erythromycin resistance has emerged and posed an increasing threat (Jacobs, 2004; Leclercq & Courvalin, 2002). The implementation of a conjugate vaccine effective against a subset of pneumococcal serotypes has had considerable benefit (Kyaw et al., 2006), but increasing resistance has been documented in serotypes not targeted by the vaccine (Farrell et al., 2007; Gertz et al., 2010).
The pneumococcus is both antigenically and genetically diverse. It can be divided by serology into more than 90 serotypes that reflect the structure of a complex polysaccharide capsule (Aanensen et al., 2007). As well as being an important virulence factor, capsule is the target antigen for conjugate vaccines. Historically, serotyping was an important epidemiological tool, and it has been known for some time that some serotypes are more likely than others to be found in cases of invasive disease. Similarly, it was noted early in studies of pneumococcal penicillin resistance that high level resistance, and the vast majority of intermediate level resistance, was concentrated in a few serotypes (Klugman, 1990). To a large degree these serotypes were the same as those that were particularly associated with carriage and disease in young children, and were later the targets for conjugate vaccination: serogroups or types such as 19, 23, 6, 9 and 14.
While serotypes remain an important part of pneumococcal biology, modern epidemiology focuses on identifying the individual clones or lineages that make up each serotype. This is particularly important in the pneumococcus, because each serotype may typically be made up of a number of clones, which are not closely related and are not equivalent in terms of antibiotic resistance.
It is known that pneumococci can receive homologous DNA from other pneumococci and indeed other oral streptococci, and incorporate it into their own genome. It has been shown conclusively that this process has led to the acquisition of resistance to penicillin mediated by penicillin binding proteins (pbps) originating in other oral streptococcal species (Dowson et al., 1993), which have then been acquired by the pneumococcus through homologous recombination. Mosaic pbp2x, which has developed in several different lineages and species, has been studied in some detail, demonstrates the importance of horizontal transfer in the evolutionary history of this locus (Chi et al., 2007), and indicates diverse oral streptococcal species as possible ancestors of the resistant pbp2x locus. Such studies should however be interpreted with some caution, because our knowledge of the population structures of related oral streptococci is so very poor, these organisms appear to be extremely diverse (Bishop et al., 2009) and as a result it is hard to pinpoint with confidence the specific source of genetic variation. Fluoroquinolone resistance may also be acquired horizontally (Balsalobre et al., 2003). This is of particular interest because recombination can transfer not only mutations causing resistance, but also additional compensatory mutations ameliorating any cost associated with that resistance (Balsalobre & de la Campa, 2008; Rozen et al., 2007). In other cases, the basis of resistance is carried on a transposon or similar genetic elements, which are also capable of horizontal transmission. Multiple resistances may be combined in single transposable elements, such as erm(B) (erythromycin) and tet(M) (tetracycline) on Tn3872 (resulting from the insertion of Tn917 into orf9 of Tn916) and Tn1545 (Varaldo et al., 2009; Cochetti et al., 2008).
The Pneumococcal Molecular Epidemiology Network
To identify important lineages/clones the customary arsenal of molecular epidemiology has been directed at pneumococcal resistance. The pneumococcal molecular epidemiology network (PMEN) combines PFGE and MLST to identify the important internationally and intercontinentally distributed clones, define their resistance phenotypes and genotypes, how they may be identified, and produce a standardized nomenclature (McGee et al., 2001). PMEN also examines the molecular causes of resistance, and records the alleles of penicillin binding proteins and, determines the presence of the mef and erm loci, which produce erythromycin resistance. Through the work of PMEN we may speak with confidence, for example, of the Spain 23F-1 clone. This is a particular lineage, originally noted as having spread from Spain to the US, with a 23F capsule exhibiting high level resistance to penicillin and with a distinctive PBP profile (Munoz et al., 1991). It has since spread to every continent, and been recorded with several other serotypes in addition to the presumably ancestral 23F. As it was the first PMEN clone it is identified as such by the suffix -1. At the time of writing PMEN recognizes 27 clones associated with at least one resistance determinant (http://www.sph.emory.edu/PMEN/).
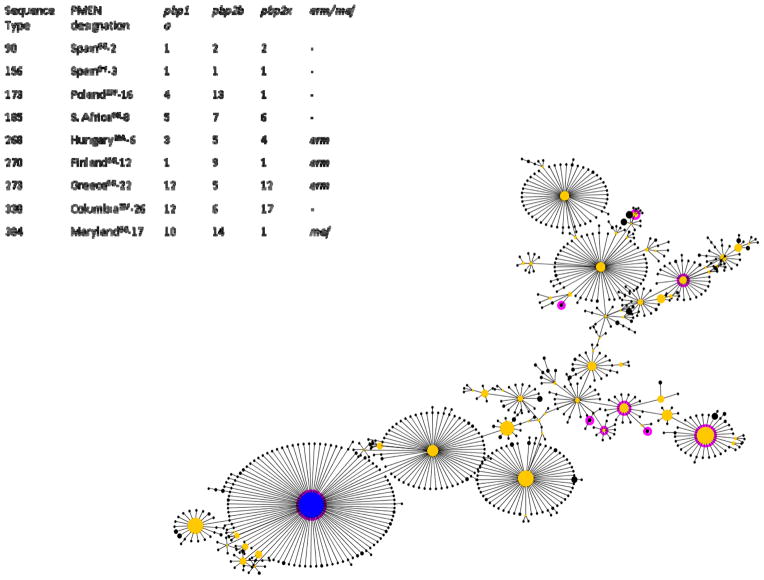
We can explore the relationships between the PMEN clones and other pneumococci using eBURST (Feil et al., 2004). Figure 3 shows the largest clonal complex presently to be found in the MLST database, which descends from ST 156 (itself the Spain9V-3 PMEN clone). Analyses of this nature must be undertaken with caution due to known biases in the submission of strains to the database, and the potential for recombination to obscure the history of a group of strains (Turner et al., 2007). Nevertheless, those clones that are closely linked by eBURST indisputably share a recent common ancestor, even if their precise relationships are difficult to discern. With this in mind it is interesting to note that no fewer than 9 of the 27 recognized PMEN clones associated with resistance are to be found in this single clonal complex. Moreover, five of these clones, all originally noted with a 6B capsule, are evident in a relatively small cluster towards the right of the diagram.
Figure 3.
The largest clonal complex in the S. pneumoniae MLST database (CC156), accessed on 6/8/2010 and visualized using eBURST. Resistant PMEN clones are highlighted in dark pink, and their ST indicated. A cluster of 6B clones is evident at right. The pbp profiles and erm/mef data are drawn from the PMEN website at http://www.sph.emory.edu/PMEN/.
Given that these five are clearly closely related, we should ask whether they are genuinely five clones with separate origins, or one clone, which has subsequently diversified. The close relationship argues in favour of the latter interpretation, yet the pbp alleles also recorded by PMEN are distinct, suggesting that they are the product of independent events (Fig. 3).
This is also evident considering another clonal complex. CC15 contains three PMEN clones (Fig. 4) all of them associated with serotype 14. Again, it appears that distinct resistance profiles have emerged from this clonal complex on three occasions. In reality, these must be considered minimal estimates, as this analysis is based on the published profiles of the PMEN clones, which may not represent all the resistance phenotypes to be found in that clone in nature. A recent project using whole genome sequencing to probe the evolution of the PMEN 1 clone has produced evidence that this lineage has acquired macrolide resistance, due to both mef and erm elements, on multiple occasions (Bentley SD, Personal communication, 2010).
Figure 4.
The 3rd largest clonal complex in the S. pneumoniae MLST database (CC15), accessed on 6/8/2010 and visualized using eBURST. Resistant PMEN clones are highlighted in dark pink, and their ST indicated. All three PMEN clones in this CC are serotype 14. The pbp profiles and erm/mef data are drawn from the PMEN website at http://www.sph.emory.edu/PMEN/.
Figure 5 shows the location of the resistant PMEN clones in the entire MLST database. As noted, they are concentrated in a few lineages. Given that resistance loci seem so ‘easy’ to obtain, it is hard to explain why this should happen in some lineages and not others, unless the cost of acquiring resistance is not constant across the pneumococcal population. This possibility is discussed further below.
Figure 5.
Population snapshot of the entire S. pneumoniae MLST database accessed on 6/8/2010 and visualized using eBURST. Resistant PMEN clones are highlighted in dark pink. CC 156 and CC 15 are indicated
Pneumococcal vaccination: old clones, new serotype
The development of a conjugate vaccine against seven pneumococcal serotypes has been a watershed in the control of pneumococcal disease. The vaccine is highly effective against invasive disease of vaccine serotype (Black et al., 2000), and with a 50% efficacy against carriage of those serotypes (Ghaffar et al., 2004). In communities with high coverage, the targeted serotypes have dwindled to the point where they are at a tiny fraction of their prior prevalence, in carriage and disease. Because these serotypes were previously associated with the majority of antibiotic resistance, their decline has had concomitant benefits in this regard (Kyaw et al., 2006).
If we assume that vaccine type strains are sufficiently disadvantaged that they have no long term future in vaccinated communities, there are two means by which any resistance loci associated with them might persist through recombination: either resistant vaccine type strains might generate escape variants through serotype switching to gain a new non-vaccine serotype, or the resistance loci themselves might be transferred into a new non-vaccine type background. Several cases of the former have been identified, and are discussed below. In the US resistant non-vaccine type (NVT) strains have been increasingly common following vaccination (Farrell et al., 2007; Gertz et al., 2010; Hanage et al., 2007; Beall et al., 2011)
Following PCV-7 introduction in the US in 2000, the Centers for Disease Control Active Bacterial Core Surveillance has undertaken surveillance of the pneumococcal population causing invasive disease in order to examine the impact of the vaccine. The results form a remarkable body of work, which can only be briefly described here for reasons of space. We will discuss three PMEN clones that were common causes of invasive disease prior to vaccination, and were also associated with antibiotic resistance: Spain23F-1, Spain9V-3 and Taiwan19F-14 (McGee et al., 2001). Using MLST these and related strains may be assigned to ‘clonal complexes’ (CCs). The respective CCs for these clones are CC 81, CC 156 and CC 236.
All three clones have persisted into the post-vaccine population with a new serotype not targeted by the vaccine, and in each the predominant serotype is 19A. The most successful and clinically significant is ST 320 (Beall et al., 2006; Beall et al., 2011; Brueggemann et al., 2007; Pai et al., 2005b; Pelton et al., 2007; Techasaensiri et al., 2010), which is a close relative of ST 236, Taiwan19F-14. In addition to an important role in invasive pneumococcal disease (IPD), ST 320 is increasingly common in carriage (unpublished observations). It maintains resistance to multiple antibiotic classes. As noted above, pneumococci are a frequent cause of otitis media, and a variant of the Spain9V-3 clone (ST 2722) has been documented which exhibits resistance to all FDA approved antimicrobials for treatment of acute otitis media (AOM) (Pichichero & Casey, 2007a). Alongside the 19A variant, 11A and 15C capsules have been found among isolates closely related to this clone (Xu et al., 2009b), illustrating that serotype switching has already produced escape variants which will not be covered by the next generation of conjugate vaccines. While 19A variants of the Spain23F-1 clone (ST 81) have been found, and indeed considerably predate the introduction of PCV-7 (Coffey et al., 1998), they do not seem common. Despite this, preliminary results of a whole genome analysis suggest that the 23F to 19A switch has occurred at least three times (Croucher et al., 2011) again underlining the capacity of resistant pneumococci to evade vaccine pressure.
In at least one case in which a successful non-vaccine type version of a PMEN clone has emerged (a 23A variant of the Columbia23F-26 clone (Pai et al., 2005a) it is clear that the vaccine escape clone was already present before vaccination. In most other cases it is unknown whether this was the case. It may be important to note that while ST 320 is a close relative of ST 236, it was not particularly common in the US with a 19F capsule prior to vaccine introduction (Gertz et al., 2003). As such it is likely to represent an import from elsewhere.
Alongside the cases of serotype switching from well-known PMEN clones, antimicrobial resistance has been increasing among NVT strains that were not previously associated with vaccine serotypes. Among these, the 19A ST 199, the 35B ST 558 and the 6C ST 1379, are particularly noteworthy (Gertz et al., 2010; Hanage et al., 2007; Richter et al., 2009).
At the time of writing, conjugate vaccines against 7 to 13 serotypes are being implemented around the world. As a result the pneumococcal population, and pneumococcal population biology, are in a state of flux. PCV-7 was introduced into the US in 2000. In the decade since then many formerly prominent vaccine type clones have almost disappeared in the face of vaccine pressure. Others have persisted through serotype switching, and yet others have emerged from obscurity. Given differences in vaccine availability, vaccination schedules, and the pneumococcal population in different parts of the world, it would be foolish to make hard and fast predictions as to which clones will be important in the future.
The survival of lineages such as ST 320, and ST 2722 through serotype switching should teach us a sobering lesson in the potential of this pathogen to respond to diverse selective pressures. When considering which clones are at high risk for dissemination of resistance, we should at least consider the possibility that some clones are a higher risk than others, not as a result of resistance itself, but as a result of their ability to acquire it.
Why resistance in S. pneumoniae?
A survey of the literature can make it seem that resistant pneumococcal isolates are overwhelmingly common, but this reflects our clinical focus. No matter how interested we might be in resistant clones, and for good reasons, the majority of pneumococci remain susceptible to all classes of antibiotics. Moreover, as shown in figures 3–5, the PMEN clones and resistance to multiple antibiotics are concentrated in a few lineages. Unlike S. aureus, pneumococcal disease is more commonly acquired in the community than in health care settings, and so it is hard to suggest that multiple resistance represents adaptation to a specific niche, or if it is, the niche in question is not so well defined.
One recent proposal is that the probability of recombination is not constant throughout the pneumococcal population (Hanage et al., 2009). If some pneumococci are more likely to undergo recombination than others, then they are more likely to acquire resistance determinants. This can be seen as an idea similar to the proposal that bursts of hypermutation can allow bacteria to scale local fitness peaks. In both cases, it is thought that maintaining a high rate of recombination or mutation is detrimental in the long term, but for a short period it may be adaptive. There is some evidence for this in the observation that strains with MLST loci that are identified as having been imported from a distinct and divergent genetic background (ie through recombination), are significantly more likely to be resistant to antibiotics (Hanage et al., 2009).
Streptococcus pyogenes
S. pyogenes is a human-specific pathogen that causes disease throughout the world, ranging in severity from mild superficial infections at the epithelium of the throat or skin (pharyngitis, impetigo), to life threatening invasive disease (egs., bacteremia, toxic shock syndrome, necrotizing fasciitis). There are an estimated 730 million cases of pharyngitis and impetigo per year caused by S. pyogenes (Carapetis et al., 2005). In addition, S. pyogenes can colonize the throat in the absence of disease; carriage rates can exceed 30% in school-aged children (Kaplan, 1980). Included among the important ecological features of S. pyogenes is its narrow range of biological hosts (i.e., humans), high prevalence of colonization and disease throughout the world, and direct person-to-person transmission.
