Abstract
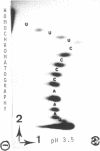
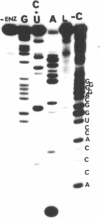
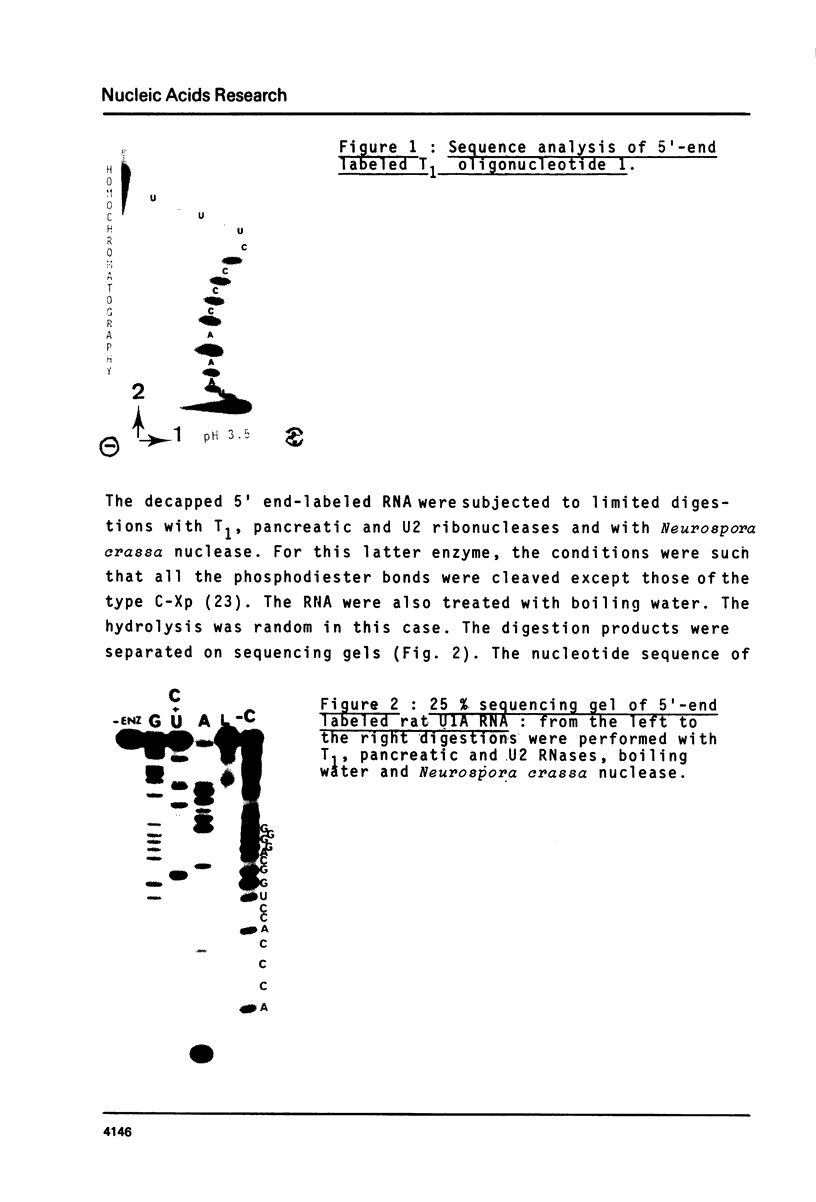
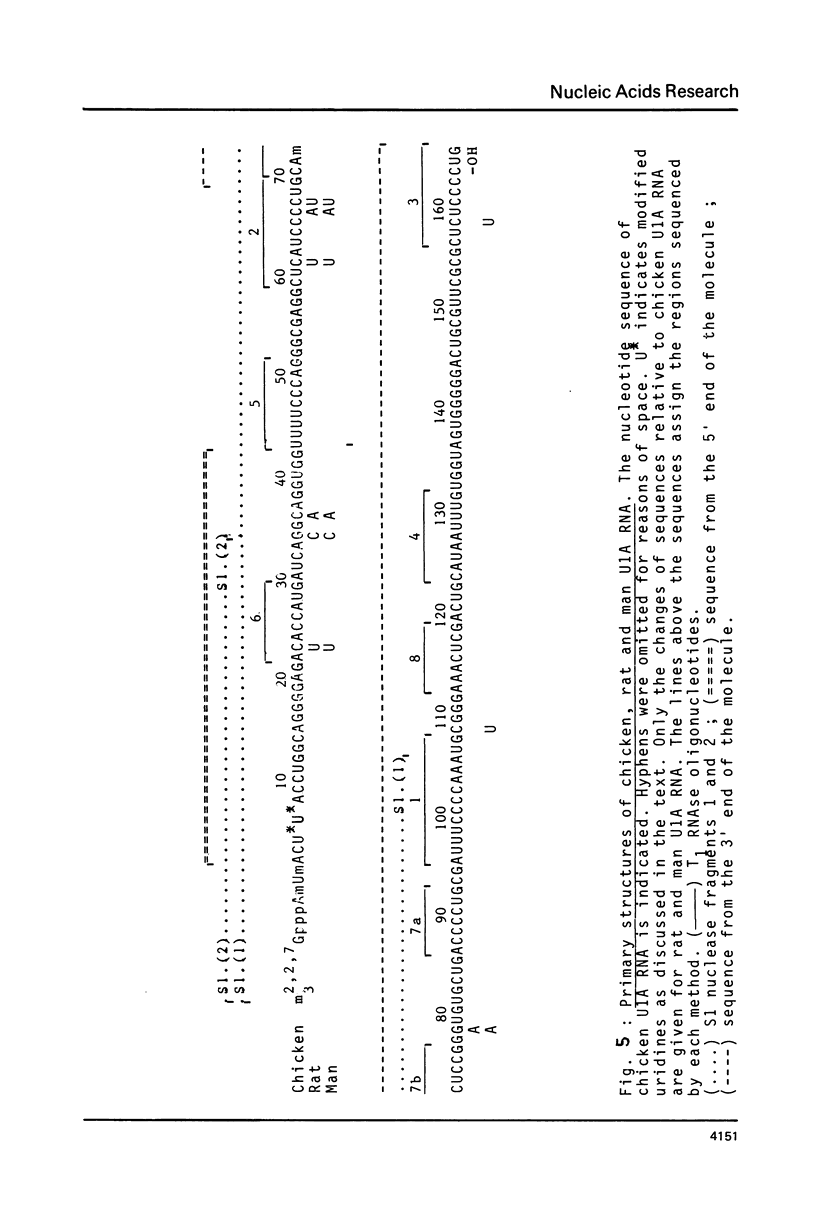
The methods of enzymatic and chemical treatment of end-labeled RNA were applied to the determination of the nucleotide sequence of chicken and man U1A RNA and to the reexamination of that of rat U1A RNA. The chemical method allowed the easy demonstration of the cap structure. All three RNA were 165 nucleotide long. Two hitherto non described modified pyrimidines were detected close to the 5' end. Only 9 base substitutions were observed from chicken to man indicating high degree of conservation of U1A RNA through evolution.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Machatt M. A., Ebel J. P. Structural study of ribosomal 23 S RNA from Escherichia coli. FEBS Lett. 1979 Nov 1;107(1):177–181. doi: 10.1016/0014-5793(79)80490-1. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Hellung-Larsen P., Frederiksen S. Isolation and DNA-RNA hybridization properties of small-molecular-weight nuclear RNA components from baby-hamster-kidney cells. Eur J Biochem. 1974 Jan 16;41(2):321–328. doi: 10.1111/j.1432-1033.1974.tb03272.x. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Flytzanis C., Alonso A., Louis C., Krieg L., Sekeris C. E. Association of small nuclear RNA with HnRNA isolated from nuclear RNP complexes carrying HnRNA. FEBS Lett. 1978 Dec 1;96(1):201–206. doi: 10.1016/0014-5793(78)81094-1. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. An evaluation of small nuclear RNA in hnRNP. FEBS Lett. 1979 Aug 1;104(1):176–182. doi: 10.1016/0014-5793(79)81110-2. [DOI] [PubMed] [Google Scholar]
