Abstract
Memory consolidation is the process by which acquired information is converted to something concrete to be retrieved later. Here we examined a potential role for brain-derived neurotrophic factor (BDNF) in mediating the enhanced memory consolidation induced by the GABAA receptor antagonist, bicuculline methiodide. With the administration of an acquisition trial in naïve mice using a passive avoidance task, mature BDNF (mBDNF) levels were temporally changed in the hippocampal CA1 region, and the lowest levels were observed 9 h after the acquisition trial. In the passive avoidance task, bicuculline methiodide administration within 1 h of training but not after 3 h significantly increased latency time in the retention trial 24 h after the acquisition trial. Concomitantly, 1 h post-training administration of bicuculline methiodide, which enhanced memory consolidation, significantly increased mBDNF levels 9 h after training compared to those of the vehicle-treated control group. In addition, exogenous human recombinant BDNF (hrBDNF) administration 9 h after training into the hippocampal CA1 region facilitated memory consolidation confirming that the increase in mBDNF at around 9 h after training plays a key role in the enhancement of memory consolidation. Moreover, the increases in latency time and immediate early gene expressions by bicuculline methiodide or hrBDNF were significantly blocked by anisomycin, a protein synthesis inhibitor, K252a, a tyrosine receptor kinase (Trk) inhibitor, or anti-TrkB IgG. These findings suggest that the increase in the level of mBDNF and its function during a restricted time window after training are required for the enhancement of memory consolidation by GABAA receptor blockade.
Keywords: GABAA receptor, bicuculline methiodide, mature brain-derived neurotrophic factor, memory consolidation, passive avoidance test
INTRODUCTION
Since the concept of memory consolidation was introduced into behavioral neuroscience, numerous efforts have been dedicated to clarify the role of consolidation during learning and memory processes (Izquierdo et al, 2008). In addition, great efforts have been made to clarify the molecular and cellular mechanisms underlying memory consolidation (Sara, 2009). Converging evidences indicate that memory consolidation requires changes in intracellular signaling pathways, gene expression, and/or de novo protein synthesis. The most extensively studied molecule in memory consolidation is brain-derived neurotrophic factor (BDNF) because it might be required for consolidation of short-term to long-term memory and for synaptic plasticity (Poo, 2001; Tyler et al, 2002). In particular, hippocampal BDNF appears to be necessary in two discrete periods, one immediately after and another 1–4 h or 3–6 h after training for long-term memory (LTM) formation in a one-trial avoidance learning (Alonso et al, 2002a, 2002b; Grecksch and Matthies, 1980; Igaz et al, 2002). In addition, it was reported that new protein synthesis and BDNF in the rat hippocampus 12 h after an acquisition of a one-trial associative learning task are critical for the persistence of LTM storage (Bekinschtein et al, 2007). Although these results suggest that BDNF is a key molecule for persistence and/or maintenance of LTM, it is still unclear whether BDNF plays a role in the enhancement of memory consolidation within a limited time of around 6–12 h after training.
It is known that α-aminobutyric acid (GABA)A receptor agonists impair memory function and that its antagonists enhance memory consolidation (McGaugh and Roozendaal, 2009). For example, GABAA receptor antagonists, including bicuculline and flumazenil, enhance performance in memory tasks (Herzog et al, 1996), and its agonists, such as muscimol and diazepam, disrupt memory formation (Castellano and McGaugh, 1989). Particularly, it was demonstrated that bicuculline increased memory consolidation when administered into the CA1 region either immediately or 1.5 h after training (Luft et al, 2004). In addition, GABAA receptor antagonists (including bicuculline) are reported to enhance BDNF expression in the hippocampus (Katoh-Semba et al, 2001; Metsis et al, 1993). If BDNF is generally used to memory consolidation, as mentioned by Bekinschtein et al, (2007), enhanced memory consolidation by GABAA receptor blockade retrieved 24 h after an acquisition trial might result from the increased BDNF levels. However, it is unclear which element(s) or which signaling pathway(s) is involved in the enhancement of memory consolidation by GABAA receptor blockade.
We hypothesized that the enhancement of memory consolidation induced by GABAA receptor blockade within a limited time window results from a GABAA receptor blockade-induced increase in BDNF levels. To test this hypothesis, we investigated using behavioral and biochemical analysis (1) whether GABAA receptor blockade using bicuculline methiodide facilitates memory consolidation, (2) whether bicuculline methiodide-induced enhancement of memory consolidation is accompanied by an increase in BDNF levels, (3) whether exogenous human recombinant BDNF (hrBDNF) administration alone enhances memory consolidation, and (4) whether inhibition of protein synthesis or blockade of BDNF receptor modulates the enhancement of memory consolidation induced by bicuculline methiodide or hrBDNF.
MATERIALS AND METHODS
Animals
Male ICR (CD-1) mice (25–30 g, 7 weeks old), which is a Swiss mouse that is used as a general-purpose stock in oncological and pharmaceutical research and is often used to investigate the mechanism of learning and memory (Banks et al, 2001; Nagai et al, 2007) were purchased from the Orient a branch of Charles River Laboratories (Seoul, Korea). Mice were housed five per cage. Animals were provided with food and water ad libitum and kept under a 12 h light/dark cycle (light on 07:00–19:00) at ambient room temperature. Animal treatment and maintenance were carried out in accordance with the Principles of Laboratory Animal Care (NIH publication No. 85–23, revised 1985) and the Animal Care and Use Guidelines of Kyung Hee University, Korea. All efforts were made to minimize the number of animals as well as their suffering.
Materials
Bicuculline methiodide, hrBDNF, anisomycin, and cycloheximide were purchased from Sigma Chemical (St. Louis, MO). K252a was obtained from Calbiochem-Novabiochem Intl. (La Jolla, CA). Zoletil 50 was purchased from Virbac laboratory (Carros, France). Anti-mature BDNF (mBDNF) antibody was purchased from Osenses Pty (SA 5159, Australia). Anti-β-actin, anti-c-Fos, anti-Zif268, anti-extracellular signal-regulated kinase (ERK), and anti-phosphorylated ERK (pERK) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-TrkB, recombinant human TrkB Fc chimera (TrkB-Fc) and goat control IgG were purchased from R&D System (Minneapolis, MN). All other materials were of the highest grade available and were obtained from normal commercial sources. Bicuculline methiodide was dissolved in a 0.9% normal saline.
Microinfusion of Drugs
Mice were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) under Zoletil 50 anesthesia (10 mg/kg, i.m.), and guide cannulae (26 G) were aimed at the dorsal hippocampal CA1 pyramidal cell layer (stereotaxic coordinates: AP, −2.10 mm; ML, ±1.00 mm; DV, −1.00 mm) using an atlas of the mouse brain (Paxinos and Franklin, 2001). The guide cannulae were fixed to the skull with dental cement and covered with dummy cannulae. Following surgery, mice were allowed to recover for seven days. Bicuculline methiodide, hrBDNF, anisomycin, K252a, anti-TrkB IgG, and goat control IgG were dissolved in 0.9% bacteriostatic saline solution before the infusion. At 15 min prior to the indicated time points after the acquisition trial, mice were carefully restrained by hand and bilaterally infused with bicuculline methiodide [1 nmol/0.5 μl/side (Ren et al, 2008)], hrBDNF [0.2 μg/0.5 μl/side, (Alonso et al, 2002a)], anisomycin [80 μg/0.5 μl/side, (Bekinschtein et al, 2007)], K252a [100 pmol/0.5 μl/side, (Liu et al, 2008)], anti-TrkB IgG [1 μg/0.5 μl/side, (Jiang et al, 2003)], goat control IgG (1 μg/0.5 μl/side), or vehicle (0.9% saline solution, 0.5 μl/side) through an injector cannula (30 G) at 0.25 μl/min (the injection time was assumed to be at the indicated time points, ie, 15 min prior to the 6 h injection). After 2 min of infusion, the infusion needle remained in the guide cannula for 1 min more to ensure proper delivery of the reagents.
