Abstract
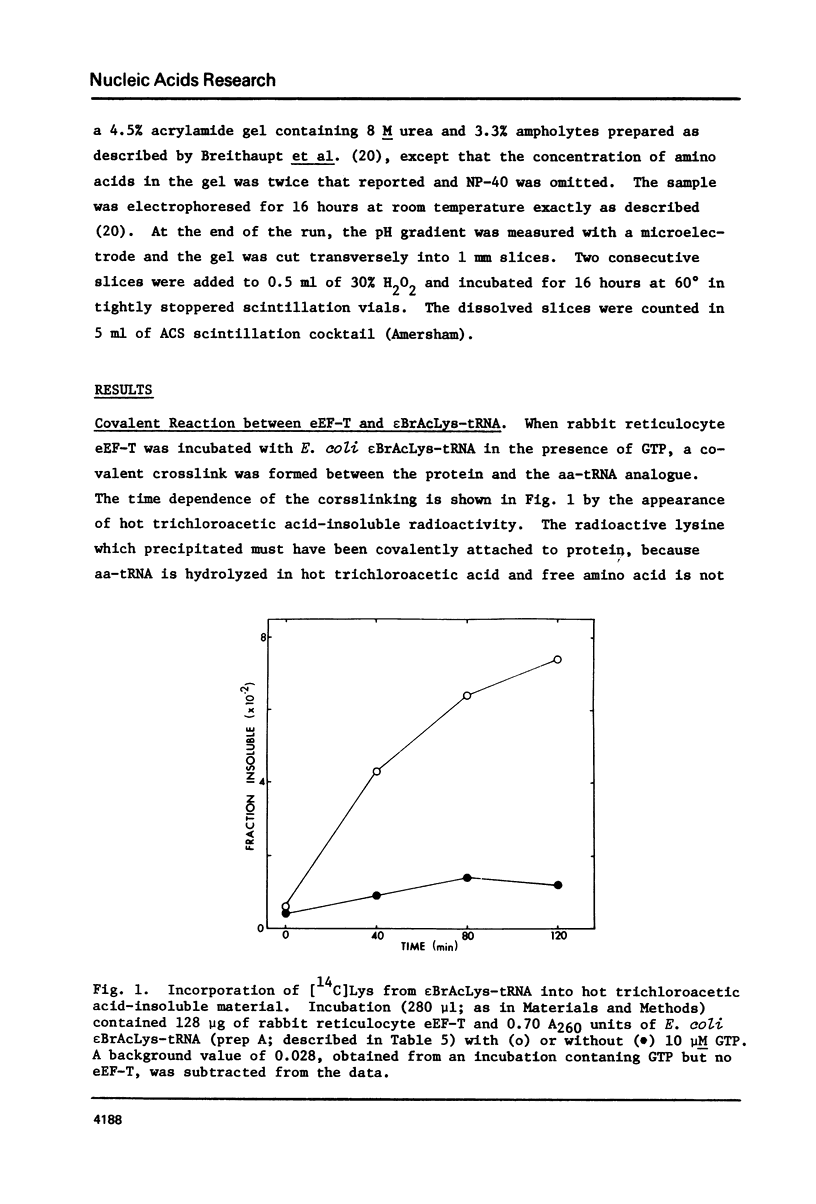
eEF-T and eEF-Tu from rabbit reticulocyte and from Artemia were affinity labeled using N epsilon-bromoacetyl-Lys-tRNA prepared with either yeast or E. coli tRNA. Only the eEF-Tu polypeptide was crosslinked when eEF-T was incubated with the reactive aminoacyl-tRNA analogue, which indicates that at least part of the aminoacyl-tRNA binding site is the same in both eEF-Tu and the multisubunit eEF-T. Complex formation (eEF-Tu x aa-tRNA x GTP) was required for crosslinking, since no covalent reaction with eEF-Tu occurred in the absence of GTP. The yield of crosslinked product was greatly reduced by adding either unmodified rabbit liver aminoacyl-tRNA or unmodified E. coli Lys-tRNA to the incubation to compete for the aminoacyl-tRNA binding site on eEF-T or eEF-Tu, indicating that the covalent reaction occurs while the N epsilon-bromoacetyl-Lys-tRNA is bound in this site. The affinity labeling of a prokaryotic and two different eukaryotic elongation factors by the same reagent suggests that there may be conservation of structure in the region of the proteins which binds the aminoacyl end of the aminoacyl-tRNA.