Resistance development in S. pyogenes
S. pyogenes stands apart from most other gram positive cocci pathogens in that it has not acquired natural resistance to the β-lactam class of antibiotics. Penicillin treatment failure is sometimes attributable to co-colonization with a β-lactamase-producing bacterium of another species (eg., S. aureus) (Quie et al., 1966; Brook & Gober, 2008). “Tolerance” of S. pyogenes to penicillin has been sporadically observed; however, the mechanistic underpinnings have yet to be explained (Pichichero & Casey, 2007b). Why S. pyogenes has (luckily) failed to evolve resistance to this drug remains a mystery (Horn et al., 1998). The lack of β-lactamase acquisition by S. pyogenes is difficult to explain by genetic barriers alone because they share with staphylococci other highly homologous resistance genes carried by mobile genetic elements. The failure of S. pyogenes to evolve genes encoding penicillin-binding proteins having a lower binding affinity for the drug might be explained by reduced fitness (eg., impaired peptidoglycan biosynthesis) that exacts a large biological cost. β-lactams remain the antibiotics of choice for the treatment of most infections due to S. pyogenes.
Macrolides are the drug of choice for patients with β-lactam allergies or a prior treatment failure. Clindamycin (a lincosamide) is preferred for patients with life-threatening soft tissue infections such as toxic shock syndrome or necrotizing fasciitis because it has good tissue penetration and quickly halts synthesis of the exotoxin that is responsible for much of the disease pathology. Clinically significant macrolide resistance in S. pyogenes emerged during the mid-1970s and has gradually become widespread (Seppala et al., 1997; Gerber, 1996; Perez-Trallero et al., 1999; Yan et al., 2000; Cha et al., 2001; Espinosa de los Monteros et al., 2001; Syrogiannopoulos et al., 2001; Cresti et al., 2002; Martin et al., 2002; Alos et al., 2003). In local surveys, resistance rates sometimes exceed 30% and can climb even higher. Importantly, there is a strong correlation between macrolide consumption and levels of resistance among S. pyogenes (Fujita et al., 1994; Seppala et al., 1997; Freeman & Shulman, 2002; Garcia-Rey et al., 2002; Albrich et al., 2004; Gagliotti et al., 2006; Hsueh et al., 2006).
Resistance to macrolides in S. pyogenes is mostly attributable to the erm(A), erm(B) and mef(A) genes; less common determinants are other forms of erm and mef genes and ribosomal mutations. The erm gene products lead to ribosome modification and the MLS phenotype that includes resistance to lincosamides, whereas the mef gene confers macrolide efflux and the M phenotype, resulting in resistance to macrolides but susceptibility to lincosamide and streptogramin B antibiotics (Sutcliffe et al., 1996). Mobile genetic elements harboring one of the macrolide resistance genes, sometimes coupled with a gene for tetracycline resistance, have been well characterized (Banks et al., 2004; Beres & Musser, 2007; Varaldo et al., 2009; Brenciani et al., 2010). In common with the above sections dealing with enterococci and pneumococci, many of the genetic elements are present in other streptococcal species, indicative of a larger network for interspecific movement of resistance genes.
Assigning S. pyogenes clones based on sequenced-based emm typing and MLST
The S. pyogenes population displays a very high level of genetic diversity (Bessen, 2009). The hair-like surface fibrils, known as M proteins, are targets of a protective host immune response; organisms undergo immune escape via diversifying selection of emm genes. M proteins provide the basis for classical serotyping of S. pyogenes. More recently; this scheme was replaced with emm sequence-based typing (www.cdc.gov/ncidod/biotech/strep/strepindex.htm) (Beall et al., 1996) and today, emm typing is almost universally used for molecular analysis of S. pyogenes isolates obtained via population-based epidemiological surveillance, with > 200 emm types identified.
In a large multicenter study of pediatric cases of pharyngitis in the USA over 3 years (2000–2003), ~3,000 S. pyogenes isolates were obtained and of these, 4.1% were macrolide resistant; 70% of resistant isolates harbored the mef(A) gene (Tanz et al., 2004). The most prevalent emm type among the macrolide resistant isolates was emm12, followed by emm75 and emm4, which together accounted for more than half of the resistant isolates; the emm75 isolates all harbor erm(A), whereas emm12 and emm4 isolates are associated with > 1 resistance gene type, indicative of distinct clones. Two parallel but independent studies show dominance of emm75, emm12, emm4 and/or emm58 genotypes among macrolide resistant S. pyogenes isolates, with overall resistance rates of ~6% (Richter et al., 2005; Green et al., 2006). Other dominant emm types associated with macrolide resistance in local surveys include emm1, emm2, emm6, emm22, emm77, and emm89 (Jasir et al., 2001; Dicuonzo et al., 2002; Martin et al., 2002; Katz et al., 2003; Zampaloni et al., 2003; Creti et al., 2007; Chan et al., 2009; Michos et al., 2009; Meisal et al., 2010).
For many strains, emm type does not correlate with clone (Enright et al., 2001; Bessen et al., 2008). Clones of S. pyogenes are more precisely defined by a combination of emm type and sequence type (ST) based on alleles at multiple housekeeping loci. To date, MLST of S. pyogenes reveals 485 STs, organized into 73 clonal complexes with 207 singleton STs, as predicted by eBURST (http://spyogenes.mlst.net/).
For macrolide-resistant S. pyogenes, the combination of emm type, ST and resistance gene type provides a good working definition of clone. The MLST database, which is publicly available, contains genotyping data for > 400 macrolide resistant isolates, deposited by numerous investigators (Perez-Trallero et al., 2004; Reinert et al., 2004; Szczypa et al., 2004; Littauer et al., 2006; Montes et al., 2006; Robinson et al., 2006; Silva-Costa et al., 2006; Perez-Trallero et al., 2007; Strakova et al., 2007; Silva-Costa et al., 2008). According to the MLST database (http://spyogenes.mlst.net/), 100 unique combinations of ST, emm type and resistance gene type [erm(A), erm(B) and/or mef] can be identified. Based on analysis of single locus variants (SLVs), as many as 73 genotypes may represent independent acquisitions of a resistance gene, whereas 27 of the clones may have arisen from another resistant clone via diversification of housekeeping alleles (Robinson et al., 2006). Thus, the population of macrolide-resistant S. pyogenes has extensive diversity, whereby new resistant clones have emerged on a wide variety of genetic backgrounds.
Phylogeography of antibiotic resistant S. pyogenes clones
The geographic location from which a macrolide resistant clone was isolated can provide the basis for estimating the relative prevalence of individual clones, whereby a wide geographic distribution for a clone is an indicator of its transmission success. Of the fully genotyped macrolide-resistant S. pyogenes in the current MLST database (http://spyogenes.mlst.net/), isolates were recovered from Europe (70.5%), North America (12.8%), Asia (11%), South America (3.2%), Oceania (1.4%), and Africa (1.1%); overall, 35 countries are represented. Only one clone [emm94/ST89/erm(A)] was recovered from all six major continents (Table 1). Other widely dispersed clones, each recovered from five continents, are emm4/ST39/mef(A) (17 countries), emm12/ST36/mef(A) (16 countries) and emm58/ST176/erm(A) (10 countries). The emm4/ST39/mef(A) clone is the predicted founder of a clonal complex having five additional STs, whereby all clones harbor emm4 and mef(A); thus, the emm4/ST39/mef(A) clone has diversified at multiple housekeeping loci, perhaps signifying that it is among the oldest and most successful of macrolide resistant S. pyogenes clones. All three resistance gene types are represented among widely dispersed clones of macrolide resistant S. pyogenes, and two emm-ST genotypes are associated with > 1 resistance gene type (emm12-ST36 and emm22-ST46) (Table 1). The oldest clone in the MLST collection is emm12/ST36/erm(B), recovered in Canada in 1976. Within more localized outbreaks, there is often a single highly prevalent strain, such as emm11/ST43/erm(B) in Gipuzkoa, Spain (Perez-Trallero et al., 2007) and emm6/ST382/mef(A) in Pittsburgh, USA (Martin et al., 2002; Banks et al., 2003).
Table 1.
The 12 most widely dispersed clones of macrolide resistant S. pyogenes.
| emm type | Resistance gene | ST | No. of countries | No. of continents | SLVs sharing the same emm type and resistance gene as the founder clone |
|---|---|---|---|---|---|
| 94 | erm(A) | 89 | 12 | 6 | None |
| 4 | mef(A) | 39 | 17 | 5 | ST38,373,421,422,423 |
| 12 | mef(A) | 36 | 16 | 5 | None |
| 58 | erm(A) | 176 | 10 | 5 | None |
| 12 | erm(B) | 36 | 9 | 3 | None |
| 22 | erm(B) | 46 | 8 | 3 | None |
| 73 | erm(A) | 331 | 5 | 3 | None |
| 1 | erm(B) | 28 | 5 | 3 | None |
| 75 | mef(A) | 49 | 5 | 3 | ST388,548 |
| 22 | mef(A) | 46 | 3 | 3 | ST389 |
| 28 | erm(B) | 52 | 11 | 2 | ST244 |
| 77 | erm(A) | 63 | 10 | 2 | ST369 |
Nine of the 10 emm types (all except for emm1) belonging to the widely dispersed, macrolide-resistant strains (Table 1) are typically associated with the sof gene, which lies ~10 kb upstream from the emm locus and is present in ~50% of strains. Sof encodes a multifunctional surface protein that can bind human fibronectin (note - the sof gene may not encode a fully functional product in emm12 strains). A strong correlation between the sof gene and macrolide resistance in S. pyogenes was previously noted (Dicuonzo et al., 2002). Perhaps related to the strong association with sof is the finding that erm-positive strains bear significant positive correlations with other fibronectin-binding proteins and the ability to invade epithelial cells, and negative correlations with biofilm formation (Facinelli et al., 2001; Baldassarri et al., 2007; Hotomi et al., 2009), in at least some studies.
Fluoroquinolone resistance has recently emerged among S. pyogenes, due to mutations in the parC and/or gyrA locus. A highly prevalent, non-susceptible emm6/ST382 clone was identified in numerous studies in many parts of the world, although most isolates appear to have low-level resistance (Alonso et al., 2005; Orscheln et al., 2005; Powis et al., 2005; Yan et al., 2008; Malhotra-Kumar et al., 2009; Smeesters et al., 2009; Montes et al., 2010; Pires et al., 2010). Other emm types associated with fluoroquinolone resistance include emm1, emm5, emm28, emm75 and emm89. The close relative of S. pyogenes - Streptococcus dysgalactiae subspecies equisimilis - may be a source for some parC and gyrA mutations (Pinho et al., 2010).
It is unusual for tetracycline to be administered for the treatment of a S. pyogenes infection, however, the widespread use of this antibiotic in general creates strong selection pressures for the emergence of a resistant clone. Indeed, in one study on a genetically diverse set of S. pyogenes strains, tetracycline resistance is estimated to have been acquired in > 80 independent genetic events (Ayer et al., 2007). Because of its minimal clinical impact, relatively little strain typing has been done on tetracycline-resistant S. pyogenes, and it remains largely unknown as to which tetracycline-resistant clones are among the most prevalent. The majority of tetracycline resistance in S. pyogenes is conferred by the tet(M) or tet(O) genes; these genes have been found within the same mobile genetic element as erm or mef genes (Varaldo et al., 2009; Brenciani et al., 2010). Because resistance to tetracycline is often more prevalent among S. pyogenes as compared to macrolide resistance, it follows that tetracycline usage for other diseases may facilitate the acquisition of macrolide resistance via co-inheritance of resistance genes (Nielsen et al., 2004).
Since macrolide consumption in the community is a strong predictor of the prevalence of macrolide resistant strains, the resistant clones that have come to dominate may have emerged simply by being in the right place at the right time. A meta-analysis of >38,000 S. pyogenes global isolates reveals that the most common emm types (in decreasing order) are emm1, emm12, emm28, emm3 and emm4 (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm) (Steer et al., 2009); with the exception of emm3, the highly prevalent emm types are also associated with macrolide resistance (Table 1). However, the opposite does not always hold true. The most widely dispersed macrolide-resistant clone is emm94; this emm type is relatively rare throughout the world when all GAS isolates - susceptible and resistant - are considered. Thus, the finding on emm94 clones is consistent with the idea that, at least in this one instance, macrolide resistance coincides with a large leap in transmission success.
Staphylococcus aureus
S. aureus asymptomatically colonises the skin and anterior nares (nostrils) of approximately one third of the human population at any given point in time (Peacock et al., 2001; Nulens et al., 2005; van Belkum et al., 2006). Although primarily a commensal species, asympomatic nasal carriage is known to be associated with infection (Deleo et al., 2010), which leads to a range of conditions from boils to life-threatening endocarditis. S. aureus is traditionally considered a nosocomial pathogen, as serious infections are more common within health-care settings, such as hospitals and nursing homes, than in the community. However, S. aureus is not limited to colonizing humans, and can cause infection in livestock and companion animals including cows, sheep, goats, buffalo, pigs and chickens (Aires-de-Sousa et al., 2007; Vanderhaeghen et al., 2010; van Belkum et al., 2008; Lowder et al., 2009). Although the true ecological range in non-human hosts is not known, there is increasing awareness of the possible risks of zoonotic transmission from livestock (Catry et al., 2010).
Disease management within health-care settings and, increasingly, within the community is substantially hampered by the rapid spread of resistance to β-lactam antibiotics. The first penicillinase producing S. aureus strains isolated from hospitalized patients were observed in 1942, just two years after penicillin was first used to treat wounded soldiers, and reported in 1944 (Kirby, 1944). The frequency of pencillin resistance rose rapidly after the World War II (Barber & Rozwasowska-Dowzenko, 1948); by 1947, 25% of strains from hospitalized patients were resistant, and this figure rose to >80% by the late 1950s (Jessen et al., 1969). For reasons that remain unclear, this frequency then levelled off, and has remained broadly consistent at 80% up to the present day. Resistance to penicillin in the community followed a similar trajectory but with an approximate 20 year lag. By the late 1950s to early 1960s, ~ 25% of isolates recovered from sporadic community-acquired infection were resistant to penicillin, by the mid 1970s this figure had risen to 70–85% (Chambers, 2001).
MRSA, emergence of a “superbug”
Methicillin was first introduced in 1959, but the first methicillin resistant S. aureus (MRSA) isolates were reported only two years later (Barber, 1961; Jevosn & Parker, 1964). Resistance to methicillin is conferred via the horizontal acquisition of a large (20–60Kb) chromosomal cassette (SCCmec), which is thought to have been introduced from naturally resistance commensal Staphylococcal species on multiple occasions (Enright et al., 2002). Different “types” of SCCmec element have been described, and the origin and evolution of these elements has been extensively discussed elsewhere (Oliveira & de Lencastre, 2002; Chongtrakool et al., 2006; Hanssen & Ericson Sollid, 2006; Deurenberg & Stobberingh, 2008; Deurenberg & Stobberingh, 2009). The increase of MRSA appears to be generally following a parallel trend to that observed previously for penicillinase producing strains; resistant strains initially gained a foothold within health-care settings, then over time resistance has also subsequently become an increasing problem in the community (Chambers, 2001; Deleo et al., 2010; Chambers & Deleo, 2009; Deleo et al., 2010).