Passive Avoidance Task
The passive avoidance task was carried out as described elsewhere (Kim and Ryu, 2008). At the first training day, for the acquisition trial, a mouse was initially placed in the light compartment and the door between the two compartments was opened 10 s later. When the mouse entered the dark compartment, the door automatically closed and an electric foot shock (0.25 mA, 3 s) was delivered through the grid floor. For the retention trial, mice were again placed in the light compartment 24 h after the acquisition trial, and the time before entering the dark compartment was recorded. The passive avoidance task was conducted in Experiments 1–4. In all of the experiments, animals were used only one time.
Brain Section Preparation and Cresyl Violet Staining
To confirm the guide cannulae position, we carried out Nissl staining. The mice were immediately anesthetized with Zoletil 50 anesthesia (10 mg/kg, i.m.) after each experiment and perfused transcardially with 0.1 M phosphate buffer (pH 7.4) followed by ice-cold 4% paraformaldehyde. Brains were removed and postfixed in phosphate buffer (0.05 M, pH 7.4) containing 4% paraformaldehyde overnight and then immersed in 30% sucrose solution (in 0.05 M phosphate-buffered saline) and stored at 4 °C until sectioning. Frozen brains were coronally sectioned on a cryostat at 30 μm and then stored in storage solution at 4 °C. After mounting sections onto gelatin-coated slides, they were stained with 0.5% cresyl violet, dehydrated through graded alcohols (70, 80, 90, and 100%), placed in xylene, and coverslipped using Histomount medium.
Western Blot Analysis
The mice were sacrificed at each designated time point, and brains were removed. Hippocampal tissue was isolated and homogenized in ice-chilled buffer [20 mM Tris-HCl (pH 7.4) containing 0.32 M sucrose, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 μg/ml aprotinin, 15 μg/ml leupeptin, 10 μg/ml bacitracin, 10 μg/ml pepstatin, 15 μg/ml trypsin inhibitor, 50 mM NaF, and 1 mM sodium orthovanadate]. Samples of homogenates (30 μg of protein for mBDNF and 15 μg of protein for c-Fos and Zif268) were subjected to SDS-PAGE (12% gel for mBDNF and 8% gel for c-Fos, Zif268, ERK, and pERK) under reducing conditions. Proteins were transferred onto PVDF membranes in transfer buffer (25 mM Tris, 192 mM glycine, 20% v/v methanol) for 2 h at 100 V and 4 °C. Western blots were performed by incubating membranes first with anti-mBDNF, anti-Zif268, anti-c-Fos, anti-ERK, or anti-pERK antibody (1 : 1000 dilution), then stripping and incubating with anti-β-actin antibody (1 : 5000, dilution). Film densitometric analysis was performed by using the Quantity One Image Analysis System (version 4.6.3, Bio-Rad Laboratories, Hercules, CA). mBDNF, c-Fos, and Zif268 levels were normalized to actin levels in the same membrane, pERK levels were normalized to ERK levels, and the mean values were referred to control values taken as 1.0.
Experiment 1
Experiment 1 was conducted to test the effects of bicuculline methiodide on the memory consolidation phase. First, bicuculline methiodide (1.25, 2.5, 5, or 10 mg/kg, i.p.) was administered immediately after an acquisition trial to determine the most effective dose of bicuculline methiodide for memory consolidation in the passive avoidance task (0.25 mA, 3 s). Second, we wanted to investigate the effective time window for bicuculline methiodide-enhanced memory consolidation. Bicuculline methiodide (5 mg/kg, i.p.) was administered immediately (0 h), 1, 3, or 6 h after the acquisition trial.
Experiment 2
To measure the temporal profile of mBDNF levels after electric foot shock, mice were introduced to one-trial passive avoidance task (0.25 mA, 3 s) and sacrificed at designated time points. Brains were removed for western blot analysis. In addition, mice were administered bicuculline methiodide (5 mg/kg, i.p.) without any electric foot shock to investigate its effects on the mBDNF level in naïve mice. Mice were sacrificed at designated time points, and brains were removed for western blot analysis.
Experiment 3
In experiment 3, we wanted to investigate the reasons why the enhancement of memory consolidation disappears when bicuculline methiodide is administered 3 h after the acquisition trial. First, mice were systemically administered bicuculline methiodide 1 or 3 h after the acquisition trial and were sacrificed 6, 9, or 12 h after the acquisition trial. In a separate experiment, to confirm the effect of hippocampal GABAA receptor blockade, bicuculline methiodide (1 nmol/side) was injected into the hippocampus 1 h after the acquisition trial, and the mice were sacrificed 6, 9, or 12 h after the acquisition trial. Western blot analysis was also conducted to assess the effects of bicuculline methiodide on mBDNF levels. In addition, we investigated the effect of exogenous hrBDNF infused into the hippocampus at specific time points (6, 9, or 12 h) after the acquisition trial on memory consolidation to confirm whether exogenously infused hrBDNF enhances memory consolidation in the passive avoidance task. Mice were injected with hrBDNF 6, 9, or 12 h after the acquisition trial and subjected into the retention trial 24 h after the acquisition trial.
Experiment 4
If mBDNF levels at specific time point(s) induced by bicuculline methiodide play a crucial role in the enhancement of memory consolidation, blockade of the interaction between mBDNF and its receptor could attenuate the enhancement of memory consolidation. TrkB blockers (K252a or TrkB IgG, to inhibit the interaction between mBDNF and its receptor) or protein synthesis inhibitor (anisomycin, to inhibit protein synthesis which could be occurring after the interaction between mBDNF and its receptor) was administered to the mice treated with bicuculline methiodide 1 h after the acquisition trial. Mice were co-administered bicuculline methiodide (systemic injection, 5 mg/kg; intra-hippocampal injection, 1 nmol/side) and anisomycin (80 μg/side), K252a (100 pmol/side), or anti-TrkB IgG (1 μg/side) 1 h and 9 h, respectively, after the acquisition trial and were subjected to the retention trial 24 h after the acquisition trial. In a separate experiment, these mice were sacrificed 12 h after the acquisition trial for measuring c-Fos and Zif286 expression levels.
Statistics
Results from the passive avoidance task (except data analyzed by Student's t-test) were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test for multiple comparisons. The latency times obtained by exogenous infusion of hrBDNF were analyzed by Student's t-test. Results regarding the effective time window for bicuculline methiodide in the passive avoidance task and the interactions between bicuculline methiodide and anisomycin, between bicuculline methiodide or hrBDNF and K252a, and between bicuculline methiodide and anti-TrkB IgG were analyzed by two-way ANOVA followed by Bonferroni's post hoc test for multiple comparisons. Statistical significance was set at P<0.05.
RESULTS
Bicuculline Methiodide Increases Memory Consolidation
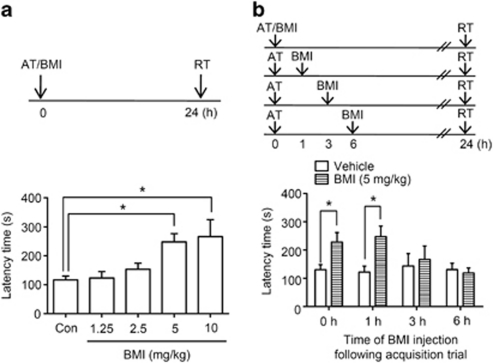
Bicuculline methiodide (1.25, 2.5, 5 or 10 mg/kg) or vehicle was administered immediately after the acquisition trial to assess its effects on memory consolidation. Step-through latency in the passive avoidance task observed 24 h after the acquisition trial displayed significant group effects [F(4, 36)=6.120, P<0.05, Figure 1a]. Mice receiving bicuculline methiodide (5 and 10 mg/kg) immediately after the acquisition trial exhibited longer latency times in the retention trial than vehicle-treated controls (5 mg/kg, +113% respect to control, P<0.05, n=8; 10 mg/kg, +29% respect to control, P<0.05, n=5, Figure 1a); however, the mice treated with 10 mg/kg of bicuculline methiodide exhibited stage 2 seizure-like activity (Stage 2, nodding and wet dog shaking) based on Racine's score (Racine, 1972). Therefore, to avoid unpredictable histological changes in the hippocampus, which is vulnerable to seizure activity induced by pro-convulsive drugs (Lado et al, 2002), we chose 5 mg/kg bicuculline methiodide for further experiments.