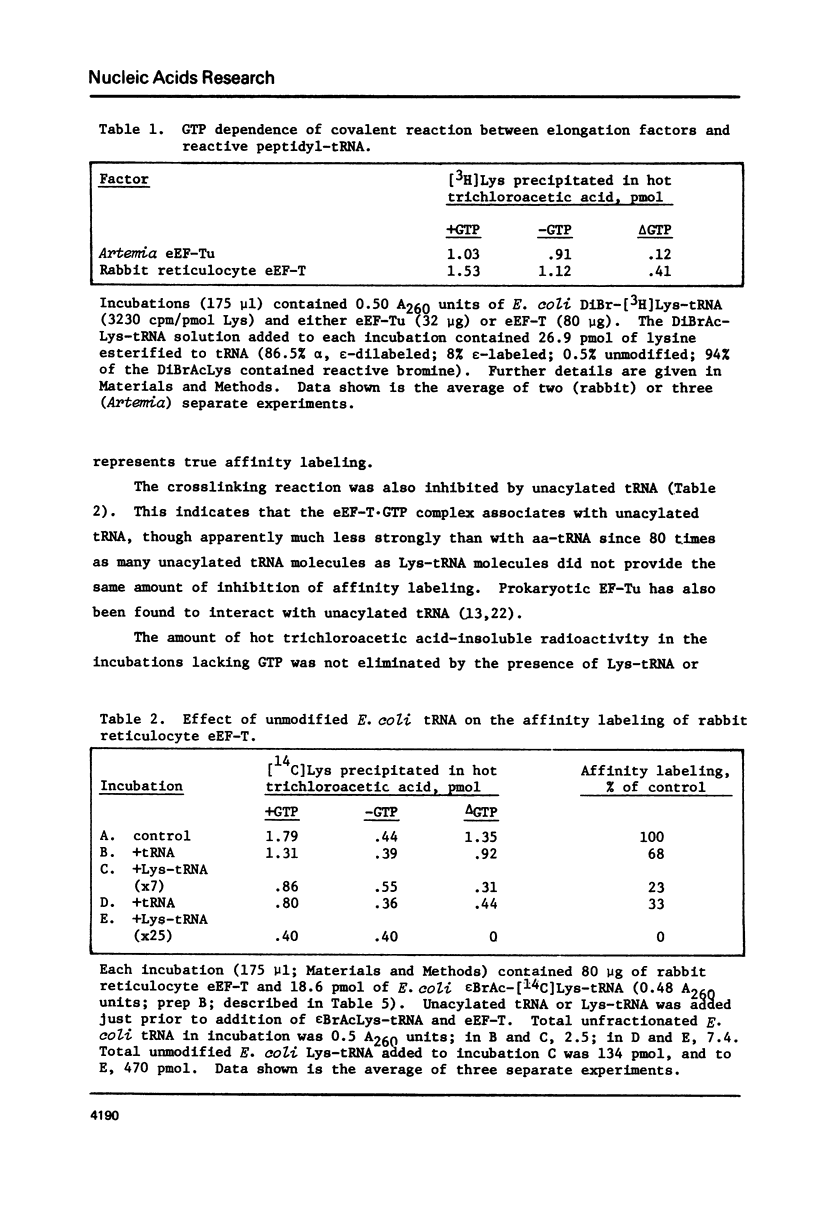
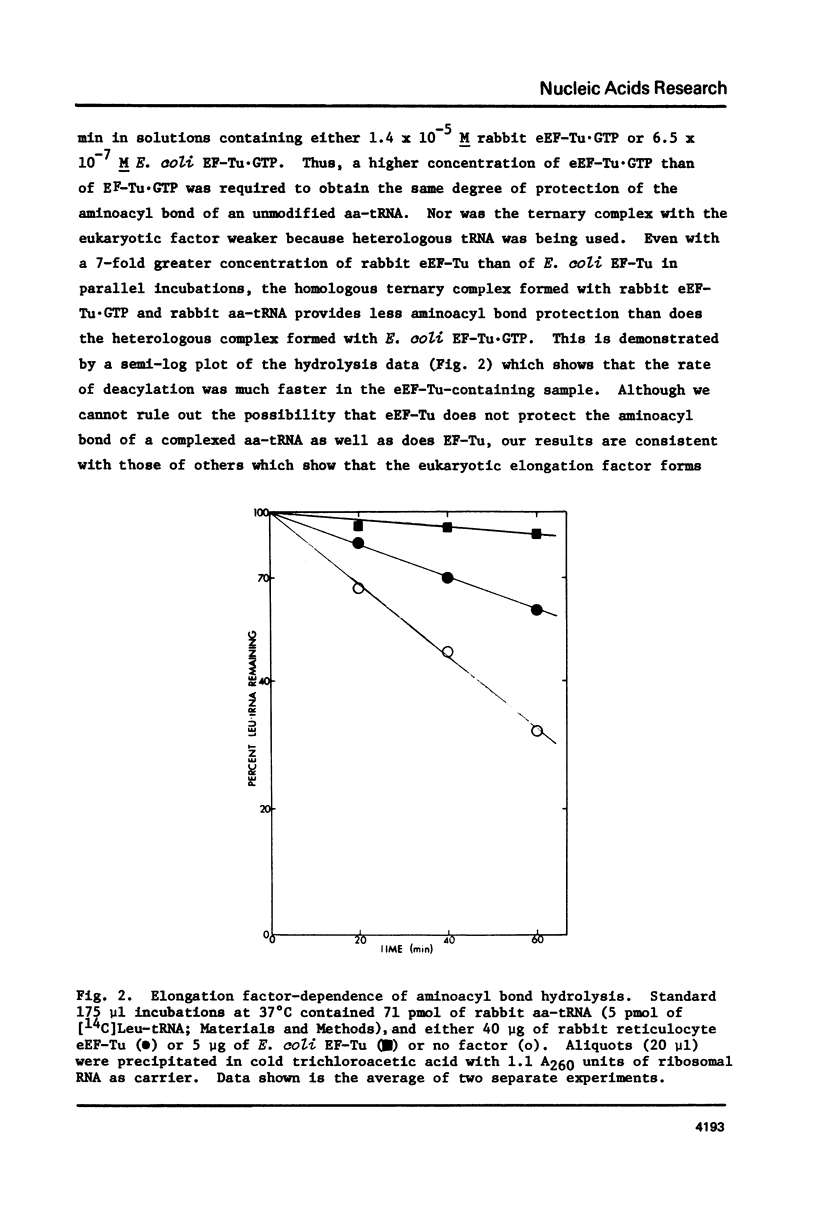
Full text
PDF




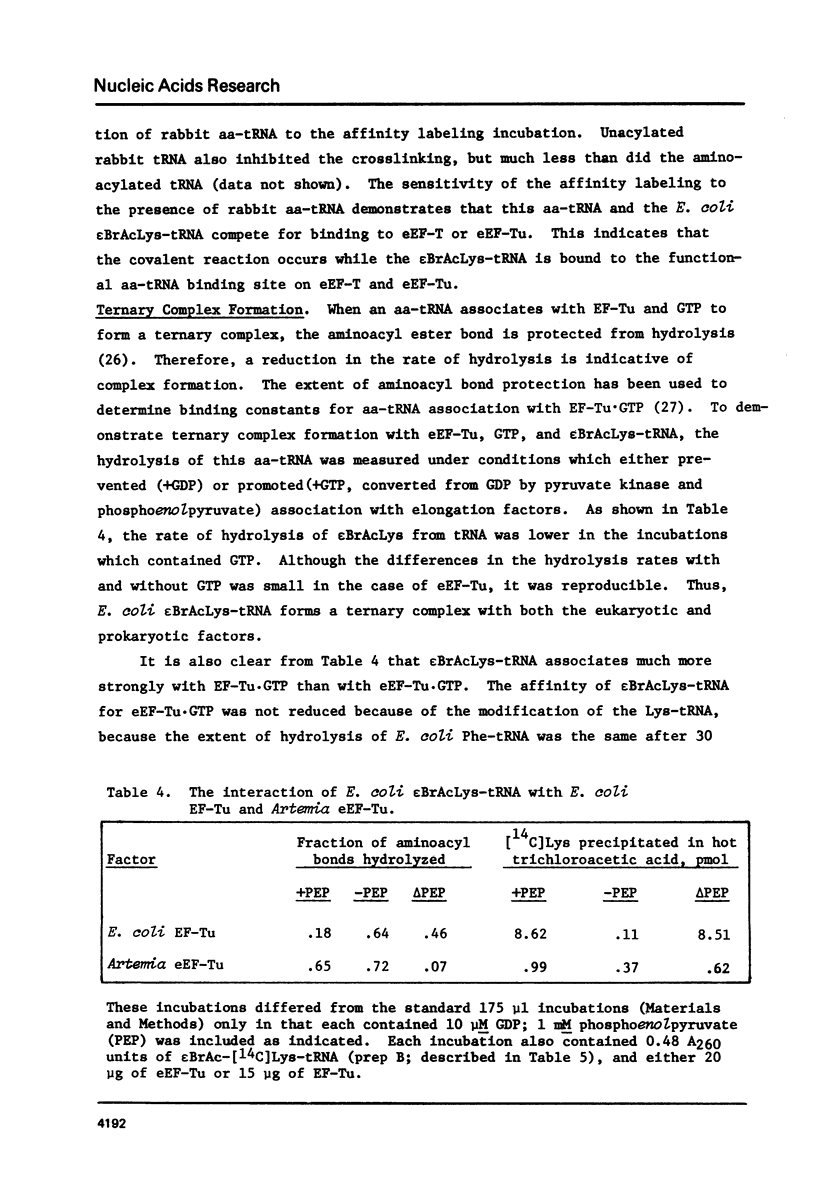




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beres L., Lucas-Lenard J. Studies on the fluorescence of the Y base of yeast phenylalanine transfer ribonucleic acid. Effect of pH, aminoacylation, and interaction with elongation factor Tu. Biochemistry. 1973 Sep 25;12(20):3998–4002. doi: 10.1021/bi00744a033. [DOI] [PubMed] [Google Scholar]
- Breithaupt T. B., Nystrom I. E., Hodges D. H., Jr, Babitch J. A. Composition of an isoelectric focusing gel yielding a broad pH gradient. Anal Biochem. 1978 Feb;84(2):579–582. doi: 10.1016/0003-2697(78)90078-7. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Differential rate of ribosomal protein synthesis in Escherichia coli B/r. J Mol Biol. 1974 Apr 15;84(3):407–422. doi: 10.1016/0022-2836(74)90449-5. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G., Weisblum B., Benzer S. THE FUNCTION OF sRNA AS AMINO ACID ADAPTOR IN THE SYNTHESIS OF HEMOGLOBIN. Proc Natl Acad Sci U S A. 1963 May;49(5):669–675. doi: 10.1073/pnas.49.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasmuk H., Nolan R. D., Drews J. The isolation and characterization of elongation factor eEF-Ts from Krebs-II mouse-ascites-tumor cells and its role in the elongation process. Eur J Biochem. 1978 Dec;92(2):479–490. doi: 10.1111/j.1432-1033.1978.tb12770.