The problems associated with methicillin resistance vary considerably across Europe and other parts of the world (Tiemersma et al., 2004). Data from the European Antimicrobial Resistance Surveillance System (EARSS; http://www.rivm.nl/earss/) for 2008 show the prevalence of MRSA from all cases of invasive disease ranging from <1% (Scandinavian countries and The Netherlands), to >50% (Portugal and Malta). Although the reasons behind this regional variation are not precisely known, differences in antibiotic usage and in the effectiveness of local infection control strategies in health care settings are likely to be important.
S. aureus has also evolved resistance to the glycopeptides vancomycin and teicoplanin, which are among the most important drugs for treating MRSA infections. These resistant strains emerged in the 1980s by two distinct mechanisms. First, chromosomal mutatations have been identified which confer a moderate increase to glycopeptides by increasing the thickness of the cell wall. (VISA/GISA phenotypes; (Howden et al., 2010)). More marked gycopetide resistance is noted in the VRSA strains, first reported in 2002 (Sievert et al., 2008). These strains have evolved though the acquisition of a van gene, which results in the synthesis of a modified peptidoglycan precursor. The prevalence of both VISA/GISA and VRSA strains have remained very low, probably due to the fitness costs associated with these genetic changes.
The newest drugs to treat MRSA are linezolid, daptomycin and tigecycline, and these remain broadly effective against VISA and VRSA strains. Although very rare, mutations in the 23S rRNA gene have been identified which reduce susceptibility to linezolid, and increasingly so as more gene copies are mutated (Besier et al., 2008). Perhaps more worringly, linezolid resistance can also be acquired horizontally through the acquisition of the cfr gene which encodes a protein which modifies (methylates) the target ribosomal gene (Long et al., 2006; Locke et al., 2010). Cfr-positive strains have recently caused nosocomial outbreaks in Spain (Sanchez Garcia et al., 2010; Morales et al., 2010). Further, this modification confers cross-resistance to phenicols, streptogramin A, lincosamides, oxazolidinones and pleuromultilins, which raises the problem of co-selection in both medical and veterinary settings where anti-ribosomal agenets may be used. It is noteworthy in this context that the plasmid harbouring the cfr gene was first noted in a strain of animal origin (Kehrenberg & Schwarz, 2006). There is also evidence for a link between reduced daptomycin susceptibility and the VISA phenotype (Wootton et al., 2006), possible related to changes in the thickness of the cell wall (Cui et al., 2006), although the potential clinical impact of this remains equivocal (Nannini et al., 2010).
Typing schemes used for S. aureus
Several typing methods have been used for epidemiological surveillance of this species, including pulse-field gel electrophoresis (PFGE), MLST (Enright et al., 2000; Cookson et al., 2007) and MLVA (multiple loci VNTR analysis, where VNTR stands for variable number of tandem repeats) (Melles et al., 2009; Schouls et al., 2009). VNTR loci are hyper-variable microsatellites, the most notable example in S. aureus being the spa gene which has been used extensively for typing studies in this species (Mellmann et al., 2008; Basset et al., 2009). Typing techniques based on variation within the different SCCmec elements is also widely used for differentiating different MRSA clones (Oliveira & de Lencastre, 2002; Chongtrakool et al., 2006). Whilst these methods present a range of utility for more detailed evolutionary analyses, they (more or less) consistently delimit the S. aureus population in to the same discrete clusters, or clonal complexes. There are exceptions however, such as agr groups (Robinson et al., 2005), and SCCmec typing. The latter approach is based on a highly mobile accessory element so does not always accurately reflect the genetic background (Nubel et al., 2008). Ultimately, a pluralistic approach of different approaches (e.g. MLST + spa typing + SCCmec typing), along with as much appropriate metadata as possible, provides the optimal utility (van Belkum et al., 2007).
The low rate of homologous recombination in S. aureus (Feil et al., 2003) is thought to explain in part how (if not why) the discrete clusters have emerged and been maintained. The consistent delineation of the same clonal complexes, even from gene content data generated using microarrays (Lindsay et al., 2006), underline that they are real biological entities, representing different fitness peaks, and as such are both of evolutionary interest and of epidemiological utility (Feil et al., 2003; Turner & Feil, 2007). These clusters can be visualised using UPGMA dendrograms or eBURST (Feil et al., 2004) (eburst.mlst.net). Extensive typing studies have revealed that hospital-acquired (HA-) MRSA isolates are particularly clonal, in fact since the 1960s only 5 clonal complexes (CCs 5, 8, 22, 45 and 30 can account for the vast majority of nosocomial infection world-wide (Crisostomo et al., 2001; Aires de Sousa et al., 2005; Gomes et al., 2006; Conceicao et al., 2007). These five clonal complexes are visualized using eBURST in Figure 6.
Figure 6.
The five major HA-MRSA containing clonal complexes as visualised by MLST/eBURST (CCs 45, 5, 8, 30, 22 shown in red). Names given to MRSA clones corresponding to highlighted STs are given in parentheses. Each circle is an ST. Linked STs differ at only one locus out of 7. The size of the circle reflects the frequency of the ST. Blue circles represent clonal founders, yellow circles are sub-founders. Full details concerning eBURST can be found at eburst.mlst.net.
Hospital and community acquired MRSA: Clinical, epidemiological and genotypic comparisons
Whereas HA-MRSA isolates have evolved from a small number of globally disseminated clonal groups, community-acquired (CA-) methicillin sensitive S. aureus (MSSA) and CA-MRSA isolates are more diverse than HA-MRSA. A recent impressive study by the European Staphylococcal Reference laboratory Working Group, led by Hajo Grundmann, characterized 2,890 MSSA and MRSA isolates from 26 European countries by spa typing (Grundmann et al., 2010). These data revealed fundamental differences in the geographic structure and diversity of MRSA and MSSA. The MSSA spa types were uniformly diverse and homogenously mixed throughout Europe, such that a large proportion of the overall variation was recovered on a local scale. Such a pattern is indicative of an endemic pathogen which has become established in a population over a long period of time. In contrast substantial geographic structure was apparent in the spa data for the MRSA isolates, such that specific types tend to be predominantly observed in particular countries, and only a small fraction of the overall diversity is observed on a local scale. This structuring was particularly apparent for the HA-MRSA isolates, and illustrates how these clones have spread through recent and rapid epidemic expansions through public health networks on a local scale.
In addition to being distinct from MSSA, HA-MRSA have historically been genetically and epidemiologically distinct from CA-MRSA, although these distinctions are now being eroded. The earliest HA-MRSA clones, such as those corresponding to ST239 (UK EMRSA-1, -4, -7, -11, the Brazilian, Portuguese and Viennese clone)are multiply resistant due to the acquisition of the large type III SCCmec element, and the rarity with which they are observed outside of health-care settings points to their adaptation to a hospital environment. In contrast, CA-MRSA isolates are more likely to be susceptible to non-β-lactam antibiotics as they harbour smaller SCCmec elements (e.g. type IV) (Feng et al., 2008; Deurenberg & Stobberingh, 2009). However, these smaller cassettes may impose a smaller fitness burden, both in vitro and in vivo (Okuma et al., 2002; Lee et al., 2007; Diep et al., 2008). Thus, to use an analogy, if HA-MRSA is burdened by a suit of armour, CA-MRSA can be thought of as wearing a bullet-proof vest, as a means of optimizing the trade-off between protection and competitiveness. For an excellent and comprehensive review of the distinct “waves” of MRSA infection, see (Chambers & Deleo, 2009).
Whilst ST239 remains the most common HA-MRSA clone globally, it has long since been replaced by other clones in Western Europe. Currently disseminated HA-MRSA clones in Europe include ST22 (EMRSA15), ST36 (EMRSA16), ST225 and ST228. These are less multiply resistant than the earliest HA-MRSA, showing resistance mostly to β-lactams, fluoroquinolones, and Macrolide-Lincosamide-Streptogramin (MLS), and have more commonly acquired SCCmec elements other than the large stereotypical HA-MRSA types I-III. The resistance profiles of these currently circulating HA-MRSA clones are broadly similar to the most common CA-MRSA clones, such as the epidemic USA300 (ST8), and the clones making up the more hetergenous population of CA-MRSA currently circulating in Europe. The most common European CA-MRSA clone is currently ST80-IV (the European clone) (Otter & French, 2010) which has been reported throughout central Europe (Vandenesch et al., 2003; Denis et al., 2005; Witte et al., 2005) Scandanavia (Hanssen et al., 2005; Fang et al., 2008) and Greece (Katopodis et al., 2010). This clone is characteristically resistant to fusidic acid, tetracycline, kanamycin and variably resistant to ciprofloxacin. It is PVL-positive and possibly originated from the Middle East or North Africa (Goering et al., 2009; Otter & French, 2010). Other CA-MRSA clones of note circulating in Europe include the PVL-positive ST152 clone (Perez-Roth et al., 2010) which is circulating throughout central Europe and the Balkans. The high frequency of ST152 in asymptomatic carriage in Mali (Ruimy et al., 2008) and from clinical isolates in Nigeria (Okon et al., 2009) points to sub-Saharan Africa as the possible origin of this clone, a scenario which points to the acquisition of an SCCmec element into an already PVL-positive background (Perez-Roth et al., 2010).
Typically there are predisposing risk factors for HA-MRSA infection. Although risk factors such as socioeconomic status and drug usage are clearly implicated in CA-MRSA (Bratu et al., 2006), these infections commonly cause serious skin and soft tissue infections in otherwise healthy individuals (Young et al., 2004). In the United States, CA-MRSA isolates recovered from sporadic cases of disease before 2001 corresponded to CC1 (USA400). This group has subsequently been largely replaced by USA300 (CC8), which is currently the leading cause of CA bacterial infections in this country (Kennedy et al., 2008; Miller & Diep, 2008). USA300 isolates have acquired elements which have been suggested to play a key role in virulence, notably the prophage encoded toxin, Panton-Valentine Leukocidin (PVL) and the arginine catabolic mobile element (ACME) (Diep et al., 2006; Diep et al., 2008). However, it has recently been reported that the USA300 lineage (and its progenitor lineage USA500) acquired increased virulence through changes in expression of core virulence genes, a finding which challenges the paradigm that virulence primarily results from the acquisition of new genes (Li et al., 2009)). USA300 has also acquired increased resistance, and has begun to spread outside of the USA (Tenover & Goering, 2009). In regions where cases of CA-MRSA are high, such as Taiwan and the USA, an increasing number of hospital-acquired infections are being caused by typical CA-MRSA clones such as USA300 (Klevens et al., 2006; Huang et al., 2007), a clear sign that the epidemiological distinction between HA-MRSA and CA-MRSA is beginning to break down (Deurenberg & Stobberingh, 2008).
Livestock-associated MRSA, a new threat?
In addition to HA-MRSA and CA-MRSA, recent attention has begun to focus on livestock-associated (LA-) MRSA. Of particular note is ST398 which is a common LA-MRSA associated with pigs. First reported in 2005, the distribution of ST398 has hitherto largely been restricted to Holland, Belgium, Northwest Germany and Denmark, although this clone has started to spread to other parts of the world (Golding et al., 2010). In addition to SCCmec, LA-MRSA ST398 have also typically acquired resistance to tetracycline, an antibiotic intensively used in pig farming. Nasal colonization of ST398 has been recorded in farmers and veterinarians, who come into close contact with pigs (Wulf & Voss, 2008), although the public health risk associated with this clone is unclear as a recent report has suggested it is poorly transmissible within the hospital environment (Wassenberg et al., 2010). Interestingly, a high frequency of asymptomatic paediatric carriage of MSSA ST398 has been noted in China (Fan et al., 2009), raising the possibility that a susceptible predecessor of this clone was imported from this country.
Next-generation sequencing illuminates the microevolution and transmission of MRSA clones
The current typing schemes have been successful in revealing the changing frequencies of the clusters over time and space (over years and decades, and on both national and international scales). However, these datasets still tend to lack the discrimination required for tracking strain transmission patterns on local scales (within and between hospitals) over short time scales (weeks and months). This is because a very small number of genotypes tend to predominate at a given location at any given point in time, a result of sequential waves of infection (de Lencastre et al., 2007). Thus more powerful techniques are required to resolve sufficient variation within clonal complexes. Nubel et al addressed this using a mutation discovery procedure to identify SNPs in ~45.5Kb (1.6% of the genome) in 135 S. aureus CC5 isolates from 22 countries (Nubel et al., 2008). These data pointed to multiple independent acquisitions of the SCCmec element, even within a single clonal group. The authors argued against the prevailing model of the rapid global dissemination of a few MRSA strains, in favour of a model of frequent emergence of “home-grown” locally restricted MRSA clones, some of which happen to all belong to the same globally disseminated clone. Such a model is consistent with the strong geographic structuring of MRSA across Europe (Grundmann et al., 2010)). A more recent paper using similar methodology focused on a single sub-cluster within the broader CC5 group, called ST225 (Nubel et al., 2010). The universal presence of a defining deletion within SCCmec showed that this cluster corresponds to a single acquisition of this element, and the authors were able to posit that this sub-group was introduced into central Europe from the USA approximately a decade ago, and has subsequently spread rapidly between hospitals.
The first clone to be characterized on a genome-wide basis using next-generation sequencing was ST239 (Harris et al., 2010). All ST239 isolates are resistant to methicillin (MRSA) and possess the large type III SCCmec cassette which confers multiple resistance. ST239 is responsible for ~90 % of hospital-acquired MRSA infection throughout most of mainland Asia (from the Middle-East to China), and much of South America (Diekema et al., 2001; Chongtrakool et al., 2006; Xu et al., 2009a). ST239 was also the predominant MRSA clone in Western Europe during the 1980s and 1990s, although it has subsequently been largely replaced by other strains (Conceicao et al., 2007) it is still circulating in Eastern Europe. Harris et al characterized 62 ST239 isolates, 42 of which were globally representative, whilst the remaining 20 were recovered from patients in a single hospital in northeast Thailand over a 7 month period (Harris et al., 2010). The study was thus designed to address both the global diversity of this clone and the utility of next-generation sequencing for very localised epidemiology.
These data point to a European origin of ST239 in the mid 1960s, and a single introduction of ST239 into South America followed by dramatic clonal spread. The data also confirmed that a wave of infection caused by ST239 in Portugal in the late 1990s was sparked by a transmission event from South America. More surprisingly, the data pointed to a South East Asian origin of the sequenced reference strain (TW20) which recovered from an outbreak in a London hospital (Edgeworth et al., 2007; Holden et al., 2010). Most notably, however, the study supports the possibility that this approach could inform on transmission chains even at the scale of a single hospital, which has clear implications for infection control (Harris et al., 2010). These data also point to an unusually high transmissibility of this clone within and between hospitals. When combined with multiple resistance and high virulence (Amaral et al., 2005; Edgeworth et al., 2007), it is highly likely that ST239 currently represents a greater public health burden globally than any other MRSA clone (Smyth et al., 2010).
By synthesizing the data from the recent studies discussed, the dynamics of HA-MRSA clones are clearly characterized by occasional international or intercontinental transmission combined with subsequent rapid localized clonal expansion. However, the relative importance of the de novo emergence of new HA-MRSA variants over transmission into a locale from elsewhere remains contentious, and may even vary between clonal lineages. Whereas the data of Nubel point to rapid and recurrent acquisitions of SCCmec variants within a single clonal group (Nubel et al., 2008), the data of Harris et al point to a stable association between a type III SCCmec type and ST239, a globally disseminated global lineage, over at least four decades (Harris et al., 2010). Although sub-variants are detected within the type III SCCmec, this observation probably reflects the high transmissibility, virulence and resistance particular to the ecology and epidemiology of ST239.