- Gattoni R., Stevenin J., Jacob M. Comparison of the nuclear ribonucleoproteins containing the transcripts of adenovirus-2 and HeLa cell dna. Eur J Biochem. 1980;108(1):203–211. doi: 10.1111/j.1432-1033.1980.tb04713.x. [DOI] [PubMed] [Google Scholar]
- Guimont-Ducamp C., Sri-Widada J., Jeanteur P. Occurrence of small molecular weight RNAs in Hela nuclear ribonucleoprotein particles containing HnRNA. Biochimie. 1977;59(8-9):755–758. doi: 10.1016/s0300-9084(77)80259-9. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Krupp G., Gross H. J. Rapid RNA sequencing: nucleases from Staphylococcus aureus and Neurospora crassa discriminate between uridine and cytidine. Nucleic Acids Res. 1979 Aug 10;6(11):3481–3490. doi: 10.1093/nar/6.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. J., Galibert F., Lelong J. C., Boiron M. Caractérisation dans la fraction nucléaire de cellules KB d'un ARN métaboliquement stable de faible poids moléculaire. C R Acad Sci Hebd Seances Acad Sci D. 1967 Mar 13;264(11):1523–1526. [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Murray V., Holliday R. Mechanism for RNA splicing of gene transcripts. FEBS Lett. 1979 Oct 1;106(1):5–7. doi: 10.1016/0014-5793(79)80682-1. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestayko A. W., Busch H. Low molecular weight RNA of the chromatin fraction from Novikoff hepatoma and rat liver nuclei. Biochim Biophys Acta. 1968 Dec 17;169(2):327–337. doi: 10.1016/0005-2787(68)90041-5. [DOI] [PubMed] [Google Scholar]