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Motoyoshi K., Nagata S., Kaziro Y. Purification and properties of a new polypeptide chain elongation factor, EF-1beta, from pig liver. J Biol Chem. 1976 Mar 25;251(6):1843–1845. [PubMed] [Google Scholar]
- Iwasaki K., Nagata S., Mizumoto K., Kaziro Y. The purification of low molecular weight form of polypeptide elongation factor 1 from pig liver. J Biol Chem. 1974 Aug 10;249(15):5008–5010. [PubMed] [Google Scholar]
- Jacobson K. B. A test of tRNA as amino acid adaptor in hemoglobin synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:719–722. doi: 10.1101/sqb.1966.031.01.092. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Cantor C. R. Elongation factor-dependent affinity labeling of Escherichia coli ribosomes. J Mol Biol. 1980 Apr;138(2):273–297. doi: 10.1016/0022-2836(80)90287-9. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Miller D. L., Cantor C. R. Functional covalent complex between elongation factor Tu and an analog of lysyl-tRNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3075–3079. doi: 10.1073/pnas.75.7.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. E., Woodward W. R., Herbert E., Menninger J. R. Nepsilon-acetyllysine transfer ribonucleic acid: a biologically active analogue of aminoacyl transfer ribonucleic acids. Biochemistry. 1976 Feb 10;15(3):569–575. doi: 10.1021/bi00648a018. [DOI] [PubMed] [Google Scholar]