Figure 1.
Effect of a single administration of bicuculline methiodide (BMI) on memory consolidation in the passive avoidance. a, The effect of BMI on memory consolidation in one-trial passive avoidance task. BMI (1.25, 2.5, 5, or 10 mg/kg, i.p.) was administered immediately after an acquisition trial. The control group was treated with vehicle solution (Con). The retrieval trial was carried out 24 h after the acquisition trial. The latency time of the BMI-treated group (5 or 10 mg/kg, i.p.) was significantly greater than that of the vehicle-treated group (*P<0.05; one-way ANOVA followed by Tukey's post hoc test). Data are presented as means±SEM (n=5–8/group). b, The effective time window of BMI on one-trial passive avoidance task. To investigate the effective time window of BMI for memory consolidation, BMI (5 mg/kg, i.p.) was administered immediately (0 h), 1, 3, or 6 h after the acquisition trial. The retrieval trial was carried out 24 h after the acquisition trial. The latency time of the BMI-treated group (0 or 1 h post-training administration) was significantly greater than that of the vehicle-treated group (*P<0.05; two-way ANOVA followed by Bonferroni's post hoc test). Data are presented as means±SEM (n=6–8/group).
To determine the effective time window for enhancement of memory consolidation by bicuculline methiodide, mice were treated with bicuculline methiodide or vehicle at designated time points after the acquisition trial. Two-way ANOVA revealed that there is significant group effect in treatment [F(1, 48)=5.924, P<0.05]. As shown in Figure 1b, mice receiving bicuculline methiodide (5 mg/kg) immediately and 1 h after the acquisition trial exhibited longer latency times than vehicle-treated controls (0 h, +85% respect to vehicle-treated control, P<0.01, n=7; 1 h, +88% respect to vehicle-treated control, P<0.05, n=7; Figure 1b) but not at 3 or 6 h. These results indicated that the reason why the effects of bicuculline methiodide are observed with administration at 1 h after the acquisition trial but not at 3 or 6 h might be due to the expression of consolidated-related molecule(s).
Because we employed single behavior task to investigate memory consolidation, we examined the effect of muscimol, a GABAA receptor agonist, on memory consolidation and the interactions between muscimol and bicuculline methiodide to confirm the experimental behavioral paradigm. Muscimol administration at 1 h after the acquisition trial significantly impaired memory consolidation [F(3, 36)=4.942, P<0.05, n=9–12, Supplementary Figure S1A]. Moreover, co-administration of bicuculline methiodide with subeffective dose of muscimol (0.5 mg/kg) significantly blocked the effect of bicuculline methiodide on memory consolidation [F(3, 34)=8.691, P<0.05, n=8–10, Supplementary Figure S1B]. In addition, significant interactions were observed between muscimol and bicuculline methiodide [F(1, 34)=5.199, P<0.05, n=8–10, Supplementary Figure S1B]. These results suggest that blockade of GABAA receptor facilitates memory consolidation.
Bicuculline Methiodide Increases mBDNF Level in the Hippocampus
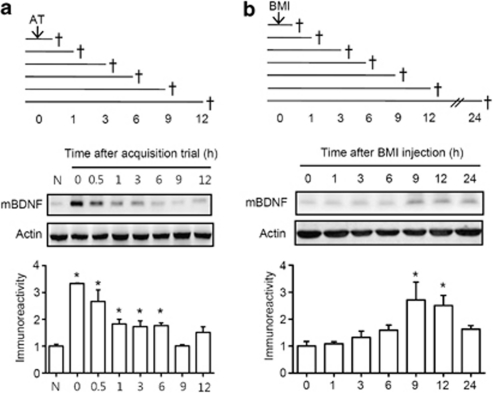
BDNF has been implicated in the modulation of synaptic plasticity (Schinder and Poo, 2000) and memory consolidation (Yin et al, 2002). Thus, we investigated the temporal changes of mBDNF levels in the hippocampus after the acquisition trial using western blots (Figure 2a). Mice were administered the one-trial passive avoidance task, and thereafter, brain tissue was subjected to western blot analysis at the indicated time points after the acquisition trial (0, 0.5, 1, 3, 6, 9, or 12 h). Naive mice were treated neither drugs nor acquisition trial. mBDNF (14 kD) levels were markedly higher in the mouse hippocampus within 1 h after the acquisition trial, and the elevated mBDNF levels gradually declined until 9 h after the acquisition trial (Figure 2a). The mBDNF levels 9 h after the acquisition trial were similar to the basal level of normal naïve mice. Thereafter, mBDNF levels were slightly increased 12 h after the acquisition trial, but not significant [0 h, +233% with respect to naive group, P<0.05, n=4; 1 h, +166% with respect to naive group, P<0.05, n=4; 3 h, +73% with respect to naive group, P<0.05, n=4; 6 h, +77% with respect to naive group, P<0.05, n=4; 9 h, +1% with respect to naive group, P>0.05, n=4; 12 h, +52% with respect to naive group; P>0.05, n=4; F(7, 24)=15.46, P<0.05]. These results were also confirmed by the immunohistochemical data obtained at 0 and 9 h after the acquisition trial in both the CA1 and dentate gyrus (DG) region [CA1, P<0.05, n=3, F(2, 6)=9.373; DG, P<0.001, n=3, F(2, 6)=6.876] (Supplementary Figure S2).
Figure 2.
Temporal profiles of mBDNF levels in the hippocampus after the passive avoidance training or bicuculline methiodide (BMI) administration. a, To determine the temporal profile of mBDNF levels after the acquisition trial without any drug administration, mice were subjected to the one-trial passive avoidance task (0.25 mA, 3 s) and then sacrificed (†) at designated time points [immediately (0), 0.5, 1, 3, 6, 9, or 12 h] after the acquisition trial for the western blotting. Naïve mouse group (N) did not received any administration of drug or training. Data are presented as means±SEM (n=4/group). *P<0.05, compared to the normal group (one-way ANOVA followed by Tukey's post hoc test). b, To investigate the temporal profiles of mBDNF levels after BMI administration, mice were treated with BMI (5 mg/kg, i.p.) and sacrificed (†) at designated time points [immediately (0), 1, 3, 6, 9, 12, or 24 h] after the administration for western blotting. Data are presented as means±SEM (n=3/group). *P<0.05, compared with the 0 h group (one-way ANOVA followed by Tukey's post hoc test).