Concluding remarks
MLST schemes developed and applied to diverse strain collection from various ecological sources and geographic locations has not only improved world-wide tracking of clinically relevant (i.e particularly virulent or resistant) circulating enterococcal, pneumococcal, group A streptococcal and staphylococcal clones but has also provided a more detailed insight into the population structure of these pathogens. MLST revealed extensive genetic variation within these low GC Gram-positive bacteria with profound differences in the mechanisms driving genetic variation. In S. aureus genetic variation is mainly driven by mutation which means that the core genome of S. aureus clonal lineages is relatively stable with gene flow structured according to clonal complexes and with rare recombination events between clonal complexes. This implies that S. aureus clonal complexes defined by MLST probably represent ancient lineages in which, over time virulent and or resistant variants have arisen through changes in the accessory genome resulting in a wide range of resistance and virulence potential (Turner & Feil, 2007). One might even argue that these ancient clonal lineages correspond to ‘biological species’ which may explain the existence of host-specific clonal complexes like CC151 and CC133, predeominantly associated with cows and small ruminents (sheep and goat) (Guinane et al., 2010). In contrast, high rates of recombination drive genetic diversity in the strict human pathogens S. pyogenes and S. pneumoniae. In these species each ST represent lineage of recent age and therefore more accurately reflect virulence and or resistance potential. In both species hyper-virulent or resistant clones can be distinguished (e.g. S. pneumoniae PMEN1 or S. pyogenes M1T1), that have evolved rapidly the last 40 years through recombination. Natural competence explains high rates of recombination in S. pneumoniae while the mechanisms underlying genetic change in S. pyogenes involve transduction and conjugation. The biological characteristics of enterococci resemble that of S. aureus as well as that of S. pneumoniae and S. pyogenes. Like S. aureus, enterococci exhibit a broad host range but like S. pneumoniae and S. pyogenes, diversification has largely been driven by recombination rather than mutation. Genetic exchange in enterococci mainly involves mobilization and conjugation of plasmids that can be accompanied by chromosome-chromosome transfer of resistance and virulence but also of MLST genes (Manson et al., 2010). High recombination rates in enterococci leading to a rapidly changing core genome, combined with the presence of host-specific STs, at least in E. faecium, indicates that STs in enterococci represent relatively recent lineages and that these genetic lineages are more likely to reflect recent changes in the accessory genome, such as acquisition of adaptive elements required for succesful maintenance in the hospitalized patients in the typical hospital-derived clones.
A systems biology approach integrating transcriptome, proteome, metabolome analysis and functional studies including animal infection models and human patient studies will increasingly give us insights in the population structure of E. faecium, E. faecalis, S. pneumoniae, S. pyogenes, and S. aureus, will reveal the extent of lateral gene transfer and identify genetic lineages that are particularly enriched in antibiotic resistant isolates that are clinically relevant. In addition and boosted by the rapid expansion in sequencing capacity and reduction in costs through the development of novel next-generation sequencing techniques, an increasing number of staphylococcal, streptococcal and enterococcal whole genome sequences will become available providing the opportunity to obtain insights into population genomics of these species and molecular correlates for disease specificity. This may open up avenues for novel intervention approaches.
Acknowledgments
We are thankful to M. Bonten and W. van Schaik for critical reading and helpful discussions. This publication made use of the Multi Locus Sequence Typing website (http://www.mlst.net) at Imperial College London developed by David Aanensen and funded by the Wellcome Trust. RW work was supported by a FP6 grant of the European Commission (LSHE-CT-2007-037410). DEB received support from the National Institutes of Health (AI-061454, GM-60793) and EJF by a FP7 grant (HEALTH #223031[TROCAR]). WPH received support from the National Insititutes of Health (GM088558-01).
Reference List
- 1.2004 National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 2.Aanensen DM, Mavroidi A, Bentley SD, Reeves PR, Spratt BG. Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J Bacteriol. 2007;189:7856–76. doi: 10.1128/JB.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aires de Sousa M, Conceicao T, Simas C, de Lencastre H. Comparison of genetic backgrounds of methicillin-resistant and -susceptible Staphylococcus aureus isolates from Portuguese hospitals and the community. J Clin Microbiol. 2005;43:5150–7. doi: 10.1128/JCM.43.10.5150-5157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aires-de-Sousa M, Parente CE, Vieira-da-Motta O, Bonna IC, Silva DA, de Lencastre H. Characterization of Staphylococcus aureus isolates from buffalo, bovine, ovine, and caprine milk samples collected in Rio de Janeiro State, Brazil. Appl Environ Microbiol. 2007;73:3845–9. doi: 10.1128/AEM.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albrich WC, Monnet DL, Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis. 2004;10:514–7. doi: 10.3201/eid1003.030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso R, Mateo E, Galimand M, Garaizar J, Courvalin P, Cisterna R. Clonal spread of pediatric isolates of ciprofloxacin-resistant, emm type 6 Streptococcus pyogenes. J Clin Microbiol. 2005;43:2492–3. doi: 10.1128/JCM.43.5.2492-2493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alos JI, Aracil B, Oteo J, Gomez-Garces JL. Significant increase in the prevalence of erythromycin-resistant, clindamycin- and miocamycin-susceptible (M phenotype) Streptococcus pyogenes in Spain. J Antimicrob Chemother. 2003;51:333–7. doi: 10.1093/jac/dkg100. [DOI] [PubMed] [Google Scholar]
- 8.Amaral MM, Coelho LR, Flores RP, Souza RR, Silva-Carvalho MC, Teixeira LA, Ferreira-Carvalho BT, Figueiredo AM. The predominant variant of the Brazilian epidemic clonal complex of methicillin-resistant Staphylococcus aureus has an enhanced ability to produce biofilm and to adhere to and invade airway epithelial cells. J Infect Dis. 2005;192:801–10. doi: 10.1086/432515. [DOI] [PubMed] [Google Scholar]
- 9.Appelbaum PC. World-wide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol. 1987;6:367–77. doi: 10.1007/BF02013089. [DOI] [PubMed] [Google Scholar]
- 10.Ayer V, Tewodros W, Manoharan A, Skariah S, Luo F, Bessen DE. Tetracycline resistance in group a streptococci: emergence on a global scale and influence on multiple-drug resistance. Antimicrob Agents Chemother. 2007;51:1865–8. doi: 10.1128/AAC.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldassarri L, Creti R, Imperi M, Recchia S, Pataracchia M, Orefici G. Detection of genes encoding internalization-associated proteins in Streptococcus pyogenes isolates from patients with invasive diseases and asymptomatic carriers. J Clin Microbiol. 2007;45:1284–7. doi: 10.1128/JCM.02119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balsalobre L, de la Campa AG. Fitness of Streptococcus pneumoniae fluoroquinolone-resistant strains with topoisomerase IV recombinant genes. Antimicrob Agents Chemother. 2008;52:822–30. doi: 10.1128/AAC.00731-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balsalobre L, Ferrandiz MJ, Linares J, Tubau F, de la Campa AG. Viridans group streptococci are donors in horizontal transfer of topoisomerase IV genes to Streptococcus pneumoniae. Antimicrob Agents Chemother. 2003;47:2072–81. doi: 10.1128/AAC.47.7.2072-2081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks DJ, Porcella SF, Barbian KD, Beres SB, Philips LE, Voyich JM, DeLeo FR, Martin JM, Somerville GA, Musser JM. Progress toward characterization of the group A Streptococcus metagenome: complete genome sequence of a macrolide-resistant serotype M6 strain. J Infect Dis. 2004;190:727–38. doi: 10.1086/422697. [DOI] [PubMed] [Google Scholar]
- 15.Banks DJ, Porcella SF, Barbian KD, Martin JM, Musser JM. Structure and distribution of an unusual chimeric genetic element encoding macrolide resistance in phylogenetically diverse clones of group A Streptococcus. J Infect Dis. 2003;188:1898–908. doi: 10.1086/379897. [DOI] [PubMed] [Google Scholar]
- 16.Baquero F. From pieces to patterns: evolutionary engineering in bacterial pathogens. Nat Rev Microbiol. 2004;2:510–8. doi: 10.1038/nrmicro909. [DOI] [PubMed] [Google Scholar]
- 17.Barber M. Methicillin-resistant staphylococci. J Clin Pathol. 1961;14:385–93. doi: 10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber M, Rozwasowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;2:641–4. doi: 10.1016/s0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- 19.Basset P, Hammer NB, Kuhn G, Vogel V, Sakwinska O, Blanc DS. Staphylococcus aureus clfB and spa alleles of the repeat regions are segregated into major phylogenetic lineages. Infect Genet Evol. 2009;9:941–7. doi: 10.1016/j.meegid.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–8. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beall B, McEllistrem MC, Gertz REJ, et al. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J Clin Microbiol. 2006;44:999–1017. doi: 10.1128/JCM.44.3.999-1017.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beall BW, Gertz RE, Hulkower RL, Whitney CG, Moore MR, Brueggemann AB. Shifting Genetic Structure of Invasive Serotype 19A Pneumococci in the United States. J Infect Dis. 2011 doi: 10.1093/infdis/jir052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beres SB, Musser JM. Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PLoS One. 2007;2:e800. doi: 10.1371/journal.pone.0000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besier S, Ludwig A, Zander J, Brade V, Wichelhaus TA. Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob Agents Chemother. 2008;52:1570–2. doi: 10.1128/AAC.01098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bessen DE. Population biology of the human restricted pathogen, Streptococcus pyogenes. Infect Genet Evol. 2009;9:581–93. doi: 10.1016/j.meegid.2009.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bessen DE, McGregor KF, Whatmore AM. Relationships between emm and multilocus sequence types within a global collection of Streptococcus pyogenes. BMC Microbiol. 2008;8:59. doi: 10.1186/1471-2180-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billstrom H, Top J, Edlund C, Lund B. Frequent occurrence of multidrug-resistant CC17 Enterococcus faecium among clinical isolates in Sweden. J Appl Microbiol. 2010;108:1810–6. doi: 10.1111/j.1365-2672.2009.04585.x. [DOI] [PubMed] [Google Scholar]
- 28.Bishop CJ, Aanensen DM, Jordan GE, Kilian M, Hanage WP, Spratt BG. Assigning strains to bacterial species via the internet. BMC Biol. 2009;7:3. doi: 10.1186/1741-7007-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Bonora MG, Olioso D, Lo Cascio G, Fontana R. Phylogenetic analysis of vancomycin-resistant Enterococcus faecium genotypes associated with outbreaks or sporadic infections in Italy. Microb Drug Resist. 2007;13:171–7. doi: 10.1089/mdr.2007.739. [DOI] [PubMed] [Google Scholar]
- 31.Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis. 2001;1:314–25. doi: 10.1016/S1473-3099(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 32.Bonten MJM, Hayden MK, Nathan C, van Voorhis J, Matushek M, Slaughter S, Rice T, Weinstein RA. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–1619. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 33.Booijink CC, El-Aidy S, Rajilic-Stojanovic M, Heilig HG, Troost FJ, Smidt H, Kleerebezem M, de Vos WM, Zoetendal EG. High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol. 2010 doi: 10.1111/j.1462-2920.2010.02294.x. [DOI] [PubMed] [Google Scholar]
- 34.Boyce JM, Opal SM, Chow JW, Zervos MJ, Potter Bynoe G, Sherman CB, Romulo RL, Fortna S, Medeiros AA. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J-Clin-Microbiol. 1994;32:1148–53. doi: 10.1128/jcm.32.5.1148-1153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd DA, Willey BM, Fawcett D, Gillani N, Mulvey MR. Molecular characterization of Enterococcus faecalis N06-0364 with low-level vancomycin resistance harboring a novel D-Ala-D-Ser gene cluster, vanL. Antimicrob Agents Chemother. 2008;52:2667–72. doi: 10.1128/AAC.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bratu S, Landman D, Gupta J, Trehan M, Panwar M, Quale J. A population-based study examining the emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 in New York City. Ann Clin Microbiol Antimicrob. 2006;5:29. doi: 10.1186/1476-0711-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenciani A, Bacciaglia A, Vignaroli C, Pugnaloni A, Varaldo PE, Giovanetti E. Phim46.1, the main Streptococcus pyogenes element carrying mef(A) and tet(O) genes. Antimicrob Agents Chemother. 2010;54:221–9. doi: 10.1128/AAC.00499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brook I, Gober AE. Failure to eradicate streptococci and beta-lactamase producing bacteria. Acta Paediatr. 2008;97:193–5. doi: 10.1111/j.1651-2227.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 39.Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 2007;3:e168. doi: 10.1371/journal.ppat.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgos MJ, Lopez RL, Abriouel H, Omar NB, Galvez A. Multilocus sequence typing of Enterococcus faecalis from vegetable foods reveals two new sequence types. Foodborne Pathog Dis. 2009;6:321–7. doi: 10.1089/fpd.2008.0169. [DOI] [PubMed] [Google Scholar]
- 41.Camargo IL, Gilmore MS, Darini AL. Multilocus sequence typing and analysis of putative virulence factors in vancomycin-resistant and vancomycin-sensitive Enterococcus faecium isolates from Brazil. Clin Microbiol Infect. 2006;12:1123–1130. doi: 10.1111/j.1469-0691.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 42.Caplin JL, Hanlon GW, Taylor HD. Presence of vancomycin and ampicillin-resistant Enterococcus faecium of epidemic clonal complex-17 in wastewaters from the south coast of England. Environ Microbiol. 2007 doi: 10.1111/j.1462-2920.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 43.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 44.Carias LL, Rudin SD, Donskey CJ, Rice LB. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J Bacteriol. 1998;180:4426–4434. doi: 10.1128/jb.180.17.4426-4434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catry B, Van Duijkeren E, Pomba MC, et al. Reflection paper on MRSA in food-producing and companion animals: epidemiology and control options for human and animal health. Epidemiol Infect. 2010;138:626–44. doi: 10.1017/S0950268810000014. [DOI] [PubMed] [Google Scholar]
- 46.Cha S, Lee H, Lee K, Hwang K, Bae S, Lee Y. The emergence of erythromycin-resistant Streptococcus pyogenes in Seoul, Korea. J Infect Chemother. 2001;7:81–6. doi: 10.1007/s101560100013. [DOI] [PubMed] [Google Scholar]