- Kemper W. M., Merrick W. C., Redfield B., Liu C. K., Weissbach H. Purification and properties of rabbit reticulocyte elongation factor 1. Arch Biochem Biophys. 1976 Jun;174(2):603–612. doi: 10.1016/0003-9861(76)90389-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Assays for eukaryotic protein synthesis. Methods Enzymol. 1979;60:108–123. doi: 10.1016/s0076-6879(79)60011-3. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Elongation factor Tu and the aminoacyl-tRNA-EFTu-GTP complex. Methods Enzymol. 1974;30:219–232. doi: 10.1016/0076-6879(74)30024-9. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Krauss G., Peters F., Maass G. Ternary complex formation between elongation factor Tu, GTP and aminoacyl-tRNA: an equilibrium study. Eur J Biochem. 1977 Sep;78(2):403–409. doi: 10.1111/j.1432-1033.1977.tb11752.x. [DOI] [PubMed] [Google Scholar]
- Ravel J. M., Dawkins R. C., Jr, Lax S., Odom O. W., Hardesty B. Interaction of rabbit reticulocyte elongation factor 1 with guanosine-triphosphate and aminoacyl-transfer ribonucleic acid. Arch Biochem Biophys. 1973 Apr;155(2):332–341. doi: 10.1016/0003-9861(73)90122-7. [DOI] [PubMed] [Google Scholar]
- Roobol K., Möller W. The role of guanine nucleotides in the interaction between aminoacyl-tRNA and elongation factor 1 of Artemia salina. Eur J Biochem. 1978 Oct 16;90(3):471–477. doi: 10.1111/j.1432-1033.1978.tb12626.x. [DOI] [PubMed] [Google Scholar]