After then, bicuculline methiodide was administered to mice without any training trial to investigate its effects on mBDNF levels in the hippocampus at various time points (sacrificing at 1, 3, 6, 9, 12, or 24 h after bicuculline methiodide administration). The mBDNF levels in the hippocampus gradually increased [F(6, 14)=5.805, P<0.05, Figure 2b] and were significantly higher at 9 and 12 h after administration (9 h, +170% with respect to 0 h group, P<0.05, n=3; 12 h, +150% with respect to 0 h group, P<0.05, n=3). In addition, bicuculline methiodide treatment immediately after the acquisition trial significantly increased mBDNF levels at 0, 0.5, 6, 9, and 12 h after the acquisition trial compared to those of the normal group [0 h, +178% with respect to normal controls, P<0.05, n=3; 0.5 h, +136% with respect to normal controls, P<0.001, n=4; 1 h, +44% with respect to normal controls, P>0.05, n=3; 3 h, +35% with respect to normal controls, P>0.05, n=4; 6 h, +139% respect to normal controls, P<0.05, n=3; 9 h, +129% respect to normal controls, P<0.05, n=3; 12 h, +101% respect to normal controls, P<0.05, n=3; F(7, 18)=3.462, P<0.05; Supplementary Figure S3]. Comparing the results of the temporal profiles of mBDNF levels after the acquisition trial without administration of bicuculline methiodide, after the administration of bicuculline methiodide without the acquisition trial treatment, and after the administration of bicuculline methiodide with the acquisition trial treatment, we hypothesized that mBDNF at 6, 9, or 12 h after an acquisition trial plays a crucial role in the bicuculline methiodide-induced enhancement of memory consolidation in the passive avoidance task.
Increased mBDNF Levels Induced by Bicuculline Methiodide or by Exogenous Administration of hrBDNF Play a Role in the Enhancement of Memory Consolidation
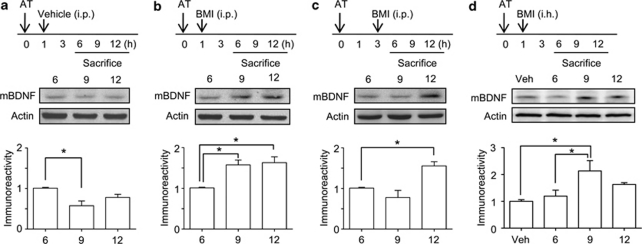
We compared the effects of bicuculline methiodide on mBDNF levels at different administration times (1 or 3 h) after the acquisition trial to examine the role of mBDNF at 6, 9, and 12 h after the acquisition trial. In the control group, which received only the acquisition trial, there was a significant decline in mBDNF level 9 h after the acquisition trial compared with that at 6 h [F(2, 9)=20.45, P<0.05, Figure 3a], as shown in Figure 2a. However, the mBDNF levels at 6 and 12 h after the acquisition trial were not significantly different. With the 1 h post-training administration of bicuculline methiodide, a significant increase in mBDNF level was observed at 9 and 12 h after the acquisition trial compared with that at 6 h [F(2, 9)=10.24, P<0.05, Figure 3b]. With the intra-hippocampal administration of bicuculline methiodide 1 h after the acquisition trial, the mBDNF level was similar to that of systemic administration of bicuculline methiodide [F(3, 10)=5.537, P<0.05, Figure 3d]. The mBDNF profile at 9 h in the 3 h post-training administration of bicuculline methiodide group was slightly decreased compared to that at 6 h, but not significant (Figure 3c). However, the mBDNF level at 12 h after the acquisition trial was significantly higher than that at 6 h [F(2, 9)=11.40, P<0.05, Figure 3c].
Figure 3.
mBDNF levels in hippocampal tissue at specific time points after an acquisition trial with or without bicuculline methiodide (BMI). Mice were administered BMI 1 or 3 h after an acquisition trial (AT) and sacrificed 6, 9, and 12 h after the acquisition trial as shown in the experimental scheme (upper panels). a, mBDNF levels at 6, 9, and 12 h after the acquisition trial in the acquisition trial only mice. b, mBDNF levels at 6, 9, and 12 h after the acquisition trial in the acquisition trial with BMI (5 mg/kg, i.p.) mice (1 h post-training). c, mBDNF levels at 6, 9, and 12 h after the acquisition trial in the acquisition trial with BMI (5 mg/kg, i.p.) mice (3 h post-training). d, mBDNF levels at 6, 9, and 12 h after the acquisition trial in the acquisition trial with bilaterally intra-hippocampal BMI (1 nmol/side) mice (1 h post-training). Vehicle (Veh) (0.9% saline) was also bilaterally infused. The data shown are the ratio relative to 6 h and are presented as means±SEM (n=4/group). *P<0.05, compared with the 6 h level (one-way ANOVA followed by Tukey's post hoc test).
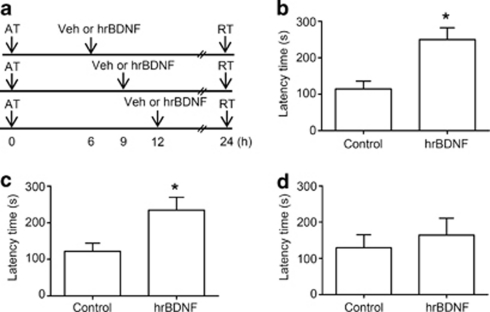
Bicuculline methiodide enhanced memory consolidation when it was administered 1 h after the acquisition trial but not 3 h after. The major differences from the results of 1 h or 3 h post-administration of bicuculline methiodide experiments were the mBDNF levels at 9 h after the acquisition trial. Because BDNF is required for consolidation of short-term to long-term memory and for synaptic plasticity (Poo, 2001; Tyler et al, 2002), we hypothesized that the mBDNF increased by bicuculline methiodide at 9 h after the acquisition trial plays a crucial role in the enhancement of memory consolidation, possibly explaining the effective time window of bicuculline methiodide. In order to test this hypothesis, we examined whether hrBDNF exogenously administered into the bilateral dorsal hippocampal CA1 region also facilitates memory consolidation (Figure 4a and Supplementary Figure S4A). Before the test, we confirmed the effect of hrBDNF on TrkB receptor signaling. hrBDNF infusion into the hippocampual CA1 region significantly increased ERK activity at 15 min after injection [F(2, 8)=8.364, P<0.05, n=3–4, Supplementary Figure S4B]. Moreover, co-infusion of hrBDNF with anti-TrkB IgG significantly blocked hrBDNF-induced increase of ERK phosphorylation [F(3, 11)=7.717, P<0.05, n=3–4, Supplementary Figure S4C]. These results suggest that intra-hippocampal administration of hrBDNF induces phosphorylation of ERK which is downstream of BDNF-TrkB signaling. Exogenous hrBDNF both 6 and 9 h after the acquisition trial significantly increased the latency time compared with that of vehicle-treated controls [6 h (control, 114.4±22.2, n=6; hrBDNF, 250.3±32.2, n=8, t(12)=3.234, P<0.05, Figure 4c); 9 h (control, 121.9±22.3, n=10; hrBDNF, 234.7±35.1, n=10, t(18)=2.712, P<0.05, Figure 4d)]. However, there were no significant differences between the 12 h post-training hrBDNF-treated group and the vehicle-treated control group [control, 129.4±36.1, n=5; hrBDNF, 164.1±47.2, n=5, t(8)=0.582, P>0.05] (Figure 4e). Therefore, elevated mBDNF levels in the hippocampus at a specific time, such as at 6 or 9 h, are sufficient to enhance memory consolidation induced by bicuculline methiodide in the passive avoidance task.
Figure 4.
Exogenous human recombinant BDNF (hrBDNF) administration enhances memory consolidation. a, Experimental scheme used in this experiment. b–d, Effect of hrBDNF on memory consolidation. hrBDNF (0.2 μg/0.5 μl/side) or vehicle (Veh, 0.9% saline solution) was bilaterally infused into hippocampus 6 (b), 9 (c), or 12 (d) h after an acquisition trial (AT), and the mice were subjected to the retrieval trial (RT) 24 h after the acquisition trial. Data are presented as means±SEM (b and c, n=8–10/group; d, n=5/group). *P<0.05, compared with the vehicle-treated control group (Student's t-test).