- 47.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–41. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan JC, Chu YW, Chu MY, Cheung TK, Lo JY. Epidemiological analysis of Streptococcus pyogenes infections in Hong Kong. Pathology (Phila) 2009;41:681–6. doi: 10.3109/00313020903257723. [DOI] [PubMed] [Google Scholar]
- 50.Chi F, Nolte O, Bergmann C, Ip M, Hakenbeck R. Crossing the barrier: evolution and spread of a major class of mosaic pbp2x in Streptococcus pneumoniae, S. mitis and S. oralis. Int J Med Microbiol. 2007;297:503–12. doi: 10.1016/j.ijmm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Chiang PC, Wu TL, Su JY, Huang YC, Chiu YP, Chia JH, Kuo AJ, Su LH. Unusual increase of vancomycin-resistant Enterococcus faecium but not Enterococcus faecalis at a university hospital in Taiwan. Chang Gung Med J. 2007;30:493–503. [PubMed] [Google Scholar]
- 52.Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, Jamklang M, Chavalit T, Song JH, Hiramatsu K. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother. 2006;50:1001–12. doi: 10.1128/AAC.50.3.1001-1012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clewell DB, Yagi Y, Dunny GM, Schultz SK. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J-Bacteriol. 1974;117:283–9. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochetti I, Tili E, Mingoia M, Varaldo PE, Montanari MP. erm(B)-carrying elements in tetracycline-resistant pneumococci and correspondence between Tn1545 and Tn6003. Antimicrob Agents Chemother. 2008;52:1285–90. doi: 10.1128/AAC.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coffey TJ, Enright MC, Daniels M, Wilkinson P, Berron S, Fenoll A, Spratt BG. Serotype 19A variants of the Spanish serotype 23F multiresistant clone of Streptococcus pneumoniae. Microb Drug Resist. 1998;4:51–5. doi: 10.1089/mdr.1998.4.51. [DOI] [PubMed] [Google Scholar]
- 56.Conceicao T, Aires-de-Sousa M, Fuzi M, Toth A, Paszti J, Ungvari E, van Leeuwen WB, van Belkum A, Grundmann H, de Lencastre H. Replacement of methicillin-resistant Staphylococcus aureus clones in Hungary over time: a 10-year surveillance study. Clin Microbiol Infect. 2007;13:971–9. doi: 10.1111/j.1469-0691.2007.01794.x. [DOI] [PubMed] [Google Scholar]
- 57.Cookson BD, Robinson DA, Monk AB, et al. Evaluation of molecular typing methods in characterizing a European collection of epidemic methicillin-resistant Staphylococcus aureus strains: the HARMONY collection. J Clin Microbiol. 2007;45:1830–7. doi: 10.1128/JCM.02402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coque TM, Willems R, Canton R, Del Campo R, Baquero F. High occurrence of esp among ampicillin-resistant and vancomycin- susceptible Enterococcus faecium clones from hospitalized patients. J Antimicrob Chemother. 2002;50:1035–1038. doi: 10.1093/jac/dkf229. [DOI] [PubMed] [Google Scholar]
- 59.Coque TM, Willems RJ, Fortun J, Top J, Diz S, Loza E, Canton R, Baquero F. Population structure of Enterococcus faecium causing bacteremia in a Spanish university hospital: setting the scene for a future increase in vancomycin resistance? Antimicrob Agents Chemother. 2005;49:2693–2700. doi: 10.1128/AAC.49.7.2693-2700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis. 2006;42(Suppl 1):S25–34. doi: 10.1086/491711. [DOI] [PubMed] [Google Scholar]
- 61.Courvalin PM, Carlier C, Croissant O, Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol-Gen-Genet. 1974;132:181–92. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- 62.Cresti S, Lattanzi M, Zanchi A, Montagnani F, Pollini S, Cellesi C, Rossolini GM. Resistance determinants and clonal diversity in group A streptococci collected during a period of increasing macrolide resistance. Antimicrob Agents Chemother. 2002;46:1816–22. doi: 10.1128/AAC.46.6.1816-1822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Creti R, Imperi M, Baldassarri L, Pataracchia M, Recchia S, Alfarone G, Orefici G. emm Types, virulence factors, and antibiotic resistance of invasive Streptococcus pyogenes isolates from Italy: What has changed in 11 years? J Clin Microbiol. 2007;45:2249–56. doi: 10.1128/JCM.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crisostomo MI, Westh H, Tomasz A, Chung M, Oliveira DC, de Lencastre H. The evolution of methicillin resistance in Staphylococcus aureus: similarity of genetic backgrounds in historically early methicillin-susceptible and -resistant isolates and contemporary epidemic clones. Proc Natl Acad Sci U S A. 2001;98:9865–70. doi: 10.1073/pnas.161272898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crook DW, Brueggemann AB, Sleeman KL, Peto TEA. Pneumococcal Carriage. In: Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG, editors. The Pneumococcus. American Society for Microbiology; Washington, D.C: 2004. pp. 136–148. [Google Scholar]
- 66.Croucher NJ, Harris SR, Fraser C, et al. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011;331:430–4. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui L, Tominaga E, Neoh HM, Hiramatsu K. Correlation between Reduced Daptomycin Susceptibility and Vancomycin Resistance in Vancomycin-Intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:1079–82. doi: 10.1128/AAC.50.3.1079-1082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dahl KH, Mater DD, Flores MJ, Johnsen PJ, Midtvedt T, Corthier G, Sundsfjord A. Transfer of plasmid and chromosomal glycopeptide resistance determinants occurs more readily in the digestive tract of mice than in vitro and exconjugants can persist stably in vivo in the absence of glycopeptide selection. J Antimicrob Chemother. 2007;59:478–486. doi: 10.1093/jac/dkl530. [DOI] [PubMed] [Google Scholar]
- 69.Damani A, Klapsa D, Panopoulou M, et al. A newly described vancomycin-resistant ST412 Enterococcus faecium predominant in Greek hospitals. Eur J Clin Microbiol Infect Dis. 2010;29:329–31. doi: 10.1007/s10096-009-0847-9. [DOI] [PubMed] [Google Scholar]
- 70.Damborg P, Sorensen AH, Guardabassi L. Monitoring of antimicrobial resistance in healthy dogs: First report of canine ampicillin-resistant Enterococcus faecium clonal complex 17. Vet Microbiol. 2008;132:190–6. doi: 10.1016/j.vetmic.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 71.Damborg P, Top J, Hendrickx AP, Dawson S, Willems RJ, Guardabassi L. Dogs are a reservoir of ampicillin-resistant Enterococcus faecium lineages associated with human infections. Appl Environ Microbiol. 2009;75:2360–5. doi: 10.1128/AEM.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Lencastre H, Oliveira D, Tomasz A. Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Curr Opin Microbiol. 2007;10:428–35. doi: 10.1016/j.mib.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–68. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denis O, Deplano A, De Beenhouwer H, Hallin M, Huysmans G, Garrino MG, Glupczynski Y, Malaviolle X, Vergison A, Struelens MJ. Polyclonal emergence and importation of community-acquired methicillin-resistant Staphylococcus aureus strains harbouring Panton-Valentine leucocidin genes in Belgium. J Antimicrob Chemother. 2005;56:1103–6. doi: 10.1093/jac/dki379. [DOI] [PubMed] [Google Scholar]
- 75.Deplano A, Denis O, Nonhoff C, Rost F, Byl B, Jacobs F, Vankerckhoven V, Goossens H, Struelens MJ. Outbreak of hospital-adapted clonal complex-17 vancomycin-resistant Enterococcus faecium strain in a haematology unit: role of rapid typing for early control. J Antimicrob Chemother. 2007;60:849–54. doi: 10.1093/jac/dkm270. [DOI] [PubMed] [Google Scholar]
- 76.Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8:747–63. doi: 10.1016/j.meegid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Deurenberg RH, Stobberingh EE. The molecular evolution of hospital- and community-associated methicillin-resistant Staphylococcus aureus. Curr Mol Med. 2009;9:100–15. doi: 10.2174/156652409787581637. [DOI] [PubMed] [Google Scholar]
- 78.Dicuonzo G, Fiscarelli E, Gherardi G, Lorino G, Battistoni F, Landi S, De Cesaris M, Petitti T, Beall B. Erythromycin-resistant pharyngeal isolates of Streptococcus pyogenes recovered in Italy. Antimicrob Agents Chemother. 2002;46:3987–90. doi: 10.1128/AAC.46.12.3987-3990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Didelot X, Falush D. Inference of bacterial microevolution using multilocus sequence data. Genetics. 2007;175:1251–66. doi: 10.1534/genetics.106.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S114–32. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 81.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 82.Diep BA, Stone GG, Basuino L, et al. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2008;197:1523–30. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 83.Dowson CG, Coffey TJ, Kell C, Whiley RA. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol Microbiol. 1993;9:635–43. doi: 10.1111/j.1365-2958.1993.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 84.Dunne WM, Wang W. Clonal dissemination and colony morphotype variation of vancomycin-resistant Enterococcus faecium isolates in metropolitan Detroit, Michigan. J Clin Microbiol. 1997;35:388–392. doi: 10.1128/jcm.35.2.388-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Durbin WJ. Pneumococcal infections. Pediatr Rev. 2004;25:418–24. [PubMed] [Google Scholar]
- 86.Edgeworth JD, Yadegarfar G, Pathak S, Batra R, Cockfield JD, Wyncoll D, Beale R, Lindsay JA. An outbreak in an intensive care unit of a strain of methicillin-resistant Staphylococcus aureus sequence type 239 associated with an increased rate of vascular access device-related bacteremia. Clin Infect Dis. 2007;44:493–501. doi: 10.1086/511034. [DOI] [PubMed] [Google Scholar]
- 87.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci U S A. 2002;99:7687–92. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Enright MC, Spratt BG, Kalia A, Cross JH, Bessen DE. Infect Immun. 2001;69:2416–27. doi: 10.1128/IAI.69.4.2416-2427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ergani-Ozcan A, Naas T, Baysan BO, Ogunc D, Inan D, Colak D, Nordmann P. Nosocomial outbreak of vancomycin-resistant Enterococcus faecium in a paediatric unit at a Turkish university hospital. J Antimicrob Chemother. 2008;61:1033–9. doi: 10.1093/jac/dkn066. [DOI] [PubMed] [Google Scholar]
- 91.Espinosa de los Monteros LE, Bustos IM, Flores LV, Avila-Figueroa C. Outbreak of scarlet fever caused by an erythromycin-resistant Streptococcus pyogenes emm22 genotype strain in a day-care center. Pediatr Infect Dis J. 2001;20:807–9. doi: 10.1097/00006454-200108000-00019. [DOI] [PubMed] [Google Scholar]
- 92.Facinelli B, Spinaci C, Magi G, Giovanetti E, Varald EP. Association between erythromycin resistance and ability to enter human respiratory cells in group A streptococci. Lancet. 2001;358:30–3. doi: 10.1016/s0140-6736(00)05253-3. [DOI] [PubMed] [Google Scholar]
- 93.Fan J, Shu M, Zhang G, et al. Biogeography and virulence of Staphylococcus aureus. PLoS One. 2009;4:e6216. doi: 10.1371/journal.pone.0006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang H, Hedin G, Li G, Nord CE. Genetic diversity of community-associated methicillin-resistant Staphylococcus aureus in southern Stockholm, 2000–2005. Clin Microbiol Infect. 2008;14:370–6. doi: 10.1111/j.1469-0691.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- 95.Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J. 2007;26:123–8. doi: 10.1097/01.inf.0000253059.84602.c3. [DOI] [PubMed] [Google Scholar]
- 96.Feil EJ, Cooper JE, Grundmann H, et al. How clonal is Staphylococcus aureus? J Bacteriol. 2003;185:3307–16. doi: 10.1128/JB.185.11.3307-3316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186:1518–30. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Y, Chen CJ, Su LH, Hu S, Yu J, Chiu CH. Evolution and pathogenesis of Staphylococcus aureus: lessons learned from genotyping and comparative genomics. FEMS Microbiol Rev. 2008;32:23–37. doi: 10.1111/j.1574-6976.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 99.Fenoll A, Jado I, Vicioso D, Perez A, Casal J. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996) J Clin Microbiol. 1998;36:3447–54. doi: 10.1128/jcm.36.12.3447-3454.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Freeman AF, Shulman ST. Macrolide resistance in group A Streptococcus. Pediatr Infect Dis J. 2002;21:1158–60. doi: 10.1097/00006454-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 101.Freitas AR, Novais C, Ruiz-Garbajosa P, Coque TM, Peixe L. Clonal expansion within clonal complex 2 and spread of vancomycin-resistant plasmids among different genetic lineages of Enterococcus faecalis from Portugal. J Antimicrob Chemother. 2009;63:1104–11. doi: 10.1093/jac/dkp103. [DOI] [PubMed] [Google Scholar]
- 102.Freitas AR, Tedim AP, Novais C, Ruiz-Garbajosa P, Werner G, Laverde-Gomez JA, Canton R, Peixe L, Baquero F, Coque TM. Global spread of the hylEfm colonization-virulence gene in megaplasmids of the Enterococcus faecium CC17 polyclonal subcluster. Antimicrob Agents Chemother. 2010;54:2660–5. doi: 10.1128/AAC.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fujita K, Murono K, Yoshikawa M, Murai T. Decline of erythromycin resistance of group A streptococci in Japan. Pediatr Infect Dis J. 1994;13:1075–8. doi: 10.1097/00006454-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Gagliotti C, Nobilio L, Milandri M, Moro ML. Macrolide prescriptions and erythromycin resistance of Streptococcus pyogenes. Clin Infect Dis. 2006;42:1153–6. doi: 10.1086/501460. [DOI] [PubMed] [Google Scholar]
- 105.Galloway-Pena JR, Nallapareddy SR, Arias CA, Eliopoulos GM, Murray BE. Analysis of clonality and antibiotic resistance among early clinical isolates of Enterococcus faecium in the United States. J Infect Dis. 2009;200:1566–73. doi: 10.1086/644790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia-Rey C, Aguilar L, Baquero F, Casal J, Martin JE. Pharmacoepidemiological analysis of provincial differences between consumption of macrolides and rates of erythromycin resistance among Streptococcus pyogenes isolates in Spain. J Clin Microbiol. 2002;40:2959–63. doi: 10.1128/JCM.40.8.2959-2963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gerber MA. Antibiotic resistance: relationship to persistence of group A streptococci in the upper respiratory tract. Pediatrics. 1996;97:971–5. [PubMed] [Google Scholar]
- 108.Gertz REJ, Li Z, Pimenta FC, Jackson D, Juni BA, Lynfield R, Jorgensen JH, Carvalho Mda G, Beall BW. Increased penicillin nonsusceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J Infect Dis. 2010;201:770–5. doi: 10.1086/650496. [DOI] [PubMed] [Google Scholar]
- 109.Gertz REJ, McEllistrem MC, Boxrud DJ, et al. Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J Clin Microbiol. 2003;41:4194–216. doi: 10.1128/JCM.41.9.4194-4216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghaffar F, Barton T, Lozano J, Muniz LS, Hicks P, Gan V, Ahmad N, McCracken GHJ. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin Infect Dis. 2004;39:930–8. doi: 10.1086/423379. [DOI] [PubMed] [Google Scholar]