- Schulman R. G., Hilbers C. W., Miller D. L. Letters to the editor: Nuclear magnetic resonance studies of protein-RNA interactions. I. The elongation factor Tu-GTP aminoacyl-tRNA complex. J Mol Biol. 1974 Dec 15;90(3):601–607. doi: 10.1016/0022-2836(74)90237-x. [DOI] [PubMed] [Google Scholar]
- Slobin L. I. Eucaryotic elongation factors Ts is an integral component of rabbit reticulocyte elongation factor 1. Eur J Biochem. 1979 May 15;96(2):287–293. doi: 10.1111/j.1432-1033.1979.tb13039.x. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Möller W. Purification and properties of an elongation factor functionally analogous to bacterial elongation factor Ts from embryos of Artemia salina. Eur J Biochem. 1978 Mar;84(1):69–77. doi: 10.1111/j.1432-1033.1978.tb12142.x. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Möller W. Purification of elongation factor 1 from embryos of Artemia salina. Methods Enzymol. 1979;60:685–703. doi: 10.1016/s0076-6879(79)60064-2. [DOI] [PubMed] [Google Scholar]
- Smith D. W., McNamara A. L. Specialization of rabbit reticulocyte transfer RNA content for hemoglobin synthesis. Science. 1971 Feb 12;171(3971):577–579. doi: 10.1126/science.171.3971.577. [DOI] [PubMed] [Google Scholar]
- Weissbach H., Ochoa S. Soluble factors required for eukaryotic protein synthesis. Annu Rev Biochem. 1976;45:191–216. doi: 10.1146/annurev.bi.45.070176.001203. [DOI] [PubMed] [Google Scholar]