Bicuculline Methiodide-induced Enhancement of Memory Consolidation is Due to the Function of mBDNF Through its Receptor in the Hippocampus
Within the hippocampus, BDNF regulates synaptic plasticity related to learning and memory, and this effect results from an interaction between mBDNF and its functional receptor TrkB. Upon binding, BDNF-TrkB activation triggers a number of intracellular signaling pathways (Martinowich and Lu, 2008) and in turn, de novo protein synthesis related to c-fos or zif268 gene expression, which participates in synaptic plasticity and memory consolidation (Alder et al, 2003; Jones et al, 2001; Yasoshima et al, 2006). Therefore, to investigate whether elevated mBDNF induced by bicuculline methiodide participates in the facilitation of memory consolidation induced by bicuculline methiodide, we decided to block protein synthesis or the TrkB receptor in the dorsal hippocampus following bicuculline methiodide administration.
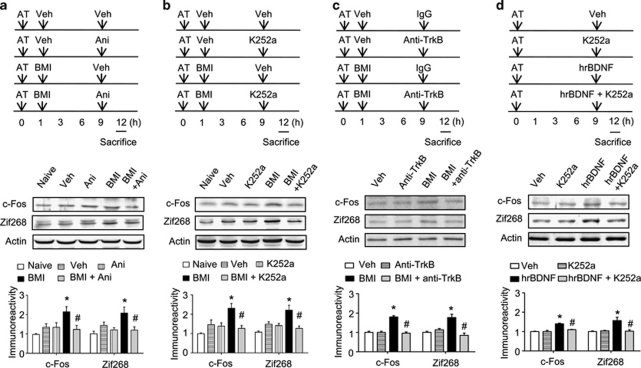
If the administration of a protein synthesis inhibitor at 6 or 9 h attenuates bicuculline methiodide-induced enhancement of memory consolidation, this would indicate that the de novo protein synthesis at that time point plays a role in the enhancement of memory consolidation. In the case of protein synthesis inhibition using anisomycin at 6 h after the acquisition trial, we observed that the latency time in the anisomycin-treated group was significantly shorter compared to the vehicle-treated group with the acquisition trial (Supplementary Figure S5B). Similar results were also observed in another protein synthesis inhibitor-treated group (3 h post-training treatment) (Supplementary Figure S5A) and in the group systemically treated with cycloheximide, a protein synthesis inhibitor, 3 or 6 h after the acquisition trial (P<0.05, Supplementary Figure S6). However, memory consolidation was not inhibited by the administration of cycloheximide 9 or 12 h after the acquisition trial (P>0.05). In the case of bicuculline methiodide administration mentioned above (Supplementary Figure S5), the increased latency time induced by bicuculline methiodide administration was reversed by anisomycin administration 3 or 6 h after the acquisition trial. However, there were no interactions between anisomycin and bicuculline methiodide [3 h, F(1, 23)=19.821, P>0.05, Supplementary Figure S5A; 6 h, F(1, 24)=2.973, P>0.05, Supplementary Figure S5B, two-way ANOVA].
The enhancement of memory consolidation induced by 1 h post-administration of bicuculline methiodide was reversed by the administration of anisomycin 9 h after the acquisition trial [Vehicle/vehicle, 104.7±7.1, n=6; vehicle/anisomycin, 113.7±19.0, n=6; bicuculline methiodide/vehicle, 227.5±13.5, n=8; bicuculline methiodide/anisomycin, 104.0±16.5, n=7, F(3, 23)=18.50, Figure 5a]. Similar results were obtained with the administration of cycloheximide [Vehicle/vehicle, 145.8±12.6, n=10; vehicle/cycloheximide, 131.6±20.8, n=10; bicuculline methiodide/vehicle, 322.1±32.2, n=10; bicuculline methiodide/cycloheximide, 223.3±17.2, n=10; F(3, 36)=15.88, P<0.05] (Supplementary Figure S7). In addition, anisomycin administration 9 h after the acquisition trial reversed the increased latency time induced by exogenous hrBDNF injection 9 h after the acquisition trial [Vehicle, 132.5±11.7, n=6; anisomycin, 142.3±21.7, n=6; hrBDNF, 230.2±28.4, n=6; hrBDNF/anisomycin, 145.0±14.6, n=6; F(3, 20)=5.08, P<0.05, Supplementary Figure S8]. These results from the inhibition of protein synthesis using anisomycin or cycloheximide suggest that protein synthesis at 9 h after the acquisition trial without any drug administration does not affect memory consolidation, but it does at 3 or 6 h, and that the increased mBDNF level at 9 h induced either by GABAA receptor blockade (1 h post-administration of bicuculline methiodide) or exogenous hrBDNF plays a crucial role in the enhancement of memory consolidation.
Figure 5.
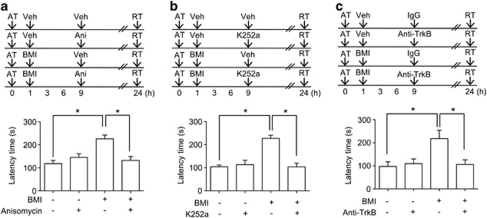
Blockade of protein synthesis or tyrosine receptor kinase B (TrkB) attenuates the enhancement of memory consolidation induced by bicuculline methiodide (BMI). a, Effect of the blockade of protein synthesis on BMI-induced enhancement of memory consolidation. BMI (5 mg/kg, i.p.) or vehicle (Veh) was administered 1 h after an acquisition trial (AT) and anisomycin (Ani, 80 μg/0.5 μl/side) or vehicle was infused into hippocampus 9 h after the acquisition trial. The mice were subjected to the retrieval trial (RT) 24 h after the acquisition trial. Data are represented as means±SEM (n=6–8/group). P values were obtained by two way ANOVA followed by Tukey's post hoc test. *P<0.05. b and c, Effect of TrkB inhibition on the BMI-induced enhancement of memory consolidation. BMI (5 mg/kg, i.p.) or vehicle was administered 1 h after the acquisition trial and K252a (100 pmol/0.5 μl/side, b) or anti-TrkB IgG (1 μg/0.5 μl/side, c) was infused into hippocampus 9 h after the acquisition trial. The mice were subjected to the retrieval trial 24 h after the acquisition trial. Data are presented as means±SEM (n=6–10/group). P values were obtained by two-way ANOVA followed by Tukey's post hoc test. *P<0.05.
To investigate whether mBDNF contributes to the enhancement of memory consolidation through interactions with its receptor, first we used a tyrosine receptor kinase inhibitor, K252a. K252a administration at 9 h after the acquisition trial had no effect on memory consolidation by itself, but it significantly attenuated the enhanced memory consolidation induced by bicuculline methiodide administration at 1 h after the acquisition trial [Vehicle/vehicle, 118.5±13.9, n=6; vehicle/K252a, 145.5±16.2, n=6; bicuculline methiodide/vehicle, 226.0±16.74, n=7; bicuculline methiodide/K252a, 132.4±17.0, n=7; F(3, 22)=9.205, P<0.05] (Figure 5b). Similar results were also observed with the intra-hippocampal injection of bicuculline methiodide in the same protocol (Supplementary Figure S9). Moreover, we observed significant interactions between bicuculline methiodide and K252a [F(1, 26)=5.496, P<0.05, Supplementary Figure S9]. To address the involvement of TrkB receptor and BDNF itself in memory consolidation, we blocked receptor-ligand binding with anti-TrkB IgG or scavenged BDNF with anti-TrkB-Fc. Anti-TrkB IgG or anti-TrkB-Fc administration at 9 h after the acquisition trial had no effect on memory consolidation by itself as shown in K252a study, but they significantly attenuated the enhanced memory consolidation induced by bicuculline methiodide administration at 1 h after the acquisition trial [(Anti-TrkB IgG: Vehicle/control IgG, 97.5±20.2, n=10; vehicle/anti-TrkB IgG, 109.8±20.1, n=10; bicuculline methiodide/control IgG, 218.1±36.7, n=10; bicuculline methiodide/anti-TrkB IgG, 106.4±19.9, n=9; F(3, 35)=5.072, P<0.05, Figure 5c), (Anti-TrkB-Fc: Vehicle/control IgG, 130.3±8.4, n=7; vehicle/anti-TrkB-Fc, 122.8±8.4, n=8; bicuculline methiodide/control IgG, 239.1±19.3, n=8; bicuculline methiodide/anti-TrkB-Fc, 146.6±21.9, n=8; F(3, 27)=8.371, P<0.05, Supplementary Figure S10)]. These results suggested that the increased mBDNF level induced by bicuculline methiodide at 9 h after the acquisition trial functions through interactions with its TrkB receptor and enhances memory consolidation through protein synthesis after the mBDNF-TrkB receptor interaction.