- 111.Goering RV, Larsen AR, Skov R, Tenover FC, Anderson KL, Dunman PM. Comparative genomic analysis of European and Middle Eastern community-associated methicillin-resistant Staphylococcus aureus (CC80:ST80-IV) isolates by high-density microarray. Clin Microbiol Infect. 2009;15:748–55. doi: 10.1111/j.1469-0691.2009.02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Golding GR, Bryden L, Levett PN, et al. Livestock-associated methicillin-resistant Staphylococcus aureus sequence type 398 in humans, Canada. Emerg Infect Dis. 2010;16:587–94. doi: 10.3201/eid1604.091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomes AR, Westh H, de Lencastre H. Origins and evolution of methicillin-resistant Staphylococcus aureus clonal lineages. Antimicrob Agents Chemother. 2006;50:3237–44. doi: 10.1128/AAC.00521-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goossens H. Spread of vancomycin-resistant enterococci: differences between the united states and europe. Infect Control Hosp Epidemiol. 1998;19:546–551. doi: 10.1086/647871. [DOI] [PubMed] [Google Scholar]
- 115.Grayson ML, Eliopoulos GM, Wennersten CB, Ruoff KL, De Girolami PC, Ferraro MJ, Moellering RC., Jr Increasing resistance to beta-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob-Agents-Chemother. 1991;35:2180–4. doi: 10.1128/aac.35.11.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Green MD, Beall B, Marcon MJ, et al. Multicentre surveillance of the prevalence and molecular epidemiology of macrolide resistance among pharyngeal isolates of group A streptococci in the USA. J Antimicrob Chemother. 2006;57:1240–3. doi: 10.1093/jac/dkl101. [DOI] [PubMed] [Google Scholar]
- 117.Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, Friedrich AW. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 2010;7:e1000215. doi: 10.1371/journal.pmed.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guinane CM, Ben Zakour NL, Tormo-Mas MA, et al. Evolutionary genomics of Staphylococcus aureus reveals insights into the origin and molecular basis of ruminant host adaptation. Genome Biol Evol. 2010;2:454–66. doi: 10.1093/gbe/evq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanage WP, Fraser C, Tang J, Connor TR, Corander J. Hyper-recombination, diversity, and antibiotic resistance in pneumococcus. Science. 2009;324:1454–7. doi: 10.1126/science.1171908. [DOI] [PubMed] [Google Scholar]
- 120.Hanage WP, Huang SS, Lipsitch M, Bishop CJ, Godoy D, Pelton SI, Goldstein R, Huot H, Finkelstein JA. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J Infect Dis. 2007;195:347–52. doi: 10.1086/510249. [DOI] [PubMed] [Google Scholar]
- 121.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh KV, Murray BE, Wolff J, Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin-Infect-Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 122.Hanssen AM, Ericson Sollid JU. SCCmec in staphylococci: genes on the move. FEMS Immunol Med Microbiol. 2006;46:8–20. doi: 10.1111/j.1574-695X.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 123.Hanssen AM, Fossum A, Mikalsen J, Halvorsen DS, Bukholm G, Sollid JU. Dissemination of community-acquired methicillin-resistant Staphylococcus aureus clones in northern Norway: sequence types 8 and 80 predominate. J Clin Microbiol. 2005;43:2118–24. doi: 10.1128/JCM.43.5.2118-2124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harris SR, Feil EJ, Holden MT, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327:469–74. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjord A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin Microbiol Infect. 2010;16:541–54. doi: 10.1111/j.1469-0691.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- 126.Hendrickx AP, van Wamel WJ, Posthuma G, Bonten MJ, Willems RJ. Five genes encoding surface-exposed LPXTG proteins are enriched in hospital-adapted Enterococcus faecium clonal complex 17 isolates. J Bacteriol. 2007;189:8321–32. doi: 10.1128/JB.00664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 128.Holden MT, Lindsay JA, Corton C, Quail MA, Cockfield JD, Pathak S, Batra R, Parkhill J, Bentley SD, Edgeworth JD. Genome sequence of a recently emerged, highly transmissible, multi-antibiotic- and antiseptic-resistant variant of methicillin-resistant Staphylococcus aureus, sequence type 239 (TW) J Bacteriol. 2010;192:888–92. doi: 10.1128/JB.01255-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Homan WL, Tribe D, Poznanski S, Li M, Hogg G, Spalburg E, Van Embden JD, Willems RJ. Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol. 2002;40:1963–1971. doi: 10.1128/JCM.40.6.1963-1971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Horn DL, Zabriskie JB, Austrian R, et al. Why have group A streptococci remained susceptible to penicillin? Report on a symposium. Clin Infect Dis. 1998;26:1341–5. doi: 10.1086/516375. [DOI] [PubMed] [Google Scholar]
- 131.Hoshuyama T, Moriguchi H, Muratani T, Matsumoto T. Vancomycin-resistant enterococci (VRE) outbreak at a university hospital in Kitakyushu, Japan: case-control studies. J Infect Chemother. 2008;14:354–60. doi: 10.1007/s10156-008-0628-x. [DOI] [PubMed] [Google Scholar]
- 132.Hotomi M, Billal DS, Togawa A, et al. Distribution of fibronectin-binding protein genes (prtF1 and prtF2) and streptococcal pyrogenic exotoxin genes (spe) among Streptococcus pyogenes in Japan. J Infect Chemother. 2009;15:367–73. doi: 10.1007/s10156-009-0724-6. [DOI] [PubMed] [Google Scholar]
- 133.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hsieh YC, Lee WS, Ou TY, Hsueh PR. Clonal spread of CC17 vancomycin-resistant Enterococcus faecium with multilocus sequence type 78 (ST78) and a novel ST444 in Taiwan. Eur J Clin Microbiol Infect Dis. 2010;29:25–30. doi: 10.1007/s10096-009-0810-9. [DOI] [PubMed] [Google Scholar]
- 135.Hsueh PR, Shyr JM, Wu JJ. Changes in macrolide resistance among respiratory pathogens after decreased erythromycin consumption in Taiwan. Clin Microbiol Infect. 2006;12:296–8. doi: 10.1111/j.1469-0691.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- 136.Huang YH, Tseng SP, Hu JM, Tsai JC, Hsueh PR, Teng LJ. Clonal spread of SCCmec type IV methicillin-resistant Staphylococcus aureus between community and hospital. Clin Microbiol Infect. 2007;13:717–24. doi: 10.1111/j.1469-0691.2007.01718.x. [DOI] [PubMed] [Google Scholar]
- 137.Iwen PC, Kelly DM, Linder J, Hinrichs SH, Dominguez EA, Rupp ME, Patil KD. Change in prevalence and antibiotic resistance of Enterococcus species isolated from blood cultures over an 8-year period. Antimicrob Agents and Chemother. 1997;41:494–495. doi: 10.1128/aac.41.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jacobs MR. Worldwide trends in antimicrobial resistance among common respiratory tract pathogens in children. Pediatr Infect Dis J. 2003;22:S109–19. doi: 10.1097/00006454-200308001-00002. [DOI] [PubMed] [Google Scholar]
- 139.Jacobs MR. Streptococcus pneumoniae: epidemiology and patterns of resistance. Am J Med. 2004;117(Suppl 3A):3S–15S. doi: 10.1016/j.amjmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 140.Jasir A, Tanna A, Efstratiou A, Schalen C. Unusual occurrence of M type 77, antibiotic-resistant group A streptococci in southern Sweden. J Clin Microbiol. 2001;39:586–90. doi: 10.1128/JCM.39.2.586-590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jessen O, Rosendal K, Bulow P, Faber V, Eriksen KR. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med. 1969;281:627–35. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- 142.Jevosn MP, Parker MT. The evolution of new hospital strains of Staphylococcus aureus. J Clin Pathol. 1964;17:243–50. doi: 10.1136/jcp.17.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaplan EL. The group A streptococcal upper respiratory tract carrier state: an enigma. J Pediatr. 1980;97:337–45. doi: 10.1016/s0022-3476(80)80178-8. [DOI] [PubMed] [Google Scholar]
- 144.Katopodis GD, Grivea IN, Tsantsaridou AJ, Pournaras S, Petinaki E, Syrogiannopoulos GA. Fusidic acid and clindamycin resistance in community-associated, methicillin-resistant Staphylococcus aureus infections in children of Central Greece. BMC Infect Dis. 2010;10:351. doi: 10.1186/1471-2334-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Katz KC, McGeer AJ, Duncan CL, et al. Emergence of macrolide resistance in throat culture isolates of group a streptococci in Ontario, Canada, in 2001. Antimicrob Agents Chemother. 2003;47:2370–2. doi: 10.1128/AAC.47.7.2370-2372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kawalec M, Pietras Z, Danilowicz E, Jakubczak A, Gniadkowski M, Hryniewicz W, Willems RJ. Clonal structure of Enterococcus faecalis isolated from Polish hospitals: characterization of epidemic clones. J Clin Microbiol. 2007;45:147–153. doi: 10.1128/JCM.01704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kehrenberg C, Schwarz S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant Staphylococcus isolates. Antimicrob Agents Chemother. 2006;50:1156–63. doi: 10.1128/AAC.50.4.1156-1163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kennedy AD, Otto M, Braughton KR, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A. 2008;105:1327–32. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khan MA, van der Wal M, Farrell DJ, Cossins L, van Belkum A, Alaidan A, Hays JP. Analysis of VanA vancomycin-resistant Enterococcus faecium isolates from Saudi Arabian hospitals reveals the presence of clonal cluster 17 and two new Tn1546 lineage types. J Antimicrob Chemother. 2008;62:279–83. doi: 10.1093/jac/dkn173. [DOI] [PubMed] [Google Scholar]
- 150.Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant Staphylococci. Science. 1944;99:452–3. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 151.Kislak JW, Razavi LM, Daly AK, Finland M. Susceptibility of pneumococci to nine antibiotics. Am J Med Sci. 1965;250:261–8. doi: 10.1097/00000441-196509000-00003. [DOI] [PubMed] [Google Scholar]
- 152.Klare I, Konstabel C, Badstubner D, Werner G, Witte W. Occurrence and spread of antibiotic resistances in Enterococcus faecium. Int J Food Microbiol. 2003;88:269–90. doi: 10.1016/s0168-1605(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 153.Klare I, Konstabel C, Mueller-Bertling S, et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur J Clin Microbiol Infect Dis. 2005;24:815–825. doi: 10.1007/s10096-005-0056-0. [DOI] [PubMed] [Google Scholar]
- 154.Klevens RM, Morrison MA, Fridkin SK, et al. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis. 2006;12:1991–3. doi: 10.3201/eid1212.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–96. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ko KS, Baek JY, Lee JY, Oh WS, Peck KR, Lee N, Lee WG, Lee K, Song JH. Molecular characterization of vancomycin-resistant Enterococcus faecium isolates from Korea. J Clin Microbiol. 2005;43:2303–2306. doi: 10.1128/JCM.43.5.2303-2306.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koh TH, Hsu LY, Chiu LL, Lin RV. Emergence of epidemic clones of vancomycin-resistant Enterococcus faecium in Singapore. J Hosp Infect. 2006;63:234–236. doi: 10.1016/j.jhin.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 158.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–63. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 159.Larsen J, Schonheyder HC, Lester CH, Olsen SS, Porsbo LJ, Garcia-Migura L, Jensen LB, Bisgaard M, Hammerum AM. Porcine-origin gentamicin-resistant Enterococcus faecalis in humans, Denmark. Emerg Infect Dis. 2010;16:682–4. doi: 10.3201/eid1604.090500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leavis H, Top J, Shankar N, Borgen K, Bonten M, Van Embden J, Willems RJ. A novel putative enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J Bacteriol. 2004;186:672–682. doi: 10.1128/JB.186.3.672-682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leavis HL, Willems RJ, Top J, Bonten MJ. High-level ciprofloxacin resistance from point mutations in gyrA and parC confined to global hospital-adapted clonal lineage CC17 of Enterococcus faecium. J Clin Microbiol. 2006;44:1059–1064. doi: 10.1128/JCM.44.3.1059-1064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leavis HL, Willems RJ, Top J, Spalburg E, Mascini EM, Fluit AC, Hoepelman A, de Neeling AJ, Bonten MJ. Epidemic and nonepidemic multidrug-resistant Enterococcus faecium. Emerg Infect Dis. 2003;9:1108–15. doi: 10.3201/eid0909.020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leavis HL, Willems RJ, van Wamel WJ, Schuren FH, Caspers MP, Bonten MJ. Insertion sequence-driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Pathog. 2007;3:75–96. doi: 10.1371/journal.ppat.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leclercq R, Courvalin P. Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2002;46:2727–34. doi: 10.1128/AAC.46.9.2727-2734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 166.Lee K, Kim YA, Park YJ, Lee HS, Kim MY, Kim EC, Yong D, Chong Y. Increasing prevalence of vancomycin-resistant enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant gram-negative bacilli: a KONSAR study in 2002. Yonsei Med J. 2004;45:598–608. doi: 10.3349/ymj.2004.45.4.598. [DOI] [PubMed] [Google Scholar]
- 167.Lee SM, Ender M, Adhikari R, Smith JM, Berger-Bachi B, Cook GM. Fitness cost of staphylococcal cassette chromosome mec in methicillin-resistant Staphylococcus aureus by way of continuous culture. Antimicrob Agents Chemother. 2007;51:1497–9. doi: 10.1128/AAC.01239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lester CH, Olsen SS, Schonheyder HC, et al. Typing of vancomycin-resistant enterococci obtained from patients at Danish hospitals and detection of a genomic island specific to CC17 Enterococcus faecium. Int J Antimicrob Agents. 2010;35:312–4. doi: 10.1016/j.ijantimicag.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 169.Lester CH, Sandvang D, Olsen SS, Schonheyder HC, Jarlov JO, Bangsborg J, Hansen DS, Jensen TG, Frimodt-Moller N, Hammerum AM. Emergence of ampicillin-resistant Enterococcus faecium in Danish hospitals. J Antimicrob Chemother. 2008;62:1203–6. doi: 10.1093/jac/dkn360. [DOI] [PubMed] [Google Scholar]
- 170.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:5883–8. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Libisch B, Lepsanovic Z, Top J, Muzslay M, Konkoly-Thege M, Gacs M, Balogh B, Fuzi M, Willems RJ. Molecular characterization of vancomycin-resistant Enterococcus spp. clinical isolates from Hungary and Serbia. Scand J Infect Dis. 2008;40:778–84. doi: 10.1080/00365540802165740. [DOI] [PubMed] [Google Scholar]
- 172.Lindsay JA, Moore CE, Day NP, Peacock SJ, Witney AA, Stabler RA, Husain SE, Butcher PD, Hinds J. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol. 2006;188:669–76. doi: 10.1128/JB.188.2.669-676.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Littauer P, Caugant DA, Sangvik M, Hoiby EA, Sundsfjord A, Simonsen GS. Macrolide-resistant Streptococcus pyogenes in Norway: population structure and resistance determinants. Antimicrob Agents Chemother. 2006;50:1896–9. doi: 10.1128/AAC.50.5.1896-1899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Locke JB, Morales G, Hilgers M, GCK, Rahawi S, Jose Picazo J, Shaw KJ, Stein JL. Elevated linezolid resistance in clinical cfr-positive Staphylococcus aureus isolates is associated with co-occurring mutations in ribosomal protein L3. Antimicrob Agents Chemother. 2010;54:5352–5. doi: 10.1128/AAC.00714-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. The Cfr rRNA methyltransferase confers resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A antibiotics. Antimicrob Agents Chemother. 2006;50:2500–5. doi: 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lopez M, Saenz Y, Alvarez-Martinez MJ, Marco F, Robredo B, Rojo-Bezares B, Ruiz-Larrea F, Zarazaga M, Torres C. Tn1546 structures and multilocus sequence typing of vanA-containing enterococci of animal, human and food origin. J Antimicrob Chemother. 2010;65:1570–5. doi: 10.1093/jac/dkq192. [DOI] [PubMed] [Google Scholar]