- Reddy R., Ro-Choi T. S., Henning D., Busch H. Primary sequence of U-1 nuclear ribonucleic acid of Novikoff hepatoma ascites cells. J Biol Chem. 1974 Oct 25;249(20):6486–6494. [PubMed] [Google Scholar]
- Reddy R., Ro-Choi T. S., Henning D., Shibata H., Choi Y. C., Busch H. MOdified nucleosides of nuclear and nucleolar low molecular weight ribonucleic acid. J Biol Chem. 1972 Nov 25;247(22):7245–7250. [PubMed] [Google Scholar]
- Ro-Choi T. S., Henning D. Sequence of 5'-oligonucleotide of U1 RNA from Novikoff hepatoma cells. J Biol Chem. 1977 Jun 10;252(11):3814–3820. [PubMed] [Google Scholar]
- Ro-Choi T. S., Redy R., Henning D., Takano T., Taylor C. W., Busch H. Nucleotide sequence of 4.5 S ribonucleic acid of Novikoff hepatoma cell nuclei. J Biol Chem. 1972 May 25;247(10):3205–3222. [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Shibata H., Ro-Choi T. S., Reddy R., Choi Y. C., Henning D., Busch H. The primary nucleotide sequence of nuclear U-2 ribonucleic acid. The 5'-terminal portion of the molecule. J Biol Chem. 1975 May 25;250(10):3909–3920. [PubMed] [Google Scholar]
- Stevenin J., Gallinaro-Matringe H., Gattoni R., Jacob M. Complexity of the structure of particles containing heterogeneous nuclear RNA as demonstrated by ribonuclease treatment. Eur J Biochem. 1977 Apr 15;74(3):589–602. doi: 10.1111/j.1432-1033.1977.tb11428.x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]