c-Fos and Zif268 are Possible Downstream Effectors of mBDNF Induced by Bicuculline Methiodide
c-Fos is an immediate early gene (IEG) associated with synaptic activity and memory processing (Cammarota et al, 2000; Countryman et al, 2005; Yasoshima et al, 2006). Another IEG that has been consistently implicated in synaptic plasticity and memory processing is Zif268 (Malkani et al, 2004; Worley et al, 1993). Given that c-Fos and Zif268 expression can be regulated by BDNF (Glorioso et al, 2006) and induced by BDNF in hippocampal neurons (Alder et al, 2003), we asked if blockade of protein synthesis or endogenous mBDNF binding to its receptor at 9 h after the acquisition trial affects c-Fos and/or Zif268 expression. A learning-associated increase in c-Fos or Zif268 immunoreactivity in hippocampal homogenates 12 h after the acquisition trial was not detected. This result suggests that both c-Fos and Zif268 have returned to basal levels at 12 h after the acquisition trial. However, we observed that the higher c-Fos expression levels in the hippocampus of mice receiving bicuculline methiodide 1 h after the acquisition trial was completely abolished by anisomycin [Vehicle/vehicle, 145% of naïve group, n=4; vehicle/anisomycin, 138% of naïve group, n=4; bicuculline/vehicle, 230% of naïve group, n=4; bicuculline/anisomycin, 127% of naïve group, n=4; F(4, 15)=5.786, P<0.05, Figure 6a] or K252a [Vehicle/vehicle, 134% of naïve group, n=4; vehicle/K252, 135% of naïve group, n=4; bicuculline methiodide/vehicle, 215% of naïve group, n=4; bicuculline methiodide/K252a, 123% of naïve group, n=4; F(4, 15)=5.786, P<0.05, Figure 6b] infusion 9 h after the acquisition trial. Moreover, we also observed similar results with Zif268 expression levels in anisomycin [Vehicle/vehicle, 145% of naïve group, n=4; vehicle/anisomycin, 121% of naïve group, n=4; bicuculline/vehicle, 208% of naïve group, n=4; bicuculline/anisomycin, 120% of naïve group, n=4; F(4, 15)=9.310, P<0.05, Figure 6a] or K252a [Vehicle/vehicle, 148% of naïve group, n=4; vehicle/K252a, 141% of naïve group, n=4; bicuculline methiodide/vehicle, 220% of naïve group, n=4; bicuculline methiodide/K252a, 127% of naïve group, n=4; F(4, 15)=4.764, P<0.05, Figure 6b] infusion. These results were also confirmed by immunohistochemistry for c-Fos [anisomycin, F(4, 13)=4.679, P<0.05, Supplementary Figure S11; K252a, F(4, 15)=4.074, P<0.05, Supplementary Figure S12] and Zif268 [anisomycin, F(4, 13)=5.321, P<0.05, Supplementary Figure S11; K252a, F(4, 15)=4.825, P<0.05, Supplementary Figure S12] expressions in the hippocampal CA1 region. Moreover, the number of c-Fos and Zif268 double-positive cells in the hippocampal CA1 region revealed similar result [anisomycin, F(4, 13)=3.903, P<0.05, Supplementary Figure S11; K252a, F(4, 15)=5.831, P<0.05, Supplementary Figure S12]. In receptor-ligand binding blockade study, anti-TrkB IgG administration blocked bicuculline methiodide-induced increases of c-Fos and Zif268 levels in the hippocampus [c-Fos, F(3, 12)=37.35, P<0.05; Zif268, F(3, 12)=13.07, P<0.05] (Figure 6c). In addition, we also observed that the higher c-Fos and Zif268 levels in the hippocampus of mice receiving hrBDNF 9 h after the acquisition trial were completely blocked by K252a co-administration [c-Fos, F(3, 12)=21.51, P<0.05; Zif268, F(3, 12)=8.762, P<0.05] (Figure 6d). Thus, inhibition of the TrkB receptor 9 h after the acquisition trial attenuated enhanced memory consolidation induced by bicuculline methiodide.
Figure 6.
Blockade of protein synthesis or tyrosine receptor kinase B (TrkB) attenuates the increase in immediate early gene expressions (c-Fos or Zif268) induced by bicuculline methiodide (BMI). Effect of blockade of protein synthesis (a) or TrkB receptor signaling (b and c) on the BMI-induced increase in c-Fos and Zif268 expressions. BMI (5 mg/kg, i.p.) or vehicle (Veh) was administered 1 h after the acquisition trial (AT) and anisomycin (Ani, 80 μg/0.5 μl/side) or vehicle was infused into hippocampal CA1 region 9 h after the acquisition trial (a). In experiment b and c, BMI (5 mg/kg, i.p.) or vehicle (Veh) was administered 1 h after the acquisition trial, and K252a (100 pmol/μl/side, b) or anti-TrkB IgG (anti-TrkB, 1 μg/0.5 μl/side, c) was infused into hippocampal CA1 region 9 h after the acquisition trial. The mice were sacrificed 12 h after the acquisition trial. d, Effect of Trk inhibition on hrBDNF-induced increase in c-Fos and Zif268 expression. Vehicle, K252a (100 pmol/0.5 μl/side), hrBDNF (0.2 μg/0.5 μl/side), or K252a +hrBDNF was infused into hippocampal CA1 region 9 h after the acquisition trial. The mice were sacrificed 12 h after the acquisition trial. Data are presented as means±SEM (n=3–4/group). *P<0.05, compared with the control group; #P<0.05, compared with the BMI-treated group (two-way ANOVA followed by Tukey's post hoc test). The naïve group was not treated with any drug or acquisition trial.
DISCUSSION
Pharmacological studies have demonstrated that post-training injections of GABAergic compounds modulate memory storage (Hatfield et al, 1999). These findings provide strong support for the view that GABAA receptors modulate post-training processes underlying memory consolidation (Brioni and McGaugh, 1988; Castellano and McGaugh, 1990). Luft et al, (2004) reported that bicuculline causes memory facilitation when infused into CA1 either immediately after training or 1.5 h post-training. Similarly, bicuculline improves memory consolidation in an invertebrate model using the crab Chasmagnathus (Carbo Tano et al, 2009). Consistent with these previous studies, we observed that bicuculline methiodide enhanced memory consolidation when systemically administered immediately or 1 h after the acquisition trial but not at 3 h after, suggesting that GABAA receptor blockade enhances memory consolidation in the one-trial passive avoidance task. However, the reasons why the effective time window for the enhancement of memory consolidation exists and the identity of signaling molecule(s) involved in bicuculline methiodide-induced enhancement of memory consolidation have remained unclear.