- 177.Lowder BV, Guinane CM, Ben Zakour NL, Weinert LA, Conway-Morris A, Cartwright RA, Simpson AJ, Rambaut A, Nubel U, Fitzgerald JR. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:19545–50. doi: 10.1073/pnas.0909285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Malhotra-Kumar S, Van Heirstraeten L, Lammens C, Chapelle S, Goossens H. Emergence of high-level fluoroquinolone resistance in emm6 Streptococcus pyogenes and in vitro resistance selection with ciprofloxacin, levofloxacin and moxifloxacin. J Antimicrob Chemother. 2009;63:886–94. doi: 10.1093/jac/dkp057. [DOI] [PubMed] [Google Scholar]
- 179.Manson JM, Hancock LE, Gilmore MS. Mechanism of chromosomal transfer of Enterococcus faecalis pathogenicity island, capsule, antimicrobial resistance, and other traits. Proc Natl Acad Sci U S A. 2010;107:12269–74. doi: 10.1073/pnas.1000139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Markogiannakis H, Pachylaki N, Samara E, Kalderi M, Minettou M, Toutouza M, Toutouzas KG, Theodorou D, Katsaragakis S. Infections in a surgical intensive care unit of a university hospital in Greece. Int J Infect Dis. 2009;13:145–53. doi: 10.1016/j.ijid.2008.05.1227. [DOI] [PubMed] [Google Scholar]
- 181.Martin JM, Green M, Barbadora KA, Wald ER. Erythromycin-resistant group A streptococci in schoolchildren in Pittsburgh. N Engl J Med. 2002;346:1200–6. doi: 10.1056/NEJMoa013169. [DOI] [PubMed] [Google Scholar]
- 182.Mato R, Delencastre H, Roberts RB, Tomasz A. Multiplicity of genetic backgrounds among vancomycin-resistant Enterococcus faecium isolates recovered from an outbreak in a New York City Hospital. Microbial Drug Resistance. 1996;2:309–317. doi: 10.1089/mdr.1996.2.309. [DOI] [PubMed] [Google Scholar]
- 183.McBride SM, Coburn PS, Baghdayan AS, Willems RJ, Grande MJ, Shankar N, Gilmore MS. Genetic variation and evolution of the pathogenicity island of Enterococcus faecalis. J Bacteriol. 2009;191:3392–402. doi: 10.1128/JB.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McBride SM, Fischetti VA, Leblanc DJ, Moellering RC, Jr, Gilmore MS. Genetic diversity among Enterococcus faecalis. PLoS ONE. 2007;2:e582. doi: 10.1371/journal.pone.0000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McGee L, McDougal L, Zhou J, et al. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J Clin Microbiol. 2001;39:2565–71. doi: 10.1128/JCM.39.7.2565-2571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Meisal R, Andreasson IK, Hoiby EA, Aaberge IS, Michaelsen TE, Caugant DA. Streptococcus pyogenes isolates causing severe infections in Norway in 2006 to 2007: emm types, multilocus sequence types, and superantigen profiles. J Clin Microbiol. 2010;48:842–51. doi: 10.1128/JCM.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Melles DC, Schouls L, Francois P, Herzig S, Verbrugh HA, van Belkum A, Schrenzel J. High-throughput typing of Staphylococcus aureus by amplified fragment length polymorphism (AFLP) or multi-locus variable number of tandem repeat analysis (MLVA) reveals consistent strain relatedness. Eur J Clin Microbiol Infect Dis. 2009;28:39–45. doi: 10.1007/s10096-008-0585-4. [DOI] [PubMed] [Google Scholar]
- 188.Mellmann A, Weniger T, Berssenbrugge C, Keckevoet U, Friedrich AW, Harmsen D, Grundmann H. Characterization of clonal relatedness among the natural population of Staphylococcus aureus strains by using spa sequence typing and the BURP (based upon repeat patterns) algorithm. J Clin Microbiol. 2008;46:2805–8. doi: 10.1128/JCM.00071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Michos AG, Bakoula CG, Braoudaki M, Koutouzi FI, Roma ES, Pangalis A, Nikolopoulou G, Kirikou E, Syriopoulou VP. Macrolide resistance in Streptococcus pyogenes: prevalence, resistance determinants, and emm types. Diagn Microbiol Infect Dis. 2009;64:295–9. doi: 10.1016/j.diagmicrobio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 190.Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D, di Grazia C, Frassoni F, Bacigalupo A, Viscoli C. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15:47–53. doi: 10.1016/j.bbmt.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 191.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:752–60. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 192.Montes M, Orden B, Tamayo E, Alos JI, Perez-Trallero E. Characterisation of the main clones of Streptococcus pyogenes carrying the ermA (subclass TR) gene in Spain. Int J Antimicrob Agents. 2006;28:408–12. doi: 10.1016/j.ijantimicag.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 193.Montes M, Tamayo E, Orden B, Larruskain J, Perez-Trallero E. Prevalence and clonal characterization of Streptococcus pyogenes clinical isolates with reduced fluoroquinolone susceptibility in Spain. Antimicrob Agents Chemother. 2010;54:93–7. doi: 10.1128/AAC.00780-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Morales G, Picazo JJ, Baos E, Candel FJ, Arribi A, Pelaez B, Andrade R, de la Torre MA, Fereres J, Sanchez-Garcia M. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis. 2010;50:821–5. doi: 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- 195.Moreno F, Grota P, Crisp C, Magnon K, Melcher GP, Jorgensen JH, Patterson JE. Clinical and molecular epidemiology of vancomycin-resistant Enterococcus faecium during its emergence in a city in southern Texas. Clin-Infect-Dis. 1995;21:1234–7. doi: 10.1093/clinids/21.5.1234. [DOI] [PubMed] [Google Scholar]
- 196.Morrison D, Woodford N, Barrett SP, Sisson P, Cookson BD. Dna banding pattern polymorphism in vancomycin-resistant Enterococcus faecium and criteria for defining strains. J Clin Microbiol. 1999;37:1084–1091. doi: 10.1128/jcm.37.4.1084-1091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Munoz R, Coffey TJ, Daniels M, et al. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991;164:302–6. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- 198.Murdoch DR, Mirrett S, Harrell LJ, Monahan JS, Reller LB. Sequential emergence of antibiotic resistance in enterococcal bloodstream isolates over 25 years. Antimicrob Agents Chemother. 2002;46:3676–3678. doi: 10.1128/AAC.46.11.3676-3678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Murray BE. The life and times of the Enterococcus. Clin-Microbiol-Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nallapareddy SR, Wenxiang H, Weinstock GM, Murray BE. Molecular characterization of a widespread, pathogenic, and antibiotic resistance-receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity island. J Bacteriol. 2005;187:5709–5718. doi: 10.1128/JB.187.16.5709-5718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nannini E, Murray BE, Arias CA. Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr Opin Pharmacol. 2010;10:516–21. doi: 10.1016/j.coph.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 202.Nelson KE, Weinstock GM, Highlander SK, et al. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–9. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nielsen HU, Hammerum AM, Ekelund K, Bang D, Pallesen LV, Frimodt-Moller N. Tetracycline and macrolide co-resistance in Streptococcus pyogenes: co-selection as a reason for increase in macrolide-resistant S. pyogenes? Microb Drug Resist. 2004;10:231–8. doi: 10.1089/mdr.2004.10.231. [DOI] [PubMed] [Google Scholar]
- 204.Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10:436–40. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 205.Nubel U, Dordel J, Kurt K, et al. A timescale for evolution, population expansion, and spatial spread of an emerging clone of methicillin-resistant Staphylococcus aureus. PLoS Pathog. 2010;6:e1000855. doi: 10.1371/journal.ppat.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nubel U, Roumagnac P, Feldkamp M, et al. Frequent emergence and limited geographic dispersal of methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2008;105:14130–5. doi: 10.1073/pnas.0804178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nulens E, Gould I, MacKenzie F, Deplano A, Cookson B, Alp E, Bouza E, Voss A. Staphylococcus aureus carriage among participants at the 13th European Congress of Clinical Microbiology and Infectious Diseases. Eur J Clin Microbiol Infect Dis. 2005;24:145–8. doi: 10.1007/s10096-004-1258-6. [DOI] [PubMed] [Google Scholar]
- 208.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 209.Okon KO, Basset P, Uba A, Lin J, Oyawoye B, Shittu AO, Blanc DS. Cooccurrence of predominant Panton-Valentine leukocidin-positive sequence type (ST) 152 and multidrug-resistant ST 241 Staphylococcus aureus clones in Nigerian hospitals. J Clin Microbiol. 2009;47:3000–3. doi: 10.1128/JCM.01119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Okuma K, Iwakawa K, Turnidge JD, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2155–61. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Oliver KR, Greene WK. Transposable elements: powerful facilitators of evolution. Bioessays. 2009;31:703–14. doi: 10.1002/bies.200800219. [DOI] [PubMed] [Google Scholar]
- 213.Orscheln RC, Johnson DR, Olson SM, Presti RM, Martin JM, Kaplan EL, Storch GA. Intrinsic reduced susceptibility of serotype 6 Streptococcus pyogenes to fluoroquinolone antibiotics. J Infect Dis. 2005;191:1272–9. doi: 10.1086/428856. [DOI] [PubMed] [Google Scholar]
- 214.Otter JA, French GL. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect Dis. 2010;10:227–39. doi: 10.1016/S1473-3099(10)70053-0. [DOI] [PubMed] [Google Scholar]
- 215.Padiglione AA, Wolfe R, Grabsch EA, Olden D, Pearson S, Franklin C, Spelman D, Mayall B, Johnson PD, Grayson ML. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother. 2003;47:2492–8. doi: 10.1128/AAC.47.8.2492-2498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pai R, Gertz RE, Whitney CG, Beall B. Clonal association between Streptococcus pneumoniae serotype 23A, circulating within the United States, and an internationally dispersed clone of serotype 23F. J Clin Microbiol. 2005a;43:5440–4. doi: 10.1128/JCM.43.11.5440-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis. 2005b;192:1988–95. doi: 10.1086/498043. [DOI] [PubMed] [Google Scholar]
- 218.Panesso D, Reyes J, Rincon S, et al. Molecular epidemiology of vancomycin-resistant Enterococcus faecium: a prospective, multicenter study in South American hospitals. J Clin Microbiol. 2010;48:1562–9. doi: 10.1128/JCM.02526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Paulsen IT, Banerjei L, Myers GS, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–4. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- 220.Peacock SJ, de Silva I, Lowy FD. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 2001;9:605–10. doi: 10.1016/s0966-842x(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 221.Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, Huang SS, Goldstein R, Hanage WP. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–72. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 222.Perez-Roth E, Alcoba-Florez J, Lopez-Aguilar C, Gutierrez-Gonzalez I, Rivero-Perez B, Mendez-Alvarez S. Familial furunculosis associated with community-acquired leukocidin-positive methicillin-susceptible Staphylococcus aureus ST152. J Clin Microbiol. 2010;48:329–32. doi: 10.1128/JCM.00622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Perez-Trallero E, Garcia C, Orden B, Marimon JM, Montes M. Dissemination of emm28 erythromycin-, clindamycin- and bacitracin-resistant Streptococcus pyogenes in Spain. Eur J Clin Microbiol Infect Dis. 2004;23:123–6. doi: 10.1007/s10096-003-1069-1. [DOI] [PubMed] [Google Scholar]
- 224.Perez-Trallero E, Marimon JM, Montes M, Orden B, de Pablos M. Clonal differences among erythromycin-resistant Streptococcus pyogenes in Spain. Emerg Infect Dis. 1999;5:235–40. doi: 10.3201/eid0502.990207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Perez-Trallero E, Montes M, Orden B, Tamayo E, Garcia-Arenzana JM, Marimon JM. Phenotypic and genotypic characterization of Streptococcus pyogenes isolates displaying the MLSB phenotype of macrolide resistance in Spain, 1999 to 2005. Antimicrob Agents Chemother. 2007;51:1228–33. doi: 10.1128/AAC.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007a;298:1772–8. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 227.Pichichero ME, Casey JR. Systematic review of factors contributing to penicillin treatment failure in Streptococcus pyogenes pharyngitis. Otolaryngol Head Neck Surg. 2007b;137:851–857. doi: 10.1016/j.otohns.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 228.Pinho MD, Melo-Cristino J, Ramirez M. Fluoroquinolone resistance in Streptococcus dysgalactiae subsp. equisimilis and evidence for a shared global gene pool with Streptococcus pyogenes. Antimicrob Agents Chemother. 2010;54:1769–77. doi: 10.1128/AAC.01377-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pires R, Ardanuy C, Rolo D, Morais A, Brito-Avo A, Goncalo-Marques J, Linares J, Santos-Sanches I. Emergence of ciprofloxacin-nonsusceptible Streptococcus pyogenes isolates from healthy children and pediatric patients in Portugal. Antimicrob Agents Chemother. 2010;54:2677–80. doi: 10.1128/AAC.01536-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Powis J, McGeer A, Duncan C, et al. Prevalence and characterization of invasive isolates of Streptococcus pyogenes with reduced susceptibility to fluoroquinolones. Antimicrob Agents Chemother. 2005;49:2130–2. doi: 10.1128/AAC.49.5.2130-2132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Quie PG, Pierce HC, Wannamaker LW. Influence of penicillinase-producing staphylococci on the eradication of group A streptococci from the upper respiratory tract by penicillin treatment. Pediatrics. 1966;37:467–76. [PubMed] [Google Scholar]
- 232.Reinert RR, Lutticken R, Sutcliffe JA, Tait-Kamradt A, Cil MY, Schorn HM, Bryskier A, Al-Lahham A. Clonal relatedness of erythromycin-resistant Streptococcus pyogenes isolates in Germany. Antimicrob Agents Chemother. 2004;48:1369–73. doi: 10.1128/AAC.48.4.1369-1373.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rice LB. Antimicrobial resistance in gram-positive bacteria. Am J Med. 2006;119:S11–9. doi: 10.1016/j.amjmed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 234.Rice LB, Carias L, Rudin S, Vael C, Goossens H, Konstabel C, Klare I, Nallapareddy SR, Huang W, Murray BE. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J Infect Dis. 2003;187:508–512. doi: 10.1086/367711. [DOI] [PubMed] [Google Scholar]
- 235.Richter SS, Heilmann KP, Beekmann SE, Miller NJ, Miller AL, Rice CL, Doern CD, Reid SD, Doern GV. Macrolide-resistant Streptococcus pyogenes in the United States, 2002–2003. Clin Infect Dis. 2005;41:599–608. doi: 10.1086/432473. [DOI] [PubMed] [Google Scholar]
- 236.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004–2005. Clin Infect Dis. 2009;48:e23–33. doi: 10.1086/595857. [DOI] [PubMed] [Google Scholar]