BDNF has been extensively studied for synaptic plasticity and memory processing, and it has been demonstrated to be essential for hippocampus-dependent long-term memory consolidation (Bekinschtein et al, 2007; Bekinschtein et al, 2008; Kang et al, 1997; Pang and Lu, 2004a; Slipczuk et al, 2009; Tyler et al, 2002). By the acquisition trial without any pharmacological manipulation, we observed that mBDNF levels rapidly increased after the acquisition trial, gradually decreased until 9 h after the acquisition trial and then increased 12 h after the acquisition trial. The mBDNF level 9 h after the acquisition trial was the lowest level observed during the 12 h after the acquisition trial, and it was almost the same as the normal level, as observed by others (Bekinschtein et al, 2007). However, it is unclear how the mBDNF levels were markedly increased immediately after the acquisition trial. BDNF is basically synthesized in a pro-form, proBDNF, which can be cleaved into mBDNF by proteases (Matsumoto et al, 2008; Seidah et al, 1996) in the intracellular or in the extracellular space (Pang et al, 2004b). Recently, it was reported that the release of proBDNF or mBDNF depends on the mode of stimulation. For instance, low-frequency stimulation induces proBDNF release at excitatory synapses (Woo et al, 2005), whereas high-frequency stimulation favors mBDNF release in the hippocampal CA1 (Nagappan et al, 2009; Yang et al, 2009). It is also possible that mBDNF is produced from proBDNF within minutes, similar to the rapid conversion from pro- to mature-form of BDNF in vitro after high frequency stimulation or KCl stimulation (Nagappan et al, 2009). Therefore, it can be speculated that the acquisition training might cause rapid release of mBDNF and conversion of proBDNF to mBDNF. However, to unravel these hypotheses, further research will be needed. In naïve mice, mBDNF levels were significantly higher 9 and 12 h after bicuculline methiodide administration, which is somewhat unusual regarding absorption time (<20 min) and half-life (<1 h) of bicuculline methiodide (Gale and Casu, 1981; Mares et al, 2000). Considering the temporal profile of mBDNF levels in training trial-treated mice without any drug administration and the temporal profile of mBDNF levels induced by bicuculline methiodide treatment without the training trial, the effective time window of bicuculline methiodide after the acquisition trial might be dependent on the mBDNF level at around 9 h after the acquisition trial. The above possibilities can be deduced as follows; the increased mBDNF level 9 h after bicuculline methiodide administration could compensate for the low level of mBDNF in the acquisition trial-treated mice without any drug administration, which might keep the mBDNF level in the hippocampus above a threshold required to enhance the consolidation of the acquired memory. To test this possibility, we compared the effects of bicuculline methiodide administration 1 or 3 h after the acquisition trial on mBDNF levels at around 9 h after the acquisition trial. The levels of mBDNF 9 h after the acquisition trial were enhanced by the administration of bicuculline methiodide 1 h but not 3 h after the acquisition trial. Similar results were also observed with the intra-hippocampal administration of bicuculline methiodide 1 h after the acquisition trial. These results suggest that the level of mBDNF 9 h after the acquisition trial is critically involved in the enhancement of memory consolidation induced by bicuculline methiodide and this represents the effective time window of bicuculline methiodide.
For the confirmation of the role of mBDNF, we used exogenous hrBDNF infusion to increase the BDNF levels at 6, 9 or 12 h after the acquisition trial. Indeed, intra-hippocampal hrBDNF infusion both 6 and 9 h after the acquisition trial increased the latency time of retention trial conducted 24 h after the acquisition trial. Bekinschtein et al, (2007) reported that late protein synthesis and BDNF expression around 12 h after training is required for the persistence of long-term memory storage but not for formation. Similar results were obtained regarding the persistence of fear memory (Ou et al, 2010). However, we did not observe the enhancement of memory consolidation by the exogenous administration of hrBDNF 12 h after the training although the mBDNF level at 12 h was similar to that at 6 h. In addition, even though mBDNF level at 12 h after the acquisition trial was enhanced by the administration of bicuculline methiodide 3 h after that trial, we did not observe any enhancement of memory consolidation. Therefore, it is likely that the increased mBDNF level 12 h after the acquisition trial is not required for the enhancement of memory consolidation retrieved 24 h after the acquisition training. However, it might be asked why hrBDNF administration 6 h after the acquisition trial enhances memory consolidation. Anisomycin alone treatment 3 or 6 h after the acquisition trial inhibited memory consolidation, whereas the same dose of anisomycin did not alter the latency time when administered 9 h after the acquisition trial. Similar results were also observed in the cycloheximide experiments. These results mean that bicuculline methiodide reverses the basal process for memory consolidation inhibited by 3 or 6 h post-training administration of anisomycin and that the level of mBDNF induced by learning at 6 h might be sufficient to consolidate acquired memory. There are several reports indicating the existence of two time windows in which long-term memory formation is sensitive to protein synthesis inhibitors: around the time of training and 3–6 h after training (Alonso et al, 2002b; Grecksch and Matthies, 1980; Igaz et al, 2002). Taken together, it is likely that the basal process of memory consolidation occurs 3–6 h after the training, and this might be enhanced by exogenous hrBDNF administration, but is inhibited by the inhibition of protein synthesis.
From the anisomycin and cycloheximide experiments with bicuculline methiodide, it can be concluded that the mBDNF level 9 h after the acquisition trial plays a crucial role in the enhancement of memory consolidation. However, it is unclear how increasing the mBDNF level 9 h after the acquisition trial enhances memory consolidation. That anisomycin administration 9 h after the acquisition trial reverts bicuculline methiodide-induced enhancement of memory consolidation to the basal level implies that protein synthesis after the binding of mBDNF to its receptor might be inhibited by anisomycin. To confirm this possibility, we used K252a and anti-TrkB IgG to inhibit the mBDNF-TrkB receptor interaction. By the administration of those drugs at 9 h after training with bicuculline methiodide (1 h post-training intraperitoneally or intra-hippocampal administration), the enhancing effects of bicuculline methiodide on memory consolidation were blocked. Moreover, the enhancement of memory consolidation induced by hrBDNF infusion into the hippocampus was totally blocked by the co-administration of anisomycin into the hippocampus 9 h after the acquisition trial, suggesting that downstream signaling after the binding of mBDNF to its receptor might contribute to the mBDNF-induced enhancement of memory consolidation. Our results do not exclude a possibility that bicuculline methiodide-induced memory consolidation might be mediated through other mechanisms. For example, bicuculline methiodide-induced increase of neuronal activity may be involved in the facilitation of memory consolidation, because a high dose of bicuculline methiodide causes seizure-like behaviors. Moreover, muscimol, which is often used for the inhibition of neuronal activity (Maren and Hobin, 2007; Oliveira et al, 2010), impaired memory consolidation and blocked the effect of bicuculline methiodide on memory consolidation. Dissecting the effect of bicuculline methiodide on neuronal activity related with memory consolidation shall be the focus of future investigations.
c-Fos and zif268 are immediate early genes regulated by mBDNF (Glorioso et al, 2006) and their gene products have roles in memory consolidation (Jones et al, 2001; Yasoshima et al, 2006). The expression levels of c-Fos and Zif268 were enhanced by the administration of bicuculline methiodide 1 h after the acquisition trial or hrBDNF 9 h after the acquisition trial. Concomitantly, these increases were reverted to the control level by the inhibition of either TrkB receptor or protein synthesis. Therefore, the ability of K252a, anti-TrkB IgG, anisomycin, or cycloheximide to block the enhancing effects of bicuculline methiodide on memory consolidation is dependent on the inhibition of protein synthesis occuring after the binding of mBDNF to its receptor TrkB. Thus, both an increase in mBDNF level at 9 h after the acquisition trial and mBDNF binding to its receptor are necessarily required to enhance the memory consolidation.