- 237.Robinson DA, Monk AB, Cooper JE, Feil EJ, Enright MC. Evolutionary genetics of the accessory gene regulator (agr) locus in Staphylococcus aureus. J Bacteriol. 2005;187:8312–21. doi: 10.1128/JB.187.24.8312-8321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Robinson DA, Sutcliffe JA, Tewodros W, Manoharan A, Bessen DE. Evolution and global dissemination of macrolide-resistant group A streptococci. Antimicrob Agents Chemother. 2006;50:2903–11. doi: 10.1128/AAC.00325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rozen DE, McGee L, Levin BR, Klugman KP. Fitness costs of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2007;51:412–6. doi: 10.1128/AAC.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ruimy R, Maiga A, Armand-Lefevre L, et al. The carriage population of Staphylococcus aureus from Mali is composed of a combination of pandemic clones and the divergent Panton-Valentine leukocidin-positive genotype ST152. J Bacteriol. 2008;190:3962–8. doi: 10.1128/JB.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ruiz-Garbajosa P, Bonten MJ, Robinson DA, et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol. 2006;44:2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rybkine T, Mainardi JL, Sougakoff W, Collatz E, Gutmann L. Penicillin-binding protein 5 sequence alterations in clinical isolates of enterococcus faecium with different levels of beta-lactam resistance. J Infect Dis. 1998;178:159–163. doi: 10.1086/515605. [DOI] [PubMed] [Google Scholar]
- 243.Sanchez Garcia M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303:2260–4. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- 244.Schouls LM, Spalburg EC, van Luit M, Huijsdens XW, Pluister GN, van Santen-Verheuvel MG, van der Heide HG, Grundmann H, Heck ME, de Neeling AJ. Multiple-locus variable number tandem repeat analysis of Staphylococcus aureus: comparison with pulsed-field gel electrophoresis and spa-typing. PLoS One. 2009;4:e5082. doi: 10.1371/journal.pone.0005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Semedo T, Santos MA, Lopes MF, Figueiredo Marques JJ, Barreto Crespo MT, Tenreiro R. Virulence factors in food, clinical and reference Enterococci: A common trait in the genus? Syst Appl Microbiol. 2003;26:13–22. doi: 10.1078/072320203322337263. [DOI] [PubMed] [Google Scholar]
- 246.Seppala H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441–6. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 247.Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis. 2008;46:668–74. doi: 10.1086/527392. [DOI] [PubMed] [Google Scholar]
- 248.Silva-Costa C, Pinto FR, Ramirez M, Melo-Cristino J. Decrease in macrolide resistance and clonal instability among Streptococcus pyogenes in Portugal. Clin Microbiol Infect. 2008;14:1152–9. doi: 10.1111/j.1469-0691.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 249.Silva-Costa C, Ramirez M, Melo-Cristino J. Identification of macrolide-resistant clones of Streptococcus pyogenes in Portugal. Clin Microbiol Infect. 2006;12:513–8. doi: 10.1111/j.1469-0691.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 250.Smeesters PR, Vergison A, Junior DC, Van Melderen L. Emerging fluoroquinolone-non-susceptible group A streptococci in two different paediatric populations. Int J Antimicrob Agents. 2009;34:44–9. doi: 10.1016/j.ijantimicag.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 251.Smyth DS, McDougal LK, Gran FW, Manoharan A, Enright MC, Song JH, de Lencastre H, Robinson DA. Population structure of a hybrid clonal group of methicillin-resistant Staphylococcus aureus, ST239-MRSA-III. PLoS One. 2010;5:e8582. doi: 10.1371/journal.pone.0008582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Solheim M, Aakra A, Snipen LG, Brede DA, Nes IF. Comparative genomics of Enterococcus faecalis from healthy Norwegian infants. BMC Genomics. 2009;10:194. doi: 10.1186/1471-2164-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611–6. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 254.Strakova L, Motlova J, Jakubu V, Urbaskova P, Kriz P. Emergence of a novel macrolide-resistant Streptococcus pyogenes emm53 strain. Clin Microbiol Infect. 2007;13:443–5. doi: 10.1111/j.1469-0691.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 255.Sun J, Song X, Kristiansen BE, Kjaereng A, Willems RJ, Eriksen HM, Sundsfjord A, Sollid JE. Occurrence, population structure, and antimicrobial resistance of enterococci in marginal and apical periodontitis. J Clin Microbiol. 2009;47:2218–25. doi: 10.1128/JCM.00388-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–24. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Syrogiannopoulos GA, Grivea IN, Fitoussi F, Doit C, Katopodis GD, Bingen E, Beratis NG. High prevalence of erythromycin resistance of Streptococcus pyogenes in Greek children. Pediatr Infect Dis J. 2001;20:863–8. doi: 10.1097/00006454-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 258.Szczypa K, Sadowy E, Izdebski R, Hryniewicz W. A rapid increase in macrolide resistance in Streptococcus pyogenes isolated in Poland during 1996–2002. J Antimicrob Chemother. 2004;54:828–31. doi: 10.1093/jac/dkh420. [DOI] [PubMed] [Google Scholar]
- 259.Tang J, Hanage WP, Fraser C, Corander J. Identifying currents in the gene pool for bacterial populations using an integrative approach. PLoS Comput Biol. 2009;5:e1000455. doi: 10.1371/journal.pcbi.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tanz RR, Shulman ST, Shortridge VD, Kabat W, Kabat K, Cederlund E, Rippe J, Beyer J, Doktor S, Beall BW. Community-based surveillance in the united states of macrolide-resistant pediatric pharyngeal group A streptococci during 3 respiratory disease seasons. Clin Infect Dis. 2004;39:1794–801. doi: 10.1086/426025. [DOI] [PubMed] [Google Scholar]
- 261.Techasaensiri C, Messina AF, Katz K, Ahmad N, Huang R, McCracken GHJ. Epidemiology and evolution of invasive pneumococcal disease caused by multidrug resistant serotypes of 19A in the 8 years after implementation of pneumococcal conjugate vaccine immunization in Dallas, Texas. Pediatr Infect Dis J. 2010;29:294–300. doi: 10.1097/INF.0b013e3181c2a229. [DOI] [PubMed] [Google Scholar]
- 262.Tenover FC, Goering RV. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother. 2009;64:441–6. doi: 10.1093/jac/dkp241. [DOI] [PubMed] [Google Scholar]
- 263.Tenover FC, McDonald LC. Vancomycin-resistant staphylococci and enterococci: epidemiology and control. Curr Opin Infect Dis. 2005;18:300–5. doi: 10.1097/01.qco.0000171923.62699.0c. [DOI] [PubMed] [Google Scholar]
- 264.Tiemersma EW, Bronzwaer SL, Lyytikainen O, Degener JE, Schrijnemakers P, Bruinsma N, Monen J, Witte W, Grundman H. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg Infect Dis. 2004;10:1627–34. doi: 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Top J, Willems R, van der Velden S, Asbroek M, Bonten M. Emergence of clonal complex 17 Enterococcus faecium in The Netherlands. J Clin Microbiol. 2008;46:214–9. doi: 10.1128/JCM.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Treitman AN, Yarnold PR, Warren J, Noskin GA. Emerging incidence of Enterococcus faecium among hospital isolates (1993 to 2002) J Clin Microbiol. 2005;43:462–463. doi: 10.1128/JCM.43.1.462-463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Turner KM, Feil EJ. The secret life of the multilocus sequence type. Int J Antimicrob Agents. 2007;29:129–35. doi: 10.1016/j.ijantimicag.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 268.Turner KM, Hanage WP, Fraser C, Connor TR, Spratt BG. Assessing the reliability of eBURST using simulated populations with known ancestry. BMC Microbiol. 2007;7:30. doi: 10.1186/1471-2180-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet. 1988;1:57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 270.Valdezate S, Labayru C, Navarro A, Mantecon MA, Ortega M, Coque TM, Garcia M, Saez-Nieto JA. Large clonal outbreak of multidrug-resistant CC17 ST17 Enterococcus faecium containing Tn5382 in a Spanish hospital. J Antimicrob Chemother. 2009;63:17–20. doi: 10.1093/jac/dkn449. [DOI] [PubMed] [Google Scholar]
- 271.van Belkum A, Melles DC, Peeters JK, van Leeuwen WB, van Duijkeren E, Huijsdens XW, Spalburg E, de Neeling AJ, Verbrugh HA. Methicillin-resistant and -susceptible Staphylococcus aureus sequence type 398 in pigs and humans. Emerg Infect Dis. 2008;14:479–83. doi: 10.3201/eid1403.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.van Belkum A, Melles DC, Snijders SV, van Leeuwen WB, Wertheim HF, Nouwen JL, Verbrugh HA, Etienne J. Clonal distribution and differential occurrence of the enterotoxin gene cluster, egc, in carriage- versus bacteremia-associated isolates of Staphylococcus aureus. J Clin Microbiol. 2006;44:1555–7. doi: 10.1128/JCM.44.4.1555-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.van Belkum A, Tassios PT, Dijkshoorn L, et al. Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin Microbiol Infect. 2007;13(Suppl 3):1–46. doi: 10.1111/j.1469-0691.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 274.van Schaik W, Top J, Riley DR, et al. Pyrosequencing-based comparative genome analysis of the nosocomial pathogen Enterococcus faecium and identification of a large transferable pathogenicity island. BMC Genomics. 2010;11:239. doi: 10.1186/1471-2164-11-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.van Schaik W, Willems RJ. Genome-based insights into the evolution of enterococci. Clin Microbiol Infect. 2010;16:527–32. doi: 10.1111/j.1469-0691.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 276.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Vanderhaeghen W, Hermans K, Haesebrouck F, Butaye P. Methicillin-resistant Staphylococcus aureus (MRSA) in food production animals. Epidemiol Infect. 2010;138:606–25. doi: 10.1017/S0950268809991567. [DOI] [PubMed] [Google Scholar]
- 278.Vankerckhoven V, Van Autgaerden T, Vael C, Lammens C, Chapelle S, Rossi R, Jabes D, Goossens H. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J Clin Microbiol. 2004;42:4473–9. doi: 10.1128/JCM.42.10.4473-4479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Varaldo PE, Montanari MP, Giovanetti E. Genetic elements responsible for erythromycin resistance in streptococci. Antimicrob Agents Chemother. 2009;53:343–53. doi: 10.1128/AAC.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.von Gottberg A, van Nierop W, Duse A, Kassel M, McCarthy K, Brink A, Meyers M, Smego R, Koornhof H. Epidemiology of glycopeptide-resistant enterococci colonizing high-risk patients in hospitals in Johannesburg, Republic of South Africa. J Clin Microbiol. 2000;38:905–9. doi: 10.1128/jcm.38.2.905-909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wassenberg MW, Bootsma MC, Troelstra A, Kluytmans JA, Bonten MJ. Transmissibility of livestock-associated methicillin-resistant Staphylococcus aureus (ST398) in Dutch hospitals. Clin Microbiol Infect. 2010 doi: 10.1111/j.1469-0691.2010.03260.x. [DOI] [PubMed] [Google Scholar]
- 282.Werner G, Coque TM, Hammerum AM, et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill. 2008;13 [PubMed] [Google Scholar]
- 283.Werner G, Fleige C, Geringer U, van Schaik W, Klare I, Witte W. IS element IS16 as a molecular screening tool to identify hospital-associated strains of Enterococcus faecium. BMC Infect Dis. 2011;11:80. doi: 10.1186/1471-2334-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Willems RJ. Population genetics of Enterococcus. In: Robinson DA, Falush D, Feil EJ, editors. Bacterial Population Genetics in Infectious Diseases. John Wiley & Sons, Inc; Hoboken, New Jersey, USA: 2010. pp. 195–216. [Google Scholar]
- 285.Willems RJ, Homan W, Top J, et al. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet. 2001;357:853–855. doi: 10.1016/S0140-6736(00)04205-7. [DOI] [PubMed] [Google Scholar]
- 286.Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, Grundmann H, Bonten MJ. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005;11:821–828. doi: 10.3201/eid1106.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Willems RJ, van Schaik W. Transition of Enterococcus faecium from commensal organism to nosocomial pathogen. Future Microbiol. 2009;4:1125–35. doi: 10.2217/fmb.09.82. [DOI] [PubMed] [Google Scholar]
- 288.Willems RJL, Top J, Van Den Braak N, Van Belkum A, Endtz H, Mevius D, Stobberingh E, Van Den Bogaard A, Van Embden JDA. Host specificity of vancomycin-resistant Enterococcus faecium. J Infect Dis. 2000;182:816–823. doi: 10.1086/315752. [DOI] [PubMed] [Google Scholar]
- 289.Witte W, Braulke C, Cuny C, Strommenger B, Werner G, Heuck D, Jappe U, Wendt C, Linde HJ, Harmsen D. Emergence of methicillin-resistant Staphylococcus aureus with Panton-Valentine leukocidin genes in central Europe. Eur J Clin Microbiol Infect Dis. 2005;24:1–5. doi: 10.1007/s10096-004-1262-x. [DOI] [PubMed] [Google Scholar]
- 290.Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59(Suppl 1):S4–16. doi: 10.1016/S0163-4453(09)60003-7. [DOI] [PubMed] [Google Scholar]
- 291.Wootton M, MacGowan AP, Walsh TR. Comparative bactericidal activities of daptomycin and vancomycin against glycopeptide-intermediate Staphylococcus aureus (GISA) and heterogeneous GISA isolates. Antimicrob Agents Chemother. 2006;50:4195–7. doi: 10.1128/AAC.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wulf M, Voss A. MRSA in livestock animals-an epidemic waiting to happen? Clin Microbiol Infect. 2008;14:519–21. doi: 10.1111/j.1469-0691.2008.01970.x. [DOI] [PubMed] [Google Scholar]
- 293.Xu BL, Zhang G, Ye HF, Feil EJ, Chen GR, Zhou XM, Zhan XM, Chen SM, Pan WB. Predominance of the Hungarian clone (ST 239-III) among hospital-acquired meticillin-resistant Staphylococcus aureus isolates recovered throughout mainland China. J Hosp Infect. 2009a;71:245–55. doi: 10.1016/j.jhin.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 294.Xu Q, Pichichero ME, Casey JR, Zeng M. Novel type of Streptococcus pneumoniae causing multidrug-resistant acute otitis media in children. Emerg Infect Dis. 2009b;15:547–51. doi: 10.3201/eid1504.071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yan JJ, Wu HM, Huang AH, Fu HM, Lee CT, Wu JJ. Prevalence of polyclonal mefA-containing isolates among erythromycin-resistant group A streptococci in Southern Taiwan. J Clin Microbiol. 2000;38:2475–9. doi: 10.1128/jcm.38.7.2475-2479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yan SS, Schreckenberger PC, Zheng X, Nelson NA, Harrington SM, Tjhio J, Fedorko DP. An intrinsic pattern of reduced susceptibility to fluoroquinolones in pediatric isolates of Streptococcus pyogenes. Diagn Microbiol Infect Dis. 2008;62:205–9. doi: 10.1016/j.diagmicrobio.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Young DM, Harris HW, Charlebois ED, Chambers H, Campbell A, Perdreau-Remington F, Lee C, Mankani M, Mackersie R, Schecter WP. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg. 2004;139:947–51. doi: 10.1001/archsurg.139.9.947. discussion 951–3. [DOI] [PubMed] [Google Scholar]
- 298.Zampaloni C, Cappelletti P, Prenna M, Vitali LA, Ripa S. emm Gene distribution among erythromycin-resistant and -susceptible Italian isolates of Streptococcus pyogenes. J Clin Microbiol. 2003;41:1307–10. doi: 10.1128/JCM.41.3.1307-1310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zheng B, Tomita H, Xiao YH, Wang S, Li Y, Ike Y. Molecular Characterization of Vancomycin-Resistant Enterococcus faecium Isolates from Mainland China. J Clin Microbiol. 2007;45:2813–8. doi: 10.1128/JCM.00457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]