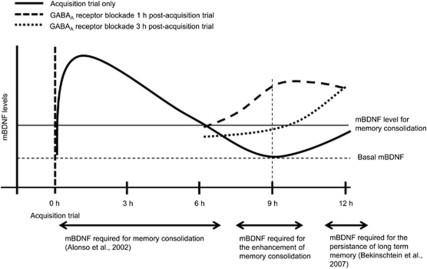
It has been reported that there is an optimal range of mBDNF levels for the enhancement of learning and memory (Cunha et al, 2009; Nakajo et al, 2008). The 1 h post-administration experiments either with systemical or intra-hippocampal injection of bicuculline methiodide, the experiments with exogenous administration of hrBDNF 9 h after the acquisition trial, and the anisomycin experiments with bicuculline methiodide or hrBDNF all support those findings. In addition, the present experiments also suggest that the level of mBDNF 9 h after training, which can be increased by GABAA receptor blockade, is critically involved in the enhancement of memory consolidation. Thus, the present results suggest that if the mBDNF level at 9 h after an acquisition trial is similar to or over the level at 6 h after the acquisition trial, memory consolidation will be enhanced (Figure 7). If, however, the mBDNF level at 12 h after the acquisition trial is similar to or over the level at 6 h after the acquisition trial, even if it is needed for the persistence of long-term memory storage (Bekinschtein et al, 2007), memory consolidation will not be enhanced.
Figure 7.
Schematic representation of the effect of GABAA receptor blockade in memory consolidation. GABAA receptor blockade enhanced memory consolidation and induced an increase in mBDNF levels 9 h after training. The increased mBDNF levels from 0 to 6 h, around 9 h, and around 12 h after training play an important role in the basal memory consolidation (Alonso et al, 2002a, 2002b), the enhancement of memory consolidation, and the persistence of long-term memory (Bekinschtein et al, 2007), respectively, in the one-trial passive avoidance task.
In summary, we found that GABAA receptor blockade enhanced memory consolidation and induced an increase in mBDNF levels at 9 h after training. In addition, we found that the increased mBDNF levels at 9 h after training play an important role in the enhancement of memory consolidation in the one-trial passive avoidance task. Taken together, these results suggest that blockade of GABAA receptor can enhance memory consolidation in the one-trial passive avoidance task by increasing hippocampal mBDNF levels within restricted time window (around 9 h after training).
Acknowledgments
This work was supported by the Korean Research Foundation Grant funded by the Korean Government (MOEHRD), (KRF-313-E00123).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23:10800–10808. doi: 10.1523/JNEUROSCI.23-34-10800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002a;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell Mol Neurobiol. 2002b;22:663–674. doi: 10.1023/A:1021848706159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Farr SA, La Scola ME, Morley JE. Intravenous human interleukin-1alpha impairs memory processing in mice: dependence on blood-brain barrier transport into posterior division of the septum. J Pharmacol Exp Ther. 2001;299:536–541. [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioni JD, McGaugh JL. Post-training administration of GABAergic antagonists enhances retention of aversively motivated tasks. Psychopharmacology (Berl) 1988;96:505–510. doi: 10.1007/BF02180032. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, et al. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: abolition by NMDA receptor blockade. Mol Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- Carbo Tano M, Molina VA, Maldonado H, Pedreira ME. Memory consolidation and reconsolidation in an invertebrate model: the role of the GABAergic system. Neuroscience. 2009;158:387–401. doi: 10.1016/j.neuroscience.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL. Retention enhancement with post-training picrotoxin: lack of state dependency. Behav Neural Biol. 1989;51:165–170. doi: 10.1016/s0163-1047(89)90797-8. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL. Effects of post-training bicuculline and muscimol on retention: lack of state dependency. Behav Neural Biol. 1990;54:156–164. doi: 10.1016/0163-1047(90)91352-c. [DOI] [PubMed] [Google Scholar]
- Countryman RA, Kaban NL, Colombo PJ. Hippocampal c-fos is necessary for long-term memory of a socially transmitted food preference. Neurobiol Learn Mem. 2005;84:175–183. doi: 10.1016/j.nlm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Cunha C, Angelucci A, D'Antoni A, Dobrossy MD, Dunnett SB, Berardi N, et al. Brain-derived neurotrophic factor (BDNF) overexpression in the forebrain results in learning and memory impairments. Neurobiol Dis. 2009;33:358–368. doi: 10.1016/j.nbd.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Gale K, Casu M. Dynamic utilization of GABA in substantia nigra: regulation by dopamine and GABA in the striatum, and its clinical and behavioral implications. Mol Cell Biochem. 1981;39:369–405. doi: 10.1007/BF00232586. [DOI] [PubMed] [Google Scholar]
- Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11:633–648. doi: 10.1038/sj.mp.4001835. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Matthies H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Res. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Stackman RW, Walsh TJ. Intraseptal flumazenil enhances, while diazepam binding inhibitor impairs, performance in a working memory task. Neurobiol Learn Mem. 1996;66:341–352. doi: 10.1006/nlme.1996.0074. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Vianna MR, Medina JH, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, da Silva WC, Bonini J, Medina JH, et al. The molecular cascades of long-term potentiation underlie memory consolidation of one-trial avoidance in the CA1 region of the dorsal hippocampus, but not in the basolateral amygdala or the neocortex. Neurotox Res. 2008;14:273–294. doi: 10.1007/BF03033816. [DOI] [PubMed] [Google Scholar]
- Jiang B, Akaneya Y, Hata Y, Tsumoto T. Long-term depression is not induced by low-frequency stimulation in rat visual cortex in vivo: a possible preventing role of endogenous brain-derived neurotrophic factor. J Neurosci. 2003;23:3761–3770. doi: 10.1523/JNEUROSCI.23-09-03761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Inaguma Y, Ichisaka S, Hata Y, Tsumoto T, et al. Induction of brain-derived neurotrophic factor by convulsant drugs in the rat brain: involvement of region-specific voltage-dependent calcium channels. J Neurochem. 2001;77:71–83. doi: 10.1046/j.1471-4159.2001.t01-1-00138.x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Ryu JH. Differential effects of scopolamine on memory processes in the object recognition test and the Morris water maze test in mice. Biomol Ther. 2008;16:173–178. [Google Scholar]
- Lado FA, Laureta EC, Moshe SL. Seizure-induced hippocampal damage in the mature and immature brain. Epileptic Disord. 2002;4:83–97. [PubMed] [Google Scholar]
- Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90:81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Luft T, Pereira GS, Cammarota M, Izquierdo I. Different time course for the memory facilitating effect of bicuculline in hippocampus, entorhinal cortex, and posterior parietal cortex of rats. Neurobiol Learn Mem. 2004;82:52–56. doi: 10.1016/j.nlm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11:617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares P, Chino M, Kubova H, Mathern P, Veliky M. Convulsant action of systemically administered glutamate and bicuculline methiodide in immature rats. Epilepsy Res. 2000;42:183–189. doi: 10.1016/s0920-1211(00)00179-0. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, et al. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci USA. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14:117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappan G, Zaitsev E, Senatorov VV., Jr, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci USA. 2009;106:1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo Y, Miyamoto S, Nakano Y, Xue JH, Hori T, Yanamoto H. Genetic increase in brain-derived neurotrophic factor levels enhances learning and memory. Brain Res. 2008;1241:103–109. doi: 10.1016/j.brainres.2008.08.080. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LC, Yeh SH, Gean PW. Late expression of brain-derived neurotrophic factor in the amygdala is required for persistence of fear memory. Neurobiol Learn Mem. 2010;93:372–382. doi: 10.1016/j.nlm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004a;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004b;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ.2001The mouse brain in stereotaxic coordinates2nd edn.Academic Press: San Diego, CA, USA [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhang FJ, Xue QS, Zhao X, Yu BW. Bilateral inhibition of gamma-aminobutyric acid type A receptor function within the basolateral amygdala blocked propofol-induced amnesia and activity-regulated cytoskeletal protein expression inhibition in the hippocampus. Anesthesiology. 2008;109:775–781. doi: 10.1097/ALN.0b013e31818a37c4. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, et al. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima Y, Sako N, Senba E, Yamamoto T. Acute suppression, but not chronic genetic deficiency, of c-fos gene expression impairs long-term memory in aversive taste learning. Proc Natl Acad Sci USA. 2006;103:7106–7111. doi: 10.1073/pnas.0600869103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.